Abstract
Behavioral responsiveness to initial cocaine use varies among individuals and may contribute to differential vulnerability to cocaine addiction. Rats also exhibit individual differences in cocaine’s effects and can be classified as low or high cocaine responders (LCRs or HCRs, respectively), based on their initial cocaine-induced locomotor activity (10 mg/kg, i.p.). Here, we used the extinction/reinstatement model to address whether or not LCRs and HCRs differ in (i) extinction/reinstatement of cocaine self-administration behavior and (ii) levels of metabotropic glutamate receptors (mGluRs) following these behaviors. During the earliest acquisition sessions, LCRs exhibited significantly greater cocaine intake (0.8 mg/kg/infusion) and cocaine-paired lever responding than HCRs, but intake and lever responding converged by the end of the cocaine self-administration portion of the study. LCRs and HCRs did not differ in cocaine seeking during the first extinction session and extinguished cocaine seeking similarly. HCRs exhibited greater reinstatement than LCRs to lower (2.5 and 5 mg/kg), but not higher (10 mg/kg), i.p. priming doses of cocaine. The effect of drug-paired cues on reinstatement following extinction was complex, with HCRs and LCRs showing the greater effect of cue depending on the order in which cue- and drug-primed tests were given. Western blot analysis revealed that mGluR5 heteromers were significantly higher in the dorsal striatum of HCRs than LCRs following reinstatement testing. Although our previous findings with the LCR/HCR model have uniformly supported the idea that lower initial cocaine-induced activation predicts more ready development of cocaine addiction-like behaviors, here, we show a more complex relationship with cocaine reinstatement.
Keywords: Individual differences, extinction/reinstatement, cocaine self-administration, metabotropic glutamate receptors, dorsal striatum
1. Introduction
One hallmark of cocaine addiction is differential susceptibility among individuals. This is highlighted by findings that despite a large number of individuals having used cocaine, only a subset of users progress to addiction (Gawin, 1991; Wagner and Anthony, 2002). Another hallmark is high vulnerability to relapse, a problem that persists even after prolonged periods of abstinence (Jaffe et al., 1989; Mahoney et al., 2007; Volkow et al., 2005; Ciccocioppo et al., 2001). Genetic (Dlugos et al., 2007; Dlugos et al., 2011; Mattay et al., 2003; Palmer et al., 2005), phenotypic (Dodge et al., 2005; Enoch, 2011; Hyman et al., 2008), and environmental (Gorwood et al., 2007) factors contribute both to the variability seen in individuals’ responses to cocaine (and other abused drugs) and to their vulnerability to addiction (Swendsen and Le Moal, 2011) and relapse (Sinha, 2011). Thus, it is important to better understand brain systems and mechanisms that underlie these individual differences, with the ultimate goal of helping to develop new strategies to prevent cocaine addiction and treat relapse.
Animal models have provided considerable evidence that individual responsiveness to drugs like cocaine can predict the likelihood of developing addiction-like behaviors. For example, our lab has long observed that a single i.p. injection of 10 mg/kg cocaine, a relatively low dose of drug, elicits a wide range of locomotor activity (LMA) in outbred Sprague-Dawley rats. We have used this range of cocaine-elicited LMA to classify rats as either low- or high-cocaine responders (LCRs or HCRs, respectively) based on the group median split (Allen et al., 2007; Gulley et al., 2003; Mandt et al., 2008, 2010, 2012a & b; Nelson et al., 2009 & 2010; Sabeti et al., 2002 & 2003). It is noteworthy that LMA differences between LCRs and HCRs are not explained by differences in brain cocaine levels, competing stereotyped behaviors or anxiety (Gulley et al., 2003; Nelson et al., 2010). However, we have found that the LCR/HCR phenotypes predict several differences in cocaine addiction-like behaviors. Specifically, LCRs more readily exhibit locomotor sensitization and conditioned place preference than HCRs in response to repeated cocaine exposure (Allen et al., 2007; Mandt et al., 2008; Nelson et al., 2009; Sabeti et al., 2003). Following acquisition of low dose cocaine self-administration, LCRs demonstrate greater motivation than HCRs to self-administer cocaine, as indicated by higher break points on a progressive ratio schedule of reinforcement (Mandt et al., 2008; but see Mandt et al., 2012b). LCRs also exhibit increased sensitivity to the discriminative stimulus properties of cocaine, as compared to HCRs (Klein and Gulley, 2009). Thus far, the results support the idea that LCRs may be the more “addiction prone” phenotype. However, whether or not LCRs and HCRs differ in reinstatement of cocaine seeking remains an important question to answer.
Pre-clinical evidence suggests a critical role for brain glutamate neuroplasticity not only in cocaine self-administration, but also during extinction of responding for cocaine and reinstatement of cocaine-seeking behaviors (for reviews see Kalivas, 2007 and 2009; Kenny et al., 2005; Knackstedt and Kalivas, 2009). Altered levels of extracellular glutamate, ionotropic glutamate receptors, and metabotropic glutamate receptors (mGluRs) are all involved. Of the eight subtypes of mGluRs identified (Nicoletti et al., 2011), the mGluR5 (group 1) and mGluR2/3 (group 2) subtypes have been most strongly linked to extinction and reinstatement of responding for cocaine (Knackstedt et al., 2010; Ghasemzadeh et al., 2009a & b). Importantly, mice lacking mGluR5s exhibit neither cocaine-induced locomotor activation nor cocaine self-administration (Chiamulera et al., 2001). Further, extinction of cocaine seeking results in lower levels of mGluR5 immunoreactivity in the nucleus accumbens (NAc) shell but increased levels in the dorsal striatum (dSTR; Ghasemzadeh et al., 2009b). Extinction training down-regulates surface levels of mGluR5 in NAc, and this may help to explain the inhibition of cue-primed reinstatement of cocaine seeking following such training (Knackstedt et al., 2010). Notably, mGluR5 antagonists, administered either systemically or locally into the NAc, reduce both cocaine- and cue-primed reinstatement of cocaine seeking (Bäckström and Hyytiä, 2006; Kumaresan et al., 2009; Lee et al., 2005; Wang et al., 2013). In contrast, positive allosteric modulation of mGluR2/3 decreases cocaine self-administration and cue-primed reinstatement of cocaine seeking (Jin et al., 2010), whereas positive allosteric modulation of mGluR5 facilitates extinction learning in cocaine self-administering rats and extinction of cocaine conditioned place preference (Cleva et al., 2011; Gass and Olive, 2009). Whether levels of mGluRs differ between LCRs and HCRs basally and/or following extinction of cocaine self-administration has not been explored.
This study was designed to determine whether LCR/HCR classification predicts relapse behaviors as tested with the cocaine self-administration extinction/reinstatement model. Cue- and cocaine-primed (1.25 – 10 mg/kg, i.p.) reinstatement of cocaine seeking was measured in LCR/HCR-classified outbred male Sprague-Dawley rats. In addition, levels of mGluR5 and mGluR2/3 protein in the dSTR and NAc were measured to investigate potential mechanisms that may contribute to differences in the propensity to reinstate cocaine seeking.
2. Material and methods
2.1. Animals
Male Sprague-Dawley rats (225-250 g) were purchased from Charles River Laboratories (Wilmington, MA) for each of the three separate experiments of this study and individually housed in plastic cages with food and water provided ad libitum. Animals were maintained on a 12:12 light:dark cycle (lights on at 0600 h). Animals were habituated to the vivarium (2 groups for the self-administration experiments: University of Colorado Denver; 1 group for the LMA only experiment: University of Colorado Anschutz Medical Campus) for 1 week prior to use, and all procedures were performed during the light cycle. Animal care and use procedures were in strict accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Guide for the Care and Use of Laboratory Animals) and were approved by the University of Colorado Denver Institutional Animal Care and Use Committee. Special attention was made to minimize animal suffering and careful planning was used to minimize the number of animals used in this study.
