ABSTRACT
Background: Stent thrombosis (ST) is a rare, but extremely severe complication of PCI. Outside clinical trials, data are limited regarding the risks and the impact of this phenomenon.
Aims: To assess prevalence, predictors, and clinical outcome of ST after implantation of drug eluting stents (DES) compared with bare metal stents (BMS), in a large case-control study in a real world scenario, as well as the relation between ST and duration of combined antiplatelet treatment.
Methods: In a case-control registry we included 475 patients who received at least 1 DES (sirolimus, zotarolimus, everolimus, paclitaxel), compared with a group of 475 patients who received at least 1 BMS. We used 1.22 DES/patient vs. 1.26 BMS/patient (p=ns), treating 1.02 DES/lesion vs. 1.05 BMS/lesion (p=ns). Main outcome was ST defined by the Academic Research Consortium (ARC) as definite (acute, sub-acute, late), probable, and possible.
Results: At 15 months we found 0.8% (4) patients in the DES group vs. 1.1% (5) patients in the BMS group with definite ST (ns); 0.4% (2) patients from each group had acute ST, while 0.4% (2) vs. 0.7% (3) patients had sub-acute ST (both comparisons were ns). None of the patients from the DES group died, whereas two patients with definite ST from the BMS group died, with a case fatality rate of 40% (2/5). 0.2% (1) patient from each group had probable ST (ns) and 0.6% (3) vs. 0.4% (2) patients had possible ST (ns). Independent predictors of stent thrombosis in merged groups were antiplatelet therapy discontinuation (HR 3.8; 95%CI 1.9-7.6; p<0.01), diabetes (HR 2.15; 95%CI 1.4-5.1; p<0.01), a lower left ventricular ejection fraction (EF) (HR 1.1; 95%CI 1.0-1.9; p<0.01 for each 10% decrease), and LAD lesions (HR 1.0; 95%CI, 0.93-1.9; P<0.01).
Conclusions: ST is a rare complication (0.95%), similar after DES or BMS implantation. Premature discontinuation of antiplatelet therapy, followed by diabetes and a lower LVEF, are the independent predictors of ST.
INTRODUCTION
Context
The general concept from which this idea was launched can be found from some astonishing numbers concerning the first cases of late ST (ST) published in Lancet, early 2004 (1-2). Later on, in 2005 a very interesting meta-analysis was published, concerning the two existing types of drug eluting stents (DES) at the time, sirolimus eluting stent (SES) and paclitaxel eluting stent (PES). This meta-analysis shows an unexpectedly high rate of ST at 9 month follow up with a cumulative rate of 1.3%. However, ST was not found to be a relevant problem in the randomized trials in which clopidogrel was given with Aspirin in different periods: 2 months in E-SIRIUS, 3 months in SIRIUS and 6 months in TAXUS. Rates of ST were similar between DES and bare metal stents (BMS) - 0.6-1.4%. However, between 6 months and 2 years of follow up, a slight increase in the rate of ST was recorded, given the names late or very late ST for DES groups (3-4).
Despite meta-analyses that monitored patients treated with DES vs. BMS, it is quite difficult to draw a definitive conclusion about the risk of ST. As the main objectives of these studies were to bring out the risk of myocardial infarction and cardiac mortality or overall mortality, and not that of ST, nevertheless we can outline some conclusions:
-
1.
Due to the different patients profiles included in registries or in randomized trials, there are differences between data reported related to myocardial infarction, mortality and implicated ST. Also, the two registry types differ in terms of reported frequency of clinical objectives. If randomized trials show the same rate of incidence of MI and mortality for the use of DES vs. BMS, the registries could tilt the balance in favor of DES, in terms of mortality. Both studies concluded that target lesion revascularization (TLR) and target vessel revascularization (TVR) are reduced through the use of DES.
-
2.
In the first year of follow up, no statistical differences exists in the incidence of ST between DES and BMS; after the first year, this incidence is in favor of DES.
-
3.
Dual antiplatelet therapy has proved extremely important in the first 6 months after DES implantation, the ideal time duration is yet to be defined based on data that have not yet been published. Recommendation for treatment duration longer than 12 months is based on multiple analyses, which might be due to statistical combinations and manipulations, so the choice of appropriate duration of antiplatelet therapy must be clearly defined for each patient based on clinical presentation, associated comorbidities, how the procedure been undergone and the type of stent used.
In the absence of AMI, coronary lesions with visible thrombi or residual dissections, intra procedural ST is a rare event (0.7%) (5). A series of several prospective trials have demonstrated the benefits of GP IIbIIIa inhibitors in interventional revascularization. Administration of abciximab has significantly reduced the incidence of acute thrombotic complications with a considerable improved prognoses both short and long term, with maintained benefits for 3 years (6-7). In the EPIC trial, abciximab administration benefits were independent of lesion characteristics that were considered to be adverse (8).
Definitions
The lack of a universal definition for ST made it difficult to compare data when this problem began to be considered. Academic Research Consortium (ARC) took in consideration for the definition of ST the time when it occurred, and the certainty of occurrence. And so, the ARC definition was imposed and is the most used definition in literature so far. In terms of certainty of ST it is divided into:
-
-
Definite thrombosis, which has a high level of certainty, the diagnosis is made on angiographic or post-mortem evidence of thrombosis.
-
-
Probable thrombosis defined as sudden death in the first 30 days after implantation of a stent or any MI in a previously stented myocardial territory, regardless of the time of occurrence.
-
-
Possible thrombosis defined as any sudden death after 30 days of stent implantation.
Although the ARC definitions were imposed, they remain an imperfect point of balance between sensitivity and specificity: definite thrombosis is highly specific, but its true frequency is underestimated, while possible thrombosis has a higher sensitivity, but the certainty of diagnosis is very low. The latest studies exclude possible thrombosis term and select its targets using terms definite and probable to put a balance between sensitivity and specificity.
