Abstract
Purpose
To determine if single nucleotide polymorphisms (SNPs) of the Corticotrophin-Releasing Hormone Binding Protein (CRHBP, rs10055255) and CRH receptor-Type 1 (CRHR1, rs1876831) were associated with posttraumatic stress disorder (PTSD) and depressive symptoms following medical-surgical intensive care unit (ICU) hospitalization.
Materials and Methods
We extracted DNA for genotyping from saliva samples of 93 ICU patients enrolled in a prospective cohort investigation. Follow-up interviews conducted 3 and 12-months post-ICU included assessment of PTSD symptoms with the PTSD Checklist-Civilian Version and depressive symptoms with the Patient Health Questionnaire-9.
Results
Homozygosity for the CRHBP rs10055255 T allele was associated with significantly fewer post-ICU PTSD (beta: −10.8, 95%Confidence Interval [95%CI]: −17.7, −3.9; P = 0.002) and depressive symptoms (beta: −3.7, 95%CI: −6.7, −0.7; P = 0.02). Carrying a CRHR1 rs1876831 C allele was associated with significantly more post-ICU depressive symptoms compared to T/T homozygotes (C/T heterozygtes: beta: 6.9, 95%CI: 1.2, 12.6; P = 0.02; C/C homozygotes: beta: 5.8, 95%CI: 0.2, 11.3, P = 0.04). These associations remained significant after adjustment for age, race, illness severity, and in-ICU steroid exposure.
Conclusions
Despite a small sample size, our findings suggest a potential role for genetic variants of CRHBP and CRHR1 in the development of post-ICU psychiatric morbidity.
Keywords: critical care, posttraumatic stress disorder, depression, corticotrophin-releasing hormone binding protein, corticotrophin-releasing hormone receptor-type 1
INTRODUCTION
Advances in critical care medicine have led to increasing survival rates for the millions of patients hospitalized annually in intensive care units (ICUs) for the treatment of critical illnesses [1]. As interest has grown in quality of survivorship following critical illnesses, an emerging body of literature has established that critical illness survivors may face substantial mental health morbidities. Three systematic reviews of 24 studies of general ICU and acute respiratory distress syndrome (ARDS) survivors have identified that 22% and 28% of critical illness survivors have substantial posttraumatic stress disorder (PTSD) and depressive symptoms, respectively [2–4]. High rates of these psychiatric disorders among critical illness survivors are an important public health concern since PTSD and major depression have been shown to be independently associated with risk of adverse medical outcomes and increased healthcare costs [5–7].
Although psychiatric morbidity in critical illness survivors has become increasingly recognized, relatively little is known about the etiology of these adverse post-ICU outcomes. Increased understanding of the mechanisms by which psychiatric disorders may develop in the aftermath of critical illnesses could lead to the development of candidate biomarkers that may identify patients at greatest risk for these outcomes.
One potential common pathway between critical illnesses and psychiatric disorders such as PTSD and major depression is the hypothalamic-pituitary-adrenal (HPA) axis [2]. Critical illnesses have been shown to induce multiple changes in cortisol homeostasis across all aspects of the HPA axis [8]. Furthermore, HPA axis hyperactivity, particularly involving corticotrophin-releasing hormone (CRH), has been theorized to play a key role in the etiology of mood and anxiety disorders [9]. Single nucleotide polymorphisms (SNPs) of the Corticotrophin-Releasing Hormone Receptor Type 1 (CRHR1) gene have been shown to be associated with risk of PTSD and major depression in the context of extreme stress [10, 11]. In addition, SNPs of the Corticotrophin-Releasing Hormone Binding Protein (CRHBP) gene have been shown to be associated with antidepressant response and remission of depressive symptoms [9]. However, no studies in non-injured critical illness survivors have examined the role that these important genetic variants may play in the pathogenesis of post-ICU PTSD and depressive symptoms.
The present longitudinal pilot investigation sought to determine if the CRHBP (rs10055255) and CRHR1 (rs1876831) SNPs were associated with risk of PTSD and depressive symptoms following medical-surgical intensive care unit (ICU) hospitalization. We hypothesized that these SNPs would have significant associations with risk of both post-ICU PTSD and depressive symptoms, and that these associations would remain present after controlling for age, race and illness severity at ICU admission.
METHODS
Study Setting and Participants
Our study cohort came from a larger prospective investigation of psychiatric and cognitive outcomes following medical-surgical ICU admission. The details of the parent study have been previously described [12]. Briefly, 150 patients admitted to an ICU for over 24 hours were prospectively recruited between September 2010 and July 2011. Key exclusion criteria were: 1) initial admission diagnosis of traumatic injury; 2) pre-existing cognitive impairment or dementia diagnosis noted in the medical record; 3) communication/language barrier; 4) ICU length of stay ≤24 hours; 5) pre-existing medical illness with life-expectancy of < 12 months; and 6) admission for a suicide attempt. The study protocol was approved by the UW Institutional Review Board, and all participants provided informed consent for all aspects of the study protocol prior to enrollment.
