ABSTRACT
Background: Secondary endocrine hypertension accounts for 5-12% of hypertension's causes. In selected patients (type 2 diabetes mellitus, sleep apnea syndrome with resistant hypertension, sudden deterioration in hypertension control), prevalence could be higher.
Objectives: To present etiology of endocrine secondary hypertension in a series of patients younger than 40 years at hypertension's onset.
Material and methods: Medical records of 80 patients (39M/41F), aged 30.1 ± 8.2 years (range: 12-40 years), with maximum systolic blood pressure=190.4 ± 29.2 mm Hg, range: 145-300 mm Hg, maximum diastolic blood pressure=107.7 ± 16.9 mm Hg, range: 80-170 mm Hg) referred by cardiologists for endocrine hypertension screening were retrospectively reviewed. Cardiac and renal causes of secondary hypertension were previously excluded. In all patients, plasma catecholamines were measured by ELISA and plasma cortisol by immunochemiluminescence. Orthostatic aldosterone (ELISA) and direct renin (chemiluminescence) were measured in 48 patients.
Results: Secondary endocrine hypertension was confirmed in 16 out of 80 patients (20%). Primary hyperaldosteronism was diagnosed in 7 (4M/3F) out of 48 screened patients (14.6%). i.e. 8.75% from whole group: 5 patients with adrenal tumors (3 left/2 right), 2 patients with bilateral adrenal hyperplasia; all patients were hypokalemic at diagnostic (average nadir K+ levels = 2.5 ± 0.5 mmol/L); four patients were hypokalaemic on diuretic therapy (indapamidum); other 3 patients were hypokalaemic in the absence of diuretic therapy. Cushing's syndrome was diagnosed in 6 patients (7.5%): subclinical Cushing due to 4 cm right adrenal tumour – n = 1, overt ACTH-independent Cushing's syndrome due to: macronodular adrenal hyperplasia associated with primary hyperparathyroidism – n = 1; due to adrenal carcinoma – n = 1; due to adrenal adenomas – n = 2; Cushing's disease – n = 1). Pheochromocytomas were diagnosed in 3 patients (3.75%).
Conclusion: Primary hyperaldosteronism was the most frequent cause of secondary endocrine hypertension in our series, followed by Cushing's syndrome and pheochromocytomas. Screening of young hypertensive patients for secondary causes, especially primary hyperaldosteronism, is mandatory.
Keywords: secondary hypertension, primary hyperaldosteronism, hypercortisolism, pheochromocytoma, screening
INTRODUCTION
Arterial hypertension affects up to 20% of adult population (1). Essential or idiopathic hypertension prevails, but secondary renal (renovascular and renal parenchymal disease) and endocrine hypertension accounts for up to 15-20% of hypertension's causes (2). In patients with systemic hypertension, identification of a secondary endocrine cause that is potentially surgically curable or suitable to specific drugs is very important. There are various causes of endocrine hypertension: primary hyperaldosteronism, pheochromocytoma, Cushing's syndrome, congenital adrenal hyperplasia due to 11 β hydroxylase and 17 α hydroxylase deficiency, apparent mineralocorticoid excess, acromegaly, hyperthyroidism (2). Primary hyperaldosteronism and pheochromocytoma are the most frequent conditions (3).
Renin–aldosterone system is a pivotal mechanism in both essential and adrenocortical causes of arterial hypertension. Several epidemiological studies suggested that primary hyperaldosteronism is the most frequent cause of secondary endocrine hypertension, around 5-12% (1). A higher prevalence (15-20%) occurred in patients with resistant hypertension and type 2 diabetes mellitus (4), sleep apnea syndrome (5).
On the other hand, Cushing's syndrome in adults is associated in up to 80% of cases with elevated blood pressure (6). Patients with resistant hypertension had a high prevalence of subclinical hypercortisolism (8%), associated with diabetes (7). Surgical treatment of adrenal incidentalomas presenting subclinical hypercortisolism improved blood pressure, weight and glucose levels (8).
Paroxysmal or sustained hypertension, associated with sinus tachycardia, is the cardinal sign of pheochromocytoma. Despite low prevalence, screening for pheochromocytoma is mandatory in all patients with suggestive symptoms, due to possible life-threatening complications of the disease (9). ❑
AIM
To present etiology of endocrine secondary hypertension in a series of patients younger than 40 years at hypertension's onset referred by cardiologists to a tertiary endocrine centre. ❑
MATERIAL AND METHODS
Patients
Eighty patients (39M/41F), aged 30.1 ± 8.2 years (range: 12-40 years), with systemic hypertension (maximum systolic blood pressure = 190.4 ± 29.2 mm Hg, range: 145-300 mm Hg; maximum diastolic blood pressure = 107.7 ± 16.9 mm Hg, range: 80-170 mm Hg) diagnosed before 40 years, referred by cardiologists for endocrine hypertension screening were retrospectively reviewed. Maximum systolic and diastolic blood pressure values were recorded either during physical examination (average of 2 measurements) or on 24 hours ambulatory blood pressure monitoring, when available. Patients referred for typical symptoms and signs of hypercortisolism and patients from multiple endocrine neoplasia (MEN) families were not included. Cardiac and renal causes of secondary hypertension were previously excluded.
