Abstract
Dopamine (DA) replacement therapy with L-DOPA is the standard treatment for Parkinson’s disease (PD). Unfortunately chronic treatment often leads to the development of abnormal involuntary movements (AIMs) referred to as L-DOPA-induced dyskinesia (LID). Accumulating evidence has shown that compensatory plasticity in serotonin (5-HT) neurons contributes to LID and recent work has indicated that acute 5-HT transporter (SERT) blockade provides anti-dyskinetic protection. However neither the persistence nor the mechanism(s) of these effects have been investigated. Therefore the current endeavor sought to mimic a prolonged regimen of SERT inhibition in L-DOPA-primed and –naïve hemi-parkinsonian rats. Rats received 3 weeks of daily co-treatment of the selective 5-HT reuptake inhibitors (SSRIs) citalopram (0, 3, or 5 mg/kg) or paroxetine (0, 0.5, or 1.25 mg/kg) with L-DOPA (6 mg/kg) during which AIMs and motor performance were monitored. In order to investigate potential mechanisms of action, tissue levels of striatal monoamines were monitored and the 5-HT1A receptor antagonist WAY100635 (0.5 mg/kg) was used. Results revealed that prolonged SSRIs attenuated AIMs expression and development in L-DOPA-primed and –naïve subjects, respectively, without interfering with motor performance. Neurochemical analysis of striatal tissue indicated that a 3 week SERT blockade increased DA levels in L-DOPA-treated rats. Pharmacologically, anti-dyskinetic effects were partially reversed with WAY100635 signifying involvement of the 5-HT1A receptor. Collectively, these findings demonstrate that prolonged SERT inhibition provides enduring anti-dyskinetic effects in part via 5-HT1A receptors while maintaining L-DOPA’s anti-parkinsonian efficacy by enhancing striatal DA levels.
Keywords: Abnormal involuntary movements, Dopamine, Serotonin transporter, Parkinson’s disease, Selective serotonin reuptake inhibitor, 5-HT1A receptor
1. Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder typified by loss of dopaminergic (DA) neurons in the substantia nigra pars compacta that leads to cardinal motor symptoms such as resting tremor, rigidity, akinesia, and postural instability. DA replacement therapy with L-DOPA remains the standard treatment for PD; however, debilitating abnormal involuntary movements (AIMs) also known as L-DOPA-induced dyskinesia (LID) develop with chronic use (Jankovic, 2005). Although the mechanisms that underlie LID are complex, a role for the serotonin (5-HT) system has gained increasing support. For instance, 5-HT neurons from the raphe nuclei can act as surrogates to the failing DA system, taking up and converting L-DOPA into DA for release into the striatum (Arai et al., 1995; Carta et al., 2007; Navailles et al., 2010). However, this neuroplasticity can be maladaptive in later stages of the disease when LID is likely to develop as 5-HT neurons do not contain DA regulatory mechanisms, such as D2 receptors and DA transporters (DAT) leading to uncontrolled release of L-DOPA-derived DA (Carta et al., 2007; de la Fuentes-Fernandez et al., 2004). This is supported by finding that removal of 5-HT raphestriatal afferents or stimulation of 5-HT1A receptors leads to a marked attenuation of L-DOPA-derived DA and LID (Carta et al., 2007; Eskow et al., 2009; Kannari et al., 2001). Therefore, pharmacological control of 5-HT neurons may temper DA dysregulation that contributes of LID development and expression.
Recent work has implicated the 5-HT transporter (SERT) for pharmacological treatment of LID. Compelling work by Rylander et al. (2010) demonstrated that L-DOPA treatment leads to a significant up-regulation of SERT in the DA-dennervated striata of rats, non-human primates, and PD patients. In addition, L-DOPA-induced increases in SERT binding appear to be positively correlated with the expression of LID suggesting that SERT blockade may convey enduring protection against dyskinesia. This has been supported by preclinical studies. For example, acute administration of the selective 5-HT reuptake inhibitors (SSRIs) citalopram, paroxetine, and fluoxetine in L-DOPA-primed, dyskinetic animals potently attenuate L-DOPA-induced AIMs and rotations without affecting L-DOPA’s ability to reverse lesion-induced motor deficit (Bishop et al., 2012; Inden et al., 2012; Kuan et al., 2008).
Although these acute effects are promising, several translational and mechanistic questions remain. Therefore, the current investigation sought to examine the potential interventional and prophylactic anti-dyskinetic effects of prolonged treatment with citalopram and paroxetine in L-DOPA-primed and –naïve hemi-parkinsonian rats. As a means towards identifying mechanisms of action, the effects of concurrent SSRI and L-DOPA administration on striatal monoamines from L-DOPA-primed rats were measured by high performance liquid chromatography (HPLC) and the contribution of the 5-HT1A receptor to the anti-dyskinetic effects of SERT blockade was examined.
2. Materials and methods
2.1. Animals
Adult male Sprague-Dawley rats were used (N = 113; approximately 2 months old and 225–250 g upon arrival; Harlan Farms, USA). Rats were housed in plastic cages (22 cm high, 45 cm deep, and 23 cm wide) and given free access to standard lab chow (Rodent Diet 5001; Lab Diet, Brentwood, MO, USA) and water. The colony room was kept on a 12 h light/dark cycle (light on at 0700 h) and maintained at 22–23°C. Rats were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of Binghamton University and the “Guide for the Care and Use of Laboratory Animals” (Institute for Laboratory Animal Research, National Academies Press, 2011).
2.2. Experiment 1: Effects of prolonged SSRI treatment in L-DOPA-primed rats
2.2.1. Medial forebrain bundle 6-hydroxydopamine lesion surgery
One week after arrival, rats (n = 44) received unilateral 6-hydroxydopamine (6-OHDA) lesions of the left medial forebrain bundle (MFB) to destroy DA neurons. Desipramine HCl (25 mg/kg, i.p.; Sigma, St. Louis, MO, USA) was given to each rat 30 min prior to 6-OHDA injection to protect norepinephrine (NE) neurons. All rats received injections of Buprenex (buprenorphine HCl; 0.03 mg/kg, i.p.; Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA) as analgesic treatment 5 min pre-surgery. Rats were anesthetized with inhalant isoflurane (2–3%; Sigma) in oxygen (2.5 L/min), and then placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). The coordinates for 6-OHDA injections were AP: −1.8 mm, ML: +2.0 mm, DV: −8.6 mm relative to bregma, with the incisor bar positioned 5.0 mm below the interaural line (Paxinos and Watson, 1998). After a small hole was drilled at the target site, a 10 μL syringe attached to a 26 gauge needle was used to deliver 4 μL of 6-OHDA (3 μg/μL; Sigma) dissolved in 0.9% NaCl + 0.1% ascorbic acid at a rate of 2 μL/min. The needle was withdrawn 5 min later. Post-surgery, rats were pair-housed and provided with soft chow, fruit, and saline as needed to facilitate recovery.
2.2.2. Pharmacological treatments and procedure
Three weeks post-surgery, rats were primed with L-DOPA methyl ester (L-DOPA; 6 mg/kg, s.c.; Sigma) + DL-serine 2-(2,3,4-trihydroxybenzyl) hydrazine hydrochloride (benserazide; 15 mg/kg, s.c.; Sigma) dissolved in 0.9% NaCl + 0.1% ascorbic acid once a day for 14 days to produce stable AIMs expression (Putterman et al., 2007; Taylor et al., 2005). Rats were tested on the Forepaw Adjusting Steps test (FAS; see description below) on 2 separate days before daily injections to establish baseline motor performance. On days 8 and 14 of L-DOPA priming, ALO AIMs (see description below) were observed every 10 min for 3 h to establish expression of dyskinesia. Rats (n = 36) with ALO AIMs scores ≥ 25 by day 14 were organized into equally dyskinetic treatment groups (n = 7–8) by counterbalancing ALO AIMs scores from day 14. For the next 3 weeks (days 15 – 36), rats received daily treatments vehicle (20% dimethyl sulfoxide (DMSO) + 80% distilled water; s.c.), citalopram (3 or 5 mg/kg, s.c.; Sigma), or paroxetine (0.5 or 1.25 mg/kg, s.c.; Sigma) followed 30 min later by L-DOPA (6 mg/kg + benserazide, 15 mg/kg, s.c.). Doses were established by previous research (Bishop et al., 2012; Brocco et al., 2002). Rats were tested for LID expression using ALO AIMs on days 15, 22, 29, and 36 and for motor performance using FAS on days 17, 24, 31. On day 37, rats were given their respective SSRI and L-DOPA treatments and decapitated 1 h after L-DOPA treatment. Left and right striata were dissected and flash frozen to examine long term SSRI effects on monoamines and their metabolites using HPLC.
