Abstract
Purpose
Focused ultrasonic propulsion is a new non-invasive technique designed to move kidney stones and stone fragments out of the urinary collecting system. However, the extent of tissue injury associated with this technique is not known. As such, we quantitated the amount of tissue injury produced by focused ultrasonic propulsion under simulated clinical treatment conditions, and under conditions of higher power or continuous duty cycles, and compared those results to SWL injury.
Materials and Methods
A human calcium oxalate monohydrate stone and/or nickel beads were implanted (with ureteroscopy) into 3 kidneys of live pigs (45–55 kg) and repositioned using focused ultrasonic propulsion. Additional pig kidneys were exposed to SWL level pulse intensities or continuous ultrasound exposure of 10 minutes duration (ultrasound probe either transcutaneous or on the kidney). These kidneys were compared to 6 kidneys treated with an unmodified Dornier HM3 Lithotripter (2400 shocks, 120 SWs/min and 24 kV). Histological analysis was performed to assess the volume of hemorrhagic tissue injury created by each technique (% functional renal volume, FRV).
Results
SWL produced a lesion of 1.56±0.45% FRV. Ultrasonic propulsion produced no detectable lesion with the simulated clinical treatment. A lesion of 0.46±0.37% FRV or 1.15±0.49% FRV could be produced if excessive treatment parameters were used while the ultrasound probe was placed on the kidney.
Conclusions
Focused ultrasonic propulsion produced no detectable morphological injury to the renal parenchyma when using clinical treatment parameters and produced injury comparable in size to SWL when using excessive treatment parameters.
Keywords: extracorporeal shock wave lithotripsy, ultrasound, acute kidney injury, nephrolithiasis
INTRODUCTION
A recent analysis of NHANES data indicates that the prevalence of stone disease in the USA has increased markedly over the last 20 years, moving from 5.2% of the population in the early 1990s to 8.8% in 2010.1 Unfortunately, this trend will presumably be sustained into the future placing an increasing burden on health care services because of limited resources and urologist availability. Knowing that the strain of caring for stone patients is increasing highlights the need to make stone removal technology as effective and efficient as possible.
Successful removal of renal stones often requires two separate steps: fracturing of the calculus into small pieces and the subsequent removal of those stone fragments. Currently, the primary modality for fragmenting kidney stones is SWL. Many patients choose SWL because it is non-invasive and can be performed on an outpatient basis. While the overall success of this technique has been impressive, it is recognized that SWL leaves residual fragments in many patients. As an added concern, several authors have suggested that the effectiveness of SWL has declined among newer generation machines,2–5 raising the fear that the presence of residual fragments will become ever more common. Having residual fragments after treatment is not confined only to SWL, as recent reports found that as many as 38% of patients retained detectible fragments after ureteroscopic stone removal6 and up to 79% retained fragments after percutaneous nephrolithotomy.7
The presence of residual stone fragments in the urinary collecting system is a clinical problem that presents a challenge to rendering patients truly stone free. The main concern with fragmented calculi in the urinary tract is that these residual fragments increase the risk of future stone growth and recurrence of symptoms.8–11 Indeed, several studies have focused on the optimal management of stones prone to produce difficult to displace fragments, such as fragments from calculi in lower pole calices.12–14 And while procedures such as diuresis, percussion and inversion therapy are helpful in displacing fragments, measureable amounts of fragments remain resistant to removal.15–16
Focused ultrasonic propulsion is a new noninvasive technique designed to move kidney stones and residual fragments out of the urinary collecting system using focused ultrasound energy. Preliminary in-vitro and in-vivo studies using this technology have shown significant success at lifting and maneuvering implanted artificial stones and human urinary calculi out of renal calices.17–18 While this technique could be used in conjunction with any stone removal procedure to help expel residual fragments, ultrasonic propulsion, being a non-invasive procedure would be particularly attractive when coupled with SWL. However, while this technique shows promise, little is known about the potential for tissue damage with focused ultrasound under these conditions.
