Abstract
The control and eradication of neurological complications associated with AIDS continues to be an important goal in efforts toward improving the well being of HIV-infected patients. Although combined antiretroviral therapies have contributed significantly to increasing the longevity of patients by suppressing the virus burden in the systemic compartments, the prevalence of HIV-associated neurological disorders continue to be on the rise. This in turn, leads to an impaired quality of life of the infected individuals who continue to suffer from mild to moderate cognitive decline and memory loss. Developing therapeutic interventions that reverse neuronal injury in the context of HIV infection, is thus of paramount importance in the field. Our previous studies have demonstrated that platelet-derived growth factor (PDGF) has a neuroprotective potential against HIV envelope protein gp120 and Tat. Paradoxically, PDGF is also a cerebrovascular permeant with deleterious effects on the blood-brain barrier resulting in increased influx of monocytes in the CNS. Herein, we review the opposing roles of PDGF in the context of HIV-associated neurodegenerative disorder (HAND).
Keywords: PDGF, HIV, Drug Abuse
Introduction
Currently over 40 million people live with HIV worldwide. In the US, the aging population represents one of the fastest growing groups with HIV. The Center for Disease Control estimates that by the year 2015, half of all Americans living with HIV will be over the age of 50. Although combination antiretroviral therapy (cART) has improved the health of millions of those living with HIV, the penetration into the CNS of many of these drugs is limited, and the quality of life of the patients continues to be diminished by milder, residual neurocognitive impairment commonly referred to as HAND. Reversible neuronal loss, decreased neurogenesis and synaptodendritic injury are emerging as important mediators of cognitive deficits associated with HIV-associated neurodegenerative disorder (HAND).
Neurotrophic family of growth factors promote neurogenesis and thus play key roles in neuronal homeostasis (Almeida et al., 2005). Various neurotrophic factors have been implicated in the protection of neurons against cellular and viral neurotoxins including brain derived neurotrophic factor (BDNF), fibroblast growth factor (FGF), nerve growth factor (NGF) & glia cell line-derived neurotrophic factor (GDNF) (Alzheimer and Werner, 2002; Almeida et al., 2005; Deierborg et al., 2008; Colafrancesco and Villoslada, 2011). Findings from our laboratories have identified a novel neurotrophic factor, platelet derived growth factor (PDGF) that plays a crucial role in reversing neuronal toxicity mediated by HIV proteins Tat & gp120 (Yao et al., 2009). Additionally, reports on the neuroprotective potential of PDGFs in various other pathologies are also extant.
Interestingly, in addition to its neuroprotective role, PDGF has also been suggested to have a paradoxical role as a vascular permeant that results in impairment of endothelial barrier during ischemic stroke (Su et al., 2008). Notably, HIV infection of the CNS during late stages of the disease process also results in endothelial barrier disruption and the ensuing neuroinflammation. It has been suggested that HIV-1 can enter the CNS through infected leukocytes likely via the Trojan horse mechanism. Findings from our lab have demonstrated up-regulation of PDGF in newly infiltrating macrophages lining the blood vessels (Potula et al., 2004). More recently we have also validated the role of PDGF as a vascular permeant in human brain microvascular cells exposed to cocaine (Yao et al., 2011a; Yao et al., 2011b). Effect of PDGF on endothelial cells has been the subject of many studies (Sato et al., 1993; Koyama et al., 1994; Cao et al., 2002). Herein we review the opposing roles of PDGF in the context of HIV infection with focus on cell signaling.
Neuroprotective role of PDGF
TRPC channels in neuroprotection mediated by PDGF-BB against HIV Tat in tyrosine hydroxylase (TH) neurons
HIV infection selectively targets the basal ganglia region of the brain leading to dopaminergic neuronal loss (Nath et al., 2000; Mocchetti et al., 2007). Importantly, evidence on the involvement of dopamine impairment observed in HAD comes from case studies on pathological specimens of HIV-infected individuals that demonstrate specific loss of these neurons (Nath et al., 2000). Experimental studies in animals have also demonstrated that nigrostriatal neurons in the region of basal ganglia are highly susceptible to toxicity mediated by HIV-1 proteins (Zauli et al., 2000). Since neurons are not infected by HIV-1, neuronal death is likely due to toxicity exerted by viral and cellular neurotoxins released from either virus-infected or activated cells (Eugenin et al., 2003). One of the key viral toxins implicated in neuronal injury/death is the virus transactivator protein, HIV-1 Tat that not only can be secreted from infected cells but is also taken up by neighboring non-infected cells, such as neurons (Liu et al., 2000). HIV-1 Tat is known to inhibit expression of tyrosine hydroxylase in dopaminergic neurons likely resulting in cognitive abnormalities observed in patients with HAD (Zauli et al., 2000; Everall et al., 2005).
Neuronal homeostasis is a result of the fine balance between neurotrophic and neurotoxic factors. Family of neurotrophic factors, such as BDNF, NGF, and GDNF have been known to play key roles in the protection of neurons against a variety of neurotoxins (Almeida et al., 2005; Arthur et al., 2006; Deierborg et al., 2008). Our findings have identified the role of yet another neurotrophic factor, PDGF that has been demonstrated as a critical factor in the postnatal brain development of rats (Smits et al., 1991).