2.3. Experiment 2: Effects of prolonged SSRI treatment on dyskinesia development
One week after arrival, rats either received unilateral 6-OHDA lesions of the left MFB (n = 47; as described previously) or sham lesions (n = 8). Two weeks post-lesion, rats were tested on FAS to establish baseline motor performance prior to treatment. Rats were assigned to equally disabled treatment groups (n = 7–8) by counterbalancing the percent intact FAS scores from baseline. To determine if SSRI administration could prevent the development of LID, 3 weeks post-lesion, rats received daily treatments of either vehicle, citalopram (3 or 5 mg/kg, s.c.), or paroxetine (0.5 or 1.25 mg/kg, s.c.) followed 30 min later by vehicle or L-DOPA (6 mg/kg + 15 mg/kg benserazide, s.c.). Rats were tested for LID development using ALO AIMs on days 1, 8, 15, and 22 and for motor performance using FAS on days 3, 10, 17. At the end of the study rats were sacrificed and left and right striata were dissected for HPLC analysis of DA depletion.
2.4. Experiment 3: 5-HT1A receptor antagonist effects on SSRI attenuation of LID
One week after arrival, rats (n = 14) received unilateral 6-OHDA lesions of the left MFB. Three weeks post-surgery, rats were primed with L-DOPA (6 mg/kg + benserazide 15 mg/kg, s.c.) once a day for 14 days to produce stable AIMs expression. On days 1, 8, and 14 of L-DOPA priming immediately after injections, ALO AIMs were observed every 10 min for 3 h to establish expression of dyskinesia and rats that had an ALO score ≥ 25 by day 14, indicative of >95% striatal DA depletion (Taylor et al., 2005) were kept for further testing (n = 12). Using a within-subjects design, rats received the following treatment across 10 test days spaced 3–4 days apart: vehicle (0.9% NaCl) or 5-HT1A receptor antagonist N-[2-[4-(2-Methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt (WAY100635; 0.5 mg/kg, sc; Sigma); and vehicle (20% DMSO + 80% distilled water; s.c.), citalopram (3 or 5 mg/kg, s.c.; Sigma), or paroxetine (0.5 or 1.25 mg/kg, s.c.; Sigma) and L-DOPA (6 mg/kg + benserazide 15 mg/kg, s.c.). Vehicle or WAY100635 were administered 5 min prior to vehicle or SSRI treatment which was administered 30 min prior to L-DOPA. Rats were tested for ALO AIMs for 3 h immediately following L-DOPA treatment. At the end of the experiment, rats were maintained for additional studies not included here.
2.5. Data Analyses
ALO AIMs (data expressed as medians ± median absolute difference; M.A.D.) were analyzed using non-parametric Kruskal-Wallis ANOVAs at each test day in experiments 1 and 2 while Friedman ANOVAs were used for treatment differences in experiment 3. Significant differences between treatment groups were determined by Mann-Whitney and Wilcoxon post-hocs, respectively. Differences in FAS (expressed as mean percent intact (lesioned paw steps relative to steps taken by intact forepaw) ± standard mean error; S.E.M.) were measured by 1-way ANOVA within each treatment. A 1-way ANOVA was used to compare baseline scores between groups. Significant differences across test days were established using Fisher’s LSD post-hocs. In experiment 1, striatal monoamine tissue levels (pg/mg of tissue ± S.E.M.) and turnover (metabolite/monoamine ± S.E.M.) were analyzed using 2-way (2 lesion × 5 treatment) ANOVAs. One-way ANOVAs were also employed to examine differences in percent intact (monoamine levels in lesioned striatum relative to intact striatum) between treatments. When appropriate, significant differences were determined by Fisher’s LSD post-hocs. For experiment 2, striatal DA levels between the intact and lesioned hemispheres were compared using an independent samples t-test. Analyses were performed with the use of Statistica 7 (Statsoft Inc., Tulsa, OK, USA) with alpha set to p = 0.05.
2.6. Behavioral Analyses
2.6.1. Abnormal involuntary movements (AIMs)
Rats were monitored for rodent dyskinesia using a procedure previously described (Bishop et al., 2012; Dupre et al., 2011; Lundblad et al., 2002). During testing (0900–1700 h), rats were placed in clear plastic cylinders (20 × 25 cm) immediately after L-DOPA injection. After injections, a trained observer blind to treatment recorded AIMs involving axial, limb, and orolingual (ALO) regions. “Axial” AIMs include dystonic twisting of the neck and torso directed toward the side of the body that is contralateral to lesion. “Limb” AIMs refer to excessive and purposeless movements of the forelimb contralateral to lesion. “Orolingual” AIMs are repetitive openings and closings of the jaw as well as multiple tongue protrusions. ALO AIMs were rated and given a severity score (0–4) for 1 min every 10 min for 3 h: 0, not present; 1, present for less than 29 s or half the time; 2, present for 30–59 s or majority of the time; 3, present for the whole 60 s but interrupted by stimulus (tap on the cylinder); or 4, present for the whole 60 s and was not interrupted by stimulus.
2.6.2. Forepaw Adjusting Steps Test (FAS)
Lesion and treatment effects on motor performance were measured using a procedure previously described (Chang et al., 1999; Eskow et al., 2007). On test days, rats were injected with vehicle, citalopram, or paroxetine 30 min prior to L-DOPA treatment and tested 1 h post L-DOPA injection. During testing rats were moved laterally across a table at a rate of 9 cm/s across 90 cm. The hindlimbs were lifted and one forepaw was restrained by the experimenter so that weight would be imposed on the other forepaw. Each test consisted of 6 trials for each paw for both forehand (adjusting for movement toward the body) and backhand (adjusting for movement away from the body) directions. Data were presented as total steps taken by each paw as well as the sum of steps taken by the lesioned forelimb divided by the sum of steps taken by the intact forelimb and multiplying by 100 to report the percent intact thus indicating the degree of disability seen in the lesioned paw.
2.7. Neurochemical Analyses with HPLC
Upon completion of the aforementioned experiments, rats were rapidly decapitated and striatal tissue was dissected and frozen at −80°C for later analysis for monoamine levels via HPLC with electrochemical detection. Reverse-phase HPLC was performed on left and right striatal tissue obtained from rats in Experiments 1 and 2, according to the protocol of Kilpatrick et al. (1986), a method for semi-automated catecholamine analysis with coulometric detection, as reported previously (Eskow et al., 2009; Eskow-Jaunarajs et al., 2011). The limit of detection was 10−10 M for the monoamines and the metabolites measured which included NE, 3,4-Dihydroxyphenylacetic acid (DOPAC), DA, 5-Hydroxyindoleacetic acid (5-HIAA), and 5-HT. The final oxidation current values were plotted on a standard curve of known concentrations from 10−6 M to 10−9 M, adjusted to respective tissue weights and expressed as pg of monoamine or metabolite per mg tissue.