Ultrasonic propulsion has similarities to extracorporeal shock wave lithotripsy in that both techniques are transcutaneous with focused acoustic energy originating from an extracorporeal source. However, for an estimated 20–40 minute long treatment, ultrasonic propulsion generates pulses with substantially lower pressures (12 versus 35–110 MPa) and lower total energy (25 versus 100–200 J) delivered to a kidney than SWL. Because the amount of energy being delivered into a kidney by ultrasonic propulsion is considerably less than that produced by SWL, injury from ultrasonic propulsion would be expected to be lower than SWL. The purpose of this study was to compare the volume of tissue injury produced by focused ultrasonic propulsion versus that produced by SWL under situations that mimic clinical treatment conditions.
MATERIALS AND METHODS
Ultrasonic Propulsion Machines
Two prototype focused ultrasonic propulsion machines have been described previously.17–19 The original system, referred to as the research system, was developed for HIFU applications and was used to initially test and refine the ultrasonic propulsion concept. This system consisted of an 8 cm diameter water filled source head that included an annular therapy transducer and a diagnostic imaging probe. Separate systems drove the therapy probe and performed the imaging, and only the focal length could be adjusted electronically.17–18 A newer, more advanced treatment system was developed and termed the clinical prototype system. 19 It consists of an ultrasound engine (Verasonics, Redmond, WA), a commercially available ultrasound imaging probe (ATL/Philips HDI C5-2 or P4-1 probe, Philips Healthcare, Andover, MA), a computer processor, and a monitor display. The user can target, push, and observe stone movement on the touchscreen in real-time. The duration of each push attempt is selected by the user from 0 to 1 second. A 1-second push delivers a 100 μs pulse repeated every 3 ms for 1 second, or in other words a 3.3% duty cycle for 1 second. When working at a depth of 7 cm this clinical prototype produces a peak pressure of 12 MPa and a peak pulse average intensity of 2,400 W/cm2 in situ.
Animal Studies
The surgical and animal treatment protocols used to assess renal injury in this study were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Washington and the Indiana University School of Medicine.
Ultrasonic Propulsion Experiment
Ureteroscopic surgery was performed on 3 kidneys in 3 female farm pigs (45–55 kg) while under general anesthesia. Reference beads (2.5 mm diameter silver-coated nickel) were placed into the upper pole of each treated kidney (using a nitinol basket) as a positional marker in order to monitor for displacement during positioning of the pig. A calcium oxalate monohydrate stone coated in tantalum powder and/or silver-coated nickel beads (2–8 mm diameter) were placed in a middle or lower pole calyx for ultrasonic repositioning. Two beads or 1 bead and 1 stone were placed in each treated kidney. The tantalum powder or silver coating was used to improve visualization while under fluoroscopic observation. The position of the target stone/beads was confirmed under direct ureteroscopic and fluoroscopic observation.
The goal of this experiment was to use the clinical prototype machine to reposition the implanted stone/beads into the renal pelvis or ureter using only focused ultrasound energy directed at the stones. The maximum output produced by this machine was used for the simulated clinical treatment to help determine the potential for injury with this clinical prototype. Initially, the ultrasonic propulsion probe was placed on the skin and an experienced sonographer identified the target stones/beads with ultrasound. Treatment was then applied transcutaneously using the same ultrasound probe. Any subsequent stone/bead movement was recorded using fluoroscopy (GE OEC 9800, GE OEC Medical Systems Inc., Salt Lake City, UT), with direct ureteroscopic observation, and with the ultrasound imager in real-time. If several push attempts were unsuccessful, the probe was repositioned, and targeting and treatment were repeated. If these maneuvers were unsuccessful, the pig was repositioned and stone displacement attempts were repeated. Machine settings and output parameters were recorded for each stone push attempt. The data recorded included output voltage, duration of the push burst, location of the focus seen in the image selected by the user, and raw images converted to video.
Overtreatment Injury Experiments
A second set of experiments was conducted on anesthetized female farm pigs (45–55 kg) without stones/beads in place to determine the extent of injury possible with this technique under extreme failures of design or use by employing treatment parameters well beyond outputs needed for ultrasonic propulsion. In addition, the injury produced by this set of treatments would provide examples of the type of injury morphology to look for in the ultrasonic propulsion experiments. In this set of experiments the original research system was used because it could exceed the maximum outputs and ultrasound exposure length (continuous) of the clinical prototype system.