PDGF is a family of growth factor comprised of five dimeric ligands (PDGF-AA, -AB, -BB, -CC and -DD) that are assembled from four gene products (PDGF-A-D) and bind to two classical tyrosine kinase receptors, PDGF-α (PDGF-αR) and PDGF-β receptor (PDGF-βR). PDGF families of factors play multiple roles during embryogenesis, brain development and in a variety of pathologies in the adult (Andrae et al., 2008). For the sake of clarity, PDGF chains A to D indicate the respective gene, while PDGF-AA, PDGF-BB, PDGF-CC, and PDGF-DD refer to the protein isoforms. PDGF has been demonstrated to protect primary hippocampal neurons against glutamate-induced neuronal damage (Beazely et al., 2008). In our previous findings using SH-SY5Y cells we have reported that PDGF can mediate neuroprotection against the HIV envelope gp120-mediated toxicity (Peng et al., 2008b; Peng et al., 2008a).
Studies from our laboratories have demonstrated that exogenous PDGF exerted neuroprotective effects against HIV-1 Tat mediated neurotoxicity in primary midbrain neurons. This effect of PDGF in neuroprotection involved the role of transient receptor potential canonical (TRPC) channels. TRPC channels are Ca2+-permeable, nonselective cation channels that play key roles in various physiological functions including neuronal survival. Silencing of TRPC channels with either short interfering RNAs (specific for TRPC 5 and 6) or using pharmacological blockers resulted in suppression of both PDGF-mediated neuroprotection as well as elevation in intracellular Ca2+ in primary midbrain neurons. In these cells PDGF-mediated neuroprotection constituted parallel but distinct ERK/CREB and PI3K/Akt pathways. Blockage of TRPC channel also resulted in suppression of PDGF-induced Pyk2/ERK/CREB activation, but had no effect on Akt activation. Furthermore, administration of PDGF was able to restore susbtantia nigra dopaminergic neurons from Tat-mediated neurotoxicity. Pre-treatment of mice with the TRP blocker resulted in attenuation of neuroprotection, thus underscoring the novel role of TRPC channels in protection of neurons mediated by PDGF.
Role of synaptic plasticity gene Arc/Arg3.1 in neuronal plasticity mediated by PDGF-BB
Our previous findings had also dissected the role of PDGF in mediating long-term potentiation (LTP). Long-term forms of learning and memory are dependent on rapid synthesis of new RNA and proteins in the cells (Davis and Squire, 1984). Increasing evidence suggests that a set of immediate-early genes including Arc/Arg3.1 (Link et al., 1995), Homer (Brakeman et al., 1997), Narp (Tsui et al., 1996) and cpg-2 are critical for transcription-dependent plasticity. Arc/Arg3.1 gene regulation is very unique since it has been shown that in stimulated neurons Arc mRNA can translocate from the nucleus to the dendrites where it is translated and integrated into the postsynaptic zone along with other cytoskeletal proteins (Steward et al., 1998; Steward and Worley, 2001). Essential role of Arc in synaptic plasticity has also been shown in Arc/Arg3.1 knockdown studies, which leads to selective deficits in LTP (Guzowski et al., 1999). Arc expression in the neurite has thus been implicated as an index of synaptic activation.
We have demonstrated that in the rat hippocampal neurons PDGF-BB regulated the expression of Arc/Arg3.1 gene, which was confirmed in vivo in a model of PDGF injection. In these studies, PDGF-R tyrosine kinase inhibitor-STI-571 was also able to abolish PDGF-BB mediated LTP ex vivo in rat hippocampal slices. Molecular pathway involved in PDGF-mediated induction of Arc/Arg3.1 included activation of the MAPK/ERK (MEK) signaling. Upstream release of intracellular calcium stores was also critical in this process. Pharmacological blocking using inhibitors specific for either MAPK/ERK phosphorylation or calcium release pathway suggested the downstream convergence of the two pathways involving the activation of the immediate early gene (Egr-1).
TRPC1 channel is critical for PDGF-BB-mediated neurogenesis
It is well documented that not only is an increased neuronal damage/loss, but that there are also fewer adult neural stem/progenitor cells (NPCs) present in the dentate gyrus of the hippocampus of patients with HIV-associated CNS disease. A defect in reduced NPCs could in turn, account for increased prevalence of behavioral deficits observed in patients with HAND in the post-ART era. In the adult mammalian brain, new dentate granule cells are continuously generated from neural progenitor cells and are integrated into the existing hippocampal circuitry via a process termed as neurogenesis (Venkatesan et al., 2007). Adult hippocampal neurogenesis is regulated by a number of physiological as well as pathological stimuli. HIV Tat, an early viral protein,has been known to impair neurogenesis (Mishra et al., 2010). Additionally, psychostimulants such as cocaine can also negatively affect the self-renewing capacity of the hippocampus by down-regulating the proliferative capacity of NPCs (Yamaguchi et al., 2004; Yamaguchi et al., 2005; Hu et al., 2006). These findings thus raise the concern that cognitive dysfunction associated with HIV infection and drug abuse could, partially be attributable the impaired neurogenesis in the hippocampus.