2.2. Drugs and chemicals
The National Institute on Drug Abuse generously provided the (−)cocaine hydrochloride used in these experiments. For i.p. injections, cocaine was dissolved in sterile saline and administered in a volume of 1 ml/kg. For i.v. infusions, cocaine was dissolved in sterile saline and filtered. All drug weights refer to the salt and all chemicals whose sources are not given were obtained from Sigma-Aldrich (St. Louis, MO).
2.3. Surgery and catheter implantation
Catheter implantation was performed as described previously (Mandt et al., 2012a). Briefly, rats were given acetaminophen (20 mg/ml) in their drinking water 48 h pre- and post-surgery. Chronic, indwelling catheters were surgically implanted into the right jugular vein of anesthetized animals (100 mg/kg, i.m. ketamine; 10 mg/kg, i.m. xylazine) that were maintained at body temperature through use of an electronic heating pad. Immediately following surgery, animals were observed during anesthesia recovery and kept on a heating pad until they could move about the cage freely. Animals recovered from surgery for a minimum of 5 days prior to LMA testing for LCR/HCR classification. Catheters were kept patent through daily flushing with 0.3 ml bacteriostatic 0.9% sodium chloride solution containing 30 U/ml heparin following surgery, and before and after each self-administration and test session. A single sodium methohexital (10 mg/kg, i.v.) infusion was given at the end of testing to verify catheter patency.
2.4. Locomotor testing and LCR/HCR classification
Classification of animals as LCRs or HCRs by their cocaine-induced locomotor response was performed as previously described (Mandt and Zahniser, 2010). Briefly, animals were transported to the behavioral testing room in their home cages and allowed to habituate for 45-60 min. Open field activity chambers [self-administration: acrylic boxes (17” × 17”) fitted with a photobeam frame (16 beams per dimension 0.5” from floor), Med Associates, St. Albans, VT; and LMA only: acrylic boxes (16” × 16”) fitted with a photo beam frame (8 beams per dimension 0.5” from the floor), San Diego Instruments, La Jolla, CA] were used to measure habituation of LMA for 90 min and cocaine-induced (10 mg/kg, i.p.) or vehicle-induced LMA for an additional 30 min. LMA was quantified in 10-min bins consisting of consecutive horizontal beam interruptions expressed as distance traveled (cm). Both types of open field activity chambers have been used previously in LCR/HCR studies (e.g., Mandt et al., 2012a, Sabeti et al., 2003). The median split of the summed 30 min cocaine-induced LMA was used to classify animals in each group as either LCRs or HCRs.
2.5. Cocaine self-administration
Following LCR/HCR classification, animals were trained to self-administer cocaine in operant conditioning chambers (Med Associates) as described previously (Mandt et al., 2012a). Chambers were individually housed within sound attenuating wooden cabinets fitted with a ventilation fan and white noise generator (90 dB). Upon initiation of the cocaine self-administration session, the drug-paired and inactive levers extended and the stimulus light (above the drug-paired lever) was illuminated indicating drug availability. This was followed by an i.v. infusion of cocaine (0.8 mg/kg/infusion over 5 - 7 sec depending on the weight of the animal). Each infusion was paired with presentation of a cue stimulus complex that consisted of extinguishing the stimulus light, activation of the house-light (opposite back wall), and activation of a tone stimulus (Sonalert Tone Generator, 2900 Hz). This lasted for a 15 sec “time-out” period during which cocaine was not available. Following the time-out period, the stimulus light over the lever was illuminated and a subsequent depression of the drug-paired lever produced an infusion of cocaine that was paired with the cue-stimulus complex. Lever pressing during the time-out period did not result in programmed consequences but was recorded. Inactive lever pressing was recorded as non-specific activity. A single priming dose was given at the initiation of each self-administration session to facilitate acquisition of cocaine self-administration. On sessions 2 – 5, additional priming infusions of cocaine were given only to animals that had not pressed the drug-paired lever 15 min after the initiation of the session.
Animals were trained to self-administer cocaine in daily 2 h-sessions, 5 days/week on a fixed ratio 1 (FR1) schedule of reinforcement. Animals were considered to have acquired cocaine self-administration, labeled session “x” in the figures, when at least 4 mg/kg of cocaine was self-administered across 3 consecutive sessions (Mandt et al., 2012a & b). Animals were allowed to continue to self-administer cocaine for 10 additional sessions (x + 10), with the criteria for stable responding being that the average cocaine intake across three consecutive sessions varied <10% for each animal. Although not part of our acquisition criteria, animals displayed clear lever preference using this method (see Figure 1B). Conditioning, testing programs, and all behavioral responding were performed and recorded using MED-PC for Windows software (Med Associates).
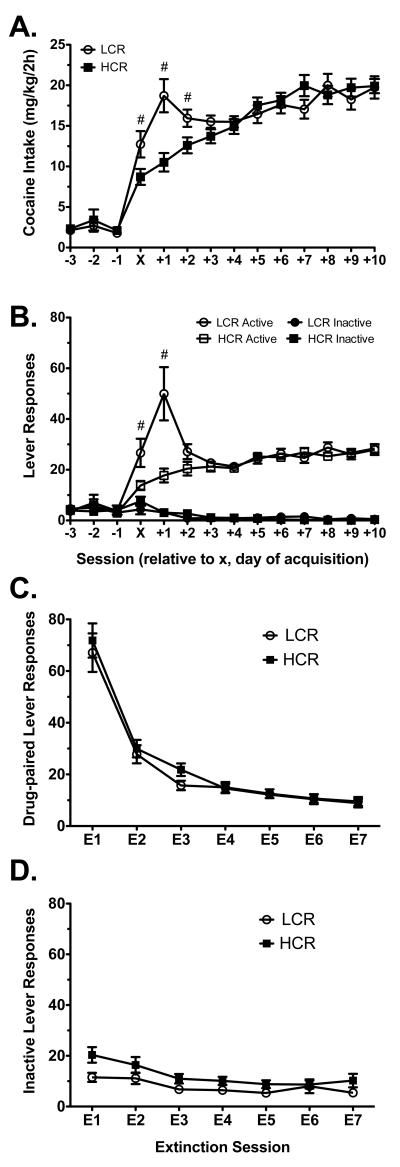
Figure 1.
Acquisition and extinction of cocaine self-administration in LCRs (n = 25) and HCRs (n = 25). Cocaine intake (A) and lever responding (B) are shown over the 3 sessions prior to, day of, and 10 post-acquisition sessions (session x). Responses on the drug-paired (C) and inactive (D) levers are shown for the 7 extinction sessions prior to reinstatement testing. Data are mean values ± SEM. White circles = LCRs, solid black squares = HCRs. #p < 0.05 LCRs vs. HCRs.
2.6. Extinction training and reinstatement testing
Extinction and reinstatement testing occurred in the absence of the cue stimulus complex and drug reinforcement unless otherwise indicated. Extinction sessions were 2 h in duration. Animals were tested under these conditions for a minimum of 7 sessions and were considered to have extinguished cocaine seeking when their responses on the drug-paired lever were <15% of their initial cocaine seeking (i.e., responses during the first extinction session) for 3 consecutive sessions. Inactive lever responses were also recorded. The same extinction criteria were used between reinstatement test sessions with the exception that animals were given a minimum of 3 extinction sessions.
Two groups of animals were tested under different reinstatement conditions. Groups SA1 and SA2 were tested sequentially, and LCRs/HCRs were classified within each group. Group SA1 (Table 1; Figure 2A) was first tested for cue-primed reinstatement of cocaine seeking; this was followed in subsequent sessions by testing for cocaine (10 mg/kg, i.p.)- or vehicle-primed reinstatement in randomized order. Cue-primed reinstatement consisted of an initial presentation of the cue stimulus complex to initiate reinstatement of cocaine seeking; each subsequent response on the drug-paired lever produced the cue stimulus complex but no cocaine infusion (contingent cue-primed reinstatement). Cocaine- and vehicle-primed reinstatement responding was measured in the absence of the cue stimulus complex (i.e., responses on the drug-paired lever had no programmed consequences). Group SA2 (Table 1; Figure 3A) was first tested for cocaine-primed reinstatement using a range of lower doses (1.25, 2.5, 5.0 mg/kg, i.p. or vehicle, 1 ml/kg) administered according to a Latin-squares design. This testing was followed by contingent cue-primed reinstatement of cocaine seeking. Lastly, animals in this group were tested for reinstatement with a 10 mg/kg i.p. cocaine priming injection. Drug-paired and inactive lever responses were recorded in all tests as measurements of cocaine-seeking and non-specific activity, respectively.
