Abstract
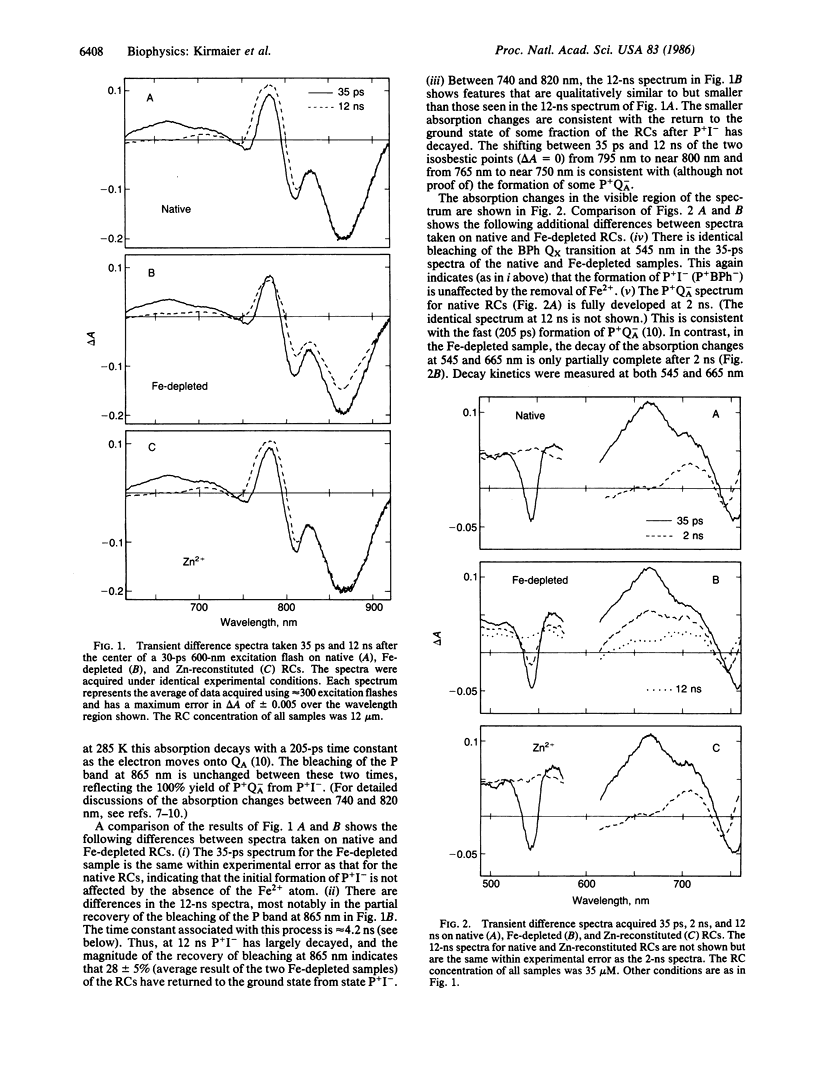
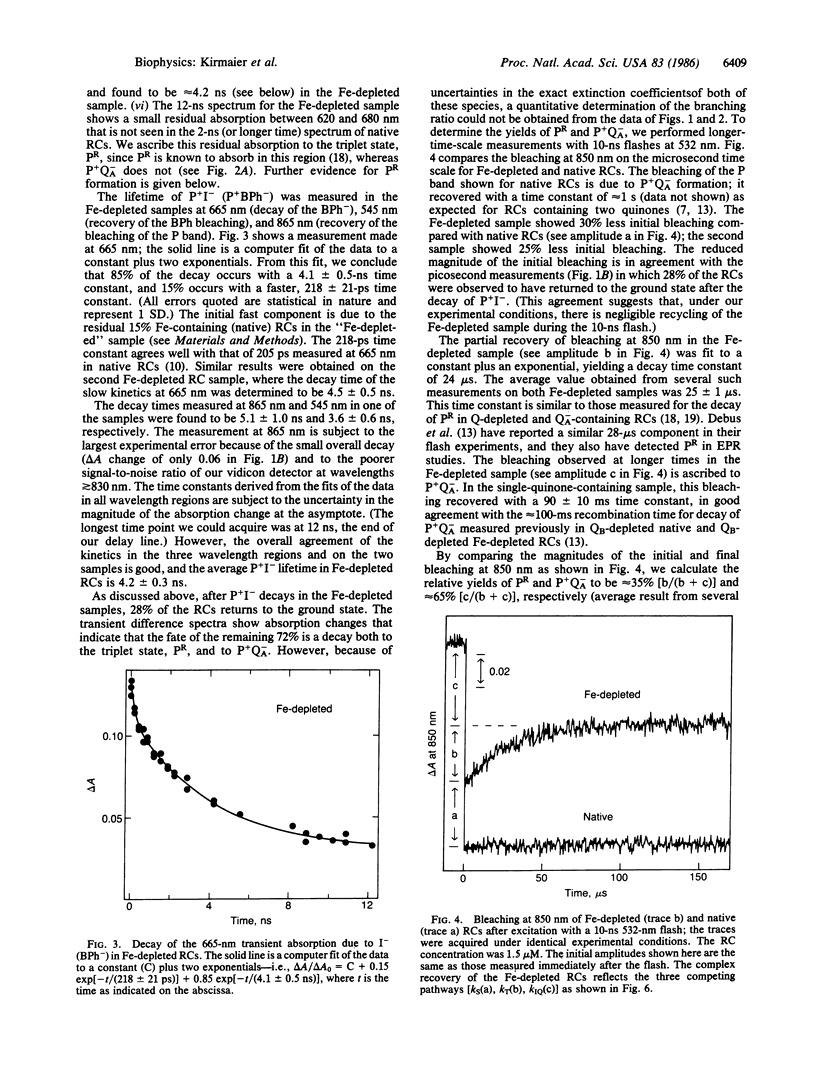
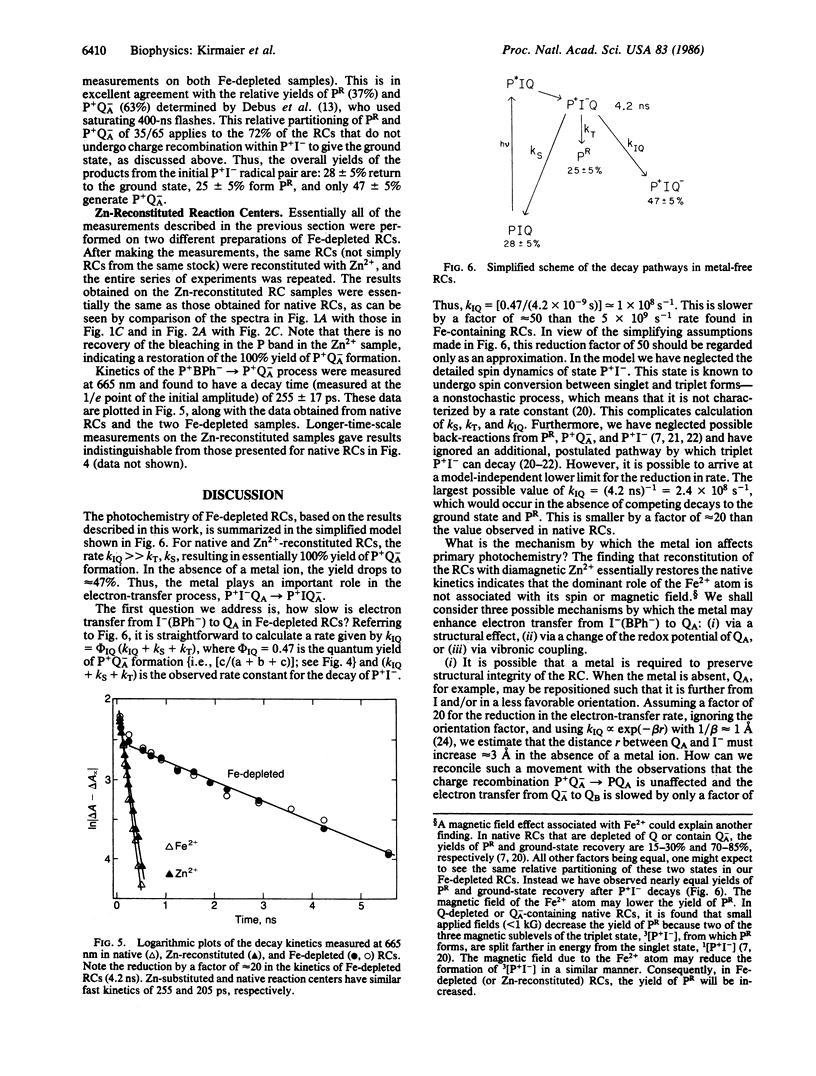
The primary photochemistry of Fe-depleted and Zn-reconstituted reaction centers from Rhodopseudomonas sphaeroides R-26.1 was studied by transient absorption spectroscopy and compared with native, Fe2+-containing reaction centers. Excitation of metal-free reaction centers with 30-ps flashes produced the initial charge-separated state P+I- (P+BPh-, where P is the primary donor and BPh is bacteriopheophytin) with a yield and visible/near-infrared absorption difference spectrum indistinguishable from that observed in native reaction centers. However, the lifetime of P+I- was found to increase approximately 20-fold to 4.2 ± 0.3 ns (compared to 205 ps in native reaction centers), and the yield of formation of the subsequent state P+QA- (QA is the primary quinone acceptor) was reduced to 47 ± 5% (compared to essentially 100% in native reaction centers). The remaining 53% of the metal-free reaction centers were found to undergo charge recombination during the P+I- lifetime to yield both the ground state (28 ± 5%) and the triplet state PR (25 ± 5%). Reconstitution of Fe-depleted reaction centers with Zn2+ restored the “native” photochemistry. Possible mechanisms responsible for the reduced decay rate of P+I- in metal-free reaction centers are discussed.