Methods
Serum/plasma cortisol was determined by immunochemiluminescence. Morning 8 a.m. cortisol basal levels and after a midnight 1 mg dexamethasone suppression test (DST) were measured (cutoff values < 1.8 µg/dL).
Plasma catecholamines were measured by ELISA (plasma metanephrines normal range: 10-90 pg/mL, plasma normetanephrines normal range: 15-180 pg/mL).
Glucocorticoid axis and catecholamines were assessed in all patients; renin-angiotensin-aldosterone axis has been assessed since 2010, in 48 patients.
Direct quantitative determination of plasma/serum aldosterone was done by enzyme immunoassay – ELISA (LDN GmbH) in 48 patients between 2010 and 2012. Method's sensitivity for aldosterone detection was 10.0 pg/mL, intra-assay precision 4.1-10.4%, inter-assay precision 9.7%, normal values: orthostatic: 40-310 pg/mL; recumbent: 10-160 pg/mL.
Quantitative determination of plasma direct renin was done by chemiluminiscence (CLIA, Liaison, DiaSorin) in 48 patients between 2010 and 2012. Method's sensitivity for renin detection was 0.3 pg/mL, intra-assay precision 2.4-5.6%, inter-assay precision 4.2-6.9%, normal values: orthostatic: 4.4-46.1 µUI/mL (2.64-27.66 pg/mL); recumbent: 2.8-39.9 µUI/mL (1.68-23.94 pg/mL).
Blood collection was done in the morning, after the patient has been standing, sitting, walking for at least 2 hours and seated for 5-15 minutes. Whenever possible, antihypertensive drugs interfering with hormonal assessment were withdrawn, according to guidelines' recommendations (10).
Statistical analysis was performed by SPSS version 12.0; data were expressed as mean ± standard deviation, median or frequencies. Comparison of different group used independent two-tailed t test and Mann-Whitney test. P <0.05 was considered to be statistically significant. ❑
RESULTS
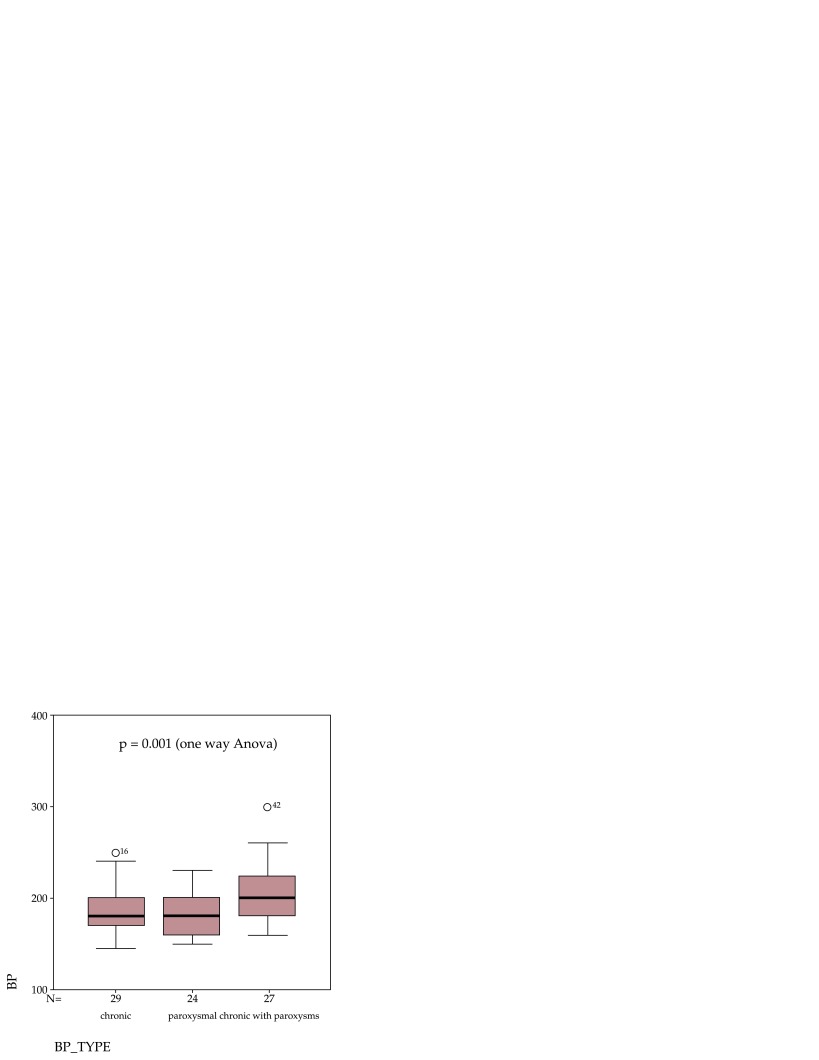
In our study, 29 patients (36.3%) had sustained hypertension, 24 patients (30%) showed paroxysmal hypertension and 27 patients (33.7%) showed chronic hypertension with paroxysms. Patients with chronic hypertension with paroxysms showed higher blood (207.1 ± 32.4 mm Hg) pressure levels than patients with sustained hypertension (183.3 ± 25.6 mm Hg), p = 0.004 (independent t test) and patients with paroxysmal hypertension (180 ± 21.1 mm Hg), p <0.001 (independent t test) (Figure 1).