Table 1. Classification of rats by cocaine-induced locomotor activity.
| Cocaine Self-Administration Groups SA1 and SA2 | ||||
|---|---|---|---|---|
| Group | Classification |
Habituation LMA
(cm/30 min) |
Cocaine LMA
(cm/30 min) |
|
| Baseline Mean | Mean | Median | ||
| SA1 | Cocaine (n = 24) | 1314 ±230 | 2506 ± 580 | |
| SA2 | Cocaine (n = 26) | 1805±230 | 2613±520 | |
| SA1 | LCR (n = 12) | 1080±290 | 956 ± 71 | 1346 |
| HCR (n = 12) | 1548±360 | 4056 ± 970 | ||
| SA2 | LCR (n = 13) | 1805±310 | 1036±140 | 1647 |
| HCR (n = 13) | 1806±340 | 4191 ±830 | ||
Comparison of cocaine-induced locomotor activity (10 mg/kg, i.p.) in all rats and rats classified as LCRs and HCRs in cocaine self-administration groups SA1 and SA2. Data are presented as mean values ± SEM and represent the sum of activity over the first 30 min post-cocaine.
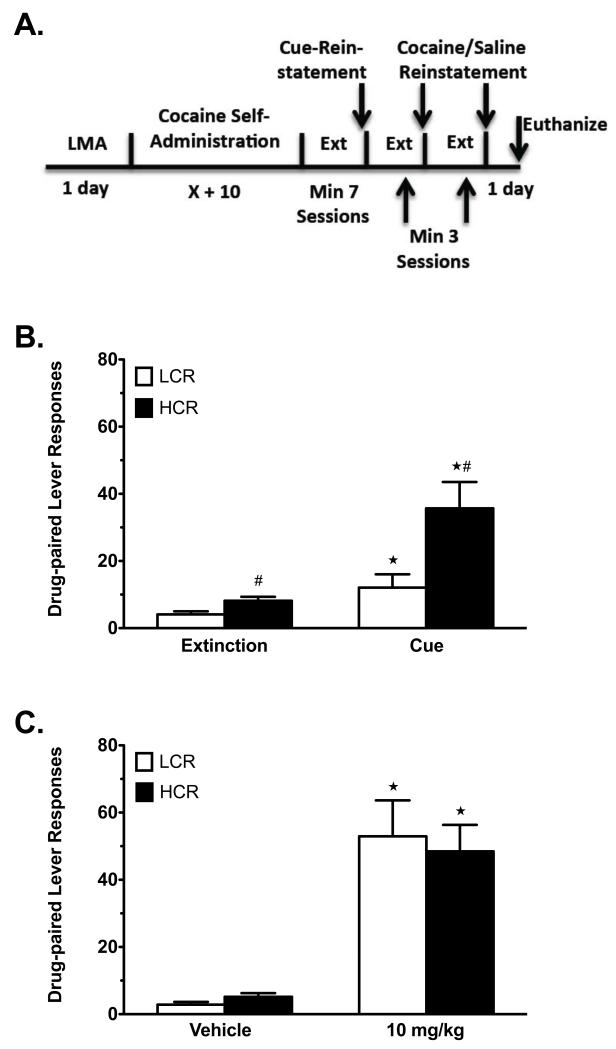
Figure 2.
Reinstatement testing in LCRs and HCRs in Group SA1. A) Timeline of reinstatement testing for LCRs and HCRs. B) Cue-primed reinstatement of cocaine seeking (LCRs n = 12, HCRs n = 12). C) Cocaine-primed reinstatement of cocaine seeking (LCRs n = 12, HCRs n = 11). Data are mean values ± SEM. White bars = LCRs, solid black bars = HCRs. #p < 0.05, LCRs vs. HCRs. *p < 0.05, test (cue or 10 mg/kg cocaine) vs. baseline (extinction or vehicle).
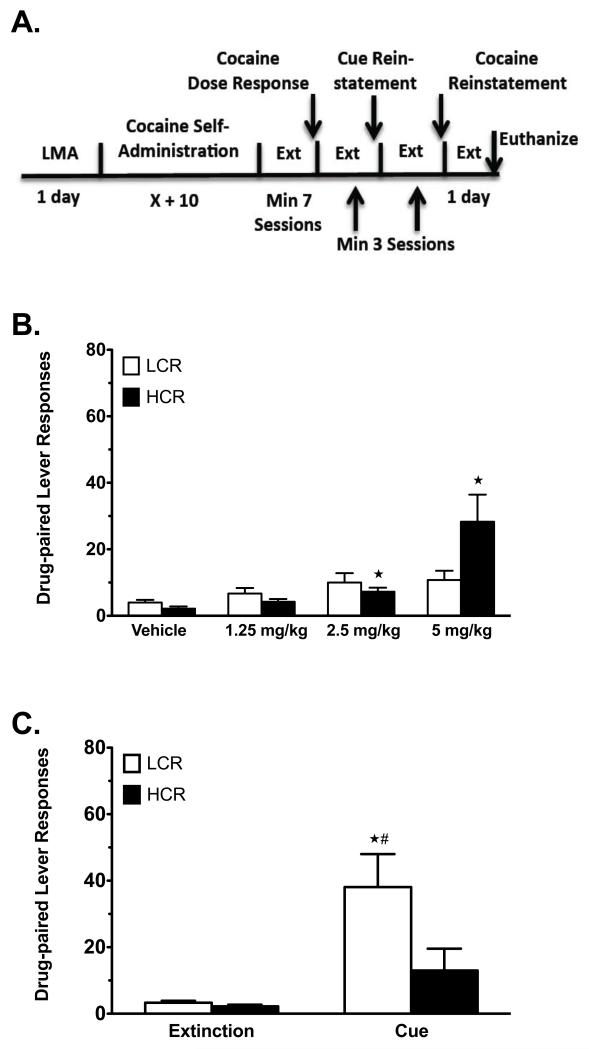
Figure 3.
Reinstatement testing in LCRs and HCRs in Group SA2. A) Timeline of reinstatement testing for LCRs and HCRs. B) Cue-primed reinstatement of cocaine seeking (LCRs n = 13, HCRs n = 13). C) Cocaine-primed reinstatement of cocaine seeking (LCRs n = 13, HCRs n = 13). Data are mean values ± SEM. White bars = LCRs, solid black bars = HCRs. #p < 0.05, LCRs vs. HCRs. *p < 0.05, test (cue, veh, 1.25 – 5 mg/kg cocaine) vs. baseline (extinction or vehicle).
2.7. Tissue collection and semi-quantitative Western blot analysis
After completion of reinstatement testing, the animals in groups SA1 and SA2 (Table 1) were extinguished for an additional session and euthanized by decapitation the following day. Two additional groups of rats were treated acutely with either cocaine (10 mg/kg, i.p.) or vehicle and then were euthanized the day after LMA testing (Table 2: Acute 1 and Acute 2). Brains were chilled in ice-cold saline and sliced into 3 mm coronal sections using a pre-chilled stainless steel rat brain matrix (Zivic Instruments, Pittsburgh, PA). The dSTR and NAc were rapidly dissected out, immediately frozen on dry ice and stored at −80°C until all animals in their respective group had completed behavioral testing.