The present study included 93 patients who provided saliva samples for DNA analyses. Saliva samples were obtained from consented patients prior to hospital discharge. There were no significant differences in baseline or clinical characteristics between the 93 patients who provided saliva samples and the other 57 patients from the parent study that did not provide a sample. Enrolled patients completed an in-person interview prior to hospital discharge and were re-interviewed via telephone at 3 and 12 months post-ICU.
Measurements and Assessments
PTSD Symptoms
PTSD symptoms at 3 and 12-months post-ICU were assessed with the PTSD Checklist-civilian version (PCL-C) [13]. The PCL-C includes questions regarding 5 symptoms in the intrusive symptom cluster (e.g., intrusive thoughts, nightmares), 7 symptoms in the avoidant symptom cluster (e.g., avoidance of thoughts or activities that remind the patient of the stressor, emotional numbing), and 5 symptoms in the arousal symptom cluster (e.g., impaired sleep, hypervigilance), and symptom severity is rated on a 5 point Likert scale [13]. Substantial PTSD symptoms can be ascertained with the PCL-C by following an algorithm that considers a score of 3 or more on at least 1 intrusive symptom, 3 avoidant symptoms, and 2 arousal symptoms as consistent with DSM-IV diagnostic criteria [13].
Depressive Symptoms
Depressive symptoms at 3 and 12-months post-ICU were assessed with the Patient Health Questionnaire-9 (PHQ-9) [14]. We defined substantial depressive symptoms as a PHQ-9 score ≥10. The PHQ-9 threshold score of 10 or more for a probable case of major depression has been found to have high sensitivity (88%) and specificity (88%) for the diagnosis of major depression compared to a structured psychiatric interview [14].
Patient and ICU-related Characteristics
Baseline patient characteristics and ICU clinical factors were obtained through medical record review and in-person interviews. Medical record-obtained characteristics included demographics (e.g., age, sex, race); ICU admission diagnosis; baseline medical comorbidity information to compute a Charlson Comorbidity Score [15]; illness severity measures at ICU admission to compute a Simplified Acute Physiology Score II (SAPS II) [16]; ICU length of stay; requirements for mechanical ventilation and duration of ventilation; requirement for major surgery or blood product transfusion; days of in-ICU exposure to benzodiazepine, opioid, and corticosteroid medications; presence of delirium in-ICU per nursing documented assessment using the Confusion Assessment Method-ICU (CAM-ICU) [17]; and presence of confusion/disorientation/difficulty following commands in critical care nursing documentation. We defined probable delirium as a documented positive CAM-ICU assessment in the ICU or nursing documented presence of confusion/disorientation/difficulty following commands at any point in the ICU.
Additional patient characteristics obtained from the baseline interviews included demographic data not obtained from medical records (e.g., marital/partnered status, education); assessment of prior trauma exposure with the National Comorbidity Survey-Replication Trauma History Screen [18] and lifetime history of major depression with the MINI International Neuropsychiatric Interview (MINI) major depressive episode module [19].
Sample Collection and Genotyping
Saliva samples were obtained from patients at the time of the in-hospital baseline interview using Oragene saliva collection kits (DNA Genotek, Ontario, Canada), and extracted following the manufacturer’s protocol. DNA samples were quantified and checked for quality on a NanoDrop instrument (Thermo Scientific, DE, USA). SNPs were genotyped using a StepOnePlus Real-Time PCR System and TaqMan SNP Genotyping Assays (Applied Biosystems, CA, USA). 50ng genomic DNA was amplified in the presence of gene-specific primers and allele-specific fluorescent probes following the manufacturer’s instructions. Genotypes were called using TaqMan Genotyper software.
Statistical Analysis
We present descriptive data as medians and interquartile ranges (IQRs) or proportions. We used χ2-tests to examine if there were deviations from Hardy-Weinberg equilibrium among the CRHBP (rs10055255) and CRHR1 (rs1876831) SNPs and found no deviations. To examine if there were significant differences in patient or clinical characteristics by CRHBP or CRHR1 genotype, we used χ2-tests or Fisher’s Exact tests for categorical variables and non-parametric K-sample equality-of-medians tests for continuous variables.
In order to test our hypotheses of interest, we used mixed-model linear regression analyses. We constructed separate general genetic models for CRHBP and CRHR1. In our analyses we retained the three distinct genotype classes for each gene as independent categorical variables, i.e., making no assumptions about how the risk for heterozygotes compares with the two homozygotes. Dependent variables were the repeated measures of PTSD symptoms and depressive symptoms in two separate regression models. Initially, we tested the associations of the CRHBP and CRHR1 genotypes with our outcomes of interest without adjustment. We then repeated our regressions and adjusted for age, race, and SAPS II scores. We implemented our regression analyses using xtmixed in STATA 12 (Stata Corporation, College Station, TX). For our primary analyses, we used two-sided significance tests for all analyses with statistical significance set at a P value of 0.05.