Previous investigation from our laboratories has suggested that pre-treatment of rat hippocampal NPCs with PDGF-BB resulted in restoration of proliferation impaired by HIV Tat & cocaine and that this involved signaling via the cognate receptors. In this study essential role of TRPC was also implicated in PDGF-BB-mediated upregulation of proliferation. Signaling pathways involved in this process included parallel but distinct ERK/CREB, PI3K/Akt pathways with the downstream activation of mTOR/4E-BP & p70S6K and NF-kB signaling. Confirmation of this pathway by silencing of TRPC1 resulted in suppression of PDGF-mediated proliferation as well as PDGF-BB-induced ERK/CREB and mTOR/4E-BP & p70S6K pathways. Consistent with this in vitro finding injection of recombinant rAAV2-PDGF-B in the hippocampi of mice resulted in restoration of impaired NPC proliferation mediated by HIV Tat & cocaine. TRPC 1 channel can thus be envisioned as a novel target that regulates cell proliferation mediated by a neurotrophic factor such as PDGF-BB and has implications for development of therapeutic intervention strategies for restoration of impaired neurogenesis mediated by viral proteins and/or drug abuse.
Neuroprotection mediated by another isoform of PDGF, PDGF-CC: Involvement of TRPC-mediated inactivation of GSK 3β
PDGF isoforms are known to exert diverse effects in various cell types. In recent years identification of yet another PDGF isoform, PDGF-CC, which has neuroprotective potential in a variety of neurological disorders is being appreciated (Tang et al., 2010). In addition to testing the role of PDGF-BB, we have also explored the involvement of another PDGF subtype-PDGF-CC, which is the newly identified third membrane of family of five PDGF chains (PDGF A-D) that dimerizes prior to receptor binding.
Neuronal survival in response to neurotrophic factors has implicated the essential role of phosphatidylinositol-3 kinase (PI-3K) (Liot et al., 2004; Zheng and Quirion, 2004; Subramaniam et al., 2005). While most of the downstream effectors of PI-3K mediating neuronal survival have not been completely identified, Akt/glycogen synthase kinase-3β (GSK3β) is strongly implicated to play an essential role in this process (Wu et al., 2007; Wang et al., 2010). Role of TRPC channels in neuronal survival is being much appreciated in recent years (Jia et al., 2007; Sossin and Barker, 2007). It has been demonstrated that PDGF-CC mediated neuroprotection against Tat toxicity involves both TRPC and Akt signaling
In our efforts to understand the effect of viral protein Tat on modulation of intrinsic expression of PDGF-CC, we demonstrated downregulation of the growth factor at both the transcription as well as the translational level in human neuroblastoma SH-SY5Y cells. Reciprocally, pretreatment with PDGF-CC abrogated Tat-induced neurotoxicity by ameliorating apoptosis as well as neurite loss. Using both pharmacological and loss of function approaches the role of PI3K/Akt signaling was suggested as being crucial in PDGF-CC-mediated neuroprotection. Additionally, involvement of TRPC1 was also critical in modulating calcium transients in PDGF-CC-mediated neuroprotection. PDGF-CC via its binding to the cognate receptor mediated inactivation of the downstream mediator (GSK3β with its phosphorylation at Ser-9). Gain and loss of function studies using cells transfected with either the wild type or mutant GSK3β constructs further confirmed the findings. Pre-treatment of cells with either the PI3K inhibitor or TRPC blocker failed to inactivate GSK3β in response to PDGF-CC, thereby implying the intersection of PI3K and TRPC signaling at the common mediator -GSK3β.
MicroRNA-29 mediated regulation of PDGF-B in Opiate and HIV neurotoxicity
Opiate dependence is emerging as a co-morbidity of HIV infection owing to intravenous route of injection. In fact, there is a strong connection between opiate usage and HIV neuropathogenesis as evidenced by accelerated incidence and progression of HAND in HIV-infected opiate users. Various reports have indicated that opiates such as morphine can potentiate HIV transactivation protein Tat-mediated toxicity in both human neurons and neuroblastoma cells. Validation of these findings in SIV/macaque model of opiate dependence demonstrated reduced expression of the tropic factor PDGF-B with a concomitant increase in miR-29b in the basal ganglia compared with the SIV-infected controls. Molecular mechanisms involved in downregulation of PDGF-BB implied transport of miR29b via exosomal shuttling from HIV Tat and morphine treated astrocytes to neurons resulting in downregulation of the target gene- PDGF-B with a concomitant decrease in neuronal cell viability. These findings thus shed light on miRNA mediated regulation of the neurotropic genes such as PDGF and that downregulation of these vital tropic factors could in turn, lead to neuronal dysfunction/death manifesting as cognitive decline observed in HAND
Similar to most chemokines and growth factors, PDGF also exerts paradoxical effects in various cell types. The deleterious effects of PDGF in the context of HIV infection will be the discussed below.