Figure 1. Blood pressure (BP) values (mmHg) in studied population according to hypertension pattern.
Regarding hypertension etiology, secondary endocrine hypertension was confirmed in 16 out of 80 patients (20%), with the limitation of measuring aldosterone/renin ratio only in 48 patients.
Hormonal assessment in our series revealed: median 8 a.m. cortisol levels after 1 mg overnight dexamethasone suppression test (DST) = 0.9 µg/dL (25th percentile = 0.6 µg/dL, 75th percentile = 1.4 µg/dL); median plasma metanephrines = 26 pg/mL (25th percentile = 15 pg/mL, 75th percentile = 45.8 pg/mL); median plasma normetanephrines = 68 pg/mL (25th percentile = 31.3 pg/mL, 75th percentile = 94.5 pg/mL); median upright morning aldosterone = 165.2 pg/mL (25th percentile = 97.6 pg/mL, 75th percentile = 229.5 pg/mL), with median upright renin = 17.3 pg/mL (25th percentile = 6.4 pg/mL, 75th percentile = 37.7 pg/mL).
Positive screening for primary hyperaldosteronism, defined as plasma aldosterone concentration (ng/dL) / direct renin concentration (ng/L) greater than 7.7 (equivalent to plasma aldosterone concentration (ng/dL)/ plasma renin activity (ng/mL h) >40) was found in 7 out of 48 screened patients (14.6%); confirmatory tests used were captopril challenge test and saline infusion test. Cushing's syndrome was diagnosed in 6 patients (8.6%): subclinical Cushing due to 4 cm right adrenal tumor – n = 1, overt ACTH-independent Cushing's syndrome due to: macronodular adrenal hyperplasia associated with primary hyperparathyroidism – n = 1; due to adrenal carcinoma – n = 1; due to adrenal adenomas – n = 2; Cushing's disease – n = 1). Pheochromocytomas were diagnosed in 3 patients (3.75%).
For imaging, adrenal ultrasound was used as screening test. When presence of an adrenal tumor was suspected on ultrasound or hormonal screening was positive for secondary endocrine hypertension, adrenal computed tomography and/or magnetic resonance imaging were performed (n=45 patients). Adrenal imaging revealed 28 cases of adrenal tumors (21 left/7 right side, 8 micronodules/20 macronodules) and 12 cases of adrenal hyperplasia (11 micronodular/1 macronodular); median tumor diameter at diagnosis was 1.2 cm (range: 0.3-13 cm, 25th percentile = 0.8 cm, 75th percentile = 3.2 cm). Adrenal imaging according to hormonal secretion is presented in Table 1.
Table 1.
Adrenal imaging in 80 young patients screened for secondary endocrine hypertension.
