Summary
Because the number of patients waiting for organ transplants exceeds the number of organs available, a better understanding of how transplantation affects the distribution of residual lifetime is needed to improve organ allocation. However, there has been little work to assess the survival benefit of transplantation from a causal perspective. Previous methods developed to estimate the causal effects of treatment in the presence of time-varying confounders have assumed that treatment assignment was independent across patients, which is not true for organ transplantation. We develop a version of G-estimation that accounts for the fact that treatment assignment is not independent across individuals to estimate the parameters of a structural nested failure time model. We derive the asymptotic properties of our estimator and confirm through simulation studies that our method leads to valid inference of the effect of transplantation on the distribution of residual lifetime. We demonstrate our method on the survival benefit of lung transplantation using data from the United Network for Organ Sharing.
Keywords: Causal Inference, G-Estimation, Lung Transplantation, Martingale Theory, Structural Nested Failure Time Models
1. Introduction
Lung transplantation is the definitive therapy for many end-stage lung diseases including chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), and idiopathic pulmonary fibrosis (IPF) (Kotloff and Thabut, 2011). Despite poor unadjusted one- and three-year survival rates after lung transplantation, 83.8 and 63.2 percent, respectively, the number of lung transplantations in the United States grew from 959 in 2000 to 1,770 in 2010 (based on Organ Procurement and Transplantation Network (OPTN) data as of March 30, 2012).
An understanding of the effect of transplantation on survival is needed to improve organ allocation and justify the intensive use of health care resources and high cost. Prior research has focused on the survival benefit within a particular native disease, e.g., COPD only, or from an older era of transplantation (see Liou et al., 2001; Thabut et al., 2003; Lahzami et al., 2010; Kotloff and Thabut, 2011, and references therein). To quantify the causal effect of transplantation, most clinical research has used a proportional hazards model with a time-dependent covariate for transplantation. This modeling approach only consistently estimates the causal effect in the absence of time-dependent confounding (Robins, 1992); however, patients with worse waitlist prognosis have priority in organ allocation.
Several methods that have been proposed, including structural nested failure time models (SNFTMs) fitted by G-estimation, to quantify the causal effect of treatment in the presence of time-dependent confounding (Robins, 1989, 1992, 2005). Applying these methods to organ transplantation is not straightforward. G-estimation requires modeling the probability of receiving treatment each time it could be offered. Prior applications of SNFTMs, such as Robins et al. (1992), have typically indexed time from when the patient entered the study and assumed that treatment assignment was independent across patients. Because a national waitlist is used to offer organs to patients as donor organs become available, the likelihood a patient is transplanted a certain number of days after listing depends on the prognosis of all other patients active on the waiting list.
In Section 2, we introduce a potential outcomes framework in which to represent the question of transplant benefit and present a structural failure time model to relate the residual survival distribution if a patient were transplanted to the distribution of residual lifetime if never transplanted. In Section 3, we describe a class of estimators for parameters in this structural failure time model, derive the asymptotic properties of the class, and discuss reasonably efficient estimators within the class. In Section 4, we give computational strategies to mitigate difficulties encountered with censored failure times. Section 5 presents the results of a simulation study. Section 6 demonstrates our method in an analysis of the survival benefit of lung transplantation using data from the United Network for Organ Sharing (UNOS).
2. Statistical Framework
We consider a hypothetical population of subjects eligible for organ transplantation. Let T*(∞) be the potential time from being listed on the waitlist until death for a randomly selected individual in the population if that patient were to never receive an organ transplant. Characteristics of the donor organ may affect post-transplant survival; denote by Q a random vector of donor organ characteristics. Define T*(t, q) to be the time from listing until death for a random patient awaiting organ transplantation were s/he to be transplanted t days after entering the waitlist with donor organ characteristics Q = q assuming that s/he had not already received an organ and was alive on the waitlist then. As all of T*(∞) and T*(t, q) may not be observed on a random subject, these random variables are referred to as potential outcomes. Define (X̄) * (t) to be the history of time-dependent covariates through t days after listing assuming the patient had not been transplanted and had not died prior to time t. The history of time-dependent covariates are also potential outcomes.
If we let
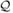 be the set of all possible donor organ characteristics, then the set of all potential outcomes for subject i is
. We refer to
and
as the residual (potential) lifetime at time t had the patient, possibly contrary to fact, never received an organ and received an organ at time t with organ characteristics Q = q, respectively. The above quantities are only defined if the patient would have survived on the waitlist for t days, i.e., T*(∞) ≥ t.
be the set of all possible donor organ characteristics, then the set of all potential outcomes for subject i is
. We refer to
and
as the residual (potential) lifetime at time t had the patient, possibly contrary to fact, never received an organ and received an organ at time t with organ characteristics Q = q, respectively. The above quantities are only defined if the patient would have survived on the waitlist for t days, i.e., T*(∞) ≥ t.
The effect of transplantation on residual lifetime can now be characterized in terms of potential outcomes. We are interested in comparing the distribution of the residual lifetime at time t had the patient received an organ transplant at time t, , to the distribution of the residual lifetime had the patient never been transplanted, .
We consider transformation models to relate the distributions of the residual potential lifetimes. We assume that there is a known function γt{z, X̄ * (t),Q; θ}, which is an increasing function of z and is indexed by a p-dimensional parameter θ, such that
| (1) |
Thus, (1) relates the conditional distribution of a known transformation of given past covariate history and organ characteristics, to that of . These transformation models are known as structural nested failure time models (SNFTMs). We define the SNFTM in terms of potential outcomes here and link this model to the observed treatment history in Section 3. Because the parameter θ describes the relationship between the distribution of potential outcomes, it has a causal interpretation. We parameterize (1) such that γt{z, X̄* (t), Q; 0} = z; therefore, a p-dimensional test of θ = 0 is a test of no effect.
A common transformation model used in survival analysis and one that we will consider extensively, is the scale accelerated failure time model given by
| (2) |
In (2), the residual lifetime can be increased or decreased proportionately as a function of covariate history and organ characteristics, where X̄*(t)Q denotes any interaction between X̄*(t) and Q. If is negative, then transplantation increases lifespan for those values of the time-dependent covariates and donor characteristics.