RESULTS
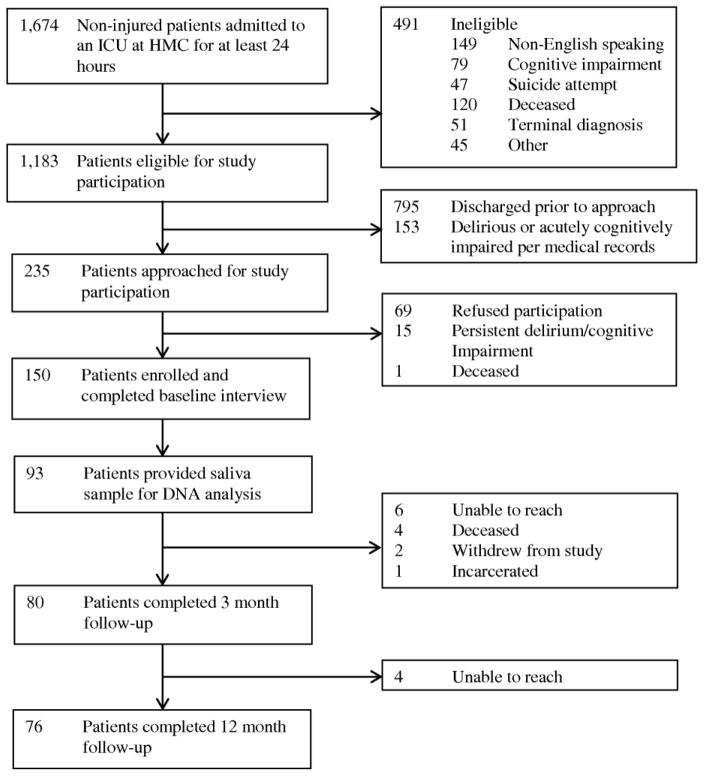
Of the 93 patients who provided saliva samples during their hospitalization, 76 (82%) completed a 12-month telephone follow-up interview (Figure 1). Table 1 presents the baseline and clinical characteristics of patients who provided saliva samples. Their median age was 50.0 (IQR: 37.5, 58.0) and slightly over half were male. Nearly one-third had a lifetime history of major depression based on the MINI. Patients’ median ICU length of stay was 5.0 days (IQR: 3.0, 9.0). Half required mechanical ventilation during their ICU admission with a median duration of 2.0 days (IQR: 1.0, 4.0). There were no significant differences among patient baseline or clinical characteristics by CRHBP or CRHR1 genotype except for a significant difference in days of ICU corticosteroid exposure by CRHBP genotype (χ2 = 7.78, degrees of freedom = 2, P = 0.02). Patients homozygous for the CRHBP (rs10055255) T allele received corticosteroids in the ICU for a median of 2 days (IQR: 0, 4) compared to a median of 0 days (IQR: 0, 2) for A/T heterozygotes and 0 days (IQR: 0, 0) for A homozygotes.
Figure 1.
Study Flow Diagram
Table 1.
Baseline and clinical characteristics of medical-surgical intensive care unit survivors participating in genetic study
| Variables | N = 93 |
|---|---|
| Baseline patient characteristics | |
|
| |
| Age | 50.0 (37.5, 58.0) |
| Female | 40 (43.0%) |
| Non-white | 25 (26.9%) |
| < High school graduate | 14 (15.1%) |
| Married/Partnered | 46 (49.5%) |
| Lifetime major depression | 30 (32.3%) |
| Prior traumatic event exposures | 4.0 (2.0, 6.0) |
| Charlson Comorbidity Score | 1.0 (0, 3.0) |
|
| |
| ICU clinical characteristics | |
|
| |
| ICU LOS (days) | 5.0 (3.0, 9.0) |
| SAPS II | 24.0 (15.0, 40.0) |
| Admission Diagnosis | |
| Cardiovascular | 8 (8.6%) |
| Pulmonary | 15 (16.1%) |
| Infectious Disease | 26 (28.0%) |
| Neurologic | 26 (28.0%) |
| Vascular Surgery | 11 (11.8%) |
| Gastrointestinal | 11 (11.8%) |
| Endocrine/Renal | 4 (4.3%) |
| Orthopedic | 5 (5.4%) |
| Oncologic | 2 (2.2%) |
| Other | 4 (4.3%) |
| Mechanical ventilation | 47 (50.5%) |
| Duration of mechanical ventilation (days) | 2.0 (1.0, 4.0) |
| Major surgery | 40 (43.0%) |
| Blood product transfusion | 23 (24.7%) |
| Probable delirium in ICU | 19 (20.4%) |
| Days of benzodiazepines | 1.0 (0, 3.0) |
| Days of opioids | 4.0 (2.0, 6.0) |
| Days of corticosteroids | 0 (0, 1.0) |
All values are medians (IQRs) or N (%) unless otherwise indicated.