| Adrenal tumors (n=28) | Adrenal hyperplasia (n=12) | No visible tumor (n=40) | |
|---|---|---|---|
| Primary hyperaldosteronism (n=7) | 5 | 2 | - |
| ACTH independent Cushing's sd. (n=5) | 4 | 1 (bilateral macronodular) | - |
| ACTH dependent Cushing's sd. (n=1) | - | 1 | - |
| Pheochromocytoma (n=3) | 3 | - | - |
| Idiopathic hypertension (n=64) | 16 | 8 | 40 |
Clinical and biochemical features in patients with primary hyperaldosteronism were presented in Table 2. Hypertension was sustained in 3 patients, paroxysmal in 1 patient and chronic with paroxysms in 3 patients. All patients were initially hypokalaemic (2.5 ± 0.5 mmol/L). Four patients were hypokalaemic on diuretic therapy (indapamidum); other 3 patients were hypokalaemic in the absence of diuretics. Before screening, potassium normalization was achieved by administering potassium supplements. During screening and performing confirmatory tests, spironolactone and potassium wasting diuretics were withdrawn for at least 4 weeks, according to guidelines recommendations (10). For hypertension control, verapamil slow-release and prazosin were administered in the majority of screened patients. In 5 screened patients with severe hypertension, additional drugs were needed (angiotensin converting enzyme inhibitors in 2 patients, angiotensin II receptor blockers in 2 patients and moxonidine in one patient). In these patients, 50 mg Captopril challenge test was performed as confirmatory test, to avoid increasing blood pressure during saline infusion test.
Table 2.
Clinical and biochemical data in patients with primary hyperaldosteronism (PA).
| PA (n=7) | All other cases (n=73) | P | |
|---|---|---|---|
| Gender | 4M/3F | 35M/38F | Ns |
| Age at HT onset (years) | 36.8 ± 4.8 | 29.5 ± 8.1 | 0.005 |
| BMI (kg/m2) | 27.4 ± 4.1 | 27.4 ± 6.3 | Ns |
| K+ (mmol/L) | 2.5 ± 0.5 | 4.4 ± 0.6 | 0.0001 |
| Systolic BP (mm Hg) | 202.8 ± 26.3 | 189.1 ± 29.4 | Ns |
| Orthostatic plasma aldosterone (pg/mL) | 313.6 ± 130.4 | 159.8 ± 97.9# | 0.02 |
| Median plasma aldosterone concentration (PAC ng/dL)/ direct renin concentration (DRC ng/L) | 20.1 | 0.9# | <0.0001 |
# Aldosterone and aldosterone/renin ratio available in 41 patients.
In cases with primary hyperaldosteronism, hypertension was associated with other major cardiovascular risk factors and clinical conditions: impaired glucose metabolism was present in 3 out of 7 patients (2 cases with type 2 diabetes mellitus and 1 case of impaired glucose tolerance); dyslipidemia was present in 5 patients, resistant hypertension in 5 patients (systolic blood pressure > 140 mm Hg and diastolic blood pressure > 90 mm despite treatment with 3 antihypertensive drugs), chronic kidney disease (GFR MDRD = 53 and 58 ml/min/1.73 cm2, two assessments at 4 months interval) in 2 patients and ischaemic stroke in one patient. Normocalcaemic hyperparathyroidism was present in 2 cases. Three patients had left adrenal tumors, 2 patients had right adrenal tumors and 2 patients had bilateral adrenal hyperplasia (Figure 2); bilateral adrenal venous sampling with aldosterone measurement was not performed.
Figure 2. Abdominal computed tomography scan showing bilateral adrenal hyperplasia in a patient with primary hyperaldosteronism.
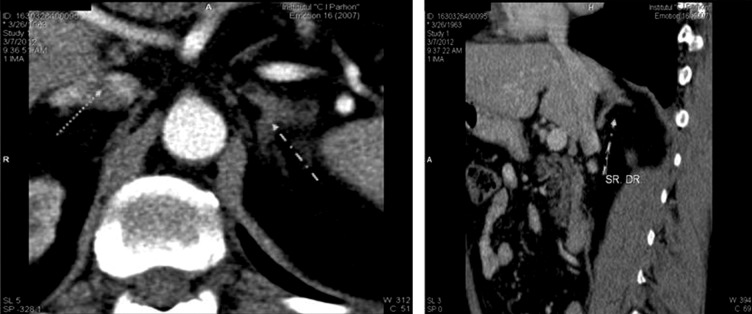
Patient E.A, male; basal aldosterone level was 209.2 pg/mL, direct renin concentration was 1.9 pg/mL, with aldosterone (ng/dL)/renin (ng/L) ratio of 11. Following 50 mg captopril challenge test, aldosterone failed to suppress more than 30% (175.75 pg/mL).
Adrenal surgery was performed in 4 out of 7 patients with primary hiperaldosteronism, with pathology confirmation of diagnosis, normalization of hypokalaemia and significant improvement in blood pressure control (cure of hypertension in one case, decrease in number and dose of antihypertensive drugs in 3 patients). Two patients with bilateral adrenal hyperplasia and one patient with left adrenal tumor, who denied surgery, were successful treated by supplementation with mineralocorticoid antagonists (spironolactone 25-50 mg/day in 2 patients, and eplerenone 100 mg/day in one patient) of antihypertensive therapy.