In terms of definite thrombosis, occurrence time it would be divided as follows:
METHODS
Objectives of the study: In this study we proposed tracking and evaluating the incidence of ST in patients treated with DES compared with BMS, and finding the relationship between ST and duration of antiplatelet therapy.
Coronary angiography was repeated for patients previously stented following the standard indications. Thus, all patients who had acute coronary syndrome, defined as any form of unstable angina or acute myocardial infarction underwent coronarography again. Patients who didn't have ACS underwent angiography according to the indications mentioned in AHA guidelines / ACC (12) at the time.
Lot Features: The registry was a case-control study in which were included consecutive patients, who received at least one DES, compared with patients who received at least one BMS, with matching gender and age; the incidence of ST was tracked using ARC definition of stent. The study was conducted between 2003 and 2008 and included 950 patients divided into two groups. The first group with a number of 475 consecutive patients each received at least one DES (578 DES were used, 268 were SES, 152 zotarolimus eluting stent – ZES, 122 everolimus eluting stent EES and 36 PES), the second group with a number of 475 consecutive patients, each received at least one BMS (598 BMS were used, BMS classification was not considered to be necessary). Having in mind the urgency of certain cases (acute coronary syndrome) we were unable to use DES in this type of patients, due to the local acquisition protocol of this type of prosthesis in our cath-lab. The study also did not include these types of patients in the BMS group for proper lot equilibrium. All patients were assessed at 1 month (clinical) at 6 months (by telephone) and 12 months (clinical) from the index procedure. For those patients who did not return to the clinic for follow-up information had their information taken by telephone. During follow-up (clinical and telephone) we inquired about recent adverse events and during the clinical visit we performed a standard clinical examination and 12 leads ECG. The study design dictated that the longest follow-up period to be of 1 year. Due to data published, relating to the high incidence of late and very late ST in DES patients, we later decided to extend the period of follow up to more than a year, with a telephone follow-up at 15 months since study inception.
Investigation Protocol: Eligible patients were fully informed about the purpose, procedures, risks and benefits of this registry and, in case of enrolling acceptance, were asked to sign and date the routine informed consent form for invasive procedures, which is standard protocol in our cath-lab. The study protocol aligns with ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institution's research committee where the research was conducted. Patients were treated in an academic hospital namely University Hospital of Bucharest. Clinical and demographic data, type of lesions, procedural complications, and proposed treatment were noted in the standard follow up forms.
Clinical examination: The following clinical parameters were précised: height, weight, waist-hip index, NYHA functional class, systolic and diastolic blood pressure and heart rate. Blood pressure was determined by following the current guidelines. Heart rate was measured using the radial pulse at the patient seated, with the upper limb in supine position.
Laboratory tests: Blood samples were collected in the Cardiology Clinic, University Hospital of Bucharest, not aimed at a specific protocol. The tests followed the standard protocol from the observation sheets. Whenever myocardial infarction was suspected after stent implantation, the diagnosis was thereby made based on increasing levels of Troponin I (TnI), and to avoid false positive results the 99th percentile was used as cut-off value. If TnI was not available, the diagnosis of myocardial infarction was based on elevated creatine kinase (CK) and creatine kinase MB (CK-MB) levels >1.5 times above normal during the first 48 hours or more than 3 times upper normal limit after 48 hours (13). Myocardial necrosis marker level assessment was accepted from any authorized laboratory, given the likelihood of a patient to present with AMI in another hospital than the one in which the stent was mounted.
Resting ECG: 12-lead ECG was performed before and after stent implantation (s) as a standard procedure in the Cardiology Clinic, University Hospital of Bucharest.
Echocardiography: was used to assess conventional structural parameters, parameters of global systolic and diastolic function (14). Data was collected from ultrasound analysis reports from the observation sheets.
Coronary angiography and angioplasty. During all the procedures performed, in our cath-lab, some cases required lesions preparation (predilation), thus the balloon size chosen were very close but slightly smaller than the stent. The stents used were pre-mounted. In this registry we used four types of DES: SES (Cordis®), PES (Boston Scientific®), ZES (Medtronic®), EES (Guidant® - later Abbot Vascular®) conform the internal policy of DES implantation in SUUB. All DES were implanted at high pressure (≥12 atm), without exceeding the rated burst pressure (RBP). If a suboptimal result was obtained, judging from visual estimation or quantitative coronary angiography (QCA) (15), the stent would be subsequently post dilated with a non-compliant balloon larger in size than the stent. Depending on vessel diameter, tortuosity, lesion length, location and operator preferences several BMS were used on occasion. All patients received unfractionated heparin bolus at a dose of 70-100 IU/kg before starting angioplasty. In case of procedural time extensions an ACT check would be needed and the patients would receive another dose of unfractionated heparin according to the standard protocol. The interventional strategy for periprocedural IIbIIIa glycoprotein use and antithrombotic medication was left to be decided by principal operator. Thrombotic stent occlusion was fully documented angiographically as complete thrombotic occlusion (TIMI flow 0 or 1), or restricted flow due to the presence of a thrombus with TIMI flow 1 or 2 in the artery treated with one or more stents in index procedure (16-18).
Statistics. For statistical data processing we used the SPSS program (Statistical Package for the Social Sciences) version 15 (19), and the statistical methods used were:
-
1.
Descriptive parametric statistics and non-parametric average with standard deviation or percentile using "unpaired Student t test";
-
2.
Data comparison with ANOVA (for discrete variables) or chi-square test and rank test (for non discrete variables);
-
3.
Univariate and multivariate regression analysis for needed correlations to be established specifically to identify independent predictors of MACE and long term restenosis;
-
4.
Odds ratio (OR) and 95% confidence intervals (CIs) were reported with probability value "two-tailed";
-
5.