Our initial attempts to produce a lesion in the kidney with the probe resting on the skin were unsuccessful, even when treating at the maximum power level setting of 240 W (10 minutes, 3.3% duty cycle) of the research machine. While it was reassuring to know that the peak pressures and pulse intensities produced by this high power level did not result in renal parenchymal damage, we still wanted to explore the morphology of lesions produced under extreme treatment conditions. As such, we decided to continue the experiments by placing the treatment probe on the kidney itself to avoid attenuation of ultrasound pulse strength as it passed through the skin and muscle of the body wall. The following information describes those experiments.
Once the pigs were anesthetized, the abdomen was opened and the intestines were positioned to one side of the abdomen to expose the kidney to be treated. The peritoneal lining covering the kidney was removed and the abdominal cavity was filled with degassed phosphate buffered saline to assist with coupling of the coupling cone of the transducer to the kidney. The kidney was immobilized with wet gauze, taking care to avoid placing the gauze in the ultrasound path. The ultrasound transducer was then positioned and the renal parenchyma was targeted for treatment. The focus was maintained between 1 and 1.5 cm below the kidney surface, which corresponds to a 6–6.5 cm total treatment head to focus depth. One pole of 1 kidney of each pig was treated with the maximum exposure of the research system (240 W) for 10 minutes at a 3.3% duty cycle (100 μs pulses every 3 ms). This setting produces a calculated p+ of 93 MPa and derated SPPA intensity of 26,130 W/cm2 in situ at a 6 cm focal depth. Derated values were calculated according to methods described by Bessonova et al.20 As part of this group of experiments the opposite pole was also treated while the kidney was exposed. However, in these treatments the opposite pole was subjected to focused ultrasound for 10 minutes as before, but a lower power setting was used (80 W) while the ultrasound was allowed to run continuously (100% duty cycle). This setting produces a p+ of 47 MPa at a derated SPPA intensity of 9,320 W/cm2 in situ.
SWL Experiment
The design of the SWL experiment and all surgical procedures employed during the experiment followed the same methods used in previously published studies.21–22 Six female farm pigs, weighing ≈ 45 kg each (Hardin Farms, Danville, IN), were anesthetized and a lower pole calyx of the left kidney was targeted for lithotripsy treatment. The targeted kidney received 2400 SWs delivered at 120 SWs/minute and 24 kV of energy from an unmodified Dornier HM-3 lithotripter (Dornier Medical Systems, Kennesaw, GA).
Lesion Analysis
At the end of ultrasound treatment (with or without stones) or SWL treatment, the kidneys were perfusion-fixed in-situ with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH = 7.4). After the kidneys were removed, they were submerged in fresh fixative for subsequent determination of lesion size. Later, Microfil (Flow Tech Inc., Carver, MA) was injected into the vasculature of the kidneys and the kidneys were embedded in paraffin, and then serially sectioned into 40 μm-thick slices. Digital photographs were taken of the slices every 120 um. The hemorrhagic lesion observed in the sections was used to determine lesion volume and expressed as percent of FRV of renal parenchyma.23 Mean lesion size ± SEM was calculated in each group of pigs. Comparison of lesion sizes between the ultrasound treated and SWL treated groups was performed with a t-test. The criterion for statistical significance was set at P < 0.05.
RESULTS
Ultrasonic Propulsion
The results of the ultrasonic propulsion experiment are summarized in Table 1. All stone/beads were found to be visible with ultrasound and all (100%) treated stone/beads underwent at least some movement from their original position, if only moving from a minor calyx into another minor calyx or shifting around within a calyx (33%). Overall, 67% of the stone/beads were successfully moved out of the initial calyx and were relocated to the renal pelvis (33%) or were moved all the way into the ureter (33%). In contrast, none of the upper pole reference beads moved from their upper pole position during the experiment. Treatment parameters for the ultrasonic propulsion group averaged 123±21 (mean ± SD) push bursts for an average total push burst time of 1.7±0.1 min/kidney using a 3.3% duty cycle. This was the time required to move the stone/beads from each kidney lower pole. Some push attempts resulted in no movement or the stone/bead moved, but fell back into the same calyx. Most often however, just a few effective push attempts were needed to clear a stone/bead from a calyx. We were unable to move 1 of the beads, and the only implanted stone, to the renal pelvis or into the ureter. Since previous studies successfully moved calcium oxalate stones,17–19 we do not believe that this failure was due to stone composition. Instead, we think that the position of the stone in the calyx (narrow recess) or geometry of the calyceal system (for the bead) prevented them from being properly relocated. It should be noted that the other beads placed in those same calyces (in kidneys B and C) were successfully moved to the pelvis or ureter. We suspect that fractured stone particles, being smaller in size than the implanted stone/beads, would more easily navigate the urine collecting system and would be successfully directed out of the kidney.