Table 2. Classification of rats by cocaine-induced locomotor activity.
| Acute Cocaine Groups Acute 1 and Acute 2 | ||||
|---|---|---|---|---|
| Group | Classification |
Habituation LMA
(cm/30 min) |
Cocaine LMA
(cm/30 min) |
|
| Baseline Mean | Mean | Median | ||
| Acute 1 | Vehicle (n = 8) | 83 ± 36 | 342 ± 65 | |
| Acute 2 | Vehicle (n = 6) | 109 ± 50 | 569±140 | |
| Acute 1 | Cocaine (n = 16) | 135 ± 48 | 880±120 | |
| Acute 2 | Cocaine (n = 18) | 78 ± 41 | 1088±330 | |
| Acute 1 | LCR (n = 8) | 226±120 | 347±102 | 772 |
| HCR (n = 8) | 95 ± 59 | 1413±180 | ||
| Acute 2 | LCR (n = 9) | 74 ± 64 | 429 ± 74 | 813 |
| HCR (n = 9) | 81 ± 55 | 1747 ± 590 | ||
Comparison of cocaine-induced locomotor activity (10 mg/kg, i.p.) in all rats and rats classified as LCRs and HCRs in groups Acute 1 and Acute 2. Data are presented as mean values ± SEM and represent the sum of activity over the first 30 min post-injection.
For Western blot analysis, tissue was homogenized in ice-cold buffer (1% sodium dodecyl sulfate (SDS), 1 mM EDTA, 150 mM NaCl, 10 mM Tris, pH 8.0) containing protease and phosphatase inhibitors (1 mM sodium orthovanadate, 1 mM sodium fluoride, and 1 ug/ml each of aprotinin, pepstatin, and leupeptin) and centrifuged at 15,339 × g for 10 min to remove undissolved nuclei and membrane particles. Protein concentrations in the supernatant were determined with the BCA protein assay (Thermo Fisher Scientific, Rockford, IL). Samples (20 ug protein; within the linear response range for each antibody used) were subjected to SDS-7.5% polyacrylamide gel electrophoresis under reducing conditions, transferred to Polyscreen PVDF transfer membranes, blocked (5% non-fat dry milk and/or 3% BSA in Tris buffered saline (140 mM NaCl, 20 mM Tris; pH 7.6) with 0.1% Tween) for 1 h at room temperature and then probed with primary antibodies for mGluR5 (1:2000; Millipore, Temecula, CA, or 1:2500; LBSBio, Seattle, WA), mGluR2/3 (1:2000; Millipore), and alpha-tubulin (1:2000, Santa-Cruz Biosciences, Santa Cruz, CA). All primary antibody incubations were performed at 4°C overnight. Incubations with secondary antibody (1:20,000 goat anti-rabbit or 1:15,000 goat anti-mouse; Bio-Rad, Hercules, CA) were performed at room temperature for 1 h. Membranes were imaged using Pierce SuperSignal West Pico or Femto chemiluminesence (Thermo Fisher Scientific). mGluR5 and mGluR2/3 protein levels were normalized using a standard curve generated with internal standards on each membrane and subsequently the loading control alpha-tubulin, and then quantified using ImageJ software (NIH, Bethesda, MD). Monomeric and dimeric forms of mGluR5 and mGluR2/3 were distinguished based on their apparent molecular weights (~120 kDa and ~250 kDa for mGluR5 and ~100 kDa and ~200kDa for mGluR2/3, respectively (Knackstedt et al., 2010; Xi et al., 2002).
2.8. Data analysis
Data are expressed as mean values ± S.E.M. Graphing and statistical analyses, including one- and two-way analysis of variance (ANOVA), repeated measures ANOVA (RMANOVA), and Student t-tests were conducted using Prism 5 (GraphPad Software Inc., La Jolla, CA) or PASW Statistics (IBM Corp., Somers, NY, USA) software. Cocaine intake was measured across 19 consecutive self-administration sessions within which all animals completed X+10 sessions, where × represents the first day that an animal self-administered at least 4 mg/kg cocaine. LMA, cocaine self-administration, extinction, and reinstatement tests were analyzed by RMANOVA with session or test as the repeated measure, or two-way ANOVA followed by LSD post-hoc tests where relevant. When the assumption of sphericity was violated for a particular repeated-measures analysis, as revealed by Mauchly’s test statistic, tests of significance were based on the more conservative Huynh-Feldt corrected degrees of freedom. The symbol, a, indicates Huynh-Feldt corrected values throughout the text. mGluR5 and mGluR2/3 levels in dSTR and NAc were compared between groups using Student t-tests. The level of significance was set at p < 0.05.
3. Results
3.1. Classification of animals as LCRs or HCRs and cocaine self-administration
Two groups of animals were classified as LCRs or HCRs, and each was used for one of the two cocaine self-administration/reinstatement experiments (SA1 and SA2, n = 32 each; Table 1), where 24 and 26 rats (SA1 and SA2, respectively) completed at least a portion of the reinstatement study. The 14 animals excluded from the original groups had lost catheter patency (n = 6), failed to acquire cocaine self-administration (n = 6), or failed to extinguish cocaine seeking to baseline criteria (n = 2). The baseline and cocaine-induced LMA values for SA1 and SA2 are shown in Table 1. RMANOVA of locomotor activity scores revealed only a main effect of test (baseline versus cocaine: (F(2,96) = 65.1, p < 0.001), but not group (SA1 versus SA2) revealing the effect of cocaine but not cohort on locomotor response. The median split values for the cocaine-induced LMA of these two groups were 1346 and 1647 cm/30 min, respectively, and HCRs exhibited ~4-fold higher cocaine-induced LMA than LCRs in both groups (Table 1).
For the third experiment measuring LMA and mGluR protein levels, rats from two additional groups of animals were either classified as LCRs or HCRs following an acute cocaine injection or were used as controls following an acute vehicle injection (Table 2: Acute 1, n = 16 cocaine and n = 8 vehicle; Acute 2, n = 18 cocaine and n = 6 vehicle). Using a different set of open field chambers than those used for the reinstatement study (see Methods), the median split values for these two experimental group’s cocaine-induced LMA were 772 cm/30 min (Acute 1) and 813 cm/30 min (Acute 2), with the HCRs again exhibiting ~4-fold higher cocaine-induced LMA than LCRs (Table 2). Tables 1 and 2 also show the baseline LMA during the final 30 min of habituation to the test chamber, as well as the overall vehicle- or cocaine-induced LMA for all of these groups.
3.2. Acquisition and Extinction of cocaine self-administration
The combined SA1 and SA2 group acquisition and extinction data are presented in Figure 1. Figure 1A shows cocaine intake for LCRs and HCRs during acquisition of cocaine self-administration. A RMANOVA of classification (LCR or HCR) and session (X through X+10) revealed a significant interaction between these variables (aF(10, 460) = 6.40, p < 0.001), as well as main effects of session (aF(10, 460) = 21.04, p < 0.001) and classification (F(1,48) = 4.49, p = 0.039). Post-hoc tests revealed that LCRs self-administered significantly more cocaine than HCRs during sessions X, X+1, and X+2, but there were no between group differences thereafter when cocaine intake had stabilized.
Figure 1B shows active and inactive lever responding by LCRs and HCRs during the acquisition phase of the experiment. RMANOVA revealed a significant three-way interaction between session (X through X+10), lever (active, inactive), and classification [LCR, HCR; (aF(10, 440) = 5.11, p = 0.002)]. In short, follow-up RMANOVA of active lever responses mirrored the intake analysis, with LCRs responding significantly more than HCRs during the earliest sessions (X and X+1 in the active lever analysis). In contrast, there were no significant LCR/HCR differences revealed by RMANOVA of inactive lever responding. Further, post-hoc analysis of lever data revealed that active lever responses were significantly higher than inactive lever responses beginning with session X, revealing the clear discrimination between active and inactive levers.