To estimate the parameters in the SNFTM, we observe a random sample of n patients listed for organ transplantation at calendar times o1,…,on and a sample of m organs that are transplanted at times s1,…,sm with characteristics q1,…,qm, respectively. For our analysis, we will always condition on these quantities but will suppress the notation for convenience. Define tij = sj− oi, the time since the ith patient entered the waitlist until the jth organ transplant for all (i, j) such that sj− oi ≥ 0. For each organ j = 1,…, m, we let Aij be the indicator for whether or not the ith patient received the organ. Define Yij as the indicator for whether or not the ith patient is at risk, i.e., currently active on the waitlist, to receive the jth organ. Let Ti and Ui be the observed time from listing until patient i dies and is transplanted, respectively; if the patient is never transplanted, we let Ui= ∞ by convention. Thus, Ui= tij if Aij= 1, and Ui = ∞ if Aij′ = 0 for all j′ = 1,…,m. Note that if Yij= 1 then oi ≤ sj, tij ≤ Ui and tij ≤Ti. However, the converse is not true because patients may be removed from the waitlist. For now, we will assume that the survival time is observed for all subjects and consider censored event times in Section 4. Let be the characteristics of the organ the ith person actually receives. The history of the observed covariate process through time t is denoted by X̄i(t); the covariate process is collected until min (Ui,Ti). To obtain valid inference, X̄(t); and Q must contain at a minimum all covariates and organ characteristics that influence the likelihood of receiving an organ and are prognostic. Note that Aij, Yij, Ti, Ui, Qi, and X̄{min(Ui,Ti)} are all observed for all i = 1,…, n and j = 1,…, m. We make the consistency assumption (C) that observed random variables equal the potential outcomes for the treatments received, i.e., and .
To succinctly index all available information just prior to the allocation of the jth organ, we define the filtration
. We assume that given
 j, the probability that the ith subject receives the jth organ is proportional to the propensity score
, which may depend on a vector of parameters φ, so that
j, the probability that the ith subject receives the jth organ is proportional to the propensity score
, which may depend on a vector of parameters φ, so that
| (3) |
Because the propensity must be non-negative, a natural form for α(·) would be an exponentiated linear combination of covaraites collected prior to sj and organ characteristics. Each time an organ becomes available, the OPTN determines an ordered list of patients to whom the organ will be offered. In spite of the deterministic manner in which procured organs are offered to patients, we argue that the stochastic model (3) is reasonable. Because the “turndown” rate of available organs is high and the ordering of patients on the waitlist depends on factors not related to prognosis, comparatively healthy patients, though less likely, do receive the available organ and organ allocation can be viewed as stochastic. We discuss the merits of model (3) and other models for organ allocation in Web Appendix A.
We assume sequential ignorability (SI); the probability of receiving an organ at any time depends only on the observed data up until that time and not additionally on any potential outcomes. This implies P(Aij= 1|
 j,
j,
 ; φ) = P(Aij= 1|
; φ) = P(Aij= 1|
 j; φ), where
j; φ), where
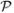 = (
= (
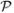 1,…,
1,…,
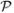 n).
n).
To aid the reader, we have included a chart in Web Appendix A that summarizes the notation for the observed and potential outcome data.
3. Class of Estimators
We define the transformed lifetime as T˜i(θ) = I(Ui<∞)[Ui+γUi{Ti−Ui, X̄i(Ui), Qi; θ}]+I(Ui=∞)Ti and the transformed residual lifetime at the time of the jth organ (for patients at risk for the jth organ) as R˜ij(θ)=T˜i(θ)−tij. Let hij{R˜ij(θ),
 j} be a p-dimensional vector that only depends on R˜ij(θ) and
j} be a p-dimensional vector that only depends on R˜ij(θ) and
 j and not on any other function of the observed survival or future covariates. We consider the class of estimators for θ given by the solution to
j and not on any other function of the observed survival or future covariates. We consider the class of estimators for θ given by the solution to
| (4) |
Subsequently, we show that all estimators in this class are consistent and asymptotically normal regardless of the choice of the p-dimensional vector hij(·) so long as we have correctly specified πij(
 j;φ). A discussion of how to choose hij(·) in practice is given at the end of the Section and in Web Appendix B. The estimators in this class are semiparametric in the sense that we have only assumed that we have correctly specified the probability of receiving a transplanted organ in (3) and the transformation model given in (1), but have not made any distributional assumptions on the survival outcomes or the time-dependent covariates.
j;φ). A discussion of how to choose hij(·) in practice is given at the end of the Section and in Web Appendix B. The estimators in this class are semiparametric in the sense that we have only assumed that we have correctly specified the probability of receiving a transplanted organ in (3) and the transformation model given in (1), but have not made any distributional assumptions on the survival outcomes or the time-dependent covariates.
We emphasize that θ characterizes the relationship between distributions of potential outcomes; we relate the joint distribution of observed data and potential outcomes in the following Theorem. We give the proof in Web Appendix B.
Theorem 1
Under the C and SI assumptions described in Section 2, where θ0 is the true value of the parameter in the transformation model given in (1) and means that the joint distribution of the random vector A is the same as the joint distribution of B.
If we let the residual lifetime for the ith patient after the jth transplant had the patient never been transplanted, then Theorem 1 implies that
| (5) |
Substituting the observed transformed residual lifetime for the (potential) residual lifetime had the patient never been transplanted allows us to derive the asymptotic properties of the estimating function evaluated at θ = θ0.
If we have correctly specified the model for the organ allocation in (3),
where the sequential ignorability (SI) assumption gives E {Aij|
 j,
j,
 } = πij(
} = πij(
 j;φ).
j;φ).
Because if one patient receives an organ everyone else on the waitlist necessarily does not, Aij is not independent of Ai′j. Therefore, we cannot use a Central Limit Theorem for independent data to argue that, as n → ∞, converges in distribution to a mean-zero normal random variable.