Abbreviations (in alphabetic order): ICU = intensive care unit; LOS = length of stay; SAPS II = Simplified Acute Physiology Score II.
At 3 months post-discharge from the ICU (median: 93.5 days, IQR: 85.0, 107.0), 17% (95%CI: 9%, 25%) of patients in the sub-cohort had substantial PTSD symptoms, while 34% (95%CI: 24%, 45%) had substantial depressive symptoms. One year post-ICU (median: 354.0 days, IQR: 345.0, 364.0), the prevalence of substantial PTSD symptoms decreased to 14% (95%CI: 6%, 21%), while the prevalence of substantial depressive symptoms decreased to 17% (95%CI: 9%, 26%).
Table 2 illustrates the genotype frequencies for CRHBP (rs10055255) and CRHR1 (rs1876831) SNPs by 3 and 12-month post-ICU substantial PTSD and depressive symptoms. No patients with either substantial PTSD or depressive symptoms at either follow-up time point were homozygous for the CRHBP T allele. Twenty-five (33%) patients and 13 (17%) patients with substantial depressive symptoms at 3 and 12-months post-ICU had at least one CRHR1 C allele. Twelve (16%) patients and 11 (14%) patients with substantial PTSD symptoms at 3 and 12-months post-ICU had at least one CRHR1 C allele.
Table 2.
Distribution of genotype frequencies for CRHBP(rs1005525) and CRHR1(rs1876831) by 3 and 12-month post-ICU posttraumatic stress disorder and depression status
| Polymorphism | Genotype | Genotype Frequency (n) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 3-month substantial PTSD symptoms | 3-month substantial depressive symptoms | 12-month substantial PTSD symptoms | 12-month substantial depressive symptoms | ||||||
| Yes | No | Yes | No | Yes | No | Yes | No | ||
| CRHBP(rs1005525) (n = 74) | A/A | 7 | 23 | 9 | 21 | 6 | 24 | 4 | 26 |
| A/T | 6 | 30 | 15 | 21 | 5 | 30 | 9 | 26 | |
| T/T | 0 | 8 | 0 | 8 | 0 | 8 | 0 | 8 | |
|
| |||||||||
| CRHR1(rs1876831) (n = 76) | T/T | 1 | 1 | 0 | 2 | 0 | 2 | 0 | 2 |
| C/T | 5 | 17 | 8 | 14 | 4 | 19 | 4 | 19 | |
| C/C | 7 | 45 | 17 | 35 | 7 | 43 | 9 | 41 | |
Abbreviations (in alphabetic order): CRHBP = Corticotrophin Releasing Hormone Binding Protein; CRHR1 = Corticotrophin
Releasing Hormone Receptor; PTSD = posttraumatic stress disorder.
In unadjusted analyses, being homozygous for the CRHBP (rs10055255) T allele was associated with significantly fewer post-ICU PTSD (beta: −10.8, 95%Confidence Interval [95%CI]: −17.7, −3.9; P = 0.002) and depressive symptoms (beta: −3.7, 95%CI: −6.7, −0.7; P = 0.02) (Table 2). Carrying a CRHR1 (rs1876831) C allele was associated with significantly more post-ICU depressive symptoms compared to T/T homozygotes (C/T heterozygtes: beta: 6.9, 95%CI: 1.2, 12.6; P = 0.02; C/C homozygotes: beta: 5.8, 95%CI: 0.2, 11.3, P = 0.04), though this SNP was not associated with post-ICU PTSD symptoms (C/T heterozygotes: beta: 3.2, 95%CI: −9.8, 16.1; P = 0.63; C/C homozygotes: beta: −0.8, 95%CI: −13.4, 11.8, P = 0.90). Since we found that days of in-ICU exposure to corticosteroids differed by CRHBP genotype, we also adjusted for in-ICU corticosteroid exposure in our fully adjusted regression models. As days of in-ICU corticosteroid exposure were not normally distributed, we adjusted for any in-ICU corticosteroid exposure. The unadjusted associations we found remained significant in our fully adjusted regression models that controlled for age, race, SAPS II score at ICU admission, and in-ICU corticosteroid exposure (Table 3).
Table 3.