Statistical significance was accepted at a p <0.05. ❑
RESULTS
Clinical Features. The general characteristics of all patients considered to receive DES compared with those treated with BMS according to local policy University Hospital of Bucharest are shown in Tables 1 and 2. There were no statistically significant differences between the 2 groups in terms of age, sex, height, weight, body mass index, NYHA functional class, systolic and diastolic blood pressure values and heart rate. There were no significant differences for risk factors other than diabetes (27% vs. 12%, p <0.01) and a history of MI (68% vs. 46%, p <0.01) when compared to BMS group. This data shows an indication expansion DES stenting for our hospital aligning with what is happening in hospitals in which the acquisition process for DES different, but not yet reaching their level. There was no drug related standardization received by patients during the periprocedural and follow up periods except for antiplatelet and anticoagulant medication. As follows:
Table 1.
The demographic characteristics of the studied groups.
| DES | BMS | p | |
|---|---|---|---|
| Age (years) | 59 ± 11 | 60 ± 11 | ns |
| Sex (% sex ♀) | 26% | 27% | ns |
| BMI (kg/m2) | 28 ± 5.8 | 27 ± 5.1 | ns |
| NYHA class | I-II | I-II | ns |
| Systolic BP (mmHg) | 129 ± 14 | 133 ± 17 | ns |
| Diastolic BP (mmHg) | 76 ± 9 | 78 ± 9 | ns |
| Heart rate (bpm) | 69 ± 9 | 72 ±10 | ns |
*BMI = body mas index; BP = blood pressure; NYHA = New York Heart Association.
Table 2.
Cardiovascular risc factor distribution for atherosclrosis.
| DES | BMS | p | |
|---|---|---|---|
| Smoking | 79% | 76% | ns |
| DM | 27% | 12% | <0.01 |
| Hypertension | 79% | 77% | ns |
| Dislipidemia | 85% | 83% | ns |
| Family history | 19% | 20% | ns |
| MI history | 68% | 46% | <0.01 |
| CVA history | 4.2% | 5% | ns |
* DM = diabetes mellitus; MI = miocardial infarction; CVA = cerebro-vascular accident.
- Aspirin (ASA) was administered as a loading dose of 250 mg in patients naive and a maintenance dose of 75-100 mg/day. Treatment duration was for life. All patients received ASA in the DES group, something we have considered mandatory in such patients, given the raised concerns about the likely incidence of ST in DES. Patients who had proven allergy to ASA didn't received DES, this being our domestic policy in our catheterization laboratory. Of those treated with BMS group 99% received aspirin. The 5 patients who did not receive ASA had proven intolerance and clopidogrel was recommended indefinitely. No major event related to ST (definite, probable or possible) occurred to this category of patients.
- The thienopyridines available during the study were ticlopidine and clopidogrel. Ticlopidine was never used. Clopidogrel was administered according the following defined protocol: patients who were not receiving this medication at all received a loading dose of 300 mg (if the procedure took place in ≥6 hours) or 600 mg (if the procedure took place in <6 h) prior to the procedure; patients who were on chronic clopidogrel therapy the 75 mg / day had their daily dose unaltered. Besides the acknowledged indications at that time in some difficult cases considered by us to be of high thrombosis risk, we recommended 150 mg clopidogrel for 7-30 days depending on the case, however these cases were not more than 8.4% in DES group, or 7.4% in the BMS (p = ns). In the fall of 2010 the study results CURRENT – OASIS 7 were published coming to reinforce the idea that the ACS patients treated with PCI and double dose clopidogrel benefit was over the conventional dose of 75 mg/day within 7 days the intervention (20-21). We found no reason to not give clopidogrel in any patient of the total 950 enrolled in the study, despite some mild side effects. With the exception of 4 patients in the DES group and 5 patients in the BMS group, all were on this type of treatment for 9 months, mainly due to the national program of reimbursement. Regarding patients who discontinued the treatment prematurely (3 patients in DES group and 4 in the BMS group), stopping clopidogrel was due to objective reasons such as various unexpected surgical procedures, but throughout the same period they remained on therapy with ASA and clopidogrel resumed at a maximum of 14 days after stopping for all patients. Time of discontinuation of clopidogrel in these patients was between the 155 days and 207 days after the procedure. No major event related to ST (definite, probable or possible) occurred in this category of patients. In each group there was one patient who stopped taking clopidogrel in the first week after discharge, but remaining on aspirin. Both had subacute ST objectified angiographically. After 9 months from the procedure, adherence to clopidogrel till the end of our follow-up to month 15 was 19% in the BMS group and 58% in the DES group (p <0.01) but the tendency to push the dual antiplatelet therapy further than one year, came with a recommendation that came after 2006.
- Of the two types of available heparin, unfractionated heparin (UFH) and low molecular weight heparin (LMWH) we used only UFH as a standard protocol: we gave bolus dose of 70-100 IU/kg before the procedure in patients naive, and if the patient was on continuous infusion of UFH or when the procedure was extended then ACT check was needed and UFH was administered according to the standard protocol. All patients received UFH. We did not administrate LMWH due to our laboratory protocol and lack of data imposing this type of medication over UFH in patients without ACS.
- IIbIIIa glycoprotein antagonists were administered with rates below that of other registries of the same type as ours. The only product eptifibatide was administered at a rate of 7.1% in the DES group vs. 6.3% in the BMS (p = ns). This drug was used abiding by the main operator preference, cases being chosen as complicated angiographically.
- Products such as direct thrombin inhibitor type bivalirudin were not available to be used.
Angiographic features. The materials used were the same, no major differences between the two groups, and here we mention arterial sheaths, diagnostic catheters, guide catheters, guide wires, angioplasty balloons semi-compliant or noncompliant. From the point of view of the DES types, they were selected from all types of stents used in the Cardiology Clinic University Hospital of Bucharest, during the registry period: SES 46%, PES 7%, ZES 26%, and EES 21%. These stents were stainless steel structure (SES, PES) or cobalt chrome (ZES, EES), permanent polymer, and drugs with cytostatic and antiproliferative roles.