Neuropathological role of PDGF
Role of PDGF as a vascular permeant
As described earlier HAND remains a common complication of HIV even in presence of virus suppression in the systemic compartment. In fact, HAND etiology involves a chronic low level activation leading to age-related morbidities (Nath et al., 2000). Inability of the medications to penetrate into the CNS further results in brain becoming a sanctuary for the virus and viral proteins. This in turn, leads to the ensuing neuroinflammation and endothelial breach commonly observed in HAND (Su et al., 2008). Our previous findings have demonstrated upregulation of PDGF in the brains of macaques with SIV encephalitis specifically in newly migrating macrophages surrounding the blood vessels (Potula et al., 2004). Intriguingly endothelial cells lining the blood vessels also demonstrated upregulation of PDGF in response to virus proteins. Since PDGF is a known vascular permeant as demonstrated in studies by Su et al on ischemic stroke(Su et al., 2008), it is likely that PDGF expressed by and around the blood vessels during SIVE, could be a factor contributing to endothelial barrier breach, adding validity to this hypothesis are various reports demonstrating deleterious effects of PDGF on endothelial cells (Sato et al., 1993; Koyama et al., 1994; Cao et al., 2002).
In addition to endothelial cells, astrocytes comprising the neovascular unit are also the source of PDGF. In fact, our previous study has demonstrated that exposure of rat and human astrocytes to the HIV-1 protein Tat resulted in the induction of PDGF at both the mRNA and protein levels via activation of ERK and JNK signaling pathways and the downstream transcription factor Egr-1. Exposure of astrocytes to PDGF-BB in turn, leads to increased proliferation and the release of proinflammatory mediators such as the chemokine monocyte chemotactic protein-1 (MCP-1). MCP-1 is a key chemokine that facilitates the recruitment of monocytes into the brain contributing to neuroinflammation and BBB leakiness (Fuentes et al., 1995; Weiss et al., 1999; Eugenin and Berman, 2007). This chemokine has been extensively studied and is expressed by a number of cell types including astrocytes, microglia and neurons (Lee et al., 1993; Rock et al., 2006). Elevated expression of MCP-1 has been demonstrated in various diseases including multiple sclerosis, amyloid lateral sclerosis, lupus nephritis, peripheral neuropathy, Alzheimer's disease and HIVE (Ransohoff et al., 1993; Berman et al., 1996; Van Der Voorn et al., 1999; Mahad and Ransohoff, 2003; Yamamoto et al., 2005; Tanuma et al., 2006; Groh et al., 2010; Marks et al., 2010). Since MCP-1 is linked to disease severity, understanding its modulation by PDGF-BB could aid in understanding the proinflammatory responses in HAND. These results suggest that astrocyte activation by PDGF-BB exaggerates monocyte recruitment into the brain via MCP-1 and underscores the critical role astrocytes play in HAND.
Cocaine-mediated induction of PDGF: Implication for increased vascular permeability
Drug abuse has been implicated as a contributing risk factor for increased neuroinflammation associated with HIV-1 infection. Intriguingly, cocaine has been shown to disrupt the BBB (Fiala et al., 1998; Zhang et al., 1998; Fiala et al., 2005). Cocaine-mediated effects on the BBB are complex involving both direct pro-apoptotic effects on the endothelial cells as well as indirect paracrine effects that are manifested by pro-inflammatory modulators such as chemokines and cytokines (Zhang et al., 1998).
In keeping with the role of PDGF-CC as a vascular permeant that augmented barrier permeability during ischemic stroke (Su et al., 2008), our findings have also demonstrated a similar role of PDGF in disrupting the endothelial barrier. In our findings exposure of human brain microvascular endothelial cells (HBMECs) to cocaine resulted in induction of PDGF-BB via the binding of cocaine to its cognate sigma receptor, with subsequent activation of MAPKs and Egr-1 pathways. In vivo validation of the role of PDGF-BB in mediating brain endothelial permeability was validated in mice injected with cocaine in the presence or absence of pre-treatment with PDGF-BB neutralizing antibody.
PDGF-B chain is a novel target gene of cocaine-mediated Notch1 signaling
Notch signaling has emerged as an important regulator of neuronal homeostasis. Similar to the physiological effects of PDGF, Notch signaling also mediates intercellular signals that affect diverse physiological functions such as proliferation, survival and differentiation (MacKenzie et al., 2004; Noseda et al., 2004). Notch receptors (Notch1 to 4) are transmembrane proteins that bind to endogenous ligands such as Delta and Jagged-1 leading to proteolytic cleavage of the Notch receptors by the γ-secretase enzyme complex and a concomitant release of the Notch intracellular domain (NICD). NICD in turn, translocates to the nucleus and interacts with the DNA binding factor CSL (also known as RBP-Jk and CBF1), leading to transactivation of promoters for the Notch target genes HES and HEY. Additional immediate downstream genes of Notch signaling have also been characterized, suggesting the existence of a larger immediate Notch transcriptome. As an example, PDGF-βR has been shown to be a target of Notch signaling gene in vascular smooth muscle cells (Jin et al., 2008). More recently PDGF-B chain has also been identified as a new member of the Notch target gene in human HBMECs, thereby implicating Notch signaling as a key player in the maintenance of BBB integrity. Our previous study demonstrated that cocaine-mediated activation of Notch1 signaling leading to targeted expression of PDGF-B involved activation of the downstream effector CSL. These findings provided the first evidence of involvement of Notch1 activation in cocaine-mediated regulation of PDGF-B expression.