However, the sequence
, k = 1,…, m is adapted to the filtration (
 k+1,
k+1,
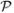 ). Furthermore, we showed above that
. Thus, Mk, k = 1,…,m is a mean-zero Martingale sequence. By the Martingale Central Limit Theorem (DasGupta, 2008) and under the regularity conditions given in Web Appendix B, as m→ ∞,
). Furthermore, we showed above that
. Thus, Mk, k = 1,…,m is a mean-zero Martingale sequence. By the Martingale Central Limit Theorem (DasGupta, 2008) and under the regularity conditions given in Web Appendix B, as m→ ∞,
Where
, I is the p × p identity matrix, and
implies the random vector Cm converges in distribution to a standard normal. We note that Aj= (A1j,…, Anj)T ∽ multinomial[πj= {π1j(
 j, φ),…, πnj(
j, φ),…, πnj(
 j, φ)}T], so var(Aj|
j, φ)}T], so var(Aj|
 j,
j,
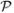 = var(Aj|
= var(Aj|
 j) = diag(πj) − πjπjT. Writing hij= hij{R˜ij(θ0),
j) = diag(πj) − πjπjT. Writing hij= hij{R˜ij(θ0),
 j} and πij= πij(
j} and πij= πij(
 j; φ) for brevity, then Bm is given by
j; φ) for brevity, then Bm is given by
Therefore, under the SI and C assumptions and also assuming that (3) is correctly specified, , where and . Technical details as well as the proof θ̂ converges in probability to θ0 are given in Web Appendix B. Although we have proven the asymptotic properties of θ̂ under the assumption that m, the number of transplantations, tends to infinity, this does not imply n, the number of patients we observe in the sample, remains constant. A regularity condition given in Web Appendix B is that there must be more than one patient at risk for each organ that is transplanted. In practicality, there are hundreds of patients available at each transplant for all solid organs in the United States. This assumption implies that n > m; and, therefore, as m→∞, n must also grow on at least the same order.
While the solution to the estimating equation in (4) is asymptotically normal regardless of the choice of hij(·), the choice of this p-dimensional vector does affect the asymptotic variance. The optimal hij(·), the p-dimensional vector that minimizes the asymptotic variance, and choices that are reasonably efficient require us to know the distribution of
. While knowledge of this distribution is unrealistic, we could posit some distribution for
in hopes that if we correctly specify the distribution, or are reasonably close, we can gain efficiency. Let
be some vector-valued function of X̄i(tij) and assume that the transformation model is the scale accelerated failure time model such that
. If we posit that
follows an exponential distribution with rate parameter exp{λ (
 j)}, a reasonable approximation for the residual lifetime of patients awaiting transplant, then we argue in Web Appendix B that an estimator that is reasonably efficient is given by the solution to
j)}, a reasonable approximation for the residual lifetime of patients awaiting transplant, then we argue in Web Appendix B that an estimator that is reasonably efficient is given by the solution to
| (6) |
Web Appendix B gives a general framework for choosing hij for other transformation models and posited conditional densities for
. We argue in Web Appendix B that if
then the estimator solving (4) is consistent even if we misspecify the model for πij(
 j; φ) and is “doubly robust” as we need only correctly specify πij(
j; φ) and is “doubly robust” as we need only correctly specify πij(
 j; φ) or specify hij so that
for the estimator to be consistent (Lunce-ford and Davidian, 2004). However, we note that specifying a sequence of distributions for
across all j that is consistent with a common data generating mechanism is practically infeasible except in trivial situations. Thus, we would expect the estimator given by the solution to (6) to be only approximately doubly robust.
j; φ) or specify hij so that
for the estimator to be consistent (Lunce-ford and Davidian, 2004). However, we note that specifying a sequence of distributions for
across all j that is consistent with a common data generating mechanism is practically infeasible except in trivial situations. Thus, we would expect the estimator given by the solution to (6) to be only approximately doubly robust.
The above discussion has derived a class of estimators for θ, the parameter in the transformation model (1), under the assumption that φ is known. To estimate φ, we could use the maximizer of the partial likelihood . Or equivalently we could solve for the root of the score of the partial likelihood, that is, the solution to
| (7) |
where we define 0/0 = 0 and the last equality follows from for all j and φ, so . To jointly estimate (θT, φT)T, one could then jointly solve the (4) and (7). The sequence of estimating functions in (7) indexed by the number of organs is also a mean-zero Martingale sequence at the true value of φ, so the joint solution will also be asymptotically normal following the preceding proof.
In addition, we may not know λ (
 j) in (6) and may posit models such that λ(
j) in (6) and may posit models such that λ(
 j,ξ) is known up to a vector of parameters ξ, e.g., λ(
j,ξ) is known up to a vector of parameters ξ, e.g., λ(
 j,ξ) = ξTXi(tij). In Web Appendix B, we argue that if the estimator for ξ is well-behaved, that is, m−1/2(ξ̂ − ξ0) = Op(1), then the asymptotic variance of θ and φ does not depend on how ξ was estimated. We discuss estimators for ξ in Web Appendix B.
j,ξ) = ξTXi(tij). In Web Appendix B, we argue that if the estimator for ξ is well-behaved, that is, m−1/2(ξ̂ − ξ0) = Op(1), then the asymptotic variance of θ and φ does not depend on how ξ was estimated. We discuss estimators for ξ in Web Appendix B.