The distribution of angiographic data for the entire study population is reported in Table 3. There were no significant differences between groups in the number of stents used, or vascular lesions treated. 1.02 stents/lesion were used in the DES group respectively 1.05 stents/lesion in the BMS group (p = ns). If we refer to the number of patients, 1.22 stents/patient were used in the DES group respectively 1.26 stents/patient in the BMS group (p = ns). The average of lesions per patient was 1.19 lesions/patient in the DES group respectively 1.20 lesions/patient in the BMS group (p = ns). Significantly more DES were used for dilation of LAD lesions (59% vs. 48%, p <0.01), as well as for ostial lesions (7% vs. 4%, p <0.01). Also, more DES were used for treatment of small caliber vessels, defined by us as being those vessels that have reference vessel diameter (RVD) <3 mm (71% vs. 41%, p <0.01) and vessels with diffuse atheromatous infiltration, defined by us as lesion length >15 mm (88% vs. 51%, p <0.01). These two definitions of diffuse vascular disease and small caliber vessels are consistent with data obtained from the related literature. Number of lesions treated for stent restenosis using another stent was overwhelmingly in favor of DES (8.0% vs. 0.4%, p <0.01). Basically a single patient in the BMS was restented also with BMS for the treatment of ISR and that due to reasons that were kept for strict patient safety. This difference in terms of ISR was expected, known as the indication there of for DES treatment. There were no statistically significant differences between the two groups when it came to bifurcation lesions, chronic total occlusion (CTO), and degenerated venous graft lesions. But, clearly we are obliged to underline the significantly lower frequency of these types of lesions treated when you compare them with those from the specific literature. The vast majority of bifurcation lesions were treated using the single stent technique in the main vessel, since it is well known that subacute ST and restenosis had a higher incidence when using two or more stents in this lesion type. All CTO had procedural success; however this doesn't show the reality of our catheterization laboratory, worthy to mention that these patients respectively were chosen from those with successful vessel opening. Patient cases with degenerated venous graft lesions were very few in number and this was mainly due to a low incidence of patients with aorto-coronary bypass addressed for angiography. All procedures were made without distal protection devices due to their lack in our laboratory, contrary to recommendations in the literature, due to the low number of such cases and strained economic conditions they were not seen necessary to equip the laboratory with such devices at that time, adding that the current circumstances are much different now.
Table 3.
Angiographic data distribution from INDEX procedure.
| DES | BMS | p | |
|---|---|---|---|
| INDEX PCI | |||
| Total number of stents | 578 | 598 | ns |
| Total number of lesions | 568 | 572 | ns |
| LAD lesions (%) | 59% | 48% | <0.01 |
| RVD <3.0 mm (%) | 66% | 44% | <0.01 |
| Lesion length >15 mm (%) | 84% | 56% | <0.01 |
| Bifurcation | 14% | 12% | ns |
| Chronic total occlusions | 6% | 6% | ns |
| Venous Grafts | 1% | 1% | ns |
| Ostial lesions | 7% | 4% | <0.01 |
| Preprocedural MLD (mm) | 0.4±0.2 mm | 0.4±0.2 mm | ns |
| Postprocedual MLD (mm) | 3.1±0.4 mm | 3.2±0.4 mm | ns |
| Acute Gain (mm) | 2.7±0.3 mm | 2.8±0.2 mm | ns |
* LAD = left anterior descending; RVD = refernce vessel diameter; MLD = minimal luminal diamter.
Main objectives results
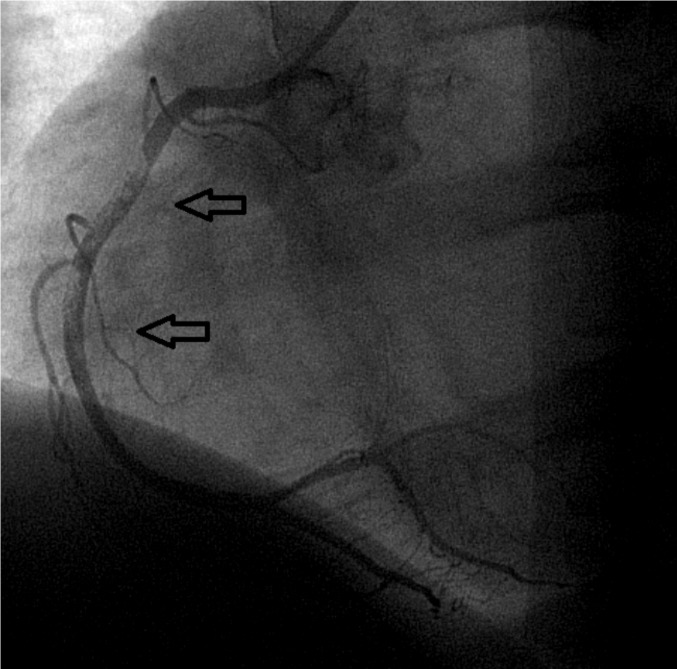
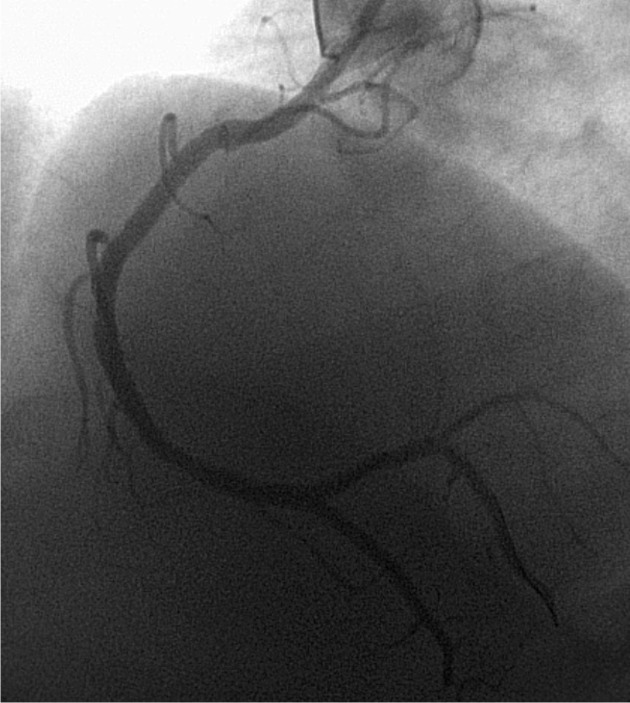
Definite thrombosis was objectified in 0.8% (4) in the DES group vs. 1.1% (5) in the BMS group (p = ns). Four patients had acute ST, two from each group (0.4%, p = ns), the remainder had subacute ST (0.4% vs. 0.7%, p = ns). There were no patients with late or very late ST. All the aforementioned cases are cases of angiographically objectified ST. Two patients with subacute ST, both located in the anterior descending coronary artery from the BMS group died during hospitalization, so rate case - fatality rate was 40% in the BMS group (2/5). One of the two dead patients stopped clopidogrel deliberated less than one week of discharge after the index procedure and was mentioned in the paragraph describing antiplatelet medication (Figures 1 and 2). In the DES group there were no deaths among those with definite ST.