Table 1.
Outcome of beads or stone placed in a kidney calyx and exposed to ultrasonic propulsion in an attempt to relocate targeted object to the renal pelvis or ureter. Data includes number of attempts to move object, actual running time of ultrasonic propulsion probe and total length of experiment.
| Kidney | Object | Result | Attempts to Move Objects | Time of Active Ultrasonic Propulsion Pushing | Total Experiment Time |
|---|---|---|---|---|---|
| A | Bead | Moved into ureter | 109 | 1.8 min | 62.7 min |
| Bead | Moved to pelvis | ||||
|
|
|||||
| B | Bead | Moved to pelvis | 95 | 1.6 min | 49.6 min |
| Bead | Moved to different calyx | ||||
|
|
|||||
| C | Bead | Moved into ureter | 164 | 1.6 min | 84.5 min |
| Stone | Moved around calyx | ||||
|
|
|||||
| Mean±SEM | 123±21 | 1.7±0.1 min | 65.6±10.2 min | ||
Description and Quantitation of Ultrasonic Propulsion Injury
The results of the ultrasonic propulsion injury experiments, along with treatment parameters, are summarized in Table 2. The ultrasonic stone propulsion technique, using parameters that actually displaced stone/beads in a living animal, did not produce a detectible lesion in the treated kidneys in that group. As shown in Figure 1 the parenchyma of a kidney from this group is free of hemorrhage. Additionally, experiments where the ultrasound pulse power was increased and the probe was resting on the skin of the animal did not produce a measureable lesion.
Table 2.
Hemorrhagic lesions of kidneys exposed to ultrasonic propulsion with clinical prototype, high power or continuous duty cycle using research ultrasound machine, or SWL.
| Transcutaneous | Probe on Kidney | SWL | |||
|---|---|---|---|---|---|
|
| |||||
| Ultrasonic Propulsion | High Power | High Power | High Duty Cycle | ||
| No. kidneys | 3 | 5 | 3 | 3 | 6 |
| Machine | Clinical | Research | Research | Research | Dornier HM-3 |
| Stone/bead | Yes | No | No | No | No |
| Power | 10 W | 240 W | 240 W | 80 W | 24 kV |
| Duty cycle | 3.3% | 3.3% | 3.3% | 100% | 120 SWs/min |
| Time (mins) | 1.7±0.1 | 10 | 10 | 10 | 20 |
| p+ (MPa) | 12 | 68 | 93 | 47 | 40–55 |
| SPPA inten. (W/cm2) | 2,400 | 15,000 | 26,130 | 9,320 | |
|
| |||||
| % lesion size | 0.0±0.0 | 0.0±0.0 | 0.46±0.37 | 1.15±0.49 | 1.56±0.45 |
FIG. 1.