Adjusted associations of CRHBP(rs1005525) and CRHR1(rs1876831) polymorphisms with post-ICU posttraumatic stress disorder and depressive symptomsa
| Post-ICU PTSD symptoms | Post-ICU depressive symptoms | |
|---|---|---|
|
| ||
| Variables | Beta (95% Confidence Interval), P Value | |
| CRHBP(rs1005525)b | ||
| A/T | −1.6 (−5.8, 2.6), P = 0.44 | 1.5 (−0.3, 3.4), P = 0.11 |
| T/T | −11.1 (−17.8, −4.7), P = 0.002 | −3.7 (−6.8, −0.6), P = 0.02 |
| Age | −0.1 (−0.3, 0.02), P = 0.09 | −0.1 (−0.1, 3.4 × 10−3), P = 0.06 |
| Non-white | 6.4 (1.6, 11.1), P = 0.009 | 1.5 (−0.6, 3.7), P = 0.16 |
| SAPS II | 0.1 (2.8 × 10−4, 0.2), P = 0.06 | 0.1 (−5.3 × 10−3, 0.1), P = 0.08 |
| In-ICU corticosteroid exposure | −0.3 (−4.9, 4.2), P = 0.89 | −0.4 (−2.5, 1.6), P = 0.68 |
|
| ||
| CRHR1(rs1876831)c | ||
| C/T | 4.0 (−8.4, 16.3), P = 0.53 | 7.2 (1.7, 12.8), P = 0.01 |
| C/C | 0.2 (−12.3, 11.9), P = 0.98 | 6.0 (0.6, 11.4), P = 0.03 |
| Age | −0.1 (−0.3, 0.02), P = 0.09 | −0.1 (−0.1, 0.02), P = 0.13 |
| Non-white | 6.3 (1.7, 10.9), P = 0.007 | 2.2 (0.1, 4.3), P = 0.04 |
| SAPS II | 0.1 (−0.04, 0.2), P = 0.18 | 0.03 (−0.03, 0.1), P = 0.19 |
| In-ICU corticosteroid exposure | −3.2 (−7.6, 1.1), P = 0.15 | −1.3 (−3.2, 0.6), P = 0.19 |
Abbreviations (in alphabetic order): CRHBP = Corticotrophin Releasing Hormone Binding Protein; CRHR1 = Corticotrophin Releasing Hormone Receptor; ICU = intensive care unit; PTSD = posttraumatic stress disorder; SAPS II = Simplified Acute Physiology Score II.
The dependent variables of the mixed-model regression analyses above are the repeated measures of PTSD and depressive symptoms at 3 and 12 months following medical-surgical intensive care unit admission. The beta coefficients can be interpreted as mean change in psychiatric symptom scale scores.
The reference group is A/A homozygotes.
The reference group is T/T homozygotes.
As a sensitivity analysis, we used Bonferroni correction of the P values from our regression models to account for multiple comparisons. In analyses without covariate adjustment, we adjusted for the number of SNPs tested (2) and phenotypes (PTSD and depressive symptoms) which led to an adjusted α= 0.01. For analyses that included adjustment for age, sex, SAPS II score, and in-ICU corticosteroid exposure, we also adjusted for the number of covariates, yielding an adjusted α = 0.003. After Bonferroni correction, the association of homozygosity for the CRHBP (rs10055255) T allele with fewer post-ICU PTSD symptoms continued to meet the threshold for statistical significance. However, the associations of homozygosity for the CRHBP (rs10055255) T allele with fewer post-ICU depressive symptoms and heterozygosity or homozygosity for the CRHR1 (rs1876831) C allele no longer met the threshold for statistical significance.
Since the lifetime history of major depression based on the MINI was high in our cohort and could impact interpretation of our findings, we conducted an additional sensitivity analysis in which we also adjusted for this covariate. In these analyses, being homozygous for the CRHBP (rs10055255) T allele was associated with significantly fewer post-ICU PTSD symptoms (beta: −9.9, 95%CI: −16.8, −3.0, P = 0.005), though the association with fewer post-ICU depressive symptoms was attenuated somewhat (beta: −2.8, 95%CI: −5.8, 0.2, P = 0.07). Carrying a single CRHR1 (rs1876831) C allele remained associated with significantly more post-ICU depressive symptoms (beta: 6.1, 95%CI: 0.8, 11.4, P = 0.03), though the association of C/C homozygosity with more post-ICU depressive symptoms was attenuated slightly (beta: 5.1, 95%CI: −0.1, 10.3, P = 0.05).
As an exploratory analysis, we examined the associations of the combined genotypes of both SNPs with levels of post-ICU PTSD or depressive symptoms. In these analyses, being homozygous for the CRHBP (rs10055255) T allele remained associated with significantly fewer post-ICU PTSD (beta: −11.3, 95%CI: −18.2, −4.5, P = 0.001) and depressive (beta: −3.4, 95%CI: −6.4, −0.4, P = 0.03) symptoms. Modeling both genotypes together resulted in further attenuation of the association of CRHR1 (rs1876831) with level of post-ICU depressive symptoms (C/T heterozygotes: beta: 5.4, 95%CI: −0.2, 11.0; P = 0.06; C/C homozygotes: beta: 3.3, 95%CI: −2.2, 8.9, P = 0.23).