Figure 1. Subacute BMS thrombosis, implanted in proximal left anterior descending artery.
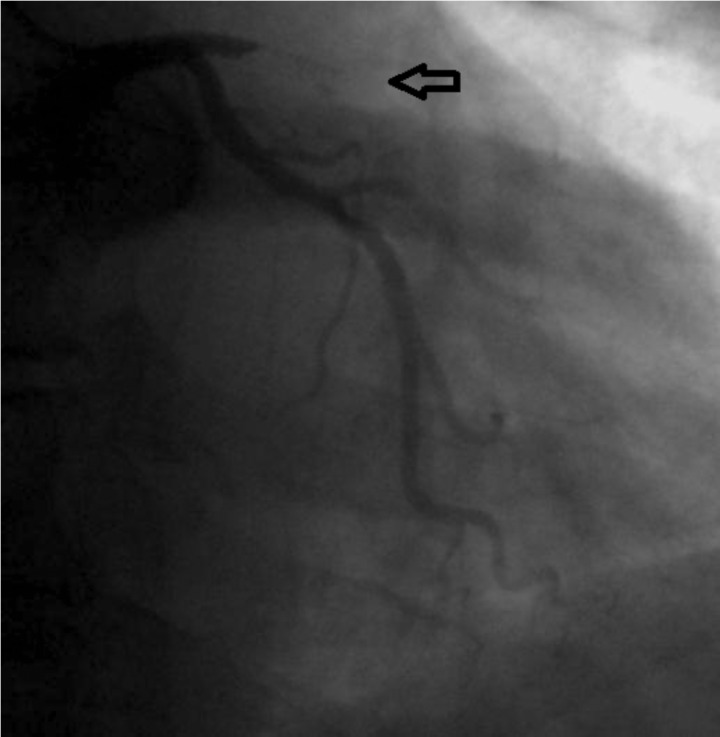
Figure 2. Balon angioplasty in the thrombozed stent in proximal LAD. The same case from Figure 1.
Probable ST was one case from each group. Thus, there was a sudden death in the first 30 days after stent implantation in the DES group and acute myocardial infarction in the BMS group with ECG changes in the territory served by the stented artery complicated with acute heart failure and death in less than 48 hours from admission. The latter patient did not have coronary angiography due to inability to ensure such a procedure neither post-mortem autopsy; therefore we eventually could not possibly objectify the presence of thrombus. Both patients were on treatment with aspirin and clopidogrel.
Possible thrombosis was reported in the DES group in 0.6% (3), and the BMS group 0.4% (2) (p = ns). Thus, in the DES group there were three sudden deaths, one on day 236 from the index procedure (patient was on dual antiplatelet treatment) and two in more than one year after stent implantation (patients were no longer on dual antiplatelet treatment, stopping clopidogrel at 9 months after the procedure). In the BMS group there were two sudden deaths, both occurred more than a year after angioplasty with stent (patients were receiving aspirin without clopidogrel). ❑
DISCUSSIONS
In 2006 the effervescence of the late ST reached its peak, following important discussions concerning the importance of the metaanalyses presented during the European Society of Cardiology held in Barcelona. The studies included in the meta analysis were RAVEL, SIRIUS, E-SIRIUS, C-SIRIUS, TAXUS II, IV, V and VI, studies that followed the first generation of DES, namely SES and PES. Those who have not accepted the idea said that the data indicate some concern that late ST is undiagnosed in patients treated with DES than with BMS, but never proven (3). In the same year, FDA raised the first questions about the long term safety of SES and PES. The survey BASKET (Basel Stent Cost-effectiveness Trial-late thrombosis events) highlighted the phenomenon of "Cath up" for late events like late myocardial infarction and sudden death in patients with premature withdrawal of double antiplatelet therapy in DES patients compared to BMS patients (4). In a meta-analysis of Maurice L. published in NEJM in 2007 after 1440 days of follow up, the incidence of ST was 1.2% for the patients treated with SES and 0.6% in the group treated with BMS (p = 0.20). As for patients treated with PES at the same follow-up period the incidence was 1.3% and 0.8% in the compared group (p = 0.24). The two curves started to breakup one year after stent implantation (5).