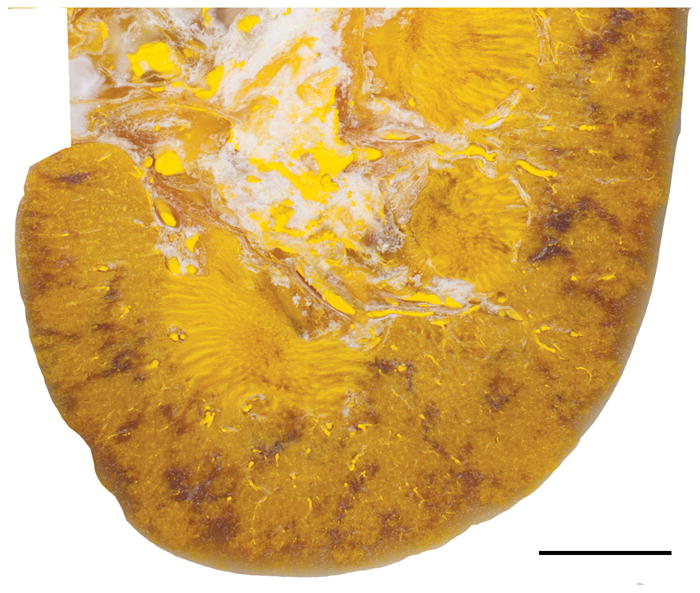
Light photograph of a cross-section of kidney from a pig treated with ultrasonic propulsion. Note the lack of hemorrhagic lesion after treatment to displace stone/beads using simulated clinical treatment parameters. The yellow color of the tissue is due to Microfil infusion of the vasculature used to mark the location of blood vessels to help distinguish vessels from tissue hemorrhage. The diffuse non-yellow regions of the cortex and medulla represent areas of imperfect Microfil infusion. Bar = 1 cm.
On the other hand, the overtreatment experiments which included using a high power setting (26,130 W/cm2) at a 3.3% duty cycle or using a lower power (9,320 W/cm2) with a 100% duty cycle for 10 minutes produced significant injury when the probe was placed on the kidney. While the size of the overtreatment lesions was variable between animals, the morphology of the lesions fell into two categories. As shown in Figure 2a, using a high power setting and low duty cycle produced a predominately hemorrhagic lesion, while the lower power and continuous duty cycle treatment produced a predominately thermal coagulation lesion. These lesions appear similar to ultrasound lesions described and studied by other groups.24–27 The hemorrhagic lesion was dark with an irregularly shaped border similar to a tissue hematoma produced by SWL. The core of the lesion seemed to be composed primarily of red blood cells with little tissue. Surrounding this core was a rim of lesser injury appearing to be composed of tissue with extravasated red blood cells. When examined in cross-section this injury was discreet and conical in shape (Fig. 2c). The thermal lesion, on the other hand, had concentric zones of injury with a center zone that was circular, white and uniform in appearance reminiscent of thermal coagulation (Fig. 2b). This circular area was surrounded by a rim of dark tissue, which was identified (based on previous studies) as an area of hemorrhage. When examined in cross-section this injury was localized to the treatment region and had a distinct border with dark red coloration extending deep into the kidney (Fig. 2d).
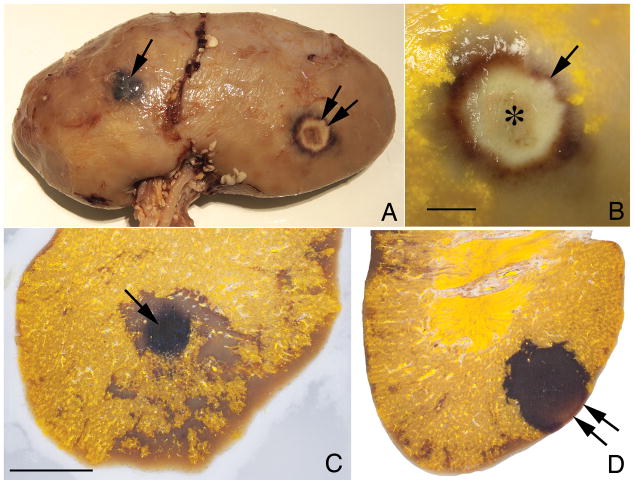
FIG. 2.
FIG. 2a. Light photograph of the surface of a pig kidney exposed to a high power level (arrow) with low duty cycle or a low power level with a continuous duty cycle (double arrow) during treatment. 2b. Higher magnification view of area exposed to continuous duty cycle resulting in a thermal coagulation lesion. Center of coagulated area marked with an asterisk (*). An arrow indicates zone of hemorrhagic tissue surrounding central region. 2c. Light photograph of a cross-section from area treated with a high power level. This lesion is dark red to black (arrow). Surrounding this lesion is an area of poor perfusion and Microfil filling resulting in pale coloration. 2d. Light photograph of a cross-section from area treated with a continuous duty cycle. This lesion is pale red to white on the surface of the kidney (double arrow). The deeper lesion is darker red in coloration indicating hemorrhage into the tissue. Note the distinct boundary between the lesion and surround tissue. Bar = 1 cm in each photograph.