4. Censored Survival Times
In most studies involving a time-to-event outcome, the end of follow-up occurs before the failure time of all subjects is observed. Denote Ci as the time from listing until the end of study for the ith subject. Similar to T˜i(θ), define the transformed follow-up time as C˜i(θ) = I(Ui< ∞) [Ui+ γUi{Ci − Ui, X̄i(Ui), Qi; θ}] + I(Ui= ∞)Ci and the transformed residual follow-up time for patients at risk for the jth organ C˜ij(θ) = C˜i(θ)—tij. The transformed residual follow-up depends on covariates collected after and treatment decisions at and after sj. Therefore, if we replace hij{R˜ij(θ),
 j} in (4) with hij[I{R˜ij(θ) < C˜ij(θ)}, min{R˜ij(θ), C˜ij(θ)},
j} in (4) with hij[I{R˜ij(θ) < C˜ij(θ)}, min{R˜ij(θ), C˜ij(θ)},
 j], then
min{R*ij(θ), C˜ij(θ)},
j], then
min{R*ij(θ), C˜ij(θ)},
 j], k = 1,…, m, is not adapted to the filtration (
j], k = 1,…, m, is not adapted to the filtration (
 k+1,
k+1,
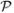 ) and does not have mean-zero increments. To obtain valid inference, we must consider a follow-up time that does not depend on future treatment (Robins et al., 1992; Joffe et al., 2012). Define the transformed artificial follow-up time,
, for patient i at risk to receive the jth organ, as follows:
) and does not have mean-zero increments. To obtain valid inference, we must consider a follow-up time that does not depend on future treatment (Robins et al., 1992; Joffe et al., 2012). Define the transformed artificial follow-up time,
, for patient i at risk to receive the jth organ, as follows:
| (8) |
where XUij = (X̄{min(U, Ci)},U, Q : f[X̄{min(U, Ci)}, U, Q:f[X̄i(tij), tij ≤ U] > 0) and f(•) is the conditional density of [X̄{min(U, Ci)}, U, Q] given {X̄(tij) = X̄i(tij), tij≤ U}. Heuristically,
is the minimum value of transformed residual follow-up over all possible future covariate and treatment trajectories given
 j. When the failure time may be administratively censored, we propose the class of estimators for θ given by the solution to
j. When the failure time may be administratively censored, we propose the class of estimators for θ given by the solution to
| (9) |
where hij(·) is a p-dimensional vector that is a function only of,
the transformed failure indicator;
, the transformed residual observation time; and
 j. Unlike
is fixed given
j. Unlike
is fixed given
 j, so the sequence
has the same distribution as a mean-zero Martingale sequence adapted to (
j, so the sequence
has the same distribution as a mean-zero Martingale sequence adapted to (
 k+1,
k+1,
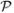 ) as in Section 3. The proof that all estimators in this class, when the failure time may be censored, are consistent and asymptotically normal is identical to the proof in Web Appendix B except
now replaces hij{R˜ij(θ),
) as in Section 3. The proof that all estimators in this class, when the failure time may be censored, are consistent and asymptotically normal is identical to the proof in Web Appendix B except
now replaces hij{R˜ij(θ),
 j}.
j}.
The natural extension of the proposed set of estimating equations (6) given in Section 3, where to gain efficiency we postulated
follows an exponential distribution with rate parameter exp{λ (
 j)} and assumed a scale accelerated failure time model, is given by
j)} and assumed a scale accelerated failure time model, is given by
| (10) |
We note that
is exponentially distributed. Thus, the proposed estimator is consistent even if we have misspecified πij(
 j; φ) if we have correctly specified the distribution of
(doubly robust). However, as noted above,
likely only approximately follows an exponential distribution.
j; φ) if we have correctly specified the distribution of
(doubly robust). However, as noted above,
likely only approximately follows an exponential distribution.
Because
some subjects with observed failure times are “artificially” censored. The use of “artificial censoring” to obtain valid causal inference when the outcome may be censored remains problematic for two main reasons (Joffe et al., 2012). First, Δij(θ,
 j) is not continuous in θ, and V˜ij(θ,
j) is not continuous in θ, and V˜ij(θ,
 j) is non-smooth in θ. Therefore, the class of estimating functions in (9), including the one proposed in (10), need not be smooth or even monotone in θ. Traditional root finding algorithms, such as Newton methods, can be difficult to implement when the objective function is non-smooth and non-monotone in θ. Second, we necessarily lose information by “artificially” censoring some subjects. We build on the work of Joffe et al. (2012) and offer some solutions that mitigate these problems in our context.
j) is non-smooth in θ. Therefore, the class of estimating functions in (9), including the one proposed in (10), need not be smooth or even monotone in θ. Traditional root finding algorithms, such as Newton methods, can be difficult to implement when the objective function is non-smooth and non-monotone in θ. Second, we necessarily lose information by “artificially” censoring some subjects. We build on the work of Joffe et al. (2012) and offer some solutions that mitigate these problems in our context.
To address the former, we consider “smooth” approximations to Δij(θ,
 j) and V˜ij(θ,
j) and V˜ij(θ,
 j) which we denote as
and
, respectively. As long as the approximations are functions of Δij(θ,
j) which we denote as
and
, respectively. As long as the approximations are functions of Δij(θ,
 j) and V˜ij(θ,
j) and V˜ij(θ,
 j) then the derived asymptotic theory is still valid. To obtain a smooth function of Δij and V˜ij we consider the following function,
j) then the derived asymptotic theory is still valid. To obtain a smooth function of Δij and V˜ij we consider the following function,
| (11) |
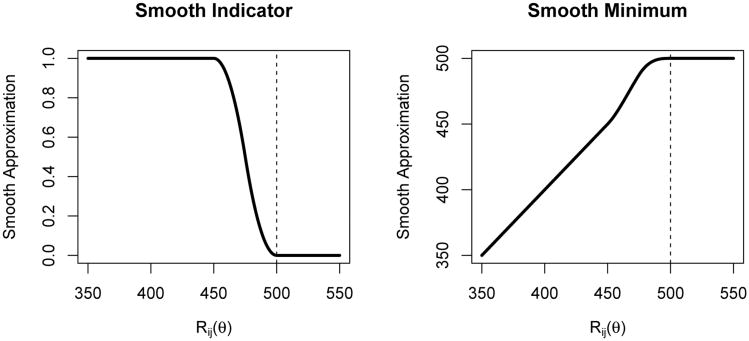
where ε > 0 controls the “smoothness.” We define equal to min . These functions, represented graphically in Figure 1, and their first derivatives are both continuous functions of R˜ij(θ) everywhere. Therefore, and are smooth functions with respect to θ so that gradient-based root-finding algorithms may be applied. We note if a large value of ε is needed, there may be little information to estimate θ and estimating θ by finding the minimum of a scaled test statistics may have better convergence and mitigate bias due to misspecification of the structural model (see Joffe et al., 2012).
Figure 1.