Due to new data published and obtained since the early use of DES and especially after 2006, as described above, related to the high incidence of late and very late ST after DES implantation, we decided to follow a large number of patients, which were all treated with DES in the Cardiology Clinic University Hospital of Bucharest (SUUB), for an initial period of 12 months, later extended to 15 months from study inception. We started from the premise that most of the literature related to ST comes from very few registries and randomized trials that had the primary objective of ST. This research assumes that real life differs significantly in certain circumstances, in comparison to randomized trials. DES use policy for all coronary lesions is difficult to implement due to current economic constraints. Thus we see necessary to pursue a controlled registry study, which includes, without exception, all patients consecutively treated with DES.
In a large cohort of consecutive patients treated with PCI with DES implantation, compared with patients treated with BMS (case control study), we reported on a period of 15 months follow-up, definite thrombosis incidence of 0.8% (4/475) in the DES group vs. 1.1% (5/475) in the BMS group (p = ns), nevertheless a substantially higher rate than the reported cases in large randomized trials (between 0.4% and 0.6%). The difference between our results and data from literature is due to the type of the studies we compare. Majority data for the literature are from randomized trials with particular inclusion/exclusion criteria. Unfortunately ST was not set as a main objective, date we got being as surrogate end-points. Our registry, as we mentioned before, has only one exclusion criteria, one which is particular for our country. So, we try to illustrate, as much as we can, the real life that we got in the cath lab, having the ST as the main objective. If you collect all cases of ST (definite, probable, possible) that being called cumulative ST, we will have an incidence of 1.7% (16/475) for each group (p = ns) (22-23). Independent predictors for cumulative ST are antiplatelet therapy discontinuation (HR 3.8; 95%CI 1.9-7.6; p<0.01), diabetes (HR 2.15; 95%CI 1.4-5.1; p<0.01), a lower left ventricular ejection fraction (EF) (HR 1.1; 95%CI 1.0-1.9; p<0.01 for each 10% decrease), and LAD lesions (HR 1.0; 95%CI, 0.93-1.9; p <0.01).
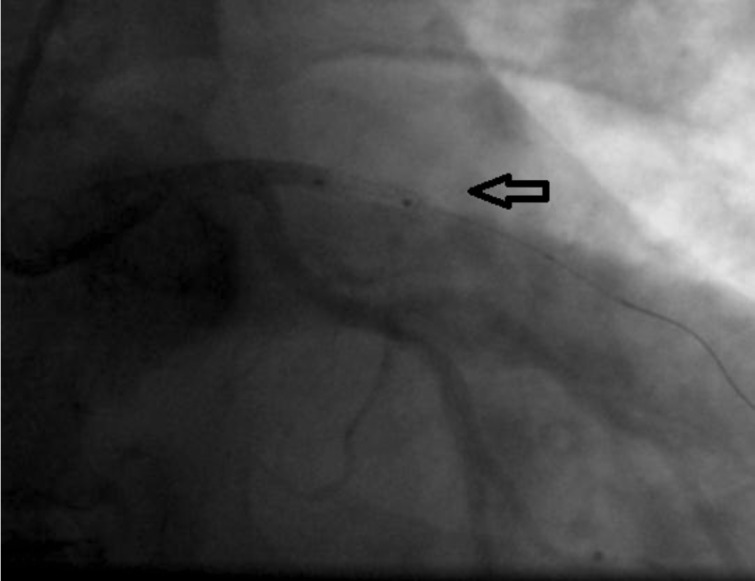
DES treatment indications have expanded in recent years, which is why more and more complex lesions, very often in patients with complex medical problems have been solved with this type of stent. Thus, more than a quarter of patients treated with DES were diabetic and over half of them had a history of an acute coronary event. Significantly more pharmacologically active stents were used for the treatment of small vessel lesions and diffuse lesions (Figures 3 and 4), which thereby increase the risk of ST. Also, the LAD lesions were preferably treated with DES, which makes the prognosis of ST in these cases to be a reserved one. Similar records that have followed patients treated with DES or BMS, discontinuation of dual antiplatelet therapy weighed more in ST, for definite (1 case of 4 i.e. 25% for the DES group and 1 case of 5 or 20 % for the BMS group, p = ns) as well as for cumulative thrombosis (3 cases out of 204, i.e. 1.5% and 3 cases of 388 i.e. 0.8%, p = 0.02), which supports idea that patients treated with DES, the kind pursued in this registry should be maintained on dual antiplatelet medication for more than one year after implantation (24). Besides discontinuation of antiplatelet agents, other factors that weighed in the balance tilting towards ST were diabetes, LVEF <30% and LAD lesions (25).
Figure 3. Subacute DES thrombosis, implanted in medio-proximal segment of right coronary artery.
Figure 4. Final vessel aspect after thromb-aspiration. Same case from Figure 3.
Diabetes is known to induce a hyper coagulant status. If we consider the association of diabetes induced nephropathy in particular, the risk of ST rises up to 4-fold (26-27). In our study, we have in the the DES group 2 cases of definite ST with diabetes out of a total of 128 cases of diabetes mellitus (2/128 1.6%, p <0.01), on the other hand in the BMS group (1/57 1.8%, p <0.01), and the cumulative thrombosis (4/128 3.1%, p <0.01) and in the BMS group (3/57 5.3%, p <0.01) (28-31).
Moderate to severe depression of LV pump function (LVEF <30%) was shown to have an important role in the increased incidence of ST. The literature data is not consistent with clear mechanisms by which depressed LV systolic function leads to ST, but the modified rheological condition could have the most important role (32-33).
LAD lesions had an important role in ST in our registry and were strongly associated especially with moderate - severe depressed LVEF (33-35).
No correlation was observed between stent type and ST. Data from the literature seem to tip the scale towards increased incidence of ST after using stents with paclitaxel. After 6 years since these ST issues were first raised, the data is still contradictory and of absolute need for randomized trials, including the most important commonly used DES in current practice, with imperative very long follow-up (35).