Following completion of the cocaine self-administration schedule, animals underwent extinction training. Cocaine seeking behavior was measured on the drug-paired lever (Figure 1C) while non-specific activity was measured on the inactive lever (Figure 1D). When behavioral responses were compared across 7 extinction sessions, LCRs and HCRs similarly extinguished cocaine seeking (Figures 1C and D). Analysis of lever responding with RMANOVA revealed significant main effects of session (aF(6,288)= 106.78, p < 0.001) and lever (aF (1,48)= 65.45, p < 0.001), and a significant session × lever interaction (aF (6,288)= 60.02, p < 0.001), but no other significant differences. Further, comparison of LCR/HCR latency to reach extinction criteria (<15% drug-paired lever responses across 3 extinction sessions), an indication of resistance to extinction, revealed no significant between-group difference (data not shown).
3.3. Cue- and cocaine-primed reinstatement
Following extinction, group SA1 was first tested for cue-primed reinstatement, followed by cocaine/saline reinstatement (see Methods). Figure 2 shows the experimental timeline (Figure 2A) and the resulting reinstatement behaviors (Figures 2B and C). The 3-extinction session baseline average and the cue-primed reinstatement of cocaine seeking responses are presented in Figure 2B. RMANOVA revealed significant main effects of test (aF(1,22) = 21.06, p < 0.001) and classification (aF(1,22) = 7.69, p = 0.011), and a significant test × classification interaction (aF(1,22) = 6.35, p = 0.019). Post-hoc analysis with paired samples t-tests revealed that both LCRs and HCRs reinstated to the cue (Figure 2B; LCRs, p = 0.032; HCRs, p = 0.002). Between-group comparisons with independent samples t-tests revealed that HCRs exhibited significantly higher responding than LCRs during both cue-primed reinstatement (p = 0.016) and at baseline (p = 0.012), though baseline responding was quite low (4.1 ± 0.94 vs. 8.2 ± 1.2 responses, LCRs vs. HCRs, respectively). Following a return to baseline extinction values, reinstatement induced by cocaine (10 mg/kg, i.p.) and saline (1 ml/kg, i.p.) was compared between LCRs and HCRs (Figure 2C). RMANOVA revealed a significant main effect of test (aF(1,21) = 47.72, p < 0.001), but neither an effect of classification nor a test × classification interaction, indicating that LCRs and HCRs reinstated equally well to this dose of cocaine, which was identical to that used initially for LCR/HCR classification.
To investigate reinstatement to a range of lower cocaine doses, group SA2 was tested for responding induced by vehicle, 1.25, 2.5 and 5 mg/kg cocaine (i.p.), followed by cue-primed and 10 mg/kg cocaine-primed (i.p.) reinstatement (Figure 3A; see Methods). Vehicle and the 3 lower doses of cocaine were administered according to Latin-squares design, with a minimum of 3 unprimed extinction sessions between each test to restore baseline extinction values. Responding on the drug-paired lever (Figure 3B) analyzed with RMANOVA revealed a significant main effect of test (aF(3,72) = 10.02, p = 0.001) and a test × classification interaction (aF(3,72) = 4.69, p = 0.027), but not a main effect of classification. Separate post-hoc analyses with one-way RMANOVA revealed a significant effect of test in HCRs (aF(3,36) = 8.43, p 0.011), but not LCRs (p = 0.072). Compared to vehicle, HCRs exhibited significant reinstatement of responding induced by 2.5 and 5 mg/kg cocaine (Figure 3B). Despite a statistical trend toward significantly greater reinstatement of responding in HCRs compared to LCRs at 5 mg/kg cocaine (p = 0.052), LCRs and HCRs did not significantly differ at any of the doses tested. After completing these 4 vehicle- and cocaine-primed reinstatement tests, cue-primed cocaine seeking was tested (Figure 3C). RMANOVA of cocaine-paired lever responses again revealed significant main effects of test (aF(1,24) = 15.69, p = 0.001) and classification (aF(1,24) = 4.48, p = 0.045), and a significant test × classification interaction (aF(1,24) = 4.35, p = 0.048). In contrast to the results with group SA1 (Figure 2B), under these conditions (Figure 3C), LCRs exhibited significantly greater reinstatement of responding compared to HCRs (p = 0.046), who did not significantly reinstate responding to cue (p = 0.110). Although the absolute values for cue-primed reinstatement in HCRs in SA1 and LCRs in SA2 are modest, it is important to note they represent 4- and 10-fold increases in responding relative to extinction, respectively. When tested for reinstatement induced by 10 mg/kg i.p. cocaine, RMANOVA revealed only a main effect of test (aF(1,24) = 30.83, p < 0.001), again indicating that LCRs and HCRs reinstated equally well to this dose of cocaine (data not shown). Inactive lever responses were not different between LCRs and HCRs in any reinstatement test (data not shown).
3.4. Striatal mGluR5 and mGluR2/3 protein levels in LCRs and HCRs
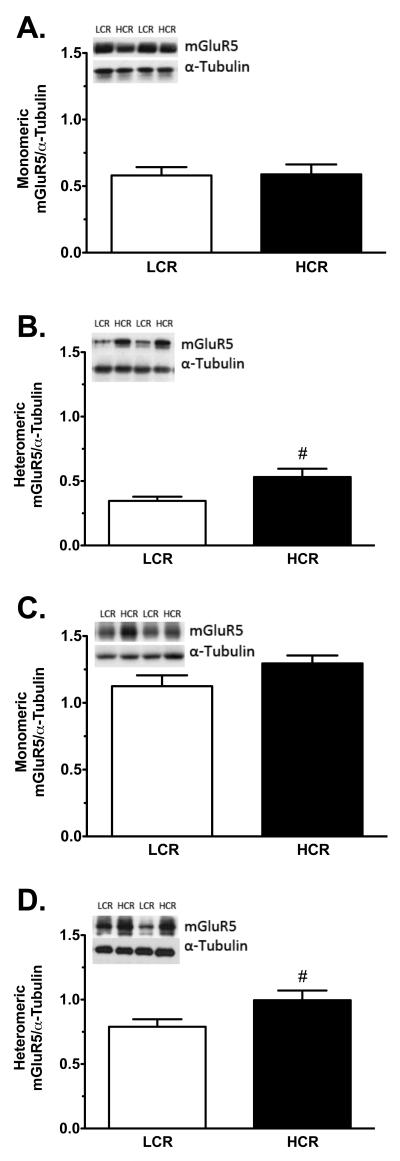
Western blot analysis was used to quantify levels of mGluR5 and mGluR2/3 proteins in the dSTR and NAc of LCRs and HCRs in both groups SA1 and SA2 following extinction/reinstatement testing. The results, expressed as a ratio of the mGluR subunit:α-tubulin, are shown in Table 3. Additionally, levels of mGluR5 in the dSTR and NAc were also measured in two additional groups of rats treated acutely with 10 mg/kg i.p. cocaine or vehicle (Acute1 and Acute 2; Table 4). In all cases, tissue was collected one day after the final experimental manipulation. Both the inactive (monomeric) and active (heteromeric) forms of these receptors were measured (see Methods). This analysis revealed only one significant LCR/HCR difference: Levels of the active form of mGluR5 in the dSTR were significantly higher by ~3-fold in HCRs than LCRs in both groups SA1 and SA2 (Table 3; Figures 4 B and D; p = 0.016 and p = 0.041, respectively). In contrast, levels of the inactive form (i.e., monomeric) of mGluR5 in the dSTR, mGluR2/3 in the dSTR, and both mGluR subtypes in the NAc were similar between LCRs and HCRs in both the SA1 and SA2 groups (Table 3; Figures 4 A and C). Likewise, levels of both forms of mGluR5 in the dSTR and NAc did not differ following an acute injection of cocaine (10 mg/kg, i.p.) compared to vehicle, nor did they differ between LCRs and HCRs (Table 4).