Plot of and the “smooth” approximations to and , respectively, as a function of R˜ij where and ε = 50.
To address the problems related to the information lost from artificial censoring, we note that the set XUij may be very large, and, as a result, . However, there may be a smaller set such that accounts for the vast majority of the conditional density, i.e. , but defined using instead of XUij in (8) is much greater. The advantage of redefining is that we increase the length of the artificial follow-up and the information to estimate θ. Unfortunately, C˜ij(θ) may be less than the redefined for a small number of (i, j) pairs. For those patients, we may not be able to evaluate Therefore, we must come up with ad hoc expressions for Δij and V˜ij(θ) (and the corresponding “smooth” approximations) for those (i, j) pairs.
To make the discussion concrete, consider the motivating example of lung transplantation. The only time-dependent covariate considered is the lung allocation score (LAS). The LAS is a composite score, ranging from 0 to 100 and updated daily, of over a dozen patient characteristics that predict waitlist mortality and post-transplant survival. The LAS prioritizes patients for organ allocation with higher scores indicating that a patient is typically “sicker” and has a higher priority for transplantation. The LAS trajectory can change precipitously; thus, . If we consider the scale accelerated failure time model given by , where xi is a vector of time-independent patient characteristics, the above discussion suggests that the “artificial” follow-up time should be given by .
If the benefit of transplantation on the multiplicative scale increases with increasing LAS, i.e., θ1< 0, and there is a survival benefit for transplantation at the maximum LAS, then , where max(LAS) = 100. While some patients do experience precipitous increases in the LAS, most patients remain relatively stable while on the waitlist. We could increase the artificial follow-up time by considering the set for some δ ≥ 0 rather than XUij in (8). This implies, if θ1< 0,
| (12) |
While using the smaller set increases the artificial follow-up time, we may not be able to evaluate because C˜ij(θ) may be less than for some (i, j) pairs. However, we could consider replacing R˜ij(θ) with min R˜ij(θ), C˜ij(θ) in the expressions for Δij and V˜ij and the “smooth” approximations of those functions. For those patients who are observed to fail or for whom , Δij and V˜ij are defined as before. Among those patients whose survival times are censored and for whom and . This introduces some bias in the estimating function given in (10), as Δij and V˜ij(θ) are no longer only functions of R˜ij and . We argue in Web Appendix C that the choice of and described above will mitigate some of the problems. We note an ongoing question is how best to chose δ. Different approaches for mitigating the loss of information associated with artificial censoring are also discussed in Web Appendix C.
5. Simulation Study
To evaluate the performance of our proposed class of estimators and methods for handling censored survival responses, we report on simulations designed to resemble the UNOS cohort described in Section 6. From the UNOS cohort, 2,000 patients were randomly selected and their listing dates, observed LAS history, and death dates if they were not transplanted were recorded. Listing dates ranged from May 4, 2005 to December 31, 2010. For the transplanted patients in UNOS cohort, we simulated their residual history had they never been transplanted by assuming the residual lifetime follows an exponential distribution with parameter depending on transplant LAS and generating a monotonically increasing residual LAS trajectory. Additional details are included in Web Appendix C.
We selected dates for 1,250 organs by drawing days without replacement from May 4, 2005 until December 31, 2010. Define LASij as the LAS score for the ith patient at the jth organ transplant. Let zij be LASij truncated from below at 30 and from above at 70. We assigned the first available organ to a patient with propensity , where Φ1= 0.20 and Φ2= –0.1625. For the patient selected to receive the organ, we transformed the residual lifetime according the scale accelerated failure time model exp{θ0 + θ1(zi1− 30)}, where θ0 = −0.1 and θ1 = −0.05. We then repeated the process of assigning organs among eligible subjects and then transforming the residual lifetime for the transplanted patient. The end of follow-up was December 31, 2010 and survival times were censored at that date. We note that we kept the same listing dates, transplant dates, and LAS history in each of the 1,000 Monte Carlo simulations as we considered these quantities fixed in Section 2.
Estimators for (θT, φT)T considered were solutions to the set of estimating equations taking the same general form
| (13) |
where , and is the restricted cubic spline transformation of LASij with knots at 32, 36, 45, and 85. In all cases, we obtained estimates of ξ by solving the set of estimating equations proposed in Web Appendix B.
The following estimators for θ were considered.
Full artificial censoring (fac): Define . Let and using the above . This estimator is asymptotically unbiased.
Minimize artificial censoring (ac+δ): Define . Because for a small number of (i, j) pairs, we replace R˜ij(θ) with min {R˜ij(θ), C˜ij(θ), as suggested in Section 4, in the definition of Δij and and the “smooth” approximations of those functions. Define and .
Ignore need for artificial censoring (ic): Consider an estimator that does not use any artificial censoring. Define Eij and Sij as the observed failure indicator and the observed minimum of transformed residual follow-up and survival.
In addition, we considered other estimators using standard causal approaches. Instead of j indexing the organ, we let j index the week since the patient was listed on the waitlist, so that πij is now the probability that the ith patient is transplanted during the jth week after listing. We estimated πij using a logistic regression model with covariates for LASij including a linear spline with knot point at 40 and a restricted cubic spline transformation of week from listing. We considered the same estimators for θ as in bullet points (1) or (2) above and denote these estimators as fac_alt and ac_alt+δ. To obtain the asymptotic variance, we assumed that observations across different subjects i were independent. This analysis is typical of those proposed in applications of structural nested failure time models but ignores that observations are correlated across subjects.
We note that the definition of the artificial follow-up implicitly assumes that θ̂1 and θ̂0 + θ̂1{min(zij) + δ − 30} are both negative, which was always true in the datasets considered in this simulation. We considered δ equal to 20, 10, and 5. The smooth approximations to the indicator and minimum functions given by (11) had ε equal to 50 days. The algorithm used to generate starting values to solve the estimating function using Newton methods is described Web Appendix C.