Clinical and biochemical features in patients with Cushing's syndrome (CS) were presented in Table 3. All patients but one showed normal potassium levels. Secondary diabetes was present in 4 out of 6 patients; all patients showed dyslipidemia; resistant hypertension was present in 1 patient. Median tumor diameter at diagnosis was 3.9 cm (range: 1-13 cm, 25th percentile = 2.6 cm, 75th percentile = 6.8 cm). Before surgery, antihypertensive treatment consisted in angiotensin converting enzyme inhibitors, β blockers, calcium channel blockers, diuretics. Spironolactone was administered in the patient presenting hypokalaemia.
Table 3.
Clinical and biochemical data in patients with Cushing's syndrome (CS)
| CS (n=6) | All other causes (n=74) | P | |
|---|---|---|---|
| Gender | 3M/3F | 36M/38F | Ns |
| Age at HT onset (years) | 34.7 ± 4.7 | 29.8 ± 8.3 | Ns |
| BMI (kg/m2) | 27.5 ± 3.3 | 27.3 ± 6.3 | Ns |
| K+ (mmol/L) | 4.2 ± 1.4 | 4.18 ± 0.7 | Ns |
| Systolic BP (mmHg) | 200 ± 42 | 189.7 ± 28.2 | Ns |
| 8 a.m. serum cortisol after DST (µg/dL) | 22 ± 6.6 | 1.1 ± 0.3 | 0.002 |
Surgical treatment consisted in unilateral adrenalectomy in 4 cases and bilateral adrenalectomy in 2 cases (one case with ACTH independent macronodular bilateral hyperplasia and one case of Cushing's disease with micronodular bilateral hyperplasia). After surgery, hypertension was cured in 4 patients and significantly improved in two patients.
Pheochromocytomas were diagnosed in 3 patients (3.75%). Adrenal imaging revealed left adrenal tumors in two cases and right adrenal tumor in one case. β blockers were avoided or progressively withdrawn for screening tests. Preoperative treatment consisted in α blockers, calcium channel blockers and adequate hydration; β blockers were added only after sustained α blockade. ❑
DISCUSSION
Etiology of secondary endocrine hypertension largely depends on studied population, screening tests applied, confirmatory tests and concomitant anti hypertensive medication interfering with renin-aldosterone system.
The present study identified etiology of secondary endocrine hypertension in a selected Romanian population younger then 40 years at hypertension's onset, referred by cardiologist to a tertiary endocrine center. Secondary endocrine hypertension was confirmed in 16 out of 80 patients (20%); primary hyperaldosteronism was most frequent cause, followed by Cushing's syndrome and pheochromocytomas.
In a series of 1001 hypertensive patients hospitalized in the endocrinology division, 65.4% had secondary hypertension (11). Adrenal hypertension was the primary cause of secondary hypertension, followed by renovascular, central, psychogenic, and renal parenchymal disease hypertension. Predictors for secondary endocrine hypertension were the presence of adrenal masses (OR = 10.1), orthostatic aldosterone value >200 pg/mL (OR = 9.7), and a ratio of orthostatic aldosterone and renin activity >40 (OR = 4.7). On the other hand, the independent predictors for essential hypertension were family history of hypertension (OR = 7.2) and body mass index above the normal range (OR = 15.1) (11).
In a series of 4642 patients with hypertension retrospectively studied, 85.24% had essential hypertension and 14.76% had secondary hypertension (12). The highest prevalence of primary hyperaldosteronism (12.12%) was found in endocrine hypertension. Among the patients with secondary hypertension, 21.9% were found in youth, and 9.85% in aged. Incidence of essential hypertension and primary hyperaldosteronism were higher in young and middle-aged male, and incidence of pheochoromocytoma was higher in females (12).
In a primary care series of 564 hypertensive patients, there were 63 cases with resistant hypertension, from whom 15 patients showed increased aldosterone/renin ratio; primary hyperaldosteronism was confirmed in 3 patients (13).
On the other hand, in 715 patients with multiple endocrine neoplasia type 1, 72 cases showed adrenal tumors larger than 1 cm; there were identified 4 cases with primary hyperaldosteronism (5.5%), 4 cases with ACTH independent Cushing's syndrome (5.5%) and 1 case with bilateral pheochromocytoma (14). Patients with multiple endocrine neoplasia were not included in this study.
Prevalence of primary hyperaldosteronism in screened patients in this study was 14.6%, due to younger patients' age. Prevalence of primary hyperaldosteronism in prospective analysis varied from 11.2% - PAPY study (15) to 7% in a recently published German study, when considering increased aldosterone/renin ratio (ARR) (16). Higher prevalence of primary hyperaldosteronism was found in subjects with resistant hypertension (11.9% increased ARR) and in stage III hypertension (18.3% increased ARR) (16).