Table 3. Western blot analysis of dSTR and NAc mGluR5 and mGluR2/3 in cocaine self-administering LCR/HCR rats following extinction/reinstatement tests.
| Group: SA1 | Striatal Subregion | |||
|---|---|---|---|---|
| dSTR | NAc | |||
|
Protein
(Ratio to α-tubulin) |
LCR | HCR | LCR | HCR |
| Monomeric mGluR5 | 0.58 ± 0.06 | 0.59 ± 0.07 | 0.97 ± 0.27 | 0.71 ± 0.12 |
| Heteromeric mGluR5 | 0.34 ± 0.03 | 0.53 ± 0.06* | 1.31 ± 0.19 | 1.20 ± 0.15 |
| Monomeric mGluR2/3 | 0.82 ± 0.23 | 0.96 ± 0.18 | ND | ND |
| Heteromeric mGluR2/3 | 1.34 ± 0.14 | 1.35 ± 0.23 | ND | ND |
| Group: SA2 | Striatal Subregion | |||
|---|---|---|---|---|
| dSTR | NAc | |||
|
Protein
(Ratio to α-tubulin) |
LCR | HCR | LCR | HCR |
| Monomeric mGluR5 | 1.12 ± 0.08 | 1.29 ± 0.06 | 1.68 ± 0.25 | 1.39 ± 0.21 |
| Heteromeric mGluR5 | 0.79 ± 0.06 | 0.99 ± 0.07* | 1.65 ± 0.23 | 1.36 ± 0.19 |
| Monomeric mGluR2/3 | 0.71 ± 0.13 | 0.51 ± 0.14 | ND | ND |
| Heteromeric mGluR2/3 | 1.09 ± 0.11 | 1.04 ± 0.11 | ND | ND |
Values shown in groups SA1 and SA2 are the ratio of mGluR protein immunoreactivity to a-tubulin loading control immunoreactivity. Data are presented as mean values ± SEM. *p < 0.05. ND = not determined.
Table 4. Western blot analysis of mGluR5 in dSTR and NAc from acute cocaine LCR, HCR, and vehicle (VEH) rats 24 h following treatment.
|
Group:
Acute 1 |
Striatal Subregion | |||||
|---|---|---|---|---|---|---|
| dSTR | NAc | |||||
|
Protein
(Ratio to α- tubulin) |
VEH | LCR | HCR | VEH | LCR | HCR |
| Monomeric | 1.90 ± 0.73 | 1.38 ± 0.33 | 0.90 ± 0.25 | 1.65 ± 0.26 | 2.00 ± 0.60 | 1.68 ± 0.40 |
| Heteromeric | 1.53 ± 0.18 | 1.40 ± 0.11 | 1.59 ± 0.27 | 2.03 ± 0.23 | 1.90 ± 0.26 | 2.19 ± 0.34 |
|
Group:
Acute 2 |
Striatal Subregion | |||||
|---|---|---|---|---|---|---|
| dSTR | NAc | |||||
|
Protein
(Ratio to α- tubulin) |
VEH | LCR | HCR | VEH | LCR | HCR |
| Monomeric | 2.35 ± 0.24 | 2.28 ± 0.38 | 2.27 ± 0.24 | 2.09 ± 0.47 | 1.89 ± 0.18 | 2.10 ± 0.15 |
| Heteromeric | 1.03 ± 0.16 | 1.12 ± 0.10 | 1.15 ± 0.11 | 1.49 ± 0.10 | 1.56 ± 0.35 | 1.65 ± 0.13 |
Values shown in groups Acute 1 and Acute 2 are the ratio of mGluR protein immunoreactivity to α-tubulin loading control immunoreactivity. Data are presented as mean values ± SEM.
Figure 4.
Western blot analysis of dSTR mGluR5 levels in LCRs and HCRs. Representative western blots and monomeric and heteromeric mGluR5 levels are shown for groups SA1 (A and B, respectively; LCRs n = 12, HCRs n = 12) and SA2 (C and D, respectively; LCRs n = 13, HCRs n = 13). Values are the ratio of mGluR protein immunoreactivity to α-tubulin loading control immunoreactivity. Data are mean values ± SEM. White bars = LCRs, solid black bars = HCRs. #p < 0.05, LCRs vs. HCRs.
4. Discussion
In this study, we found that individual differences in LMA responses to an acute dose of cocaine (10 mg/ kg, i.p.) predicted relapse behaviors in a rat model of cocaine self-administration. LCRs demonstrated higher cocaine intake and active lever responding than HCRs during the initial stages of acquisition of cocaine self-administration, but there were no differences between LCRs and HCRs during later sessions or in responding during extinction training. Only HCRs reinstated cocaine seeking to administration of lower doses of cocaine (2.5 and 5 mg/kg, i.p.), whereas both LCRs and HCRs reinstated equally well to 10 mg/kg-primed cocaine seeking. When contingent cue-primed reinstatement was tested first, HCRs demonstrated significantly greater drug-paired lever pressing than LCRs. Interestingly, however, when cue-primed reinstatement was tested following repeated cocaine-primed reinstatement tests, HCRs did not reinstate to cue, whereas LCRs exhibited robust drug-paired lever responding. HCRs were found to have higher levels of heteromeric mGluR5 in the dSTR than LCRs in both the SA1 and SA2 groups, whereas mGluR5 levels were similar in LCRs and HCRs following acute cocaine exposure. These findings suggest that cocaine self-administration, extinction, and/or reinstatement testing results in higher levels of the active heteromeric form of mGluR5 in the dSTR of animals that are initially more responsive to cocaine-induced LMA.
Previously, when LCRs and HCRs acquired self-administration behavior more slowly using a low dose of cocaine (0.25 mg/kg/infusion delivered over 12-sec), we found that LCRs subsequently exhibited increased motivation compared to HCRs (i.e., attained higher breakpoints on a progressive ratio schedule of reinforcement), to self-administer a range of cocaine doses (Mandt et al., 2008). However, there were no differences between LCRs and HCRs in acquisition of cocaine self-administration. Further, repeated exposure to 10 mg/kg i.p. cocaine prior to self-administration decreased the latency to acquire cocaine self-administration in all rats, regardless of the development of LMA sensitization only in the LCRs (Mandt et al., 2008). Here, we observed higher cocaine intake in LCRs than HCRs during the first three days of acquisition. In the present study LCRs and HCRs self-administered a higher dose of cocaine delivered more rapidly than in the previous study (i.e., 0.8 mg/kg over 5 – 7-sec vs. 0.25 mg/kg over 12-sec), and it is also possible that the priming dose(s) of cocaine used in this study may have selectively sensitized LCRs to the psychostimulant effects of cocaine, resulting in significantly higher responding for cocaine during the early stages of acquisition under these conditions. However, recently, we have demonstrated that cocaine dose and cocaine self-administration history affected sensitization to the motivational effects of cocaine independent of LCR/HCR classification when higher cocaine doses were infused more rapidly (Mandt et al., 2012b). Nonetheless, it is possible that the specific pattern of exposure to cocaine is an important variable in the development of sensitization, a variable not controlled when animals are permitted to freely self-administer cocaine. This possibility will be explored in future studies. In any case, once cocaine self-administration behavior was stable, it was equivalent in LCRs and HCRs.
LCRs and HCRs did not differ in initial cocaine seeking measured on the first day of extinction training, resistance to extinguish cocaine seeking over the first seven days of extinction training, or latency to reach extinction criteria. Interestingly, when cue-primed reinstatement was tested first during cocaine abstinence, HCRs exhibited ~3-fold greater cue-primed reinstatement than LCRs. Although we have never before found HCRs to exhibit greater addiction-like behaviors than LCRs, HCRs are more impulsive than LCRs on a delayed discounting task (Stanis et al., 2008). However, when we measured cue-primed reinstatement after repeated low-dose cocaine reinstatement testing, we found that the reverse was true. In light of the “incubation of cocaine craving” phenomenon proposed by Shaham and colleagues (e.g., Grimm et al., 2001; Lu et al., 2004; etc.), which found cue-primed reinstatement to progressively increase up to 60 days of withdrawal, these findings are intriguing. In the present study, withdrawal time from active cocaine self-administration (i.e., cue-paired cocaine infusions) prior to the cue-primed reinstatement test differed between groups SA1 and SA2 by ~5 weeks (i.e., ~7 vs. ~30 sessions of withdrawal, respectively). However, time was not the only factor that differed between groups SA1 and SA2. It is possible that dose-response tests conducted prior to the cue-primed reinstatement test (group SA2 only) contributed to the different results. Thus, until we are able to directly test the “incubation of cocaine craving” phenomenon in LCRs and HCRs, we only can conclude that the effect of cues on reinstatement of cocaine seeking in LCRs/HCRs is complex.