Study limits. A limitation of the current study was the poor angiographic documentation. However, case partitioning according to ARC definition can be considered to be very close to real life, drawing attention to those cases with a high likelihood of ST, which may otherwise be overlooked (15-16). We took the potential risk of adverse events overlooking lack of their proper reporting, but we are convinced that we have achieved a high rate of patient tracking, having no patients lost to follow-up. Initially, the study design was intended for one year follow up, later was extended with another telephone follow up three months later, this being appreciated by us as a method of increasing objective reporting. We assumed the risk of losing the very late ST patients especially in the DES group, but when we had started the registry this fact was not a reality. It was not in our protocol to test patients with ST for clopidogrel resistance, because this test was not in our local laboratory standard of care, but we referred the patients to hematology specialist for this particular test. Few and inconsistent data were available as we did not take in consideration. We tried to perform IVUS for all the definite ST, but due to the objective reason this was not possible. For the cases we did, no procedure related or human error ST related was found. No mismatch between stents and vessel diameter was found. For the case we did not perform IVUS, we reviewed the initial protocol and recorded data; high pressure deployment was used for stents and no spontaneous or provoked dissection was found. ❑
CONCLUSIONS
The cumulative incidence of ST (sum of all three entities defined by the ARC) is 1.7% (8/475) for each group. The incidence of definite and probable ST, which according to experts is the most important, is 1.1% vs. 1.3% in the DES, BMS respectively (p=ns). Stopping antiplatelet medication prematurely weighed most in favor of ST. Along with this factor, diabetes, LVEF <30% and LAD lesions are identified as independent predictors of cumulative ST. No correlation was found between ST and stent type used. There is a tendency towards correlation in terms of the stent length with thrombosis, but with no statistical significance. This trend may be due to high-pressure stent implantation and post dilation processes, factors known to decrease the incidence of thrombotic events. The clinical consequences were extremely serious. Thus, the case - fatality in the BMS group was 40%, and the majority of the other patients with ST, had nonfatal myocardial infarction. ❑
CONFLICT OF INTEREST
Claudiu Stoicescu received, in 2008, a one-year fellowship in Leicester, UK from the European Society of Cardiology/European Association of Percutaneous Coronary Intervention, which was random sponsored by Cordis, Johnson & Johnson, Miami Lakes FL 33014, USA.
FINANCIAL SUPPORT
none declared.
References
- 1.Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, Predictors, and Outcome of Thrombosis after Successful Implantation of Drug-Eluting Stents. JAMA. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 2.McFadden EP, Stabile E, Regar E, et al. Late Thrombosis in Drug-Eluting Coronary Stents after Discontinuation of Antiplatelet Therapy. Lancet. 2004;364:1519–1521. doi: 10.1016/S0140-6736(04)17275-9. [DOI] [PubMed] [Google Scholar]
- 3.Nordmann A, Briel M, Bucher H. Safety of Drug Eluting Stents: Insights from a meta-analysis.. In: European Society of Cardiology; 2006; Barcelona. [Google Scholar]
- 4.Pfisterer M, Brunner-La Rocca HP, et al. for the BASKET-LATE Investigators. Late Clinical Events After Clopidogrel Discontinuation May Limit the Benefit of Drug-Eluting Stents: An Observational Study of Drug-Eluting Versus Bare-Metal Stents; JACC. 2006;10:26–26. doi: 10.1016/j.jacc.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Topol, textbook of intervantional cardiology 5th edition 2008
- 6.Glycoprotein IIb/IIIa Receptor Inhibition with Abciximab with Lower Heparin Dosages During Percutaneous Revascularization. N Engl J Med. 1997;336:1689–1696. doi: 10.1056/NEJM199706123362401. [DOI] [PubMed] [Google Scholar]
- 7.Brener S, Barr L, Burchenal J, et al. A Randomized, Placebo Controlled Trial of Abciximab with Primary Angioplasty for Acute MI. The RAPPORT trial. Circulation. 1997;96(supplI):I–473. [Google Scholar]
- 8.Topol EJ, Ferguson JJ, Weisman HF, et al. Long-term Protection from Myocardial Ischemic Events in a Randomized Trial of Brief Integrin beta3 Blockade with Percutaneous Coronary Intervention. EPIC Investigator Group. Evaluation of Platelet Iib/IIIa Inhibition for Prevention of Ischemic Complications. JAMA. 1997;278:479–484. doi: 10.1001/jama.278.6.479. [DOI] [PubMed] [Google Scholar]
- 9.Cutlip DE, Windecker S, Mehran R, et al. Clinical End Points in Coronary Stent Trials: A Case for Standardized Definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 10.Krucoff MW. The Academic Research Consortium Governance Charter. J Am Coll Cardiol Intv. 2011;4:595–596. doi: 10.1016/j.jcin.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Smith Jr. S. ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Summary Article. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/SCAI Writing Committee to Update the 2001 Guidelines for Percutaneous Coronary Intervention. Circulation. 2006;113:156–175. doi: 10.1161/CIRCULATIONAHA.105.170815. [DOI] [PubMed] [Google Scholar]