One of major difficulties in a correct screening for secondary endocrine hypertension is selection of antihypertensive medication during diagnosis. False-positive results due to medication are possible in pheochromocytoma (17). In suspected primary hyperaldosteronism, both case detection and confirmatory tests require switching medication to non-dihydropyridine calcium channel antagonists (verapamil slow-release) and/or alpha adrenergic blockers (10). This can often worsen hypertension, precipitate hypertensive crisis, persistent hypokalaemia despite potassium supplements, atrial fibrillation, heart failure, requiring hospitalization in many cases (18). In selected patients, only thiazides, loop diuretics and aldosterone antagonists should be withdrawn, and the diagnosis can be made if serum aldosterone remains markedly elevated (≥240 pmol/L) at the end of saline infusion test (19).
Changing medication in our study was limited due to severe complications of the patients. In 5 screened patients with severe hypertension, additional drugs to verapamil and prazosin were needed (angiotensin converting enzyme inhibitors in 2 patients, angiotensin II receptor blockers in 2 patients and moxonidine in one patient). In these patients, 50 mg Captopril challenge test was performed as confirmatory test, to avoid worsening of hypertension during saline infusion test.
Patients with primary hyperaldosteronism in this study showed significant co morbidities and target-organ damage: impaired glucose metabolism (3/7), dyslipidemia (5/7), severe hypertension (5/7), and chronic kidney disease (2/7), ischaemic stroke (1/7). Primary hyperaldosteronism and hypercortisolism were associated with an increased cardiovascular risk in comparison with essential hypertension patients (3,20,21). In a series of 553 patients with primary hyperaldosteronism, prevalence of comorbidities was reported to be higher (OR = 2.4) in hypokalaemic than normokalaemic PA. The prevalence of cardiac events was significantly higher in hypokalaemic PA (OR = 2.2); there was a significantly higher prevalence of angina pectoris (OR = 4.7) and chronic cardiac insufficiency (OR = 2.8) in hypokalaemic compared with normokalaemic PA patients. Atrial arrhythmias also showed a trend toward higher prevalence in hypokalaemic PA (20), but a prospective, ongoing study (PAPPHY) aims to answer the question if PA is associated with a higher prevalence of lone (non-valvular) paroxysmal or permanent atrial flutter or fibrillation (22). Subgroup analysis of cerebrovascular events revealed a higher, but not significantly different, rate of stroke (OR= 1.90) in normokalaemic PA (20) as compared with hypokalaemic PA. Also renal damage is higher in primary hyperaldosteronism as compared with essential hypertension (23).
Also patients with adrenal incidentalomas and subclinical Cushing's syndrome (cortisol > 138 nmol/L after 1 mg dexamethasone suppression test) were reported to have an increased prevalence of coronary heart disease (OR = 6.1), type 2 diabetes (OR = 3.44), osteoporosis (OR = 5.94) and osteoporotic fractures (OR = 6.53). However, in patients with nonfunctioning adrenal tumor and in adrenal tumors with subclinical Cushing's syndrome, prevalence of arterial hypertension was similar (21).
In our study, 51 patients (63.7%) had blood pressure paroxysms. However, blood pressure variability was not determined in studied patients. Blood pressure variability was associated with target-organ damage - increased cardiovascular risk (24), stroke risk and renal impairment - independent of mean of the blood pressure. Therefore, patients with secondary endocrine hypertension had high vascular risk, demanding precocious diagnosis and treatment intervention.
There were no gender differences between patients with secondary endocrine hypertension and patients with essential hypertension. Older age at diagnosis in patients with primary hyperaldosteronism was probable due to late referral and diagnosis in these patients with long standing hypertension.
In presented series, all patients with primary hyperaldosteronism were hypokalaemic (3 patients spontaneously and 4 patients on potassium wasting diuretics). High prevalence of hypokalemia in this series revealed an under-diagnosed disease. In another Romanian study, overall prevalence of hypokalaemia in 26 patients with primary hyperaldosteronism was 57.7% and 76.4% in adenomas with aldosterone secretion (25). Significantly lower prevalence of hypokalaemia was reported in the literature (below 40%), revealing a correct screening and an earlier diagnosis of primary hyperaldosteronism (10,16,15). Awareness of both cardiologists and endocrinologists regarding endocrine hypertension should increase, in order to improve case detection rate, especially in high risk patients, who should be screened earlier, before complication's appearance (26, 27). Screening should not be applied only in hypokalaemic hypertension, but also in normokalaemic patients with mild and severe hypertension, resistant hypertension (10) and guidelines should be adapted according to patient's age and comorbidities (28). ❑
CONCLUSIONS
Primary hyperaldosteronism was the most frequent cause of secondary endocrine hypertension in our series, followed by Cushing's syndrome and pheochromocytomas. Screening of young hypertensive patients for secondary causes, especially primary hyperaldosteronism, is mandatory. An earlier assessment is less influenced by concomitant medication; proper screening and diagnosis permit improvement in hypertension's cure rate and an efficient prevention of high blood pressure's complications. ❑
ACKNOWLEDGEMENTS
The authors would like to thank to all referral cardiologists, to Ana Maria Stefanescu, PhD, Sorina Schipor, PhD for catecholamines' measurement, to Carmen Iordachescu, MD, PhD for cortisol measurements.