3. Results
3.1. Experiment 1
3.1.1. Prolonged SSRI treatment attenuates established L-DOPA-induced AIMs
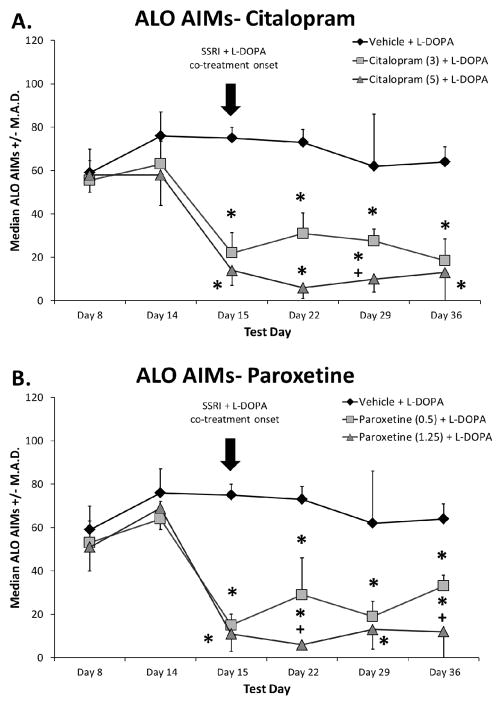
In order to establish the effect of prolonged systemic SSRI treatment on established LID, rats previously rendered dyskinetic received vehicle, citalopram, or paroxetine 30 min before L-DOPA daily for 3 weeks. Statistical analyses revealed that all groups were equally dyskinetic prior to SSRI treatment on priming days 8 and 14 (Figure 1). Importantly, introduction of citalopram and paroxetine dose-dependently attenuated ALO AIMs expression (all H2 > 10.4; all p < 0.05; Fig. 1A, B). Post-hoc analyses revealed that the anti-dyskinetic effects of SSRI pre-treatment persisted throughout the 3 weeks of testing.
Fig. 1.
Effects of prolonged selective 5-HT reuptake inhibitors citalopram (A) and paroxetine (B) on the expression of established L-DOPA-induced abnormal involuntary movements (AIMs). Rats were L-DOPA-primed for 14 days and axial, limb, and orolingual (ALO) AIMs were recorded on days Prime 8 and Prime 14. Thereafter, equally dyskinetic groups were treated with vehicle, citalopram (3 or 5 mg/kg, s.c.), or paroxetine (0.5 or 1.25 mg/kg, s.c.) 30 min prior to L-DOPA (6 mg/kg, s.c.) + benserazide (15 mg/kg, s.c.) daily for 22 days. The arrow indicates the start of SSRI and L-DOPA co-treatment. ALO AIMs were evaluated for 3 h after L-DOPA on days 15, 22, 29, and 36. Values are expressed as medians (AIMs ± median absolute difference; M.A.D.). Significant differences were determined by non-parametric Kruskal-Wallis ANOVAs at each test day with Mann-Whitney post-hocs. * p < 0.05 vs. Vehicle + L-DOPA, + p < 0.05 vs. Citalopram (3) or Paroxetine (0.5) + L-DOPA.
3.1.2. Prolonged SSRI administration does not alter L-DOPA efficacy in L-DOPA-primed rats
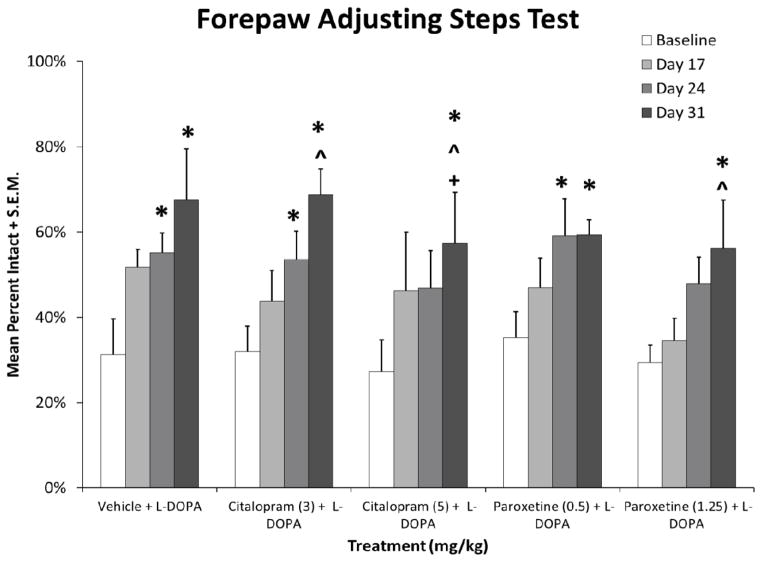
In order to determine the effects of prolonged SSRI treatment on L-DOPA’s anti-parkinsonian efficacy, motor performance was assayed using FAS. As shown in Figure 2, all groups were equally impaired at baseline. Significant effects in treatment groups demonstrated several important features (vehicle: F3,18= 4.1, p < 0.05; citalopram 3 mg/kg: F3,21= 7.5; all p < 0.05; citalopram 5 mg/kg: F3,18= 4.5; p < 0.05; paroxetine 0.5 mg/kg: F3,18= 4.3; p < 0.05; paroxetine 1.25 mg/kg: F3,18= 3.2; p < 0.05). First, chronic L-DOPA treatment reversed lesion-induced stepping by the second test day. Low doses of SSRIs were similar to L-DOPA alone. Higher doses of SSRI pretreatment appeared to temporarily affect efficacy but did not interfere with L-DOPA’s efficacy by the last day of testing.
Fig. 2.
Effects of prolonged selective 5-HT reuptake inhibitors citalopram and paroxetine on L-DOPA reversal of motor deficit on the forepaw adjustment steps test. Baseline stepping deficits were established 3 weeks post-lesion, prior to L-DOPA priming. Rats then received daily treatment with vehicle, citalopram (3 or 5 mg/kg, s.c.), or paroxetine (0.5 or 1.25 mg/kg, s.c.) 30 min prior to L-DOPA (6 mg/kg, s.c.) + benserazide (15 mg/kg, s.c.) for 22 days. Forepaw adjusting steps were evaluated 60 min after treatments on days 17, 24, and 31. Values (as means + standard mean error; S.E.M.) are expressed as percent intact vs. intact forepaw. Significant differences were determined by 1-way ANOVAs within each treatment condition. When appropriate, motor performance differences across test days were analyzed with Fisher LSD post-hocs. Baseline values were evaluated by a 1-way ANOVA to determine equivalent stepping deficit across groups. * p < 0.05 vs. Baseline, ^ p < 0.05 vs. Day 17, + p < 0.05 vs. Day 24.
3.1.3. Prolonged SSRI administration increases tissue DA levels in lesioned striatum
One hour after rats received their last L-DOPA treatment, tissue from intact and lesioned striata were dissected for HPLC analyses of lesion and treatment induced changes in levels of monoamines (DA, 5-HT), their metabolites (DOPAC, 5-HIAA), and their turnover (Table 1). Analyses identified main effects of lesion for each. Specifically, in the lesioned striatum, DA (F1,29 = 750, p < 0.05), DOPAC (F1,29 = 198, p < 0.05), and 5-HT (F1,29 = 16, p < 0.05) were decreased while 5-HIAA was elevated (F1,29 = 119, p < 0.05). DA turnover (F1,29 = 28.3, p < 0.05) and 5-HT turnover (F1,29 = 73.1, p < 0.05) were enhanced in the lesioned vs. intact striatum. To more fully examine treatment-induced changes, 1-way ANOVAs carried out on percent intact values identified a significant effect of treatment on DA levels (F4,29 = 4.17, p < 0.05). Post-hoc analysis revealed that 3 week administration of SSRIs with L-DOPA nearly doubled DA levels in the lesioned striatum compared to L-DOPA alone (all p < 0.05).
Table 1.
