ABSTRACT
Postinfectious arthritis is a relatively often encountered in pediatric practice. The authors present the most important data concerneing this pathology, with up to date informations exemplifying with case presentations. Clinical cases bring to attention the most common forms of postinfectious arthritis (reactive arthritis, postinfectious arthritis bacterial, viral, spirochete, and so on). Although highly studied and commonly found in current pediatric practice, arthritis occurring after infections remains controversial entities, especially regarding terminology. While, according to some authors, postinfectious arthritis belongs to the large group of reactive arthritis, by other authors, these joint events are independent entities.
Keywords: arthritis, postinfectious arthritis, child
INTRODUCTION
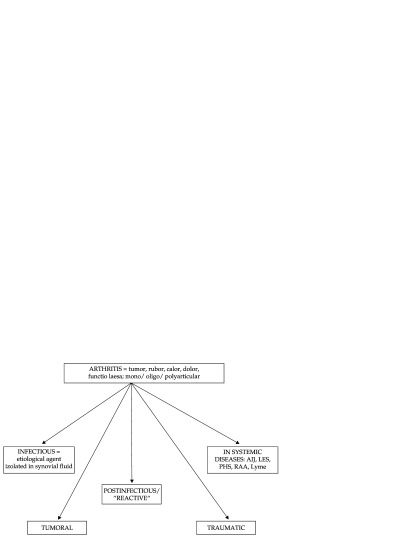
Arthritis is defined as a restriction of joint movement, associated with swelling, heat, redness and pain at mobilization. In contrast to the symptoms, arthralgia is joint pain without limitation of the joint movement, and without inflammatory phenomena. This pathology poses a diagnostic problem in pediatric practice (1). Its annual incience is 0.6-27/ 100,000 (2). With varied etiologies, arthritis is a common cause of presentation to your pediatrician (Figure 1).
Figure 1. Pediatric arthritis - classification.
The most common arthritis in children recognizes acute installed causes. Among these causes: traumatic (sprain, dislocation, fracture etc.) intra- or postinfectious causes (septic arthritis, osteomyelitis, viral arthritis, poststreptococcal arthritis etc.), inflammatory/immunological causes (transient synovitis, reactive arthritis, etc.), tumors (bone tumors, metastases, etc.), degenerative diseases (Legg Calve Perthes disease, osteochondritis dissecans etc.).
This article will present exclusively postinfectious arthritis occurring in the evolution of various infections specific in childhood. ❑
CASE REPORTS
For a better understanding of the topic, four clinical cases were selected, clinically and etiologically representative.
Case 1: a 3 years old boy was admitted into the hospital, presenting swelling of right ankle, associated with fever (39-40°C) and gastrointestinal symptomatology (vomiting, abdominal pain and bloody, mucous stools). At the presentation he had impaired general status, clinical signs of acute dehydration (5-7%), abdominal pain at palpation, swelling of the right ankle with classical signs of acute inflammation pain, heat, redness, and limitation of movement. Laboratory investigations revealed leukocytosis with neutrophilia (WBC = 13700/µL, Ne = 82%) positive inflammatory markers (CRP = 15mg/dl, ESR = 40 mm/h, fibrinogen = 550 mg/dl) hyposideremic, microcytic, hypochromic anemia (Hgb = 10.7 g/dl; serum iron = 30 ng/dL). Bacteriological studies of stool samples identified Shigella flexneri. The diagnosis was: acute gastroenteritis with Shigella flexnerii, acute dehydration, and right ankle arthritis. Laboratory investigations were completed to identify the cause of arthritis. Assessment of renal and hepatic function was normal. Determination of antinuclear antibodies (ANA) and of rheumatoid factor were normal as well as ASLO and viral serology (CMV, EBV, HSV, ECHO, Coxsackie, HIV, HCV, HBV). Serology for Mycoplasma and Chlamydia were negative. Antibiogram guided antibiotic therapy was initiated and nonsteroidal anti-inflammatory therapy. Evolution was favorable with gradual remission of digestive symptoms and of arthritis after 7 days of treatment. In the 10th day of treatment clinical evaluation of the child at was normal and stool culture was negative. It is important to mention that gastrointestinal infection caused by Shigella flexnerii can be followed after several weeks by arthritis. Shigella is one of the classical bacterial agents responsible for the occurrence of postinfectious arthritis (Reiter syndrome). The case presented here is unique because arthritis occurred after a very short period (5 days) from the onset of digestive symptoms.
Case 2: A 12 years old girl was admitted to the hospital to be investigated in order to identify the causes of right knee swelling accompanied by high fever (39°C).