DISCUSSION
In this prospective investigation of a small cohort of medical-surgical ICU survivors, we have gathered preliminary evidence that SNPs of two genes involved in the regulation of HPA axis activity may have important associations with PTSD and depressive symptoms in medical-surgical ICU survivors. Our findings suggest that being a CRHBP (rs10055255) T homozygote may be associated with significantly fewer post-ICU PTSD or depressive symptoms. At the same time, carrying a CRHR1 (rs1876831) C allele may be associated with greater severity of post-ICU depressive symptoms. However, the latter two findings were no longer statistically significant after Bonferroni correction. Our findings require replication in a larger cohort of critical illness survivors.
In light of the limited statistical power afforded by the small size of our cohort, we chose to genotype only one variant each in our two genes of interest, CRHBP and CRHR1. The choice of variants was motivated by several considerations. SNP rs10055255 in the CRHBP gene is a haplotype tagging SNP with a fairly high minor allele frequency in Caucasians (0.40), which increases the statistical power of comparisons in our sample of mostly Caucasian subjects [20]. Furthermore, this variant was previously found to be associated with antidepressant response and remission of depressive symptoms [9]. CRHR1 (rs1876831) has a reported minor allele frequency of 0.22 in Caucasians [20], and two previous studies have shown higher stress reactivity in rs1876831 C/C homozygotes [21, 22].
To our knowledge, the present investigation is only the second study to find an association between genetic variants and psychiatric morbidity in ICU survivors [23]. This prior study found that being a Glucocorticoid Receptor Gene (BclI) G homozygote was associated with greater severity of PTSD symptoms following ICU admission post-cardiac surgery [23]. These results, as well as our own, may in part be explained by the role of the HPA axis in memory. Glucocorticoids are involved in the consolidation of traumatic memories [24], and the CRH receptor in particular may have a role in consolidation of remote fear memories [25]. In addition, CRH activity in the amygdala may contribute to negative affective states and emotional dysregulation [26]. Although Hauer et al.’s findings as well as our own require replication in larger cohorts, when taken together, these results suggest an important potential role for genes that regulate cortisol homeostasis in the pathogenesis of PTSD and depression in the aftermath of critical illness.
We found that in-ICU exposure to corticosteroid medications in our cohort differed by CRHBP genotype, with T/T homozygotes receiving corticosteroids for more days than A/T heterozygotes or A/A homozygotes. Prior studies have found that critically ill patients may have an insufficiency of endogenous glucocorticoids [27], and that in-ICU corticosteroid administration may decrease the risk of post-ICU PTSD [28]. However, increased in-ICU corticosteroid exposure among patients homozygous for the CRHBP (rs10055255) T/T allele did not appear to account for the association of this genotype with fewer post-ICU PTSD symptoms seen in our sample. Additional studies in larger cohorts of critical illness survivors are needed in order to examine possible interactions between CRHBP genotype and the dose and duration of in-ICU corticosteroid exposure. These investigations could enhance understanding of the potential mechanisms linking CRHBP genotype with exogenous corticosteroid exposure and post-critical illness psychopathology.
Our findings of a possible association between the CRHBP (rs10055255) SNP and decreased severity of post-ICU psychiatric symptoms are supported by previous work showing this SNP to be associated with remission of depressive symptoms after treatment with citalopram [9]. Further, prior studies identifying associations between CRHR1 SNPs and greater risk of major depression concur with our results that the CRHR1 (rs1876831) SNP may be associated with greater severity of post-ICU depressive symptoms [29, 30].
The primary clinical implication of these results is that they suggest the possibility of a differential risk profile for post-ICU psychiatric morbidity based on variants of CRHBP and CRHR1 genes, particularly if replicated in larger studies. This information could be particularly useful to help identify, and potentially guide treatments for, critical illness survivors at greater risk for adverse longer-term psychiatric outcomes prior to hospital discharge. Indeed, a randomized controlled trial of cognitive behavioral therapy following ICU admission post-cardiac surgery is attempting to utilize such a strategy by randomizing patients homozygous for the BclI G allele into the trial [31]. Our findings suggest that utilizing CRHBP and CRHR1 allele profiles to aid in identification of at-risk critical illness survivors for evidenced-based interventions could prevent the development of longer-term psychiatric disorders and warrants additional research.