CONFLICT OF INTEREST
Partial data were presented as an oral communication at the ENS@T Symposium "Adrenal Tumors and Arterial Hypertension", Poiana Brasov, Romania, 7-9th of June 2012.
FINANCIAL SUPPORT
none declared.
References
- 1.Fagugli RM, Taglioni C. Changes in The Perceived Epidemiology of Primary Hyperaldosteronism. Int J Hypertens. 2011:162804–162804. doi: 10.4061/2011/162804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moraitis A, Stratakis C. Adrenocortical Causes Of Hypertension. Int J Hypertens. 2011:624691–624691. doi: 10.4061/2011/624691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Salameh A, Cohen R, Chanson P, et al. Update on Endocrine Hypertension. Article in French. Ann Endocrinol (Paris) 2012;73(Suppl1):S26–S35. doi: 10.1016/S0003-4266(12)70012-4. [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee JJ, Khoo CM, Thai AC, et al. Type 2 Diabetic Patients with Resistant Hypertension should be Screened For Primary Aldosteronism. Diab Vasc Dis Res. 2010;7:6–13. doi: 10.1177/1479164109350556. [DOI] [PubMed] [Google Scholar]
- 5.Di Murro A, Petramala L, Cotesta D, et al. Renin-Angiotensin-Aldosterone System in Patients with Sleep Apnoea: Prevalence of Primary Aldosteronism. J Renin Angiotensin Aldosterone Syst. 2010;11:165–172. doi: 10.1177/1470320310366581. [DOI] [PubMed] [Google Scholar]
- 6.Cicala MV, Mantero F. Hypertension in Cushing's Syndrome: From Pathogenesis to Treatment. Neuroendocrinology. 2010;92(1):44–49. doi: 10.1159/000314315. [DOI] [PubMed] [Google Scholar]
- 7.Martins LC, Conceicao FL, Muxfeldt ES, et al. Prevalence and Associated Factors of Subclinical Hypercortisolism in Patients with Resistant Hypertension. J Hypertens. 2012;30:967–973. doi: 10.1097/HJH.0b013e3283521484. [DOI] [PubMed] [Google Scholar]
- 8.Chiodini I, Morelli V, Salcuni AS, et al. Beneficial Metabolic Effects of Prompt Surgical Treatment in Patients with an Adrenal Incidentaloma Causing Biochemical Hypercortisolism. J Clin Endocrinol Metab. 2010;95:2736–2745. doi: 10.1210/jc.2009-2387. [DOI] [PubMed] [Google Scholar]
- 9.Prejbisz A, Lenders JW, Eisenhofer G, et al. Cardiovascular Manifestations of Phaeochromocytoma. J Hypertens. 2011;29:2049–2060. doi: 10.1097/HJH.0b013e32834a4ce9. [DOI] [PubMed] [Google Scholar]
- 10.Funder JW, Carey RM, Fardella C, et al. Case Detection, Diagnosis and Treatment of Patients with Primary Aldosteronism: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 11.Ye D, Dong F, Lu X, et al. Analysis of Various Etiologies Of Hypertension in Patients Hospitalized in the Endocrinology Division. Endocrine. 2012;42:174–181. doi: 10.1007/s12020-011-9588-5. [DOI] [PubMed] [Google Scholar]
- 12.Li NF, Wang L, Zhou KM, et al. Analysis of Etiology of the Patients with Hypertension from the People's Hospital of Xinjiang Uygur Autonomous Region. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:865–868. [PubMed] [Google Scholar]
- 13.Schmiemann G, Gebhardt K, Hummers-Pradier E, et al. Prevalence of Hyperaldosteronism in Primary Care Patients with Resistant Hypertension. J Am Board Fam Med. 2012;25:98–103. doi: 10.3122/jabfm.2012.01.110099. [DOI] [PubMed] [Google Scholar]
- 14.Gatta-Cherifi B, Chabre O, Murat A, et al. Adrenal Involvement in Men1. Analysis of 715 Cases from the Groupe d'etude des Tumeurs Endocrines Database. Eur J Endocrinol. 2012;166:269–279. doi: 10.1530/EJE-11-0679. [DOI] [PubMed] [Google Scholar]