As shown in Figures 2c and 2d, both overtreatment conditions produced quantifiable areas of parenchymal injury. Quantitation of the lesions under each condition is shown in Table 2. The lesion size in the overtreatment group was comparable to that found in the SWL group. There was no statistically significant difference in lesion size between these groups. In contrast, the treatment parameters used to mimic clinical treatment conditions that moved stone/beads did not produce a hemorrhagic lesion.
DISCUSSION
The ultimate goal of any surgical intervention for nephrolithiasis is to render the patient stone free. Unfortunately, it is common to have persistent residual fragments present in the kidney irrespective of the technique employed to remove the calculus.6,7,9,14 In this study we report on the successful relocation (up to 67%) of implanted stone/beads (which served as substitute fragments) using a novel method designed to displace fragments from the urinary collecting system. Unlike the indirect stone clearance assistance methods listed in the introduction, the ultrasonic propulsion system uses acoustic radiation force to act directly on objects in the collecting system to initiate movement. Because focused ultrasound waves pass through the kidney when the system is activated, there is potential for injury during treatment. However, we were unable to detect evidence of hemorrhagic injury when the ultrasonic propulsion technique was used to move implanted stone/beads in a manner similar to what would occur during a clinical treatment. SWL exposure on the other hand, produced a lesion that comprised over 1% of the functional renal volume of the treated kidney. So based on these results one could predict that the use of ultrasonic propulsion to move fragments should contribute little to nothing in the way of the total injury to the kidney when used in conjunction with SWL.
This is not to say that ultrasonic propulsion is completely without risk. As shown in a previous study where the ultrasonic propulsion probe was applied directly on the surface of the kidney,18 and from the experiments reported here with a high power levels or continuous duty cycles, significant injury can occur if there is careless selection of treatment parameters and system design. This injury can include intraparenchymal hemorrhage and thermal coagulation lesions (Fig. 2a–d). Fortunately, the treatment parameters necessary to produce these injuries are far in excess of parameters used to move the implanted stones/beads with respect to peak pressure (47–93 vs. 12 MPa), time (10 vs. 1.7 minutes) and intensity (9,320–26,130 vs. 2,400 W/cm2). In fact, the parameters used to move the implanted stone/beads were the maximum settings available on the prototype clinical system. The parameters used to cause injury in the kidneys could only be generated with the research system. This suggests that the potential for injury with this technology when using clinical treatment parameters should be small.
There are several potential limitations to this study. First, while the lack of injury noted after moving the stone/beads suggest that ultrasonic propulsion is safe and has a wide margin of safety because of the extreme measures needed to create an injury, additional safety analyses will be needed if treatment parameters are increase beyond what we have tested. For example, as noted above newer generation lithotripters appear less effective at fracturing stones than the original Dornier HM-3,2–5 and these machines may leaving behind larger stone fragments than treatment with the HM-3. This may require increasing the length of the ultrasonic propulsion treatment or increasing the power level of the machine in order to clear an increased number of large stone fragments from the urinary collecting system. In turn, this increase in treatment length or machine power may cause some renal injury. Other circumstances where treatment length or machine power might be increased to move objects in the urinary collecting system could include situations where patients stones exceed the size of the stone/beads used in this study, where stone fragments are enmeshed in a blood clot, and where a targeted stone is adhering tightly to a papilla. A second potential limitation concerns the placement of the stone/beads. Ureteroscopic implantation of the stone/beads may have dilated the urine collecting system. If this happened it could make it easier to move the stone/bead, than would occur under normal circumstances of in situ stone growth, and could inflate the success rate of the technique. A third potential limitation of this study concerns the restriction of skin to stone distance related to patient size. The current ultrasonic propulsion machine has a maximum treatment depth of 12–14 cm. Normal patients with a body wall thickness similar to that of the pigs used in this study should not present a problem when it comes to fragment movement, as the morphology of muscle and fat are similar between people and pigs. However, obese patients may present a challenge moving stone fragments if adipose tissue layers are very thick and exceed the maximum treatment depth. Efforts are currently underway to redesign the treatment probe to allow greater depth of ultrasound beam penetration through tissue.