The functionality of CRHBP (rs10055255) is unknown. CRHR1 (rs1876831) has been previously shown to alter an intronic binding site for Sp1, a transcription factor that regulates transcription activation [32]. Alteration of the Sp1 binding site could lead to differential amounts of available CRHR1 receptors [33], which could have important implications since CRH is known to mediate negative emotional responses to stress [34]. Our study has several potential limitations. Although we found associations between the CRHBP (rs10055255) and CRHR1 (rs1876831) SNPs with post-ICU depressive symptoms, these results did not retain statistical significance after Bonferroni correction, a factor most likely attributable to our small sample size. Moreover, our small sample size precludes adequate characterization of the effect sizes of the genetic variants we studied in relationship to predicting risk of post-ICU PTSD and depressive symptoms. Therefore, our findings regarding the potential role of these two specific SNPs in the development of post-ICU PTSD and depression should be interpreted with caution. In addition, while the questionnaires we used to assess post-ICU PTSD and depressive symptoms have not been specifically validated in critical illness survivors, they have been used in many relevant populations such as the family members of medical-surgical ICU survivors [35, 36]. Further, we did not obtain in-ICU serum cortisol levels and so could not make associations between the SNPs we studied with serum cortisol concentrations and subsequent risk of PTSD or depression.
In conclusion, this pilot study found that genetic polymorphisms in the CRHBP and CRHR1 genes may have important roles in the pathogenesis of PTSD and depressive symptoms following medical-surgical ICU admission. We identified that being a CRHBP (rs10055255) T homozygote was associated with significantly fewer post-ICU PTSD symptoms even after adjusting for multiple comparisons. Furthermore, we found that being a CRHBP (rs10055255) T homozygote was associated with fewer post-ICU PTSD symptoms, while carrying at least CRHR1 (rs1876831) C allele was associated with more post-ICU depressive symptoms. If replicated by larger cohort studies of critical illness survivors, these results may serve to illuminate the pathogenesis of psychiatric morbidity in the aftermath of medical illnesses as well as to inform the development of clinical biomarkers that could aid in identification of at-risk critical illness survivors to facilitate early referrals for appropriate, evidence-based treatments.
Acknowledgments
The authors thank Collin McFadden, B.A., and Jeffrey Love, B.A., for assistance with patient recruitment and data collection, and Jin Wang, Ph.D., for assistance with data cleaning.
FUNDING
This work was supported by grants KL2 TR000421, R03 AA020146-02, NRSA-T32/MH20021-12, R01 AA01602 and K24 MH086814-03 from the National Institutes of Health and grant ADAI-1009-2 from the University of Washington Alcohol and Drug Abuse Institute.
Footnotes
Disclosures: Drs. Davydow, Kohen, Hough, Zatzick, and Ms. Tracy have no relevant conflicts of interest to disclose. Dr. Katon discloses that he has received honoraria in the last 12 months for CME lectures funded indirectly by Lilly, Forest and Pfizer.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iwashyna TJ. Survivorship will be the defining challenge of critical care in the 21st century. Ann Intern Med. 2010;153:204–205. doi: 10.7326/0003-4819-153-3-201008030-00013. [DOI] [PubMed] [Google Scholar]
- 2.Davydow DS, Gifford JM, Desai SV, et al. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30:421–434. doi: 10.1016/j.genhosppsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davydow DS, Gifford JM, Desai SV, et al. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med. 2009;35:796–809. doi: 10.1007/s00134-009-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davydow DS, Desai SV, Needham DM, et al. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70:512–519. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katon WJ, Rutter C, Simon G, et al. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care. 2005;28:2668–2672. doi: 10.2337/diacare.28.11.2668. [DOI] [PubMed] [Google Scholar]
- 6.Edmonson D, Richardson S, Falzon L, et al. Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: a meta-analytic review. PLoS One. 2012;7:38915. doi: 10.1371/journal.pone.0038915. Epub 2012 Jun 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katon W, Lin E, Russo J, et al. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psychiatry. 2003;60:897–903. doi: 10.1001/archpsyc.60.9.897. [DOI] [PubMed] [Google Scholar]
- 8.Gomez-Sanchez CE. Adrenal dysfunction in critically ill patients. N Engl J Med. 2013 Mar 19; doi: 10.1056/NEJMe1302305. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Binder EB, Owens MJ, Liu W, et al. Association of polymorphisms in genes regulating the corticotropin-releasing factor system with antidepressant treatment response. Arch Gen Psychiatry. 2010;67:369–379. doi: 10.1001/archgenpsychiatry.2010.18. [DOI] [PubMed] [Google Scholar]