On admission, the patient was febrile, with impaired overall condition, significant swelling in the right knee with limitation of movement. Recently she had appendectomy and in the right iliac fossa signs of local inflammation and tenderness were present, adjacent to the linear scar. In the history of the disease it was mentioned that 10-14 days prior to the occurrence of arthritis, the child had fever (39-40°C), abdominal pain and diarrhea. Abdominal pain gradually intensified, the patient having clinical signs of appendicitis. Appendectomy was performed and antibiotherapy with ceftriaxone was administered. After 4 days of favorable evolution the child has been discharged from hospital. In the third day after discharge, arthritis of the right knee occurred and the patient was readmitted.
Laboratory investigations were repeated: blood count showed leukocytosis with neutrophilia, inflammatory markers were highly positive and stool culture was positive for Yersinia enterocolitica. Other investigations performed (viral and bacterial serology, rheumatoid factor, ANA, liver function, kidney, etc.) were normal. Clinical and laboratory reassessment led to the diagnosis of reactive arthritis following gastrointestinal infection with Yersinia enterocolitica. The patient received antibiotherapy (Trimethoprim/sulfometoxazol) and anti-inflammatory agents (NSAIDs) for 10 days with gradual remission of joint swelling.
This case of digestive infection with Yersinia enterocolitica was presented because – pseudoappendicular onset is commonly encountered in this type of infection and, in most cases, is a diagnosis pitfall. Often patients are diagnosed as having a surgical pathology and thus are undergoing surgery. We appreciate the importance of presenting this case in the light of evolution, considering it a typical postinfectious arthritis with this bacterium as the etiologic agent.
Case 3: An 18 months old girl was hospitalized for fever, watery diarrhea and vomiting. Symptoms progressively worsened which resulted in the hospitalization of the child to just 24 hours after the onset of clinical manifestations. On admission the child had an impaired general status, was febrile, pale, having clinical signs of acute dehydration of 5-7%. Laboratory investigations performed at admission revealed leukopenia with neutropenia (WBC = 2900/µL, Ne = 20%), hypoglycemia, mild metabolic acidosis respiratory compensated, hyponatremia, moderately raised inflammatory markers. Stool cultures were negative, but fecal antigen determination for Rotavirus was positive. The diagnosis of rotavirus acute gastroenteritis, acute dehydration syndrome 5-7% was established. Treatment included: endovenous rehydration and electrolyte rebalancing, diet, symptomatic medication. Progressive improvement of symptoms was recorded. Three days after digestive symptoms have been remitted bilateral knee arthritis occured. The child was readmitted and paraclinical investigation were repeated (viral serology, bacteriological studies, acute phase reactants, immunology, etc.). Laboratory investigations were normal. Treatment with NSAID induced remission of joint inflammatory symptoms after 5 days. Postviral arthritis after Rotavirus enterocolitis is uncommon. In literature there is not mentioned postinfectious arthritis as a complication of infection with rotavirus. This event imposes close supervision of children who develop rotavirus gastroenteritis to identify rare complications of the disease). In support of this hypothesis came more arguments: isolation of rotavirus in faeces in a patient with digestive infection previously to arthritis occurance, the benign clinical course of arthritis that was of short duration and not followed by sequelae, laboratory investigations that have ruled out other possible causes of arthritis (because the age of the patient was not typical, as well as the clinical picture, onset of juvenile arthritis was initially brought in discussion, but paraclinical data did not sustain this possibility, other possible causes of athritis were excluded viral serology).