Conclusions
In the brains of individuals with HAND, upregulation of chemokines in the CNS is considered a correlate of neuroinflammation. However, recent evidence raises the possibility that, in addition to their role as chemoattractants, chemokines can also functions as neurotransmitters or neuromodulators (Rostene et al., 2007). An example of this is the chemokine fractalkine that functions as an inflammatory mediator by attracting peripheral macrophages into the brain and opposingly, can also serve as a neuroprotective factor (Tong et al., 2000; Eugenin et al., 2003). Moreover, it can also function to regulate neuronal survival through its antiapoptotic effects (Meucci et al., 1998).
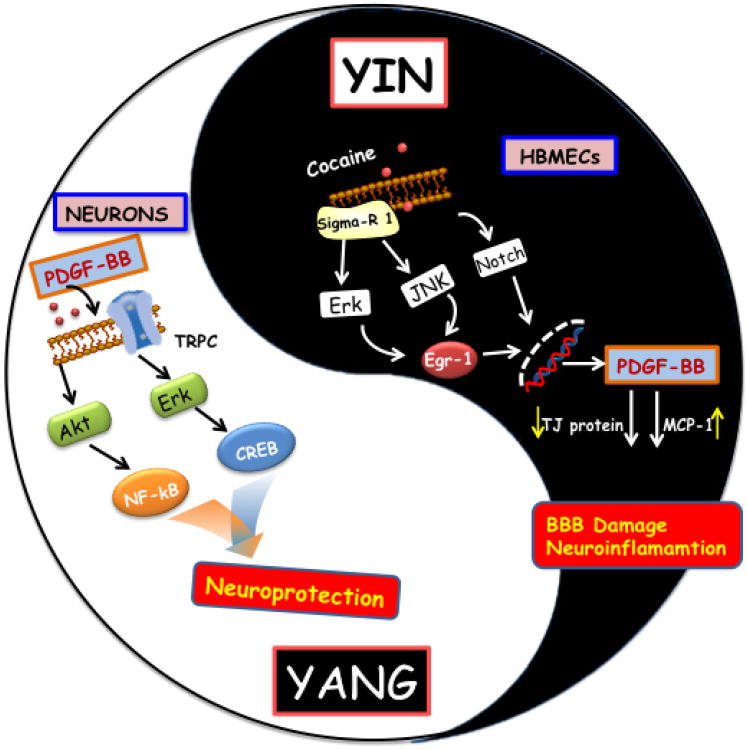
In response to cellular damage, the host is also capable of generating trophic growth factors such as PDGF which, similar to fractalkine, can also exert somewhat paradoxically diverse roles. During the progression of HIV infection PDGF upregulation by macropahges and endothelial cells can results in increased expression of MCP-1 resulting in enhanced neuroinflammation and a concomitant breach of the blood brain barrier. On the other hand, PDGF can also provide tropic support for the neurons against a variety of neurotoxic mediators (cellular and viral products). Thus, depending on the cell type within the tissue, the same host factor can manifest diverse activation responses leading to diverse outcomes. The ultimate outcome of infection in the CNS (neuronal survival versus damage) is thus a result of the ensuing shift in balance between the neurotrophic versus neurotoxic products manifested over time following infection. Figure 1 summarizes the diverse functions of PDGF, both as a neuroprotective agent and also as a vascular permeant in the setting of HIV toxicity and drug abuse. Since this sort of paradoxical regulation is a common theme of the cytokines and growth factors, caution has to be exercised in development of therapeutic targets involving these mediators.
Figure 1. Schematic illustration demonstrating the paradoxical role of PDGF-BB in the context of HAND and drug abuse.
Yang role of PDGF-BB indicated that PDGF-BB-mediated engagement of the activation of TRPC 1 channels resulting in Ca2+ influx transients with subsequent activation of ERK/CREB, but not the Akt/NF-kB pathway, culminating in neuroprotection. Yin role of PDGF-BB indicated that exposure of cocaine leads to sigma receptor-mediated activation of ERK and JNK/Egr-1 or Notch pathways, result in PDGF-BB expression with concomitant BBB damage and neuroinflammation.
Acknowledgments
This work was supported by grants DA020392, DA033150,DA024442, DA035203, DA033614 (SB) and DA030285 (HY) from the National Institutes of Health.