Effects of unilateral 6-hydroxydopamine (6-OHDA) lesions and treatment with selective 5-HT reuptake inhibitors citalopram and paroxetine on concentrations of dopamine (DA), 3,4-Dihydroxyphenylacetic acid (DOPAC), DA turnover, serotonin (5-HT), 5-Hydroxyindoleacetic acid (5-HIAA), and 5-HT turnover in the striatum of L-DOPA primed rats (n=7/group). Rats were treated with vehicle, citalopram (3 or 5 mg/kg, s.c.), or paroxetine (0.5 or 1.25 mg/kg, s.c.) 30 minutes prior to L-DOPA (6 mg/kg, s.c.) + benserazide (15 mg/kg, s.c.) consistently for 21 days. Before being sacrificed, rats received their respective treatments and were killed 60 minutes after L-DOPA.
| Treatment with L-DOPA 6 mg/kg | DA | DOPAC | DA Turnover | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Intact | Lesion | % Intact | Intact | Lesion | % Intact | Intact | Lesion | % Intact | |||||
|
| |||||||||||||
| Vehicle | 12149 ± 495 |
*
|
86 ± 18 | 0.6 ± 0.1 | 2958 ± 217 |
*
|
281 ± 86 | 9 ± 3 | 0.24 ± 0.01 |
*
|
3.87 ± 1.03 | 1620 ± 391 | |
| Citalopram 3 mg/kg | 11449 ± 494 | 177 ± 20 |
^
|
2 ± 0.2 | 3409 ± 428 | 949 ± 431 | 30 ± 15 | 0.30 ± 0.03 | 4.37 ± 1.67 | 1482 ± 601 | |||
| Citalopram 5 mg/kg | 12410 ± 739 | 161 ± 28 | 1 ± 0.2 | 3025 ± 240 | 287 ± 101 | 10 ± 5 | 0.25 ± 0.03 | 1.68 ± 0.47 | 680 ± 190 | ||||
| Paroxetine 0.5 mg/kg | 12418 ± 1416 | 162 ± 14 | 1 ± 0.1 | 3226 ± 267 | 499 ± 338 | 7 ± 2 | 0.25 ± 0.01 | 1.24 ± 0.27 | 392 ± 68 | ||||
| Paroxetine 1.25 mg/kg | 11555 ± 1184 | 151 ± 28 | 1 ± 0.2 | 2853 ± 317 | 173 ± 29 | 7 ± 1 | 0.25 ± 0.02 | 1.66 ± 0.61 | 655 ± 227 | ||||
| Treatment with L-DOPA 6 mg/kg | 5-HT | 5-HIAA | 5-HT Turnover | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Intact | Lesion | % Intact | Intact | Lesion | % Intact | Intact | Lesion | % Intact | ||||
|
| ||||||||||||
| Vehicle | 278 ± 28 |
*
|
284 ± 67 | 103 ± 18 | 423 ± 37 |
*
|
582 ± 34 | 143 ± 14 | 1.55 ± 0.07 |
*
|
2.52 ± 0.42 | 168 ± 32 |
| Citalopram 3 mg/kg | 371 ± 28 | 271 ± 29 | 75 ± 10 | 407 ± 38 | 633 ± 30 | 160 ± 11 | 1.12 ± 0.11 | 2.55 ± 0.39 | 228 ± 25 | |||
| Citalopram 5 mg/kg | 366 ± 30 | 285 ± 51 | 81 ± 12 | 375 ± 21 | 562 ± 32 | 158 ± 15 | 0.99 ± 0.05 | 2.21 ± 0.30 | 209 ± 26 | |||
| Paroxetine 0.5 mg/kg | 392 ± 45 | 280 ± 21 | 76 ± 10 | 414 ± 26 | 582 ± 15 | 143 ± 8 | 1.12 ± 0.11 | 2.14 ± 0.15 | 198 ± 15 | |||
| Paroxetine 1.25 mg/kg | 432 ± 35 | 286 ± 34 | 69 ± 9 | 420 ± 17 | 565 ± 47 | 135 ± 10 | 1.00 ± 0.08 | 2.08 ± 0.19 | 216 ± 28 | |||
Values (as means ± standard mean error; S.E.M.) are expressed as picograms monoamine or metabolite per milligram wet weight tissue, percent intact vs. intact hemisphere, and turnover estimates were determined by dividing individual subject’s metabolite by monoamine. Differences between lesion (intact vs lesioned striatum), treatments, and lesion x treatment (2 × 5) interactions were determined by 1 and 2-way ANOVAs. Individual cell differences were analyzed with Fisher LSD post-hocs.
p < 0.05 vs. Intact,
p < 0.05 vs. Vehicle.
3.2. Experiment 2
3.2.1. Prolonged SSRI treatment reduces the development of L-DOPA-induced AIMs
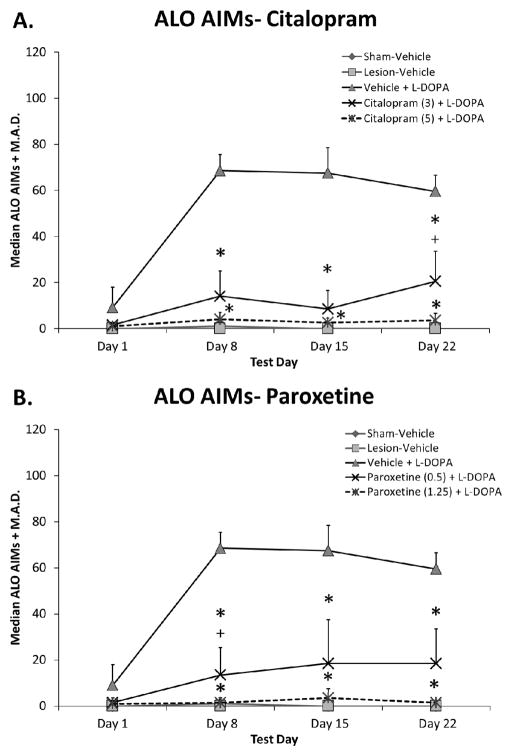
To establish whether SSRI treatment could blunt LID development, L-DOPA-naïve rats were pre-treated daily with vehicle, citalopram, or paroxetine 30 min prior to L-DOPA for 3 weeks. As shown in Figure 3, citalopram and paroxetine significantly inhibited ALO AIMs development (all H4 > 19.9; all p < 0.05; Fig. 3A, B). Post-hoc analyses demonstrated that both drugs and doses of SSRIs produced similar anti-dyskinetic effects with the exception of day 22 for citalopram and day 8 for paroxetine where higher doses were superior to lower doses (both p < 0.05).
Fig. 3.
Effects of prolonged selective 5-HT reuptake inhibitors citalopram (A) and paroxetine (B) on the development of L-DOPA-induced abnormal involuntary movements (AIMs). L-DOPA-naïve rats were treated with vehicle, citalopram (3 or 5 mg/kg, s.c.), or paroxetine (0.5 or 1.25 mg/kg, s.c.) 30 min prior to vehicle or L-DOPA (6 mg/kg, s.c.) + benserazide (15 mg/kg, s.c.) daily for 22 days. ALO AIMs were evaluated for 3 h after L-DOPA on days 1, 8, 15, and 22. Values are expressed as medians (AIMs + median absolute difference; M.A.D.). Significant differences were determined by non-parametric Kruskal-Wallis ANOVAs at each test day with Mann-Whitney post-hocs. * p < 0.05 vs. Vehicle + L-DOPA, + p < 0.05 vs. Citalopram (5) or Paroxetine (1.25) + L-DOPA.
3.2.2. Prolonged SSRI treatment does not alter L-DOPA efficacy in L-DOPA-naïve rats
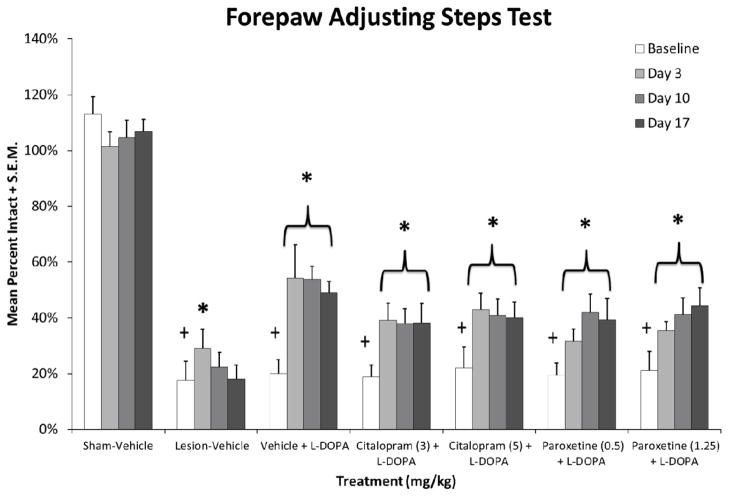
Throughout Experiment 2, motor performance was also monitored for lesion-induced stepping deficits, stepping improvement by L-DOPA, and possible changes with SSRI co-administration. As shown in Figure 4, at baseline all 6-OHDA-lesioned rats displayed severe stepping deficits (about 20% intact stepping) when compared to sham-lesioned rats (F6,48 = 35.5, p < 0.05). This motor deficit was supported by HPLC analysis in rats that received unilateral 6-OHDA (t90 = 12.9, p < 0.05) which resulted in a 96% reduction in DA compared to intact striata (data not shown). L-DOPA restored stepping alone or when combined with citalopram or paroxetine (vehicle: F3,21 = 5.7, p < 0.05; citalopram 3 mg/kg: F3,21 = 8.0, p < 0.05; citalopram 5 mg/kg: F3,21 = 8.9, p < 0.05; paroxetine 0.5 mg/kg: F3,21 = 6.9, p < 0.05; paroxetine 1.25 mg/kg: F3,21 = 5.0, p < 0.05). Post-hoc analyses revealed that L-DOPA efficacy was maintained through the 3 week testing period.