- 15.Rossi GP, Bernini G, Caliumi C, et al. A Prospective Study of the Prevalence of Primary Aldosteronism in 1,125 Hypertensive Patients. J am Coll Cardiol. 2006;48:2293–2300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 16.Hannemann A, Bidlingmaier M, Friedrich N, et al. Screening for Primary Aldosteronism in Hypertensive Subjects: Results from Two German Epidemiological Studies. Eur J Endocrinol. 2012;167:7–15. doi: 10.1530/EJE-11-1013. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Sippel RS, O'Dorisio MS, et al. The North American Neuroendocrine Tumor Society Consensus Guideline for the Diagnosis and Management of Neuroendocrine Tumors: Pheochromocytoma, Paraganglioma and Medullary Thyroid Cancer. Pancreas. 2010;39:775–783. doi: 10.1097/MPA.0b013e3181ebb4f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer E, Beuschlein F, Bidlingmaier M, et al. Commentary on the Endocrine Society Practice Guidelines: Consequences of Adjustment of Antihypertensive Medication in Screening of Primary Aldosteronism. Rev Endocr Metab Disord. 2011;12:43–48. doi: 10.1007/s11154-011-9163-7. [DOI] [PubMed] [Google Scholar]
- 19.Solar M, Malirova E, Ballon M, et al. Confirmatory Testing in Primary Aldosteronism: Extensive Medication Switching is not Needed in all Patients. Eur J Endocrinol. 2012;166:679–686. doi: 10.1530/EJE-11-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Born-Frontsberg E, Reincke M, Rump LC, et al. Cardiovascular and Cerebrovascular Comorbidities of Hypokalemic and Normokalemic Primary Aldosteronism: Results of the German Conn's Registry. J Clin Endocrinol Progressively Increased Patterns of Subclinical Cortisol Hypersecretion in Adrenal Incidentalomas Differently Predict Major Metabolic And Cardiovascular Outcomes: A Large Cross-Sectional Study. Metab. 2009;94:1125–1130. doi: 10.1210/jc.2008-2116. [DOI] [PubMed] [Google Scholar]
- 21.Di Dalmazi G, Vicennati V, Rinaldi E, et al. Eur J Endocrinol. 2012;166:669–677. doi: 10.1530/EJE-11-1039. [DOI] [PubMed] [Google Scholar]
- 22.Rossi GP, Seccia TM, Gallina V, et al. Prospective Appraisal of the Prevalence of Primary Aldosteronism in Hypertensive Patients Presenting with Atrial Flutter or Fibrillation (PAPPHY Study): Rationale and Study Design. J Hum Hypertens. 2013;27:158–163. doi: 10.1038/jhh.2012.21. [DOI] [PubMed] [Google Scholar]
- 23.Rossi GP, Bernini G, Desideri G, et al. Renal Damage in Primary Aldosteronism: Results of the PAPY Study. Hypertension. 2006;48:232–238. doi: 10.1161/01.HYP.0000230444.01215.6a. [DOI] [PubMed] [Google Scholar]
- 24.Asayama K, Kikuya M, Schutte R, et al. Home Blood Pressure Variability as Cardiovascular Risk Factor in the Population of Ohasama. Hypertension. 2013;61:61–69. doi: 10.1161/HYPERTENSIONAHA.111.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chioncel V, Paun D, Amuzescu B, et al. Evolution Features of Hypertensive Patients with Primary Aldosteronism – Prospective Study. J Med Life. 2012;5:354–359. [PMC free article] [PubMed] [Google Scholar]
- 26.Trifanescu RA, Poiana C. Update in Endocrinology - Primary Hyperaldosteronism - from Secondary Hypertension Towards Metabolic Syndrome and Beyond. Maedica (Buchar) 2012;7:90–91. [PMC free article] [PubMed] [Google Scholar]
- 27.Trifanescu RA. Primary Hyperaldosteronism - the Most Frequent Cause of Secondary Endocrine Hypertension. Acta Endo (Buc) 2012;8:523–527. [Google Scholar]
- 28.Arlt W. A Detour Guide to the Endocrine Society Clinical Practice Guideline on Case Detection, Diagnosis and Treatment of Patients with Primary Aldosteronism. Eur J Endocrinol. 2010;162:435–438. doi: 10.1530/EJE-09-0869. [DOI] [PubMed] [Google Scholar]