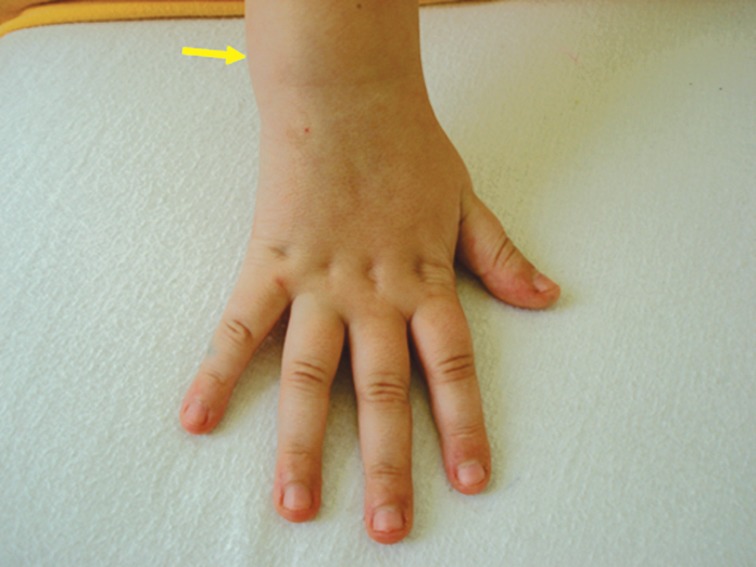
Case 4: A 6 years 11 months old girl was admitted to the hospital with localized joint swelling of the right wrist (Figure 2) associating limitation of movement. On admission the child had wrist arthritis, accompanied by maculoeritematous itchy rash. Evolution of long duration (about 6 weeks) of joint phenomena, associated with rash raised suspicion of chronic juvenile arthritis (JRA) with oligoarticular onset. Laboratory investigations (blood count, rheumatoid factor, ANA) were negative, inflammatory markers (ESR, fibrinogen, C-reactive protein) were normal, and lack of response to the treatment with nonsteroidal anti-inflammatory agents (NSAIDs), required reevaluation a possible postinfectious reactive arthritis was put in discussion. Viral serology for HSV, CMV, EBV, ECHO, Coxsackie have been performed all of them being negative. Serology to Borellia Burgdoferi confirmed by Western blot documented borelliosis. The diagnosis was Lyme disease; stage III (articular manifestations). Treatment included antibiotics associated with NSAIDs. Evolution was favorable with completely remission of joint symptoms, without squeals. ❑
Figure 2. Swelling of the right wrist in a 6 years girl (clinical case 4).
DISCUSSIONS
In 1969 terminology of reactive arthritis was for the first time adopted, to define arthritis that occur during or immediately after an extraarticular infection, without being able to isolate the etiologic agent that produced inflammatory joint fenomna. Initially, only arthritis that appeared after gastrointestinal or genitourinary infections caused by certain bacteria (Salmonella, Shigella, Chlamydia, Yersinia and so on) were included into this category (Table 1). The prevalence of reactive arthritis in yersiniosis patients was reported to 12%, in some studies, comparing to 5% articular symptoms in a reference group (3). This arthritis was responsible for the classic form of Reiter's syndrome (1,4-6). Postinfectious arthritis is considered by some authors as reactive arthritis belonging to the large group of reactive arthritis (1). Postinfectious arthritis delineation of reactive arthritis is controversial.
Table 1.
Gastrointestinal/ Urogenital pathogens associated with arthritis.
| Salmonella sp. |
| Shigella flexnerii |
| Campilobacter jejuni |
| Yersinia enterocolitica |
| Chlamydia trachomatis |
| Escherichia coli |
| Clostridium difficile |
| Chlamydia pneumoniae |
(Adapted from: David T Yu et Colab. – Formerly Reiter syndrome- Reactive Arthritis; 2012 oct.; uptodate.com)
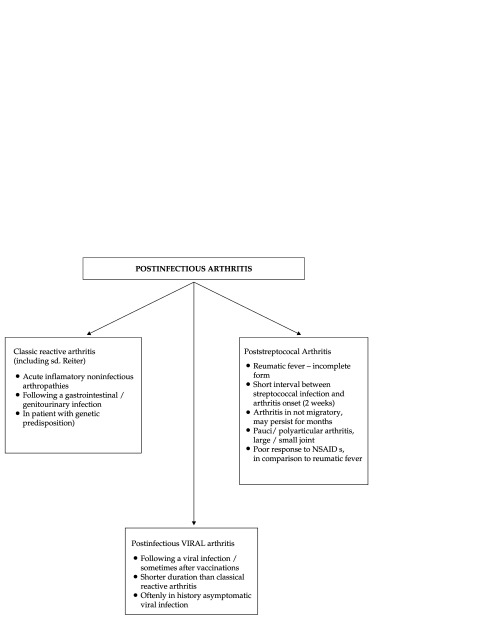
Currently cases of arthritis that begin after infections of different etiologies and different extraarticular locations have been described, other than gastrointestinal or urinary infections. In Figure 3 postinfectious arthritis classification used in pediatric practice is presented.
Figure 3. Postinfectious Arthritis – Clasiffication.
The great majority of postinfectious arthritis occur during or after an extraarticular viral infection (Rubella, Parvovirus B19, Hepatitis B, etc.) (Table 2) but also with / or after the administration of certain vaccines (rubella vaccination, influenza, etc.) (2,4,7).
Table 2.