Abbreviation
- BDNF
brain-derived neurotrophic factor
- cART
combination antiretroviral therapy
- FGF
fibroblast growth factor
- GDNF
glia cell line-derived neurotrophic factor
- GSK3β
glycogen synthase kinase-3β
- HAND
HIV-associated neurodegenerative disorder
- LTP
long-term potentiation
- MCP-1
monocyte chemotactic protein-1
- NGF
nerve growth factor
- NICD
Notch intracellular domain
- NPC
neural stem/progenitor cells
- PDGF
platelet derived growth factor
- PI-3K
phosphatidylinositol-3 kinase
- TH
tyrosine hydroxylase
- TRPC
transient receptor potential canonical
References
- Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, Carvalho RF, Carvalho AP, Duarte CB. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- Alzheimer C, Werner S. Fibroblast growth factors and neuroprotection. Adv Exp Med Biol. 2002;513:335–351. doi: 10.1007/978-1-4615-0123-7_12. [DOI] [PubMed] [Google Scholar]
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur DB, Georgi S, Akassoglou K, Insel PA. Inhibition of apoptosis by P2Y2 receptor activation: novel pathways for neuronal survival. J Neurosci. 2006;26:3798–3804. doi: 10.1523/JNEUROSCI.5338-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beazely MA, Lim A, Li H, Trepanier C, Chen X, Sidhu BR, Macdonald JF. Platelet-derived growth factor selectively inhibits NR2B-containing NMDA receptors in ca1 hippocampal neurons. J Biol Chem. 2008 doi: 10.1074/jbc.M805384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JW, Guida MP, Warren J, Amat J, Brosnan CF. Localization of monocyte chemoattractant peptide-1 expression in the central nervous system in experimental autoimmune encephalomyelitis and trauma in the rat. J Immunol. 1996;156:3017–3023. [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Cao R, Brakenhielm E, Li X, Pietras K, Widenfalk J, Ostman A, Eriksson U, Cao Y. Angiogenesis stimulated by PDGF-CC, a novel member in the PDGF family, involves activation of PDGFR-alphaalpha and -alphabeta receptors. FASEB J. 2002;16:1575–1583. doi: 10.1096/fj.02-0319com. [DOI] [PubMed] [Google Scholar]
- Colafrancesco V, Villoslada P. Targeting NGF pathway for developing neuroprotective therapies for multiple sclerosis and other neurological diseases. Arch Ital Biol. 2011;149:183–192. doi: 10.4449/aib.v149i2.1376. [DOI] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Deierborg T, Soulet D, Roybon L, Hall V, Brundin P. Emerging restorative treatments for Parkinson's disease. Prog Neurobiol. 2008;85:407–432. doi: 10.1016/j.pneurobio.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Berman JW. Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. J Neurosci. 2007;27:12844–12850. doi: 10.1523/JNEUROSCI.4154-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, D'Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. J Neurochem. 2003;85:1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Everall IP, Hansen LA, Masliah E. The shifting patterns of HIV encephalitis neuropathology. Neurotox Res. 2005;8:51–61. doi: 10.1007/BF03033819. [DOI] [PubMed] [Google Scholar]
- Fiala M, Gan XH, Zhang L, House SD, Newton T, Graves MC, Shapshak P, Stins M, Kim KS, Witte M, Chang SL. Cocaine enhances monocyte migration across the blood-brain barrier. Cocaine's connection to AIDS dementia and vasculitis? Adv Exp Med Biol. 1998;437:199–205. doi: 10.1007/978-1-4615-5347-2_22. [DOI] [PubMed] [Google Scholar]
- Fiala M, Eshleman AJ, Cashman J, Lin J, Lossinsky AS, Suarez V, Yang W, Zhang J, Popik W, Singer E, Chiappelli F, Carro E, Weinand M, Witte M, Arthos J. Cocaine increases human immunodeficiency virus type 1 neuroinvasion through remodeling brain microvascular endothelial cells. J Neurovirol. 2005;11:281–291. doi: 10.1080/13550280590952835. [DOI] [PubMed] [Google Scholar]
- Fuentes ME, Durham SK, Swerdel MR, Lewin AC, Barton DS, Megill JR, Bravo R, Lira SA. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J Immunol. 1995;155:5769–5776. [PubMed] [Google Scholar]
- Groh J, Heinl K, Kohl B, Wessig C, Greeske J, Fischer S, Martini R. Attenuation of MCP-1/CCL2 expression ameliorates neuropathy in a mouse model for Charcot-Marie-Tooth 1×. Hum Mol Genet. 2010;19:3530–3543. doi: 10.1093/hmg/ddq269. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hu S, Cheeran MC, Sheng WS, Ni HT, Lokensgard JR, Peterson PK. Cocaine alters proliferation, migration, and differentiation of human fetal brain-derived neural precursor cells. J Pharmacol Exp Ther. 2006;318:1280–1286. doi: 10.1124/jpet.106.103853. [DOI] [PubMed] [Google Scholar]
- Jia Y, Zhou J, Tai Y, Wang Y. TRPC channels promote cerebellar granule neuron survival. Nat Neurosci. 2007;10:559–567. doi: 10.1038/nn1870. [DOI] [PubMed] [Google Scholar]
- Jin S, Hansson EM, Tikka S, Lanner F, Sahlgren C, Farnebo F, Baumann M, Kalimo H, Lendahl U. Notch signaling regulates platelet-derived growth factor receptor-beta expression in vascular smooth muscle cells. Circ Res. 2008;102:1483–1491. doi: 10.1161/CIRCRESAHA.107.167965. [DOI] [PubMed] [Google Scholar]