Keywords: photosynthesis, electron transfer, reaction center
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chidsey C. E., Takiff L., Goldstein R. A., Boxer S. G. Effect of magnetic fields on the triplet state lifetime in photosynthetic reaction centers: Evidence for thermal repopulation of the initial radical pair. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6850–6854. doi: 10.1073/pnas.82.20.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debus R. J., Feher G., Okamura M. Y. Iron-depleted reaction centers from Rhodopseudomonas sphaeroides R-26.1: characterization and reconstitution with Fe2+, Mn2+, Co2+, Ni2+, Cu2+, and Zn2+. Biochemistry. 1986 Apr 22;25(8):2276–2287. doi: 10.1021/bi00356a064. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Fajer J., Brune D. C., Davis M. S., Forman A., Spaulding L. D. Primary charge separation in bacterial photosynthesis: oxidized chlorophylls and reduced pheophytin. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4956–4960. doi: 10.1073/pnas.72.12.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher G., Hoff A. J., Isaacson R. A., Ackerson L. C. ENDOR experiments on chlorophyll and bacteriochlorophyll in vitro and in the photosynthetic unit. Ann N Y Acad Sci. 1975 Apr 15;244:239–259. doi: 10.1111/j.1749-6632.1975.tb41534.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann K. J., Dutton P. L., Netzel T. L., Leigh J. S., Rentzepis P. M. Picosecond kinetics of events leading to reaction center bacteriochlorophyll oxidation. Science. 1975 Jun 27;188(4195):1301–1304. doi: 10.1126/science.188.4195.1301. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D., Okamura M. Y., Feher G. Electron-transfer kinetics in photosynthetic reaction centers cooled to cryogenic temperatures in the charge-separated state: evidence for light-induced structural changes. Biochemistry. 1984 Nov 20;23(24):5780–5786. doi: 10.1021/bi00319a017. [DOI] [PubMed] [Google Scholar]
- Martin J. L., Breton J., Hoff A. J., Migus A., Antonetti A. Femtosecond spectroscopy of electron transfer in the reaction center of the photosynthetic bacterium Rhodopseudomonas sphaeroides R-26: Direct electron transfer from the dimeric bacteriochlorophyll primary donor to the bacteriopheophytin acceptor with a time constant of 2.8 +/- 0.2 psec. Proc Natl Acad Sci U S A. 1986 Feb;83(4):957–961. doi: 10.1073/pnas.83.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris J. R., Scheer H., Katz J. J. Models for antenna and reaction center chlorophylls. Ann N Y Acad Sci. 1975 Apr 15;244:260–280. doi: 10.1111/j.1749-6632.1975.tb41535.x. [DOI] [PubMed] [Google Scholar]
- Norris J. R., Uphaus R. A., Crespi H. L., Katz J. J. Electron spin resonance of chlorophyll and the origin of signal I in photosynthesis. Proc Natl Acad Sci U S A. 1971 Mar;68(3):625–628. doi: 10.1073/pnas.68.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson W. W., Clayton R. K., Cogdell R. J. Excited states of photosynthetic reaction centers at low recox potentials. Biochim Biophys Acta. 1975 May 15;387(2):265–278. doi: 10.1016/0005-2728(75)90109-7. [DOI] [PubMed] [Google Scholar]
- Rockley M. G., Windsor M. W., Cogdell R. J., Parson W. W. Picosecond detection of an intermediate in the photochemical reaction of bacterial photosynthesis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2251–2255. doi: 10.1073/pnas.72.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury N. W., Becker M., Middendorf D., Parson W. W. Picosecond kinetics of the initial photochemical electron-transfer reaction in bacterial photosynthetic reaction centers. Biochemistry. 1985 Dec 17;24(26):7516–7521. doi: 10.1021/bi00347a002. [DOI] [PubMed] [Google Scholar]
- Woodbury N. W., Parson W. W. Nanosecond fluorescence from isolated photosynthetic reaction centers of Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1984 Nov 26;767(2):345–361. doi: 10.1016/0005-2728(84)90205-6. [DOI] [PubMed] [Google Scholar]