In conclusion, this study simulated a clinical treatment for the expulsion of residual stone fragments or small stones from their original position in a middle or lower pole calyx towards the ureter and renal pelvis. Every implanted stone/bead exhibited some displacement with 33% of the stone/beads being repositioned to the renal pelvis and 33% moved into the ureter. No hemorrhagic injury was detected in the kidneys when using these clinical treatment parameters. However, injury was detected in kidneys treated with excessively high power or with continuous duty cycle settings.
Acknowledgments
This project was supported by grants from the National Institutes of Health (P01-DK43881 and R01-DK092197) and the National Space Biomedical Research Institute through NASA (NCC 9-58).
Abbreviations and Acronyms
- NHANES
National Health and Nutrition Examination Survey
- SWL
extracorporeal shock wave lithotripsy
- MPa
megapascal
- J
joule
- W
watt
- FRV
functional renal volume
- p+
peak positive pressure
- SWs
shockwaves
- SEM
standard error of the mean
- SD
standard deviation
- SPPA
spatial peak pulse average
- HIFU
high intensity focused ultrasound
References
- 1.Scales CD, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerbl K, Rehman J, Landman J, et al. Current management of urolithiasis: progress or regress? J Endourol. 2002;16:281. doi: 10.1089/089277902760102758. [DOI] [PubMed] [Google Scholar]
- 3.Portis AJ, Yan Y, Pattaras JE, et al. Matched pair analysis of shock wave lithotripsy effectiveness for comparison of lithotriptors. J Urol. 2003;169:58. doi: 10.1016/S0022-5347(05)64034-7. [DOI] [PubMed] [Google Scholar]
- 4.Gerber R, Studer UE, Danuser H. Is newer always better? A comparative study of 3 lithotriptor generations. J Urol. 2005;173:2013. doi: 10.1097/01.ju.0000158042.41319.c4. [DOI] [PubMed] [Google Scholar]
- 5.Zehnder P, Roth B, Birkhauser F, et al. A prospective randomised trial comparing the modified HM3 with the Modulith SLX-F2 lithotripter. Eur Urol. 2011;59:637. doi: 10.1016/j.eururo.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Rippel CA, Nikkel L, Lin YK, et al. Residual fragments following ureteroscopic lithotripsy: incidence and predictors on postoperative computerized tomography. J Urol. 2012;188:2246. doi: 10.1016/j.juro.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 7.Park J, Hong B, Park T, et al. Effectivness of noncontrast computed tomography in evaluation of residual stones after percutaneous nephrolithotomy. J Endourol. 2007;21:684. doi: 10.1089/end.2006.0352. [DOI] [PubMed] [Google Scholar]
- 8.Streem SB, Yost A, Mascha E. Clinical implications of clinically insignificant stone fragments after extracorporeal shock wave lithotripsy. J Urol. 1996;155:1186. [PubMed] [Google Scholar]
- 9.Chen RN, Streem SB. Extracorporeal shock wave lithotripsy for lower pole calculi: long-term radiographic and clinical outcome. J Urol. 1996;156:1572. [PubMed] [Google Scholar]
- 10.Osman MM, Alfano Y, Kamp S, et al. 5-year-follow-up of patients with clinically lnsignificant residual fragments after extracorporeal shockwave lithotripsy. Eur Urol. 2005;47:860. doi: 10.1016/j.eururo.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Skolarikos A, Paptsoris AG. Diagnosis and Management of postpercutaneous nephrolithotomy residual stone fragments. J Endourol. 2009;23:1751. doi: 10.1089/end.2009.1546. [DOI] [PubMed] [Google Scholar]
- 12.Lingeman JE, Siegel YI, Steele B, et al. Management of lower pole nephrolithiasis: a critical analysis. J Urol. 1994;151:663. doi: 10.1016/s0022-5347(17)35042-5. [DOI] [PubMed] [Google Scholar]