- 12.King SB 3rd, Smith SC Jr, Hirshfeld JW Jr, et al. 2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee. Circulation. 2008;117:261–295. doi: 10.1161/CIRCULATIONAHA.107.188208. [DOI] [PubMed] [Google Scholar]
- 13.Alpert JS, Thygesen K, Antman E, et al. Myocardial Infarction Redefined – A Consensus Document of the Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. J Am Coll Cardiol. 2000:36959–969.969. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 14.Gottdiener JS. American Society of Echocardiography Recommendations for Use of Echocardiography in Clinical Trials. J Am Soc Echocardiogr. 2004;17:1086–1119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Nichols AB, Gabrieli CF, Fenoglio JJ Jr, et al. Quantification of Relative Coronary Arterial Stenosis by Cinevideodensitometric Analysis of Coronary Arteriograms. Circulation. 1984;69:512–512. doi: 10.1161/01.cir.69.3.512. [DOI] [PubMed] [Google Scholar]
- 16.Gibson CM, Braunwald E, et al. TIMI Frame Count: A Quantitative Method of Assessing Coronary Artery Flow. Circulation. 1996;93:879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 17.Manginas A, Cokkinos DV, et al. Estimation of Coronary Flow Reserve Using the Thrombolysis In Myocardial Infarction (TIMI) Frame Count Method. Am J Cardiol. 2000;83:1562–1565. doi: 10.1016/s0002-9149(99)00149-6. [DOI] [PubMed] [Google Scholar]
- 18.Gibson CM, Murphy S, Menown I, et al. Determinants of Coronary Blood Flow after Thrombolytic Administration. TIMI Study Group. Thrombolysis in Myocardial Infarction. J Am Coll Cardiol. 1999;34:1403–1412. doi: 10.1016/s0735-1097(99)00397-6. [DOI] [PubMed] [Google Scholar]
- 19.http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cftopic/pma/pma.cfm?num=p020026; 24 Apr 2003
- 20.The CURRENT-OASIS Investigators. Dose Comparisons of Clopidogrel and Aspirin in Acute Coronary Syndromes. N Engl J Med. 2010;363:930–942. doi: 10.1056/NEJMoa0909475. [DOI] [PubMed] [Google Scholar]
- 21.Mehta SR, Tanguay JF, Eikelboom JW, et al. Double-dose versus Standard-dose Clopidogrel and High-dose versus Low-dose Aspirin in Individuals Undergoing Percutaneous Coronary Intervention for Acute Coronary Syndromes (CURRENT-OASIS 7): A Randomised Factorial Trial. Lancet. 2010 doi: 10.1016/S0140-6736(10)61088-4. [DOI] [PubMed] [Google Scholar]
- 22.Stone GW, Ellis SG, Cox DA, et al. A Polymer-Based, Paclitaxel-Eluting Stent in Patients with Coronary Artery Disease. N Engl J Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 23.Moses JW, Leon MB, Popma JJ, et al. Sirolimus-Eluting Stents versus Standard Stents In Patients with Stenosis in A Native Coronary Artery. N Engl J Med. 2003;349:1315–23. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 24.Moussa I, Di Mario C, Reimers B, et al. Subacute Stent Thrombosis in the Era of Intravascular Ultrasound-Guided Coronary Stenting without Anticoagulation: Frequency, Predictors and Clinical Outcome. J Am Coll Cardio. 1997;29:6–12. doi: 10.1016/s0735-1097(96)00452-4. [DOI] [PubMed] [Google Scholar]
- 25.Cheneau E, Leborgne L, Mintz GS, et al. Predictors of Subacute Stent Thrombosis: Results of A Systematic Intravascular Ultrasound Study. Circulation. 2003;108:43–47. doi: 10.1161/01.CIR.0000078636.71728.40. [DOI] [PubMed] [Google Scholar]
- 26.Singer DE, Moulton AW, Nathan DM. Diabetic Myocardial Infarction: Interaction of Diabetes with Other Preinfarction Risk Factors. Diabetes. 1989;38:350–357. doi: 10.2337/diab.38.3.350. [DOI] [PubMed] [Google Scholar]
- 27.Smith JW, Marcus FI, Serokman R. Prognosis of Patients with Diabetes Mellitus after Acute Myocardial Infarction. Am J Cardiol. 1984;54:718–721. doi: 10.1016/s0002-9149(84)80196-4. [DOI] [PubMed] [Google Scholar]
- 28.Laakso M. Diabetes and Cardiovascular Disease in Type 2 Diabetes: Challenge for Treatment and Prevention. J Intern Med. 2001;249:225–235. doi: 10.1046/j.1365-2796.2001.00789.x. [DOI] [PubMed] [Google Scholar]
- 29.Juutilainen A, Laakso M, et al. Gender Difference in the Impact of Type 2 Diabetes on Coronary Heart Disease Risk. Diabetes Care. 2004;27:2898–2904. doi: 10.2337/diacare.27.12.2898. [DOI] [PubMed] [Google Scholar]
- 30.Gu K, Cowie CC, Harris MI. Diabetes and Decline in Heart Disease Mortality in US Adults. JAMA. 1999;281:1291–1297. doi: 10.1001/jama.281.14.1291. [DOI] [PubMed] [Google Scholar]
- 31.Haffner SM, Lehto S, Rönnemaa T, et al. Mortality from Coronary Heart Disease in Subjects with Type 2 DM and in non DM Subjects with and without Prior MI. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 32.Jean-Pierre Bassand J-P, Hamm CW, Ardissino D, et al. Guidelines for the Diagnosis and Treatment of Non-St-Segment Elevation Acute Coronary Syndromes. Eur Heart J. 2007;28:1598–1660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 33.Jeremias A, Sylvia B, Bridges J, et al. Stent Thrombosis after Successful Sirolimus-Eluting Stent Implantation. Circulation. 2004;109:1930–1930. doi: 10.1161/01.CIR.0000127105.99982.21. [DOI] [PubMed] [Google Scholar]
- 34.Amann K, Ritz E. Cardiac Disease in Chronic Uremia: Pathophysiology. Adv Ren Replace Ther. 1997;4:212–224. doi: 10.1016/s1073-4449(97)70030-x. [DOI] [PubMed] [Google Scholar]
- 35.Ge L, Airoldi F, Iakovou I, et al. Clinical and Angiographic Outcome after Implantation of Drug-Eluting Stents in Bifurcation Lesions with the Crush Stent Technique Importance of Final Kissing Balloon Postdilation. J Am Coll Cardiol. 2005;46:613–620. doi: 10.1016/j.jacc.2005.05.032. [DOI] [PubMed] [Google Scholar]