Although LCRs and HCRs in Group SA1 showed similar, robust 10 mg/kg-primed cocaine reinstatement, it was previously demonstrated that LCRs are more sensitive than HCRs to the discriminative stimulus effects of cocaine (Klein and Gulley, 2009). Therefore, we first tested lower cocaine doses in group SA2 with the hypothesis that LCRs would be more sensitive to the reinstating properties of cocaine. However, we found that only HCRs reinstated cocaine seeking to these lower doses (i.e., 2.5 and 5 mg/kg cocaine, i.p.). Importantly, there are significant differences between this study and the Kline and Gulley study that make direct comparison difficult. For example, in the Kline and Gulley (2009) study, the animals used were trained at the lower doses to test sensitivity whereas our animals were tested for reinstatement only once at each dose after prolonged high cocaine exposure during self-administration. Further, while it was unexpected that HCRs reinstated cocaine seeking to lower doses than LCRs, HCRs have been found to be more sensitive to the pharmacological effects of cocaine than LCRs (i.e., DAT inhibition; Sabeti et al., 2002; Nelson et al., 2009). Thus, it is possible this difference contributed to the difference in pharmacological-induced reinstatement of cocaine seeking in LCRs and HCRs. Interestingly, LCRs and HCRs in both groups SA1 and SA2 showed robust reinstatement of cocaine seeking to the 10 mg/kg i.p. cocaine, suggesting that any difference in pharmacological sensitivity is overcome by the dose initially used to distinguish LCRs from HCRs.
In this study we found higher levels of mGluR5 heteromers in dSTR of HCRs than LCRs in both self-administration groups following cocaine reinstatement testing, but not following acute cocaine exposure, suggesting that the self-administration, extinction, and/or reinstatement testing differentially regulated the amount of active mGluR5 protein in the two phenotypes. In contrast, we observed no LCR/HCR differences in mGluR2/3 heteromers. Since mGluR5s could not be monitored longitudinally in the same rats, it is unclear if the levels of heteromeric mGluR5 in the dSTR decreased in LCRs, increased in HCRs, and/or some combination of those effects. It is also possible that high cocaine exposure during cocaine self-administration significantly decreased mGluR5 expression in both LCRs and HCRs, but mGluR5 expression levels recovered more quickly in HCRs. In any case, it is interesting to note that restoring tone to excitatory postsynaptic mGluR5s in NAc core has been shown to promote drug seeking (Kupchik et al., 2012).
Interestingly, we detected the LCR/HCR differences in mGluR5s in the dSTR, but not in the NAc. Opposing changes in synaptosomal/surface mGluR5s have been observed in rat dSTR and NAc following extinction of cocaine seeking: increased levels in the dSTR and decreased levels in the NAc shell and core (Ghasemzadeh et al., 2009a & 2009b; Knackstedt et al., 2010). If mGluR5s were differentially reduced in the NAc of LCRs and HCRs following extinction, the reduction(s) may have been below our limits of detection or may have normalized following the repeated extinction/reinstatement testing in our study. In any case, our results are consistent with reports that following longer periods of cocaine seeking, there is a shift in the brain regions involved in habitual, compulsive drug-taking and -seeking from the NAc to the dSTR (Belin and Everitt, 2008; Ito et al., 2002; Pierce and Vanderschuren, 2010; See et al., 2007; Willuhn et al., 2012).
5. Conclusions
Classification of animals based on their initial locomotor response to cocaine (i.e., LCRs and HCRs) is a useful model for studying individual differences in susceptibility to the development of addiction-like behaviors. LCRs and HCRs extinguished cocaine seeking similarly, but they differed in the temporal profile of cue-primed reinstatement and sensitivity to cocaine-primed reinstatement. Whereas HCRs reinstated to cue-primed reinstatement of cocaine seeking during early testing, LCRs exhibited robust reinstatement following longer withdrawal periods. This is potentially an important finding, which we will explore in the future, as it relates to relapse following detoxification and a longer period of drug abstinence. Only HCRs reinstated to low dose cocaine priming infusions (2.5 and 5 mg/kg, i.p.), consistent with LCR/HCR differences in sensitivity to the pharmacological effects of cocaine (HCRs > LCRs). The differences in cue-primed reinstatement may be related to differential regulation of dSTR mGluR5s in LCR/HCRs, but additional testing is required to fully understand this effect. Interestingly, LCRs and HCRs reinstated equally well to 10 mg/kg primed reinstatement of cocaine seeking, indicating that the dose used to initially distinguish LCRs from HCRs was sufficient to trigger relapse behaviors regardless of LCR/HCR classification. Although our previous results with the LCR/HCR model have all supported the idea that lower initial cocaine-induced activation in individuals predicts more ready development of addiction-like behaviors, our current findings suggest that the relationship between the initial effects of cocaine and reinstatement of cocaine seeking is more complex.
Highlights.
LCRs exhibited greater initial cocaine intake
HCRs exhibited greater reinstatement to low cocaine priming doses
LCRs and HCRs differed in the temporal profile of cue-induced reinstatement
mGluR5 heteromers higher in dorsal striatum of HCRs following reinstatement testing
cue-induced reinstatement may be related to differential regulation of dSTR mGluR5s
Acknowledgements
This work was supported by NIH grants R01 DA004216 (NRZ, RMA), T32 AA007464 (DLS), F31 DA027277 (DJY), and K05DA015050 (NRZ). We would like to acknowledge Jillian Laggart for her work with the animals.