- 13.Albala DM, Assimose DG, Clayman RV, et al. Lower pole I: prospective randomized trial of extracorporeal shock wave lithotripsy and percutaneous nephrostolithotomy for lower pole nephrolithiasis--initial results. J Urol. 2001;166:2072. doi: 10.1016/s0022-5347(05)65508-5. [DOI] [PubMed] [Google Scholar]
- 14.Pearle MS, Lingeman JE, Leveillee R, et al. Prospective randomized trial comparing shock wave lithotripsy and ureteroscopy for lower pole caliceal calculi 1 cm or less. J Urol. 2005;173:2005. doi: 10.1097/01.ju.0000158458.51706.56. [DOI] [PubMed] [Google Scholar]
- 15.Pace KT, Tariq N, Dyer SJ, et al. Mechanical percussion, inversion and diuresis for residual lower pole fragments after shock wave lithotripsy: a prospective, single blind, randomized controlled trial. J Urol. 2001;166:2065. [PubMed] [Google Scholar]
- 16.Chiong E, Hwee ST, Kay LM, et al. Randomized controlled study of mechanical percussion, diuresis, and inversion therapy to assist passage of lower pole renal calculi after shock wave lithotripsy. Urology. 2005;65:1070. doi: 10.1016/j.urology.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 17.Shah A, Owen NR, Lu W, et al. Novel ultrasound method to reposition kidney stones. Urol Res. 2010;38:491. doi: 10.1007/s00240-010-0319-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah A, Harper JD, Cunit BW, et al. Focused ultrasound to expel calculi from the kidney. J Urol. 2012;187:739. doi: 10.1016/j.juro.2011.09.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harper JD, Sorensen MD, Cunit BW, et al. Safety and effecacy of a clinical prototype using focused ultrasound to expel calculi from the kidney. J Urol. 2013 doi: 10.1016/j.juro.2013.03.120. http//dx.doi.org/10.1016/j.juro.2013.03.120. [DOI] [PMC free article] [PubMed]
- 20.Bessonova OV, Khokhlova VA, Canney MS, et al. A derating mothod for therapeutic applications of high intensity focused ultrasound. Acoust Phys. 2010;56:354. doi: 10.1134/s1063771010030140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Handa RK, McAteer JA, Willis LR, et al. Dual-head lithotripsy in synchronous mode: Acute effect on renal function and morphology in the pig. BJU Int. 2007;99:1134. doi: 10.1111/j.1464-410X.2006.06736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willis LR, Evan AP, Connors BA, et al. Relationship between kidney size, renal injury, and renal impairment induced by shock wave lithotripsy. J Am Soc Nephrol. 1999;10:1753. doi: 10.1681/ASN.V1081753. [DOI] [PubMed] [Google Scholar]
- 23.Blomgren PM, Connors BA, Lingeman JE, et al. Quantitation of shock wave lithotripsy-induced lesion in small and large pig kidneys. Anat Rec. 1997;249:341. doi: 10.1002/(SICI)1097-0185(199711)249:3<341::AID-AR4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 24.Linke CA, Carstensen EL, Frizzell LA, et al. Localized tissue destruction by high-intensity focused ultrasound. Arch Surg. 1973;107:887. doi: 10.1001/archsurg.1973.01350240053015. [DOI] [PubMed] [Google Scholar]
- 25.Elbadawi A, Linke CA, Carstensen EL, et al. Histomorphologic features of ultrasonic renal injury. Arch Pathol Lab Med. 1976;100:199. [PubMed] [Google Scholar]
- 26.Paterson RF, Barret E, Siqueira TM, et al. Laparoscopic partial kidney ablation with high intensity focused ultrasound. J Urol. 2003;169:347. doi: 10.1016/S0022-5347(05)64124-9. [DOI] [PubMed] [Google Scholar]
- 27.Solomon SB, Nicol TL, Chan DY, et al. Histologic evolution of high-intensity focused ultrasound in rabbit muscle. Invest Radiol. 2003;38:293. doi: 10.1097/01.RLI.0000066421.79958.96. [DOI] [PubMed] [Google Scholar]