- Koyama N, Watanabe S, Tezuka M, Morisaki N, Saito Y, Yoshida S. Migratory and proliferative effect of platelet-derived growth factor in rabbit retinal endothelial cells: evidence of an autocrine pathway of platelet-derived growth factor. J Cell Physiol. 1994;158:1–6. doi: 10.1002/jcp.1041580102. [DOI] [PubMed] [Google Scholar]
- Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol. 1993;150:2659–2667. [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci U S A. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liot G, Gabriel C, Cacquevel M, Ali C, MacKenzie ET, Buisson A, Vivien D. Neurotrophin-3-induced PI-3 kinase/Akt signaling rescues cortical neurons from apoptosis. Exp Neurol. 2004;187:38–46. doi: 10.1016/j.expneurol.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- MacKenzie F, Duriez P, Wong F, Noseda M, Karsan A. Notch4 inhibits endothelial apoptosis via RBP-Jkappa-dependent and -independent pathways. J Biol Chem. 2004;279:11657–11663. doi: 10.1074/jbc.M312102200. [DOI] [PubMed] [Google Scholar]
- Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Semin Immunol. 2003;15:23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Marks SD, Shah V, Pilkington C, Tullus K. Urinary monocyte chemoattractant protein-1 correlates with disease activity in lupus nephritis. Pediatr Nephrol. 2010;25:2283–2288. doi: 10.1007/s00467-010-1605-z. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M, Taneja M, Malik S, Khalique H, Seth P. Human immunodeficiency virus type 1 Tat modulates proliferation and differentiation of human neural precursor cells: implication in NeuroAIDS. J Neurovirol. 2010;16:355–367. doi: 10.3109/13550284.2010.513028. [DOI] [PubMed] [Google Scholar]
- Mocchetti I, Nosheny RL, Tanda G, Ren K, Meyer EM. Brain-derived neurotrophic factor prevents human immunodeficiency virus type 1 protein gp120 neurotoxicity in the rat nigrostriatal system. Ann N Y Acad Sci. 2007;1122:144–154. doi: 10.1196/annals.1403.010. [DOI] [PubMed] [Google Scholar]
- Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, Bell J, Hauser KF, Mattson M. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol. 2000;14:222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Noseda M, Chang L, McLean G, Grim JE, Clurman BE, Smith LL, Karsan A. Notch activation induces endothelial cell cycle arrest and participates in contact inhibition: role of p21Cip1 repression. Mol Cell Biol. 2004;24:8813–8822. doi: 10.1128/MCB.24.20.8813-8822.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F, Dhillon NK, Yao H, Zhu X, Williams R, Buch S. Mechanisms of platelet-derived growth factor-mediated neuroprotection--implications in HIV dementia. Eur J Neurosci. 2008a;28:1255–1264. doi: 10.1111/j.1460-9568.2008.06444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F, Dhillon N, Callen S, Yao H, Bokhari S, Zhu X, Baydoun HH, Buch S. Platelet-derived growth factor protects neurons against gp120-mediated toxicity. J Neurovirol. 2008b;14:62–72. doi: 10.1080/13550280701809084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potula R, Dhillion N, Sui Y, Zien CA, Funa K, Pinson D, Mayo MS, Singh DK, Narayan O, Buch S. Association of platelet-derived growth factor-B chain with simian human immunodeficiency virus encephalitis. Am J Pathol. 2004;165:815–824. doi: 10.1016/S0002-9440(10)63344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Hamilton TA, Tani M, Stoler MH, Shick HE, Major JA, Estes ML, Thomas DM, Tuohy VK. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- Rock RB, Hu S, Sheng WS, Peterson PK. Morphine stimulates CCL2 production by human neurons. J Neuroinflammation. 2006;3:32. doi: 10.1186/1742-2094-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Beitz JG, Kato J, Yamamoto M, Clark JW, Calabresi P, Raymond A, Frackelton AR., Jr Platelet-derived growth factor indirectly stimulates angiogenesis in vitro. Am J Pathol. 1993;142:1119–1130. [PMC free article] [PubMed] [Google Scholar]
- Smits A, Kato M, Westermark B, Nister M, Heldin CH, Funa K. Neurotrophic activity of platelet-derived growth factor (PDGF): Rat neuronal cells possess functional PDGF beta-type receptors and respond to PDGF. Proc Natl Acad Sci U S A. 1991;88:8159–8163. doi: 10.1073/pnas.88.18.8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossin WS, Barker PA. Something old, something new: BDNF-induced neuron survival requires TRPC channel function. Nat Neurosci. 2007;10:537–538. doi: 10.1038/nn0507-537. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, Gao Y, Pietras K, Mann K, Yepes M, Strickland DK, Betsholtz C, Eriksson U, Lawrence DA. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med. 2008;14:731–737. doi: 10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Shahani N, Strelau J, Laliberte C, Brandt R, Kaplan D, Unsicker K. Insulin-like growth factor 1 inhibits extracellular signal-regulated kinase to promote neuronal survival via the phosphatidylinositol 3-kinase/protein kinase A/c-Raf pathway. J Neurosci. 2005;25:2838–2852. doi: 10.1523/JNEUROSCI.5060-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Arjunan P, Lee C, Li Y, Kumar A, Hou X, Wang B, Wardega P, Zhang F, Dong L, Zhang Y, Zhang SZ, Ding H, Fariss RN, Becker KG, Lennartsson J, Nagai N, Cao Y, Li X. Survival effect of PDGF-CC rescues neurons from apoptosis in both brain and retina by regulating GSK3beta phosphorylation. J Exp Med. 2010;207:867–880. doi: 10.1084/jem.20091704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanuma N, Sakuma H, Sasaki A, Matsumoto Y. Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol. 2006;112:195–204. doi: 10.1007/s00401-006-0083-7. [DOI] [PubMed] [Google Scholar]