Fig. 4.
Effects of prolonged selective 5-HT reuptake inhibitors citalopram and paroxetine on L-DOPA reversal of motor deficit in L-DOPA-naïve rats using the forepaw adjustment steps test. Baseline stepping deficits were established 3 weeks post-lesion. Rats then received daily treatment with vehicle, citalopram (3 or 5 mg/kg, s.c.), or paroxetine (0.5 or 1.25 mg/kg, s.c.) 30 min prior to L-DOPA (6 mg/kg, s.c.) + benserazide (15 mg/kg, s.c.) for 22 days. Forepaw adjusting steps were evaluated 60 min after treatments on days 3, 10, and 17. Values (as means + standard mean error; S.E.M.) are expressed as percent intact vs. intact forepaw. Significant differences were evaluated by 1-way ANOVAs for baseline stepping across groups and within each treatment condition. When appropriate, motor performance differences across test days were analyzed with Fisher LSD post-hocs. * p < 0.05 vs. Baseline, + p < 0.05 vs. Sham-Vehicle.
3.3. Experiment 3
3.3.1. The 5-HT1AR antagonist, WAY100635, partially reverses SSRI effects on LID
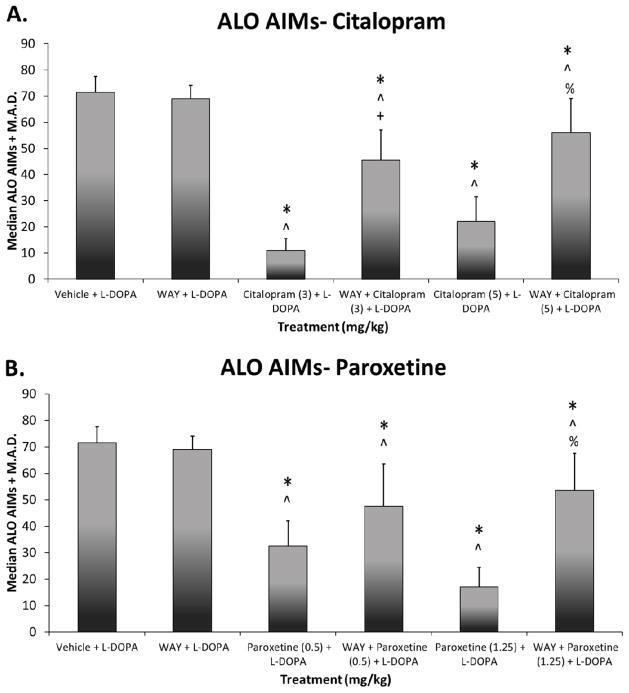
To investigate the role of 5-HT1A receptors in SSRIs’ anti-dyskinetic effects, the 5-HT1A receptor antagonist WAY100635 was employed in L-DOPA-primed hemi-parkinsonian rats. As shown in Figure 5, significant treatment effects were observed for citalopram (χ2 (5) = 48.8, p < 0.05) and paroxetine (χ2 (5) = 44.9, p < 0.05). In support of previous research, acute treatment with high and low doses of SSRIs effectively reduced AIMs expression (all p < 0.05). These anti-dyskinetic effects likely involved stimulation of 5-HT1A receptors as WAY100635 partially reversed citalopram and paroxetine effects.
Fig. 5.
Effects of the 5-HT1A receptor antagonist WAY100635 on the anti-dyskinetic effects of selective 5-HT reuptake inhibitors citalopram (A) and paroxetine (B). Rats were L-DOPA-primed for 14 days and ALO AIMs were recorded on days 1, 8, and 14. Rats were then treated with vehicle or WAY100635 (0.5, s.c.) 5 min before vehicle, citalopram (3 or 5 mg/kg, s.c.), or paroxetine (0.5 or 1.25 mg/kg, s.c.) which was given 30 min prior to L-DOPA (6 mg/kg, s.c.) + benserazide (15 mg/kg, s.c.) each test day. ALO AIMs were evaluated for 3 h after L-DOPA. Values are expressed as medians (AIMs + median absolute difference; M.A.D.). Significant differences were determined by non-parametric Friedman ANOVAs and Wilcoxon post-hocs. * p < 0.05 vs. Vehicle + L-DOPA, ^ p < 0.05 vs. WAY + L-DOPA, + p < 0.05 vs. Citalopram (3) or Paroxetine (0.5) + L-DOPA, % p < 0.05 vs. Citalopram (5) or Paroxetine (1.25) + L-DOPA.
4. Discussion
The current study provides strong preclinical evidence for prolonged SERT blockade as a viable therapeutic strategy for LID intervention and prevention as well as potential mechanisms for such actions. First, a 3 week administration of the SSRIs citalopram and paroxetine was shown to attenuate dyskinesia expression in L-DOPA-primed rats without interfering with L-DOPA’s therapeutic efficacy. Second, co-administration of SSRIs with L-DOPA commencement prevented the development of dyskinesia without modifying L-DOPA’s anti-parkinsonian effects. Third, neurochemical and pharmacological findings suggest that the effects of SSRIs were partially attributable to actions on 5-HT1A receptors and striatal DA.
A significant body of research has implicated the 5-HT system in the development and expression of LID (Carta et al., 2007; Eskow et al., 2009; Kannari et al., 2006; Navailles et al., 2010). Initial strategies targeted the 5-HT1 family of receptors to normalize exaggerated L-DOPA-derived DA release from 5-HT neurons (Bibbiani et al., 2001; Lindgren et al., 2010; Muñoz et al., 2009). Although such approaches have led to favorable clinical outcomes (Bara-Jiminez et al., 2005; Bonifati et al., 1994; Olanow et al., 2004), stimulation of 5-HT1A receptors at higher doses can also affect the anti-parkinsonian efficacy of L-DOPA (Goetz et al., 2007; Iravani et al., 2006; Kannari et al., 2001). Therefore, there exists a need for alternative strategies that target the serotonergic system. Recent evidence has suggested that SERT inhibition is a viable option as acute administration of SSRIs attenuate L-DOPA-induced side effects in hemi-parkinsonian rats (Bishop et al., 2012; Inden et al., 2012). However, the long-term efficacy of SERT inhibition on LID has yet to be systematically investigated and such findings would improve the potential translational value of compounds with such actions.
Therefore, the first goal of the current work was to examine whether daily co-administration of the SSRIs citalopram and paroxetine with L-DOPA to rats previously rendered dyskinetic would maintain positive interventional effects against AIMs expression. This was indeed the case. Both lower and higher doses of SSRIs immediately reduced AIMs by 70–80% and 80–90%, respectively, mirroring results from previous acute studies (Bishop et al., 2012). More importantly, these anti-dyskinetic effects were maintained throughout the 3 weeks of behavioral testing, indicating the potential for prolonged SSRI use as adjunctive therapy in PD patients with previously developed LID. Clinical studies directly testing anti-dyskinetic effects of SSRIs have been limited and these investigations have varied in approach. For instance, in L-DOPA responsive PD patients, fluoxetine was shown to reduce apomorphine-induced dyskinesia by nearly 50% (Durif et al., 1995). In contrast, Chung et al. (2005) found dyskinesia induced by intravenous L-DOPA was unaffected by short-term paroxetine. Clearly further clinical research is warranted.