Viruses asociated with arthritis.
| Rubella | Coxsackievirus B |
| Parvovirus B19 | Togaviruses |
| Hepatitis B | Rubivirus |
| Hepadnavirus | Ross River |
| Adenoviruses | Chikungunya |
| Adenovirus 7 | O'nyong-nyong |
| Herpesviruses | Mayaro |
| Herpes simplex | Sindbis |
| Cytomegalovirus | Ockelbo |
| Epstein-Barr | Pogosta |
| Varicella-zoster | Orthopoxviruses |
| Paramyxoviruses | Variola virus (smallpox) |
| Mumps | Alphaviruses |
| Enteroviruses | Vaccinia virus |
| Echovirus | |
| * Influenza B |
(Adapted from: Miller LM, Cassidy TJ - Postinfectious Arthritis and Related Conditions. In: Behrman RE, Kliegman RM, Jenson HB (eds) Nelson Textbook of Pediatrics, 18th end. Philadelphia, WB. Saunders 2007; * Bruck et Colab.: Transient oligoarthritis of the lower extremity following influenza B virus infection: Case report. Pediatric Rheumatology, 2010 8:4.)
The mechanism by which joint inflammatory phenomena occur is extremely complex and it is still uncompletely understood.
By definition, reactive arthritis involve an impaired inflammatory modification, acute installed, nonsuppurative, which occurs after a time from an extraarticular infection in individuals with a certain genetic predisposition and in which the causative agent cannot be isolated in synovial fluid (aseptic arthritis) (2,5,7). When it comes to arthritis occurring after gastrointestinal or genitourinary bacterial infections, most likely etiopathogenic mechanism is an autoimmune response in which the major role is played by T lymphocytes (1,7-10). At the joint, T lymphocytes react with antigens causing an inflammatory reaction. In addition, the absence of identification of bacterial DNA in synovial fluid supports the hypothesis of a cell-mediated immune reaction (1,2,5,7). Thus, it is estimated that cell-mediated immune system plays a major role. Hypothesis of immune responses is suggested by the identification of immunological similarities between HLA B27 antigen and specific bacterial antigens (10). Molecular mimicry theory is based on interaction between microbial antigen and self-proteins followed by stimulation and perpetuation of a Th2-cell–mediated autoimmune response. Th2 cytokines are also envolved in chronic joint damage (11).
On the other hand, several studies have also demonstrated the involvement of humoral immunity in the development of postiinfectious arthritis. Thus, some patients have experienced postinfectious arthritis and developed high and persistent titres of IgM and IgG antibodies against the involved germs, in contrast to patients who developed infection with the same bacteria but without the appearance of postinfectious arthritis (10).
Pathogenesis of postviral arthritis is more complex because they often do not even meet the classical definition criteria of reactive arthritis, there being some viral infection during which the virus can be isolated in synovial fluid (rubella, varicella zoster, herpes simplex v., CMV etc.) (7). In these situations it is estimated that articular manifestations are expression of real intrainfectious arthritis. With other viral infections (hepatitis B virus, adenovirus type 7) in the synovial fluid antigen-antibody type immune complexes were isolated, highlighting a possible role of these in the pathogenesis of postviral arthritis (7,10,12).
Generally, postinfectious arthritis involves joints of the lower limbs (ankles, knees) (1,7,9).
In this work poststreptococcal arthritis will not be included, although it is considered by some authors as postinfectious arthritis, but it is still a stand-alone entity. In Table 1 the most common etiological agents involved in the development of joint inflammatory phenomena (postinfectious arthritis) are listed.
Postinfectious arthritis diagnosis is often difficult. In most cases, it is based on a careful history and clinical elements observed after patient examination. Postinfectious arthritis is suspected if the patient meets the following criteria:
Has suggestive musculoskeletal changes: asymmetrical oligoarthritis especially in lower limbs, enthesitis, dactylitis (4,7,9).
-
Recent history of extraarticular infection: that may be documented by the existence of positive cultures, or microbiological studies or may be supported only on history of the disease (recent diarrheal episode, genitourinary infections, viral rash etc) (1,7,9).
The existence of a free interval of 2-4 weeks from the time of infection until the appearance of articular manifestations (this free interval may be absent) (7-9).
No other explanation for these joint manifestations: it will consider exclusion of other causes of arthritis in children (septic arthritis and other spondyloarthropathies, juvenile rheumatoid arthritis, systemic lupus erythematosus etc.) (4,8,9).
It is important to mention that the lack of identification of the causative agent (by assessing synovial fluid) does not exclude the diagnosis of postinfectious arthritis. In addition, the literature underlines that in about 50% of cases, the etiologic agent cannot be isolated (1,7) and in 25% of cases of postinfectious arthritis occurring in children, the infection was asymptomatic in history (4,7,9).