The results for the estimates of θ1, the change in the log acceleration factor per unit increase in the LAS, are given in Table 1. Convergent solutions were found for greater than 99.9 percent of all datasets. Using the observed failure indicator and observed follow-up time (ic estimator), results in highly biased and inefficient estimators. The estimators that index time from when the person entered the waitlist and do not account for the dependence in the organ allocation (fac_alt and ac_alt+δ estimators) are all highly biased and lead to poor coverage probability of Wald-type confidence intervals. Even though the full artificial censoring estimator is consistent and asymptotically normal, at this sample sizes the estimator has a slightly skewed distribution which led to a small amount of bias. The bias decreases as the number of organs in the sample increased from 1,250 to 2,500 (results reported in Web Appendix C). The amount of information lost due to artificial censoring for this estimator is extensive, with the follow-up time on the “non-transplant” scale decreased on average by more than a factor of eight. Given the maximum follow-up is 5.66 years, it is not surprising that the sampling distribution for this estimator is not symmetric at this sample size. For the proposed method of minimizing the effects of artificial censoring, there is a clear bias variance trade-off. As we consider smaller values of δ, we necessarily increase the “artificial” follow-up time and the variance of the estimator decreases. However, at the same time the proportion of (i, j) pairs where we cannot evaluatey increases, and the asymptotic bias increases as we consider ad hoc approaches for the failure indicator. The mean square error is minimized when δ was chosen to be either 10 or 5 across different simulation scenarios. The smaller bias in the estimator when δ = 10 results in 95 percent Wald-type confidence intervals that more closely attained their nominal coverage than when δ = 5. When δ = 20, the mean square error for these methods is typically greatest compared to the other values of δ considered, and the small skewness in the sampling distribution of the estimator results in Wald-type confidence intervals that typically fall short of nominal coverage. Additional simulation results are reported in Web Appendix C.
Table 1.
Summary of the parameter estimates for θ1 by estimator from 1000 Mote Carlo simulations when the number of organs allocated was 1,250. Converge: Proportion of MC datasets the estimator converged; MC mean: Monte Carlo average of the parameter estimates; MC SD: Monte Carlo standard deviation of the parameter estimates; Avg SE: Average of the standard error estimates of the parameters; Ratio Avg SE/SD: Ratio of the average of the standard error estimates to the standard deviation of the parameter estimates; Ratio MSE: Ratio of the average squared error of the fac estimates to the average squared error of the estimates of the given estimator; CP: Monte Carlo coverage probability of 95 percent Wald-type confidence intervals. Approximate standard errors for the values in the column MC Mean ranged from 0.00021 to 0.00037, the column MC SD ranged from 0.00015 to 0.00026, the column Avg SE ranged from 0.00019 to 5e-04, the column Ratio Avg SE/SD ranged from 0.045 to 0.045, the column Ratio MSE ranged from 0.028 to 0.33, the column CP ranged from 0.0036 to 0.012.
| Converge | MC Mean | MC SD | Avg SE | Ratio Avg SE/SD | Ratio MSE | CP | |
|---|---|---|---|---|---|---|---|
| fac | 0.999 | −0.0515 | 0.0117 | 0.0116 | 1.00 | 1.00 | 0.874 |
| ac+20 | 0.999 | −0.0504 | 0.0114 | 0.0098 | 0.86 | 1.07 | 0.927 |
| ac+10 | 0.999 | −0.0490 | 0.0082 | 0.0085 | 1.04 | 2.02 | 0.956 |
| ac+05 | 0.999 | −0.0477 | 0.0079 | 0.0081 | 1.03 | 2.06 | 0.954 |
| ic | 1.000 | −0.0583 | 0.0093 | 0.0157 | 1.68 | 0.89 | 0.987 |
| fac_alt | 0.999 | −0.0586 | 0.0110 | 0.0099 | 0.90 | 0.71 | 0.809 |
| ac_alt+20 | 1.000 | −0.0569 | 0.0085 | 0.0078 | 0.92 | 1.16 | 0.851 |
| ac_alt+10 | 1.000 | −0.0553 | 0.0073 | 0.0065 | 0.89 | 1.71 | 0.831 |
| ac_alt+05 | 1.000 | −0.0543 | 0.0067 | 0.0060 | 0.89 | 2.20 | 0.845 |
6. Application to UNOS Data
To assess the causal effect of lung transplantation, we use data from UNOS, which compiles data on all waitlist candidates and transplants in the United States. For our analysis, we consider all patients that were actively waiting for a lung transplantation at some point from May 4, 2005, the beginning of the LAS era, until December 31, 2010. We exclude patients waiting for multi-organ transplantation. Because organs are allocated differently for pediatric patients, we limit our analysis to those patients that were 18 years of age or older at listing. For patients that were lost to follow-up after transplantation or were removed from the waitlist prior to transplantation or death, death dates were ascertained using the Social Security Death Master File (SSDMF). Because the SSDMF contains limited data on non-United States citizens, we restrict our analysis to United States citizens. End of follow-up for this study is December 31, 2010. Although rare, some patients are re-transplanted in the study period. To avoid an overly optimistic assessment of transplantation by including the effects of a second procedure and to be consistent with prior clinical analyses, we use time to graft failure, defined as patient death or time of re-transplantation, as the endpoint.
A total of 12,637 listings and 8,222 organ transplants are included in our analysis. For the purposes of computing the LAS, native diseases are divided into four groups: A (primarily COPD), B (primarily pulmonary hypertension), C (primarily CF), and D (primarily IPF). Most patients in this cohort have native diseases that fall into groups A (36.9 percent) and D (45.9 percent) with smaller numbers in groups B (5.3 percent) and C (11.9 percent). Of those patients who never went on to be transplanted, 1,740 were observed to fail during the study period. Of the 8,222 patients transplanted, 2,509 died during the study period.
Most patients awaiting a lung transplant have an LAS between 30 and 40 (median: 33.8, Q1-Q3: 32.1-37.4). Very few patients have an LAS greater than 60 on the waitlist because these patients tend to die or to be transplanted quickly. The distribution of the LAS at transplantation remains skewed to the right although less skewed than the waitlist LAS (median: 38.9, Q1-Q3: 34.3-47.4).