In addition to interventional properties we also sought to establish the potential prophylactic effects of SERT blockade against LID in rats that were naïve to L-DOPA therapy. Under the present conditions, citalopram and paroxetine provided pronounced dose-dependent protection against the development of AIMs across the entire 3 weeks of treatment. Interestingly, given the immediate prophylactic actions of SSRIs, this would suggest that anti-dyskinetic effects are conveyed via short-term pharmacological actions (Yamato et al., 2001) that are not altered by the long-term plasticity often required for purported anti-depressant efficacy (Benmansour et al., 2002). Importantly, these effects were achieved by SSRI doses that produce antidepressant-like effects in the rat (Komorowski et al, 2012; Tuerke et al., 2009). Although humans and rats metabolize drugs differently, SSRI doses used to treat depression in humans may therefore also convey anti-dyskinetic effects. Therefore, one inadvertent and unexplored positive characteristic of SSRI treatment often prescribed for affective symptoms in early PD (Branchi et al., 2008; Eskow-Jaunarajs et al., 2011; Nilsson et al., 2001), may be an unexplored prophylaxis against LID development.
These preclinical behavioral studies strongly support SERT as a therapeutic target for the reduction and/or prevention of LID. However, the mechanism(s) by which the anti-dyskinetic effects are conveyed remains speculative. One leading candidate is indirect activation of the 5-HT1A receptor. Pharmacologically, acute SERT blockade is known to increase synaptic 5-HT (Bymaster et al., 2002; Perry and Fuller, 1992). In fact, at anti-dyskinetic doses, citalopram (5 mg/kg) has been shown to increase 5-HT levels and reduce 5-HT turnover in the dorsal raphe of hemi-parkinsonian rats (Bishop et al., 2012). Thus, SSRI-mediated increases in 5-HT may activate 5-HT1A somatodendritic autoreceptors thereby inhibiting raphe neuronal firing and 5-HT release (Blier et al., 1997; Casanovas et al., 1997; Malagie et al., 1995). In the parkinsonian brain, raphestriatal inhibition by SSRI-induced 5-HT may also regulate L-DOPA-derived DA release via 5-HT1A receptors leading to attenuated AIMs (Eskow et al., 2009; Yamato et al., 2001). In support of this, the 5-HT1A receptor antagonist WAY100635 did reverse the anti-dyskinetic effects of SSRIs, similar to previous findings with L-DOPA-induced rotations (Inden et al., 2012). However, the reversal was not complete, suggesting that other mechanisms likely contribute. One probable candidate is the 5-HT1B receptor, which act locally in the striatum rather than the raphe to modify DA release and LID (Carta et al., 2007; Jaunarajs et al., 2009; Lindgren et al., 2010). Thus, a unique feature of SERT inhibition may be indirect 5-HT1 stimulation through increased endogenous 5-HT tone resulting in the observed anti-dyskinetic efficacy. Whether the integrity of the raphe nuclei, which can be affected in PD (Halliday et al., 1990; Politis et al., 2010), modifies the effects of SERT blockade on LID remains an open question.
In the investigation of novel anti-dyskinetic agents, it is also important to consider interactions with anti-parkinsonian medications. Clinical studies of the motor effects of SSRI treatment in PD have yielded conflicting results where SSRIs have been shown to improve, worsen, or have no influence over L-DOPA’s anti-parkinsonian efficacy (Chung et al., 2005; Linazasoro 2000; Rampello et al., 2002). Our previous research demonstrated that acute administration of citalopram or paroxetine with L-DOPA did not interfere with L-DOPA-induced motor recovery (Bishop et al., 2012). Here, this was examined using prolonged regimens. In L-DOPA-primed rats, the reversal of motor deficit by L-DOPA was first observed on the 10th day of co-treatment with vehicle and low doses of citalopram and paroxetine. By day 17, all treatment groups displayed improved motor performance. By comparison, L-DOPA efficacy was observed on the first day of testing in L-DOPA-naïve rats regardless of SSRI dose and this was maintained over 3 weeks. Though adverse side effects have been reported in PD patients and rodent models treated with SSRIs no such observations were made in the present study (Linazasoro 2000; Speiser et al., 2008). From a translational point of view, that recurrently administered SSRIs not only reduced LID, but also maintained L-DOPA’s anti-parkinsonian efficacy is an attractive feature of this strategy. It also highlights a unique, but speculative, characteristic of SERT blockade in the PD brain; whereby inhibition of SERT in the absence of DAT may reduce the uptake of L-DOPA-derived DA back into 5-HT cells. Generally, this has been supported by work suggesting that there is a great deal of promiscuity between monoamine transporters (Daws, 2009; Zhou et al., 2005). In particular, SERT has been shown to be capable of clearing extracellular DA (Larsen et al., 2011) and such a mechanism may be particularly important in the DAT deficient striatum. For example, Kannari et al. (2006) demonstrated that striatal SERT blockade with fluoxetine increased local L-DOPA-derived DA. Thus, we were interested in how prolonged systemic SSRI administration would alter striatal DA tissue content in L-DOPA-primed rats. Not surprisingly, striatal DA was significantly depleted as a result of 6-OHDA lesion. However, rats co-treated with SSRIs and L-DOPA also displayed significantly elevated striatal DA content. While the observed increase was still well below intact striatal DA levels, it may reflect augmented extracellular DA levels that maintained L-DOPA efficacy while concomitantly suppressing LID (Pavese et al., 2006). How non-DA transporters in the parkinsonian brain modify DA neurotransmission has yet to be fully explored, but could be a promising mechanism for novel treatment approaches.
Overall, we show that prolonged treatment with FDA-approved SSRIs disrupts the establishment and development of L-DOPA-induced AIMs. The anti-dyskinetic effects of SSRIs are partially mediated through activation of the inhibitory 5-HT1A receptor; however the nature of this activation is unknown. Prolonged SSRI treatment also maintains L-DOPA’s anti-parkinsonian efficacy throughout the treatment period. This may be conveyed by treatment-induced increases in striatal DA by SERT blockade after L-DOPA administration. Although several questions remain regarding the neurobiological articulation of the reported effects, the current study implicates a novel role for SERT inhibition for the improved use of L-DOPA therapy in PD.
Chronic SSRI treatment disrupts L-DOPA-induced AIMs expression and development.
These anti-dyskinetic effects are partially mediated by 5-HT1A receptor activation.
Chronic SSRI treatment maintains L-DOPA’s anti-parkinsonian efficacy.
L-DOPA maintenance may be due to SSRI-induced striatal dopamine increase.
Acknowledgments
This work was supported by NIH NS059600, the Michael J. Fox Foundation and the Center for Development and Behavioral Neuroscience at Binghamton University.
Abbreviations
- DA
Dopamine
- PD
Parkinson’s disease
- AIMs
Abnormal involuntary movements
- LID
L-DOPA-induced dyskinesia
- 5-HT
Serotonin
- SERT
Serotonin transporter
- SSRI
Selective 5-HT reuptake inhibitor
- DAT
Dopamine transporter
- HPLC
High performance liquid chromatography
- 6-OHDA
6-hydroxydopamine
- MFB
Medial forebrain bundle
- NE
Norepinephrine
- Benserazide
DL-serine 2-(2,3,4-trihydroxybenzyl) hydrazine hydrochloride
- FAS
Forepaw adjusting steps test
- DMSO
Dimethyl sulfoxide
- WAY100635
N-[2-[4-(2-Methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate salt
- ALO
Axial, limb and orolingual
- DOPAC
3,4-dihydroxyphenylacetic acid
- 5-HIAA
5-hydroxyindoleacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Melissa M. Conti, Email: mconti2@binghamton.edu.
Corinne Y. Ostock, Email: costock1@binghamton.edu.
David Lindenbach, Email: dlinden1@binghamton.edu.
Adam A. Goldenberg, Email: agolden1@binghamton.edu.