Lyme arthritis was described for the first time in United States of America, in the early 1970s, in a pediatric population when a number of pediatric arthritis occurred in the region around Lyme, Connecticut (13). There are many strains of Borrelia burgdorferi, different between United States and Europe, which can explain various clinical manifestations of Lyme disease. In Romania, in recent years, numerous cases with Burgdoferi Borrelia infection in children were diagnosed. Lyme disease has extremely polymorphic clinical expression. Most patients had severe neurological manifestations (polyradiculoneuritis, facial paralysis, encephalitis, etc.), arthritis in this age group being much more rare, being another example of diagnostic pitfall. Our case expressed positive serology to Borellia Burgdoferi, which is present in one third of early stages and in all patients with late stage Lyme disease. Also, arthritis is a hallmark for late disease, while arthralgia is a common sign in early stage of Lyme disease (13). ❑
CONCLUSIONS
Postinfectious arthritis in children is a relatively common cause of presentation to medical service and often represents a diagnostic pitfall. Viral infections, which are the main infectious etiology in childhood, may be associated with arthritis that can occur concurrently with or after a free interval (2-4 weeks).
Presence of arthritis requires detailed clinical and laboratory evaluation for a correct diagnosis and for an appropriate treatment. Postinfectious arthritis has a favorable outcome with symptomatic treatment, recovery occurs within a few weeks (aproximately 6 weeks). Arthritis after enteric or genitourinary infections (Chlamydia trahomatis) may become chronic/inflammatory bowel disease may develop over time. ❑
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Andrei Zamfirescu for providing us the ultrasonography exam of the children and Dr. Nina Bratu for the surgical examination of the children.
CONFLICT OF INTEREST
none declared.
FINANCIAL SUPPORT
none declared.
References
- 1.Ravinder K, Gupta K. Reactive Arthritis in Children. JK Science. 2007;9:109–10. [Google Scholar]
- 2.Hannu T. Reactive Arthritis. Best Pract Res Clin Rheumatol. 2011;25:347–347. doi: 10.1016/j.berh.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Rosner BM, Werber D, Höhle M, et al. Clinical Aspects and Self-reported Symptoms of Sequelae of Yersinia Enterocolitica Infections in a Population-based Study, Germany 2009-2010. BMC Infect Dis. 2013;13:236–236. doi: 10.1186/1471-2334-13-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Townes JM. Reactive Arthritis after Enteric Infections in the United States: The Problem of Definition. Clinical Infectious Diseases. 2010;50:247–54. doi: 10.1086/649540. [DOI] [PubMed] [Google Scholar]
- 5.Hannu T, Inman R, Granfors K. Reactive arthritis or post-infectious arthritis? Best Pract Res Clin Rheumatol. 2006;20:419–433. doi: 10.1016/j.berh.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Ahvonen P, Sievers K, Aho K. Arthritis Associated with Yersinia Enterocolitica Infection. Acta Rheumatol Scand. 1969;15:232–232. doi: 10.3109/rhe1.1969.15.issue-1-4.32. [DOI] [PubMed] [Google Scholar]
- 7.Miller M, Cassidy JT. In Nelson Textbook of Pediatrics, 19th edition (2010) by Richard E., Md Behrman (editor), Robert M., Md Kliegman (editor), Hal B., Md Jenson (editor) by WB Saunders, 18th edition. WB. Saunders; Philadelphia: 2007. Postinfectious Arthritis and Related Conditions; cap: 147; pp. 519–522. [Google Scholar]
- 8.Yu DT. Formerly Reiter syndrome. Reactive Arthritis; 2012 oct.; uptodate.com. http://www.uptodate.com/contents/reactive-arthritis-formerly-reiter-syndrome [Google Scholar]
- 9.Bruck N, Gahr M, Pessler F. Transient Oligoarthritis of the Lower Extremity Following Influenza B Virus Infection: Case Report. Pediatric Rheumatology. 2010;8:4–4. doi: 10.1186/1546-0096-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox CJ, Kempsell KE, Gaston JS. Investigation of Infectious Agents Associated with Arthritis by Reverse Transcription PCR of Bacterial rRNA. Arthritis Res Ther. 2003;5:1–8. doi: 10.1186/ar602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter JD, Hudson AP. Reactive Arthritis: Clinical Aspects and Medical Management. Rheum Dis Clin North. doi: 10.1016/j.rdc.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Moore TL, Syed R. Specific Viruses that Cause Arthritis. 2011; uptodate.com. http://www.uptodate.com/contents/specific-viruses-that-cause-arthritis [Google Scholar]
- 13.Meyerhoff JO, Cunha BA, Brent LH, et al. Lyme Disease. Medscape. Accesed June 2013. http://emedicine.medscape.com/article/330178-overview [Google Scholar]