Kaplan-Meier survival curves for patients once they entered the waitlist and once they were transplanted by the initial and transplanted LAS, respectively, are plotted in Web Figure 2. The waitlist survival was censored at the time the patient was transplanted. This crude analysis suggests that the benefit of transplantation increases with increasing LAS and that transplantation may not be effective for patients with low LAS.
Figure 2.
Logarithm of the estimated scale acceleration factor in the transformation model for the causal effect of lung transplantation in Group A (primarily COPD, left) and Group D (primarily IPF, right) by LAS and transplantation type. The curves here are drawn for patients under 60 years of age, not in UNOS regions 6-8, and not paying for the transplant through private insurance or Medicare. We emphasize that negative (positive) values of the log scale factor suggest that transplantation increases (decreases) lifespan. The histogram in the background gives the number of patients transplanted for that range of LAS and native disease group. This figure appears in color in the electronic version of this article.
We considered a model for the propensity to receive a transplant that is an exponentiated linear combination of covariates as in the simulation. Covariates included in the model were current LAS, native disease group, anticipated payment method (private insurance, Medicare, and other public funding including Medicaid), age, UNOS region, LAS by transplant type (bilateral and unilateral) interaction, and native disease by transplant type interaction. We considered a restricted cubic spline transformation of LAS with knot points at 32, 36, 43, and 85 to allow the log propensity to vary nonlinearly with LAS.
To model the effect of transplantation, we used a scale accelerated failure time model, similar to (2), with covariates for current LAS, age, UNOS region, payment method, and native disease. We considered UNOS region as a binary variable (indicator for Regions 6-8 which include the Northwest and Midwest states) as those regions in preliminary analyses showed better post-transplant outcomes. We considered that in (2) LAS may have a different effect based on native disease (LAS by native disease group by transplant interaction). Within Group A, a linear spline with knot point at 40 was used to model the effect of LAS in the transformation model. For all other native disease groups, the relationship between LAS and the log acceleration factor was adequately modeled as linear. For native disease groups A (primarily COPD) and D (primarily IPF) we included a term for transplant type. For groups B and C, nearly all patients receive bilateral transplants. We truncated LAS from below at 30 and from above 70, as in the simulation study, due to small numbers of patients transplanted beyond these values.
We estimated the parameters in the organ propensity model and SNFTM by solving estimating equations as in (13) with and Sij= V˜ij. We parameterized and estimated ξ as is described in Web Appendix B. We defined the “artifical” follow-up time as in (12). In the simulation study both δ = 10 and δ = 5 performed well. We used δ = 5 + 0.5min{max(LASij − 40,0), 10} in this application. For those (i, j) pairs where we cannot evaluate we replace R˜ij(θ) with min{R˜ij(θ),C˜ij(θ)}.
Estimates and corresponding standard errors for parameters in the transformation model are given in Table 2. We graphically represent how the scale acceleration factor varies by LAS and transplant type for patients in Group A and Group D in Figure 2. Parameter estimates for the organ allocation model are given in Web Appendix D. Across all native diseases, the benefit of transplantation on the multiplicative scale increases with increasing LAS. With the exception of group B which includes very few patients, transplantation offers a significant survival benefit for high allocation scores. Bilateral transplant confers a significantly greater survival benefit for patients with native diseases in group D but does not confer a significant advantage for patients in group A. For all patients in group A and patients in group D that receive a single lung transplant, transplantation confers a significant survival loss for patients with low LAS values, near 30. For the other native disease groups, transplantation does not show any significant survival gains or losses for low allocation scores. A large percentage of patients are transplanted at these LAS scores that show no significant survival benefit or even significant survival loss. Patients who intended to pay for the transplantation procedure using private insurance have significantly better survival benefit compared to those patients that intend to pay for transplantation using public funds. Patient’s age and geographic region do not significantly affect survival benefit. Web Appendix D contains additional information on this analysis including diagnostics of the adequacy of the proposed transformation model and non-parametric estimates of the survival distribution.
Table 2.
Parameter estimates of the scale accelerated failure time model. Group A: primarily COPD; Group B: primarily pulmonary hypertension; Group C: primarily CF; Group D: primarily IPF; Single LTx: single lung transplantation. The reference for Private Insurance and Medicare Insurance is Medicaid or other public funding for payment. LAS has been centered at 30 so the interpretation of the intercept term in log acceleration factor at an LAS of 30.
| Parameter | Estimate | Std. Error |
|---|---|---|
| Group A Intercept | 1.077 | 0.229 |
| Group A (LAS-30) | −0.193 | 0.035 |
| Group A max(LAS-40,0) | 0.143 | 0.042 |
| Group A Single LTx | 0.059 | 0.133 |
| Group B Intercept | 0.139 | 0.312 |
| Group B (LAS-30) | −0.029 | 0.066 |
| Group C Intercept | 0.055 | 0.152 |
| Group C (LAS-30) | −0.094 | 0.008 |
| Group D Intercept | 0.007 | 0.123 |
| Group D (LAS-30) | −0.064 | 0.004 |
| Group D Single LTx | 0.277 | 0.088 |
| Age ≥ 60 at Listing | −0.014 | 0.117 |
| Private Insurance | −0.358 | 0.109 |
| Medicare Insurance | −0.150 | 0.124 |
| UNOS Regions 6-8 | −0.027 | 0.104 |
7. Discussion
We have proposed a method to evaluate the causal effect of organ transplantation on the distribution of residual lifetime. Our method conceptualizes each organ transplant as a non-randomized trial where one person receives treatment (transplantation) and the remaining people on the waitlist do not. This approach is similar to the methods of Hernán et al. (2008), to assess the effect of hormone replacement therapy on time to coronary heart disease, and Schaubel et al. (2009), to evaluate the effect of liver transplantation on survival. Although conceptually similar, our work differs in some key dimensions. Both Schaubel et al. (2009) and Hernán et al. (2008) use a Cox proportional hazards model to assess the effect of treatment as opposed to the accelerated failure time model we propose. Within a given “experiment,” Schaubel et al. (2009) and Hernán et al. (2008) must censor patients when they “cross-over” from one treatment group to another and then weight the uncensored patients by the inverse probability that they would “cross-over.” In the context of organ transplantation, “crossing-over” occurs anytime a patient moves from the waitlist group to the post-transplant group. This censoring necessarily leads to a loss of information, and inverse weighting can lead to unstable estimators. On the other hand, our approach uses G-estimation, which requires artificially censoring subjects to obtain valid inference. Importantly, we derive the asymptotic properties of our estimator as the number of “experiments” tends to infinity, which the prior work does not. Indeed, Schaubel et al. (2009) does not propose large sample inference and uses the bootstrap to obtain approximate standard errors of the regression parameters.