- Tong N, Perry SW, Zhang Q, James HJ, Guo H, Brooks A, Bal H, Kinnear SA, Fine S, Epstein LG, Dairaghi D, Schall TJ, Gendelman HE, Dewhurst S, Sharer LR, Gelbard HA. Neuronal fractalkine expression in HIV-1 encephalitis: roles for macrophage recruitment and neuroprotection in the central nervous system. J Immunol. 2000;164:1333–1339. doi: 10.4049/jimmunol.164.3.1333. [DOI] [PubMed] [Google Scholar]
- Tsui CC, Copeland NG, Gilbert DJ, Jenkins NA, Barnes C, Worley PF. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J Neurosci. 1996;16:2463–2478. doi: 10.1523/JNEUROSCI.16-08-02463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Voorn P, Tekstra J, Beelen RH, Tensen CP, Van Der Valk P, De Groot CJ. Expression of MCP-1 by reactive astrocytes in demyelinating multiple sclerosis lesions. Am J Pathol. 1999;154:45–51. doi: 10.1016/S0002-9440(10)65249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan A, Nath A, Ming GL, Song H. Adult hippocampal neurogenesis: regulation by HIV and drugs of abuse. Cell Mol Life Sci. 2007;64:2120–2132. doi: 10.1007/s00018-007-7063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yang HJ, Xia YY, Feng ZW. Insulin-like growth factor 1 protects human neuroblastoma cells SH-EP1 against MPP+-induced apoptosis by AKT/GSK-3beta/JNK signaling. Apoptosis. 2010;15:1470–1479. doi: 10.1007/s10495-010-0547-z. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Nath A, Major EO, Berman JW. HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes. J Immunol. 1999;163:2953–2959. [PubMed] [Google Scholar]
- Wu Y, Shang Y, Sun S, Liang H, Liu R. Erythropoietin prevents PC12 cells from 1-methyl-4-phenylpyridinium ion-induced apoptosis via the Akt/GSK-3beta/caspase-3 mediated signaling pathway. Apoptosis. 2007;12:1365–1375. doi: 10.1007/s10495-007-0065-9. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Seki T, Namba T, Juan R, Arai H, Hori T, Asada T. Repetitive cocaine administration decreases neurogenesis in adult rat hippocampus. Ann N Y Acad Sci. 2004;1025:351–362. doi: 10.1196/annals.1316.043. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Seki T, Namba T, Liu J, Arai H, Hori T, Shiga T. Decreased cell proliferation in the dentate gyrus of rats after repeated administration of cocaine. Synapse. 2005;58:63–71. doi: 10.1002/syn.20182. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Horiba M, Buescher JL, Huang D, Gendelman HE, Ransohoff RM, Ikezu T. Overexpression of monocyte chemotactic protein-1/CCL2 in beta-amyloid precursor protein transgenic mice show accelerated diffuse beta-amyloid deposition. Am J Pathol. 2005;166:1475–1485. doi: 10.1016/s0002-9440(10)62364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Duan M, Buch S. Cocaine-mediated induction of platelet-derived growth factor: implication for increased vascular permeability. Blood. 2011a;117:2538–2547. doi: 10.1182/blood-2010-10-313593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Duan M, Hu G, Buch S. Platelet-derived growth factor B chain is a novel target gene of cocaine-mediated Notch1 signaling: implications for HIV-associated neurological disorders. J Neurosci. 2011b;31:12449–12454. doi: 10.1523/JNEUROSCI.2330-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Peng F, Fan Y, Zhu X, Hu G, Buch SJ. TRPC channel-mediated neuroprotection by PDGF involves Pyk2/ERK/CREB pathway. Cell Death Differ. 2009;16:1681–1693. doi: 10.1038/cdd.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauli G, Secchiero P, Rodella L, Gibellini D, Mirandola P, Mazzoni M, Milani D, Dowd DR, Capitani S, Vitale M. HIV-1 Tat-mediated inhibition of the tyrosine hydroxylase gene expression in dopaminergic neuronal cells. J Biol Chem. 2000;275:4159–4165. doi: 10.1074/jbc.275.6.4159. [DOI] [PubMed] [Google Scholar]
- Zhang L, Looney D, Taub D, Chang SL, Way D, Witte MH, Graves MC, Fiala M. Cocaine opens the blood-brain barrier to HIV-1 invasion. J Neurovirol. 1998;4:619–626. doi: 10.3109/13550289809114228. [DOI] [PubMed] [Google Scholar]
- Zheng WH, Quirion R. Comparative signaling pathways of insulin-like growth factor-1 and brain-derived neurotrophic factor in hippocampal neurons and the role of the PI3 kinase pathway in cell survival. J Neurochem. 2004;89:844–852. doi: 10.1111/j.1471-4159.2004.02350.x. [DOI] [PubMed] [Google Scholar]