Elias Kampton, Email: ekampto1@binghamton.edu.
Rich Dell’isola, Email: rdelliso@binghamton.edu.
Aaron C. Katzman, Email: akatzma1@binghamton.edu.
Christopher Bishop, Email: cbishop@binghamton.edu.
References
- Arai R, Karasawa N, Geffard M, Nagatsu I. L-DOPA is converted to dopamine in serotonergic fibers of the striatum of the rat: a double-labeling immunofluoresence study. Neurosci Lett. 1995;195:195–198. doi: 10.1016/0304-3940(95)11817-g. [DOI] [PubMed] [Google Scholar]
- Bara-Jiminez W, Bibbiani F, Morris M, Dimitrova T, Sherzai A, Mouradian M, Chase T. Effects of serotonin 5-HT1A agonist in advanced Parkinson’s disease. Mov Disord. 2005;20:932–936. doi: 10.1002/mds.20370. [DOI] [PubMed] [Google Scholar]
- Benmansour S, Owens W, Cecchi M, Morilak D, Frazer A. Serotonin clearance in vivo is altered to a greater extent by antidepressant-induced downregulation of the serotonin transporter than by acute blockade of this transporter. J Neurosci. 2002;22:6766–6772. doi: 10.1523/JNEUROSCI.22-15-06766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibbiani F, Oh J, Chase T. Serotonin 5-HT1A agonist improves motor complications in rodent and primate parkinsonian models. Neurol. 2001;57:1829–1834. doi: 10.1212/wnl.57.10.1829. [DOI] [PubMed] [Google Scholar]
- Bishop C, George J, Buchta W, Goldenberg A, Mohamed M, Dickinson S, Eissa S, Eskow Jaunarajs K. Serotonin transporter inhibition attenuates l-DOPA-induced dyskinesia without compromising l-DOPA efficacy in hemi-parkinsonian rats. Eur J Neurosci. 2012;36:2839–2848. doi: 10.1111/j.1460-9568.2012.08202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, Bergeron R, de Montigny C. Selective activation of postsynaptic 5-HT1A receptors induces rapid antidepressant response. Neuropsychopharmacol. 1997;16:333–338. doi: 10.1016/S0893-133X(96)00242-4. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Fabrizio E, Cipriani R, Vanacore N, Meco G. Buspirone in levodopa-induced dyskinesias. Clin Neuropharmacol. 1994;17:73–82. doi: 10.1097/00002826-199402000-00008. [DOI] [PubMed] [Google Scholar]
- Branchi I, D’Andrea I, Armida M, Cassano T, Pèzzola A, Potenza R, Morgese M, Popoli P, Alleva E. Nonmotor symptoms in Parkinson’s disease: investigating early-phase onset of behavioral dysfunction in the 6-hydroxydopamine-lesioned rat model. J Neurosci Res. 2008;86:2050–2061. doi: 10.1002/jnr.21642. [DOI] [PubMed] [Google Scholar]
- Brocco M, Dekeyne A, Veiga S, Girardon S, Millan M. Induction of hyperlocomotion in mice exposed to a novel environment by inhibition of serotonin reuptake; a pharmacological characterization of diverse classes of antidepressant agents. Pharmacol, Biochem Behav. 2002;71:667–680. doi: 10.1016/s0091-3057(01)00701-8. [DOI] [PubMed] [Google Scholar]
- Bymaster F, Zhang W, Carter P, Shaw J, Chernet E, Phebus L, Wong D, Perry K. Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacol. 2002;160:353–361. doi: 10.1007/s00213-001-0986-x. [DOI] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Björklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- Casanovas J, Lesourd M, Artigas F. The effect of the selective 5-HT1A agonists alnespirone (S-20499) and 8-OH-DPAT on extracellular 5-hydroxytryptamine in different regions of rat brain. Br J Pharmacol. 1997;122:733–741. doi: 10.1038/sj.bjp.0701420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JW, Wachtel SR, Young D, Kang UJ. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson’s disease: studies on medial forebrain bundle and striatal lesions. Neurosci. 1999;88:617–628. doi: 10.1016/s0306-4522(98)00217-6. [DOI] [PubMed] [Google Scholar]
- Chung KA, Carlson NE, Nutt JG. Short-term paroxetine treatment does not alter the motor response to levodopa in PD. Neurol. 2005;64:1797–1798. doi: 10.1212/01.WNL.0000161841.41885.80. [DOI] [PubMed] [Google Scholar]
- Daws L. Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther. 2009;121:89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente-Fernández R, Schulzer M, Mak E, Calne DB, Stoessl A. Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson’s disease: implications for dyskinesias. Brain. 2004;127:2747–2754. doi: 10.1093/brain/awh290. [DOI] [PubMed] [Google Scholar]
- Dupre K, Ostock C, Eskow Jaunarajs K, Button T, Savage L, Wolf W, Bishop C. Local modulation of striatal glutamate efflux by serotonin 1A receptor stimulation in dyskinetic, hemiparkinsonian rats. Exp Neurol. 2011;229:288–299. doi: 10.1016/j.expneurol.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durif F, Vidailhet M, Bonnet A, Blin J, Agid Y. Levodopa-induced dyskinesias are improved by fluoxetine. Neurol. 1995;45:1855–1858. doi: 10.1212/wnl.45.10.1855. [DOI] [PubMed] [Google Scholar]
- Eskow-Jaunarajs K, Angoa-Perez M, Kuhn D, Bishop C. Potential mechanisms underlying anxiety and depression in Parkinson’s disease: consequences of l-DOPA treatment. Neurosci Biobehav Rev. 2011;35:556–64. doi: 10.1016/j.neubiorev.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskow KL, Dupre KB, Barnum CJ, Dickinson SO, Park JY, Bishop C. The role of the dorsal raphe nucleus in the development, expression, and treatment of L-dopa-induced dyskinesia in hemiparkinsonian rats. Synap. 2009;63:610–620. doi: 10.1002/syn.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskow K, Gupta V, Alam S, Park J, Bishop C. The partial 5-HT1A agonist buspirone reduces the expression and development of L-DOPA-induced dyskinesia in rats and improves L-DOPA efficacy. Pharmacol Biochem Behav. 2007;87:306–314. doi: 10.1016/j.pbb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Goetz C, Damier P, Hicking C, Laska E, Müller T, Olanow C, Rascol O, Russ H. Sarizotan as a treatment for dyskinesias in Parkinson’s disease: a double-blind placebo-controlled trial. Mov Disord. 2007;22:179–186. doi: 10.1002/mds.21226. [DOI] [PubMed] [Google Scholar]
- Halliday G, Li Y, Blumbergs P, Joh T, Cotton R, Howe P, Blessing W, Geffen L. Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease. Ann Neurol. 1990;27:373–385. doi: 10.1002/ana.410270405. [DOI] [PubMed] [Google Scholar]
- Inden M, Abe M, Minamino H, Takata K, Yoshimoto K, Tooyama I, Kitamura Y. Effect of selective serotonin reuptake inhibitors via 5-HT1A receptors on L-DOPA-induced rotational behavior in a hemiparkinsonian rat model. J Pharmacol Sci. 2012;119:10–19. doi: 10.1254/jphs.12003fp. [DOI] [PubMed] [Google Scholar]
- Iravani MM, Tayarani-Binazir K, Chu WB, Jackson MJ, Jenner P. In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates, the selective 5-hydroxytryptamine 1a agonist (R)-(+)-8-OHDPAT inhibits levodopa-induced dyskinesia but only with increased motor disability. J Pharmacol Exp Ther. 2006;319:1225–1234. doi: 10.1124/jpet.106.110429. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Motor fluctuations and dyskinesias in Parkinson’s disease: clinical manifestations. Mov Disord. 2005;20:S11–S16. doi: 10.1002/mds.20458. [DOI] [PubMed] [Google Scholar]