- 10.Amstadter AB, Nugent NR, Yang BZ, et al. Corticotrophin-releasing hormone type 1 receptor (CRHR1) variants predict posttraumatic stress disorder onset and course in pediatric injury patients. Dis Markers. 2011;30:89–99. doi: 10.3233/DMA-2011-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley RG, Binder EB, Epstein MP, et al. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davydow DS, Zatzick D, Hough CL, et al. A longitudinal investigation of posttraumatic stress and depressive symptoms over the course of the year following medical-surgical intensive care unit admission. Gen Hosp Psychiatry. 2013 Jan 28; doi: 10.1016/j.genhosppsych.2012.12.005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weathers FW, Huska JA, Keane TM. The PTSD Checklist – civilian version. Boston, MA: The National Center for PTSD, Boston VA Medical Center; 1991. [Google Scholar]
- 14.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American Multicenter Study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 17.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 18.Ramstad SM, Russo J, Zatzick DF. Is it an accident? Recurrent traumatic life events in level I trauma center patients compared to the general population. J Trauma Stress. 2004;17:529–534. doi: 10.1007/s10960-004-5802-z. [DOI] [PubMed] [Google Scholar]
- 19.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:S22–S33. [PubMed] [Google Scholar]
- 20.Manolio TA, Collins FS. The HapMap and genome-wide association studies in diagnosis and therapy. Annu Rev Med. 2009;60:443–456. doi: 10.1146/annurev.med.60.061907.093117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blomeyer D, Treutlein J, Esser G, et al. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008;63:146–151. doi: 10.1016/j.biopsych.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Schmid B, Blomeyer D, Treutlein J, et al. Interacting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds. Int J Neuropsychopharmacol. 2010;13:703–714. doi: 10.1017/S1461145709990290. [DOI] [PubMed] [Google Scholar]
- 23.Hauer D, Weis F, Papassotiropoulos A, et al. Relationship of a common polymorphism of the glucocorticoid receptor gene to traumatic memories and posttraumatic stress disorder in patients after intensive care therapy. Crit Care Med. 2011;39:643–650. doi: 10.1097/CCM.0b013e318206bae6. [DOI] [PubMed] [Google Scholar]
- 24.Kuhlmann S, Wolf OT. Arousal and cortisol interact in modulating memory consolidation in healthy young men. Behav Neurosci. 2006;120:217–223. doi: 10.1037/0735-7044.120.1.217. [DOI] [PubMed] [Google Scholar]
- 25.Thoeringer CK, Henes K, Eder M, et al. Consolidation of remote fear memories involves Corticotropin-Releasing Hormone (CRH) receptor type-1 mediated enhancement of AMPA receptor GluR1 signaling in the dentate gyrus. Neuropsychopharmacology. 2012;37:787–796. doi: 10.1038/npp.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valdez GR, Koob GF. Allostasis and dysregulation of corticotropin-releasing factor and neuropeptide Y systems: implications for the development of alcoholism. Pharmacol Biochem Behav. 2004;79:671–689. doi: 10.1016/j.pbb.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 27.Schelling G, Roozendaal B, Krauseneck T, et al. Efficacy of hydrocortisone in preventing posttraumatic stress disorder following critical illness and major surgery. Ann NY Acad Sci. 2006;1071:46–53. doi: 10.1196/annals.1364.005. [DOI] [PubMed] [Google Scholar]
- 28.Schelling G, Briegel J, Roozendaal B, et al. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biol Psychiatry. 2001;50:978–985. doi: 10.1016/s0006-3223(01)01270-7. [DOI] [PubMed] [Google Scholar]
- 29.Wasserman D, Wasserman J, Rozanov V, et al. Depression in suicidal males: genetic risk variants in the CRHR1 gene. Genes Brain Behav. 2009;8:72–79. doi: 10.1111/j.1601-183X.2008.00446.x. [DOI] [PubMed] [Google Scholar]
- 30.Utge S, Soronen P, Partonen T, et al. A population-based association study of candidate genes for depression and sleep disturbance. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:468–476. doi: 10.1002/ajmg.b.31002. [DOI] [PubMed] [Google Scholar]
- 31.Hauer D, Kolassa IT, Laubender RP, et al. A genotype-specific, randomized controlled behavioral intervention to improve the neuroemotional outcome of cardiac surgery: study protocol for a randomized controlled trial. Trials. 2013;14:89. doi: 10.1186/1745-6215-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson GM, Freytag SO. Synergistic activation of a human promoter in vivo by transcription factor Sp1. Mol Cell Biol. 1991;11:1935–1943. doi: 10.1128/mcb.11.4.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treutlein J, Kissling C, Frank J, et al. Genetic association of the human corticotropin releasing hormone receptor (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- 34.Steckler T, Holsboer F. Corticotropin-releasing hormone receptor subtypes and emotion. Biol Psychiatry. 1999;46:1480–1508. doi: 10.1016/s0006-3223(99)00170-5. [DOI] [PubMed] [Google Scholar]
- 35.Gries CJ, Engelberg RA, Kross EK, et al. Predictors of symptoms of posttraumatic stress and depression in family members after patient death in the ICU. Chest. 2010;137:280–287. doi: 10.1378/chest.09-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kross EK, Engelberg RA, Gries CJ, et al. ICU care associated with symptoms of depression and posttraumatic stress disorder among family members of patients who die in the ICU. Chest. 2011;139:795–801. doi: 10.1378/chest.10-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]