In modeling the effect of lung transplantation, we have assumed a scale accelerated failure time model. Although model diagnostics presented in Web Appendix D suggest that this model adequately represents the effect of transplantation, future research should examine other more flexible transformation models. In particular, a model that acknowledges that the increased risk surgical mortality soon after transplantation may be more appropriate.
Clinically, the results indicate that many patients are transplanted that show no statistically significant survival benefit. Given the large numbers of patients that die on the waitlist, this suggests that the waitlist order should further emphasize waitlist prognosis over geography or exact blood type match. However, survival is only one measure of patient well-being and future analysis could consider the causal effects of transplant on quality of life (Finlen Copeland et al., 2012).
This research has focused on the effect of the effect of transplantation as compared to never receiving a transplant. A related question that patients face is whether to receive a transplant now or wait until later to maximize survival. Deriving such an optimal transplantation regime is complicated by the fact that patients cannot be transplanted whenever they would like but instead must be offered an organ. Future work should examine this question.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R37AI031789, R01CA051962, R01CA085848 and P01CA142538 and by HRSA contract 234-2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Supplementary Materials: Web Appendices A-D referenced in Sections 2-7 are available with this paper at the Biometrics website on Wiley Online Library.
Contributor Information
David M. Vock, Email: vock@umn.edu, Division of Biostatistics, University of Minnesota, Minneapolis, Minnesota 55455, U.S.A.
Anastasios A. Tsiatis, Department of Statistics, North Carolina State University, Raleigh, North Carolina 27695, U.S.A
Marie Davidian, Department of Statistics, North Carolina State University, Raleigh, North Carolina 27695, U.S.A.
Eric B. Laber, Department of Statistics, North Carolina State University, Raleigh, North Carolina 27695, U.S.A
Wayne M. Tsuang, Lung Transplant Program, Department of Medicine Duke University Medical Center, Durham, North Carolina 27710, U.S.A
C. Ashley Finlen Copeland, Lung Transplant Program, Department of Medicine Duke University Medical Center, Durham, North Carolina 27710, U.S.A.
Scott M. Palmer, Lung Transplant Program, Department of Medicine Duke University Medical Center, Durham, North Carolina 27710, U.S.A
References
- DasGupta A. Asymptotic Thoery of Statistics and Probability. Springer; New York: 2008. [Google Scholar]
- Finlen Copeland CA, Vock DM, Pieper KS, Mark DB, Palmer SM. Impact of lung transplantation upon recipient quality of life: A serial, prospective, multi-center analysis through the first posttransplant year. Chest. 2012;142 doi: 10.1378/chest.12-0971. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernán MA, Alonso A, Logan R, Grodstein F, Michels KB, Willett WC, Manson JE, Robins JM. Observational studies analyzed like randomized experiments: An application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19:766–779. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe MM, Yang WP, Feldman H. G-estimation and artificial censoring: Problems, challenges, and applications. Biometrics. 2012;68:275–286. doi: 10.1111/j.1541-0420.2011.01656.x. [DOI] [PubMed] [Google Scholar]
- Kotloff RM, Thabut G. Lung transplantation. American Journal of Respiratory and Critical Care Medicine. 2011;184:159–171. doi: 10.1164/rccm.201101-0134CI. [DOI] [PubMed] [Google Scholar]
- Lahzami S, Bridevaux PO, Soccal PM, Wellinger J, Robert JH, Ris HB, Aubert JD. Survival impact of lung transplantation for COPD. European Respiratory Journal. 2010;36:74–80. doi: 10.1183/09031936.00087809. [DOI] [PubMed] [Google Scholar]
- Liou TG, Adler FR, Cahill BC, FitzSimmons SC, Huang D, Hibbs JR, Marshall BC. Survival effect of lung transplantation among patients with cystic fibrosis. Journal of the American Medical Association. 2001;286:2683–2689. doi: 10.1001/jama.286.21.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: A comparative study. Statistics in Medicine. 2004;23:29372960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- Robins J. Estimation of the time-dependent accelerated failure time model in the presence of confounding factors. Biometrika. 1992;79:321–334. [Google Scholar]
- Robins JM. The analysis of randomized and non-randomized AIDS treatment trials using a new approach to causal inference in longitudinal studies. Health Service Research Methodology: A Focus on AIDS. NCHSR, US Public Health Service; Rockville, MD: 1989. pp. 113–159. [Google Scholar]
- Robins JM. Structural Nested Failure Time Models Encyclopedia of Biostatistics. John Wiley & Sons, Ltd; 2005. pp. 4372–4389. [Google Scholar]
- Robins JM, Blevins D, Ritter G, Wulfsohn M. G-estimation of the effect of prophylaxis therapy for pneumocystis-carinii pneumonia on the survival of AIDS patients. Epidemiology. 1992;3:319–336. doi: 10.1097/00001648-199207000-00007. [DOI] [PubMed] [Google Scholar]
- Schaubel DE, Wolfe RA, Sima CS, Merion RM. Estimating the effect of a time-dependent treatment by levels of an internal time-dependent covariate: Application to the contrast between liver wait-list and posttransplant mortality. Journal of the American Statistical Association. 2009;104:49–59. [Google Scholar]
- Thabut G, Mal H, Castier Y, Groussard O, Brugiere O, Marrash-Chahla R, Leseche G, Fournier M. Survival benefit of lung transplantation for patients with idiopathic pulmonary fibrosis. Journal of Thoracic and Cardiovascular Surgery. 2003;126:469–475. doi: 10.1016/s0022-5223(03)00600-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.