- Jaunarajs K, Dupre K, Steiniger A, Klioueva A, Moore A, Kelly C, Bishop C. Serotonin 1B receptor stimulation reduces D1 receptor agonist-induced dyskinesia. NeuroReport. 2009;20:1265–1269. doi: 10.1097/WNR.0b013e3283300fd7. [DOI] [PubMed] [Google Scholar]
- Kannari K, Shen H, Arai A, Tomiyama M, Baba M. Reuptake of L-DOPA-derived extracellular dopamine in the striatum with dopaminergic denervation via serotonin transporters. Neurosci Lett. 2006;402:62–65. doi: 10.1016/j.neulet.2006.03.059. [DOI] [PubMed] [Google Scholar]
- Kannari K, Yamato H, Shen H, Tomiyama M, Toshihiro S, Matsunaga M. Activation of 5-HT1A but not 5-HT1B receptors attenuates an increase in extracellular dopamine derived from exogenously administered L-DOPA in the striatum with nigrostriatal denervation. J Neurochem. 2001;76:1346–1353. doi: 10.1046/j.1471-4159.2001.00184.x. [DOI] [PubMed] [Google Scholar]
- Kilpatrick IC, Jones MW, Phillipson OT. A semiautomated analysis method for catecholamines, indoleamines, and some prominent metabolites in microdissected regions of the nervous system: an isocratic HPLC technique employing coulometric detection and minimal sample preparation. J Neurochem. 1986;46:1865–1876. doi: 10.1111/j.1471-4159.1986.tb08506.x. [DOI] [PubMed] [Google Scholar]
- Komorowski M, Huston J, Klingenhegel I, Paulat J, Sackers J, Topic B. Distance from source of reward as a marker for extinction-induced “despair”: modulation by the antidepressants clomipramine and citalopram. Neurosci. 2012;223:152–162. doi: 10.1016/j.neuroscience.2012.07.064. [DOI] [PubMed] [Google Scholar]
- Kuan WL, Zhao JW, Barker RA. The role of anxiety in the development of levodopa-induced dyskinesias in an animal model of Parkinson’s disease, and the effect of chronic treatment with the selective serotonin reuptake inhibitor citalopram. Psychopharmacol (Berl) 2008;197:279–293. doi: 10.1007/s00213-007-1030-6. [DOI] [PubMed] [Google Scholar]
- Larsen M, Sonders M, Mortensen O, Larson G, Zahniser N, Amara S. Dopamine transport by the serotonin transporter: a mechanistically distinct mode of substrate translocation. J Neurosci. 2011;31:6605–6615. doi: 10.1523/JNEUROSCI.0576-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linazasoro G. Worsening of Parkinson’s disease by citalopram. Park Relat Disord. 2000;6:111–113. doi: 10.1016/s1353-8020(99)00050-4. [DOI] [PubMed] [Google Scholar]
- Lindgren H, Andersson D, Lagerkvist S, Nissbrandt H, Cenci M. L-DOPA-induced dopamine efflux in the striatum and the substantia nigra in a rat model of Parkinson’s disease: temporal and quantitative relationship to the expression of dyskinesia. J Neurochem. 2010;112:1465–1476. doi: 10.1111/j.1471-4159.2009.06556.x. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci. 2002;15:120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- Malagié I, Trillat A, Jacquot C, Gardier A. Effects of acute fluoxetine on extracellular serotonin levels in the raphe: an in vivo microdialysis study. Eur J Pharmacol. 1995;286:213–217. doi: 10.1016/0014-2999(95)00573-4. [DOI] [PubMed] [Google Scholar]
- Muñoz A, Carlsson T, Tronci E, Kirik D, Björklund A, Carta M. Serotonin neuron-dependent and –independent reduction of dyskinesia by 5-HT1A and 5-HT1B receptor agonists in the rat Parkinson model. Exp Neurol. 2009;219:298–307. doi: 10.1016/j.expneurol.2009.05.033. [DOI] [PubMed] [Google Scholar]
- Navailles S, Bioulac B, Gross C, De Deurwaerdère P. Serotonergic neurons mediate ectopic release of dopamine induced by L-DOPA in a rat model of Parkinson’s disease. Neurobio Disord. 2010;38:136–143. doi: 10.1016/j.nbd.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Nilsson F, Kessing L, Bolwig T. Increased risk of developing Parkinson’s disease for patients with major affective disorder: a register study. Acta Psychiatr Scand. 2001;104:380–386. doi: 10.1034/j.1600-0447.2001.00372.x. [DOI] [PubMed] [Google Scholar]
- Olanow C, Damier P, Goetz C, Mueller T, Nutt J, Rascol O, Serbanescu A, Deckers F, Russ H. Multicenter, open-label, trial of Sarizotan in Parkinson disease patients with levodopa-induced dyskinesias (the SPLENDID study) Clin Neuropharmacol. 2004;27:58–62. doi: 10.1097/00002826-200403000-00003. [DOI] [PubMed] [Google Scholar]
- Pavese N, Evans A, Tai Y, Hotton G, Brooks D, Lees A, Piccini P. Clinical correlates of levodopa-induced dopamine release in Parkinson disease: a PET study. Neurol. 2006;67:1612–1617. doi: 10.1212/01.wnl.0000242888.30755.5d. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson W. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Perry K, Fuller R. Effect of fluoxetine on serotonin and dopamine concentration in microdialysis fluid from rat striatum. Life Sci. 1992;50:1683–1690. doi: 10.1016/0024-3205(92)90423-m. [DOI] [PubMed] [Google Scholar]
- Politis M, Wu K, Loane C, Turkheimer F, Molloy S, Brooks D, Piccini P. Depressive symptoms in PD correlate with higher 5-HTT binding in raphe and limbic structures. Neurol. 2010;75:1920–1927. doi: 10.1212/WNL.0b013e3181feb2ab. [DOI] [PubMed] [Google Scholar]
- Putterman DB, Munhall AC, Kozell LB, Belknap JK, Johnson SW. Evaluation of levodopa dose and magnitude of dopamine depletion as risk factors for levodopa-induced dyskinesia in a rat model of Parkinson’s disease. J Pharmacol Exp Ther. 2007;323:277–284. doi: 10.1124/jpet.107.126219. [DOI] [PubMed] [Google Scholar]
- Rampello L, Chiechia S, Raffaele R, Vecchio I, Nicoletti F. The SSRI, citalopram, improves bradykinesia in patients with Parkinson’s disease treated with L-Dopa. Clin Neuropharmacol. 2002;25:21–24. doi: 10.1097/00002826-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Rylander D, Parent M, O’Sullivan SS, Dovero S, Lees AJ, Bezard E, Descarries L, Cenci MA. Maladaptive plasticity of serotonin axon terminals in levodopa-induced dyskinesia. Ann Neurol. 2010;68:619–628. doi: 10.1002/ana.22097. [DOI] [PubMed] [Google Scholar]
- Speiser Z, Fine T, Litinetsky L, Eliash S, Blaugrund E, Cohen S. Differential behavioral syndrome evoked in the rats after multiple doses of SSRI fluoxetine with selective MAO inhibitors rasagiline or selegiline. J Neural Transm. 2008;115:107–116. doi: 10.1007/s00702-007-0811-8. [DOI] [PubMed] [Google Scholar]
- Taylor J, Bishop C, Walker P. Dopamine D1 and D2 receptor contributions to L-DOPA-induced dyskinesia in the dopamine-depleted rat. Pharmacol Biochem Behav. 2005;81:887–893. doi: 10.1016/j.pbb.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Tuerke K, Leri F, Parker L. Antidepressant-like effects of paroxetine are produced by lower doses than those with produce nausea. Pharmacol Biochem Behav. 2009;93:190–195. doi: 10.1016/j.pbb.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Yamato H, Kannari K, Shen H, Suda T, Matsunaga M. Fluoxetine reduces L-DOPA-derived extracellular DA in the 6-hydroxydopamine-lesioned rat striatum. Neuroreport. 2001;12:1123–1126. doi: 10.1097/00001756-200105080-00015. [DOI] [PubMed] [Google Scholar]
- Zhuo F, Lang Y, Salas R, Zhang L, de Biasi M, Dani J. Corelease of dopamine and serotonin from striatal dopamine terminals. Neuron. 2005;46:65–74. doi: 10.1016/j.neuron.2005.02.010. [DOI] [PubMed] [Google Scholar]