Abbreviations
- LCR
(low cocaine responder)
- HCR
(high cocaine responder)
- LMA
(locomotor activity)
- NAc
(nucleus accumbens)
- dSTR
(dorsal striatum)
- DA
(dopamine)
- DAT
(dopamine transporter)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen RM, Everett CV, Nelson AM, Gulley JM, Zahniser NR. Low and high locomotor responsiveness to cocaine predicts intravenous cocaine conditioned place preference in male Sprague-Dawley rats. Pharmacol. Biochem. Behav. 2007;86:37–44. doi: 10.1016/j.pbb.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Chen Y, Banko JL, Sherrler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, Lindsley CW, Olive MF, Conn PJ. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 2009;34:2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–86. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat. Neuroscience. 2001;4(9):873–4. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: Reversal by D(1) antagonists. Proc. Natl. Acad. Sci. 2001;98:1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleva RM, Hicks MP, Gass JT, Wischerath KC, Plasters ET, Widholm JJ, Olive MF. mGluR5 positive allosteric modulation enhances extinction learning following cocaine self-administration. Behav. Neurosci. 2011;125(1):10–9. doi: 10.1037/a0022339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugos A, Freitag C, Hohoff C, McDonald J, Cook EH, Deckert J, de Wit H. Norepinephrine transporter gene variation modulates acute response to D-Amphetamine. Biol. Psychiatry. 2007;61(11):1296–305. doi: 10.1016/j.biopsych.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Dlugos AM, Hamidovic A, Hodgkinson C, Shen PH, Goldman D, Pamer AA, de Wit H. OPRM1 gene variants modulate amphetamine-induced euphoria in humans. Genes Brain Behav. 2011;10(2):199–209. doi: 10.1111/j.1601-183X.2010.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge R, Sindelar J, Sinha R. The role of depressive symptoms in predicting drug abstinence in outpatient substance abuse treatment. J. Subst. Abus. Treat. 2005;28:343–59. doi: 10.1016/j.jsat.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Positive allosteric modulation of mGLuR5 receptors facilitates extinction of a cocaine contextual memory. Biol. Psychiatry. 2009;65(8):717–20. doi: 10.1016/j.biopsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–6. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Region specific alterations in glutamate receptor expression and subcellular distribution following extinction of cocaine self-administration. Brain Res. 2009a;1267:89–102. doi: 10.1016/j.brainres.2009.01.047. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Neuroadaptations in the cellular and postsynaptic group 1 metabotropic glutamate receptor mGluR5 and Homer proteins following extinction of cocaine self-administration. Neurosci. Lett. 2009b;454(2):167–71. doi: 10.1016/j.neulet.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorwood P, Wohl M, Le Strat Y, Rouillon F. Gene-environment interactions in addictive disorders: epidemiological and methodological aspects. C. R. Biol. 2007;330(4):329–38. doi: 10.1016/j.crvi.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation: Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley JM, Hoover BR, Larson GA, Zahniser NR. Individual differences in cocaine-induced locomotor activity in rats: behavioral characteristics, cocaine pharmacokinetics, and the dopamine transporter. Neuropsychopharmacology. 2003;28(12):2089–101. doi: 10.1038/sj.npp.1300279. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Paliwal P, Chaplin TM, Mazure CM, Rounsaville BJ, Sinha R. Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug Alcohol Depend. 2008;92(1-3):208–16. doi: 10.1016/j.drugalcdep.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources . Guide for the Care and Use of Laboratory Animals. 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council; Washington, DC: 1996. [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J. Neurosci. 2002;22(14):6247–53. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology. 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- Jin X, Semenova S, Yang L, Ardecky R, Sheffler DJ, Dahl R, Conn PJ, Cosford ND, Markou A. The mGluR2 positive allosteric modulator BINA decreases cocaine self-administration and cue-induced cocaine-seeking and counteracts cocaine-induced enhancement of brain reward function in rats. Neuropsychopharmacology. 2010;35(10):2021–36. doi: 10.1038/npp.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Glutamate systems in cocaine addiction. Curr. Opin. Pharmacol. 2007;4(1):23–9. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate hypothesis of addiction. Nat. Rev. Neurosci. 2009;10(8):561–72. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology. 2005;179(1):247–54. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- Klein DA, Gulley JM. Reduced sensitivity to the locomotor stimulant effects of cocaine is associated with increased sensitivity to its discriminative stimulus properties. Behav. Pharmacol. 2009;20:67–77. doi: 10.1097/FBP.0b013e3283242fdd. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr. Opin. Pharmacol. 2009;9(1):59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J. Neurosci. 2010;30(23):7984–92. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav. Brain Res. 2009;202(2):238–44. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Moussawi K, Tang X-C, Wang X, Kalivas BC, Kolokithas R, Ogburn KB, Kalivas PW. The effect of N-acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biol. Psychiatry. 2012;71:978–986. doi: 10.1016/j.biopsych.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Mahoney JJ, 3rd, Kalechstein AD, De La Garza R, 2nd, Newton TF. A qualitative and quantitative review of cocaine-induced craving: The phenomenon of priming. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31:593–599. doi: 10.1016/j.pnpbp.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandt BH, Schenk S, Zahniser NR, Allen RM. Individual differences in cocaine induced locomotor activity in male Sprague-Dawley rats and their acquisition of and motivation to self-administer cocaine. Psychopharmacology. 2008;201:195–202. doi: 10.1007/s00213-008-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandt BH, Zahniser NR. Low and high cocaine locomotor responding male Sprague-Dawley rats differ in rapid cocaine-induced regulation of striatal dopamine transporter function. Neuropharmacology. 2010;58(3):605–12. doi: 10.1016/j.neuropharm.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandt BH, Johnston NL, Zahniser NR, Allen RM. Acquisition of cocaine self-administration in male Sprague-Dawley rats: Effects of cocaine dose but not initial locomotor response to cocaine. Psychopharmacology. 2012a;219(4):1089–97. doi: 10.1007/s00213-011-2438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandt BH, Gomez E, Johnston NL, Zahniser NR, Allen RM. Cocaine dose and self-administration history, but not initial cocaine locomotor responsiveness, affects sensitization to the motivational effects of cocaine in rats. J. Pharmacol. Exp. Ther. 2012b;342(1):214–21. doi: 10.1124/jpet.112.194092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR. Catechol O-Methyltransferase cal 158-met genotype and individual variation in the brain response to amphetamine. Proc. Natl. Acad. Sci. 2003;100(10):6186–91. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP. Metabotropic glutamate receptor: From the workbench to the bedside. Neuropharmacology. 2011;60:1017–1041. doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak M, Halbout B, O’Conner EC, Rodriguez Parkitna J, Su T, Chai M, Crombag HS, Bilbao A, Spanagel R, Stephens DN, Schutz G, Engblom D. Incentive learning underlying cocaine-seeking requires mGLuR5 receptors located on dopamine D1 receptor expressing neurons. J. Neurosci. 2010;30(36):11973–82. doi: 10.1523/JNEUROSCI.2550-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AA, Verbitsky M, Suresh R, Kamens HM, Reed CL, Li N, Burkhart-Kasch S, McKinnon CS, Belknap JK, Gilliam TC, Phillips TJ. Gene expression differences in mice divergently selected for methamphetamine sensitivity. Mamm. Genome. 2005;16(5):291–305. doi: 10.1007/s00335-004-2451-8. [DOI] [PubMed] [Google Scholar]
- Nelson AM, Larson GA, Zahniser NR. Low or high cocaine responding rats differ in striatal extracellular dopamine levels and dopamine transporter number. J. Pharmacol. Exp. Ther. 2009;331(3):985–97. doi: 10.1124/jpet.109.159897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AM, Kleschen MJ, Zahniser NR. Individual differences in cocaine-induced locomotor activity of male Sprague-Dawley rats are not explained by plasma corticosterone levels. Neurosci. Lett. 2010;476:9–13. doi: 10.1016/j.neulet.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Vanderschuren LJMJ. Kicking the habit: the neural basis of ingrained behaviors in cocaine addiction. Neuroscience & Biobehavioral reviews. 2010;35:212–219. doi: 10.1016/j.neubiorev.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Acute cocaine differentially alters accumbens and striatal dopamine clearance in low and high cocaine locomotor responders: behavioral and electrochemical recordings in freely moving rats. J. Pharmacol. Exp. Ther. 2002;302:1201–1211. doi: 10.1124/jpet.102.035816. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Individual differences in cocaine-induced locomotor sensitization in low and high cocaine locomotor-responding rats are associated with differential inhibition of dopamine clearance in nucleus accumbens. J. Pharmacol. Exp. Ther. 2003;305:180–90. doi: 10.1124/jpet.102.047258. [DOI] [PubMed] [Google Scholar]
- Sinha R. New findings on the biological factors predicting addiction relapse vulnerability. Current Psychiatry Reports. 2011;13(5):398–405. doi: 10.1007/s11920-011-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanis JJ, Burns RM, Sherrill LK, Gulley JM. Disparate cocaine-induced locomotion as a predictor of choice behavior in rats trained in a delay-discounting task. Drug Alcohol Depend. 2008;98:54–62. doi: 10.1016/j.drugalcdep.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen J, Le Moal M. Individual vulnerability to addiction. Annals of the New York Academy of Sciences. 2011;1216:73–85. doi: 10.1111/j.1749-6632.2010.05894.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: Relevance to addiction. J. Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner FA, Anthony JC. From first drug use to drug dependence: developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Wang X, Moussawi K, Knackstedt L, Shen H, Kalivas PW. Role of mGluR5 neurotransmission in reinstated cocaine-seeking. Addict. Biol. 2013;18(1):40–9. doi: 10.1111/j.1369-1600.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Everitt BJ, Phillips PEM. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc. Natl. Acad. Sci. 2012;109(50):20703–8. doi: 10.1073/pnas.1213460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z-X, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of Group II metabotropic glutamate receptor signaling by chronic cocaine. J. Pharmacol. Exp. Ther. 2002;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]