Abstract
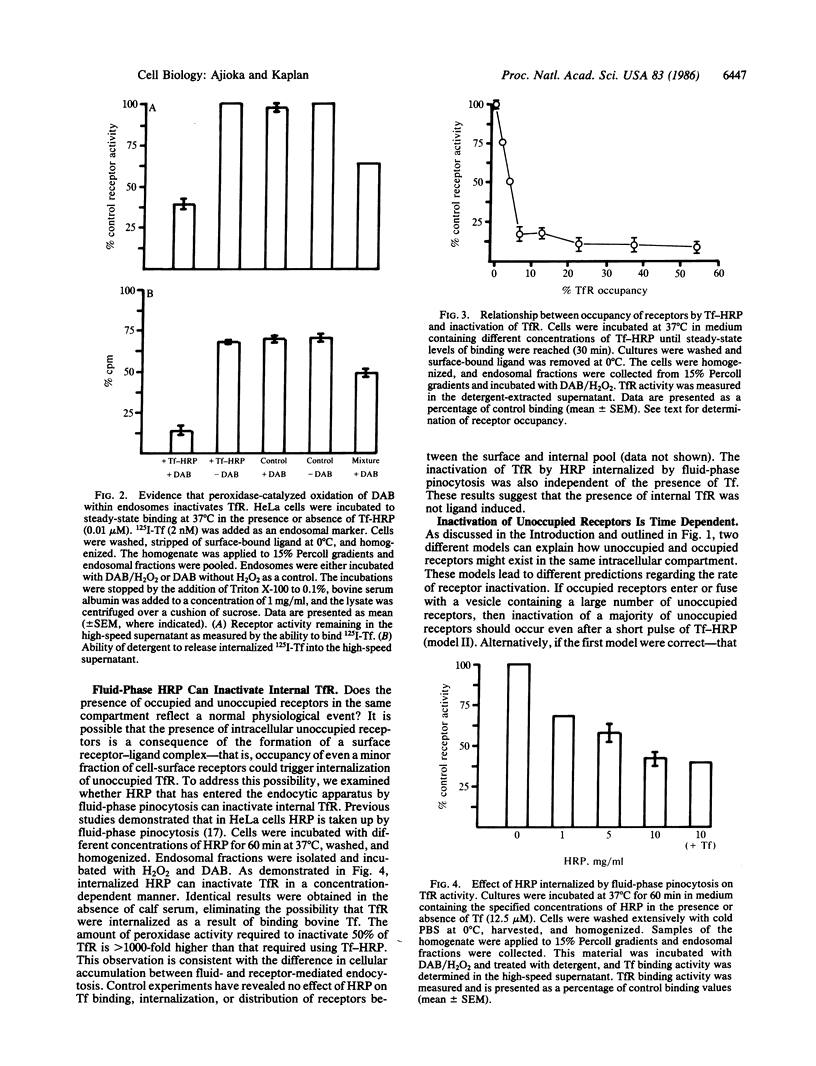
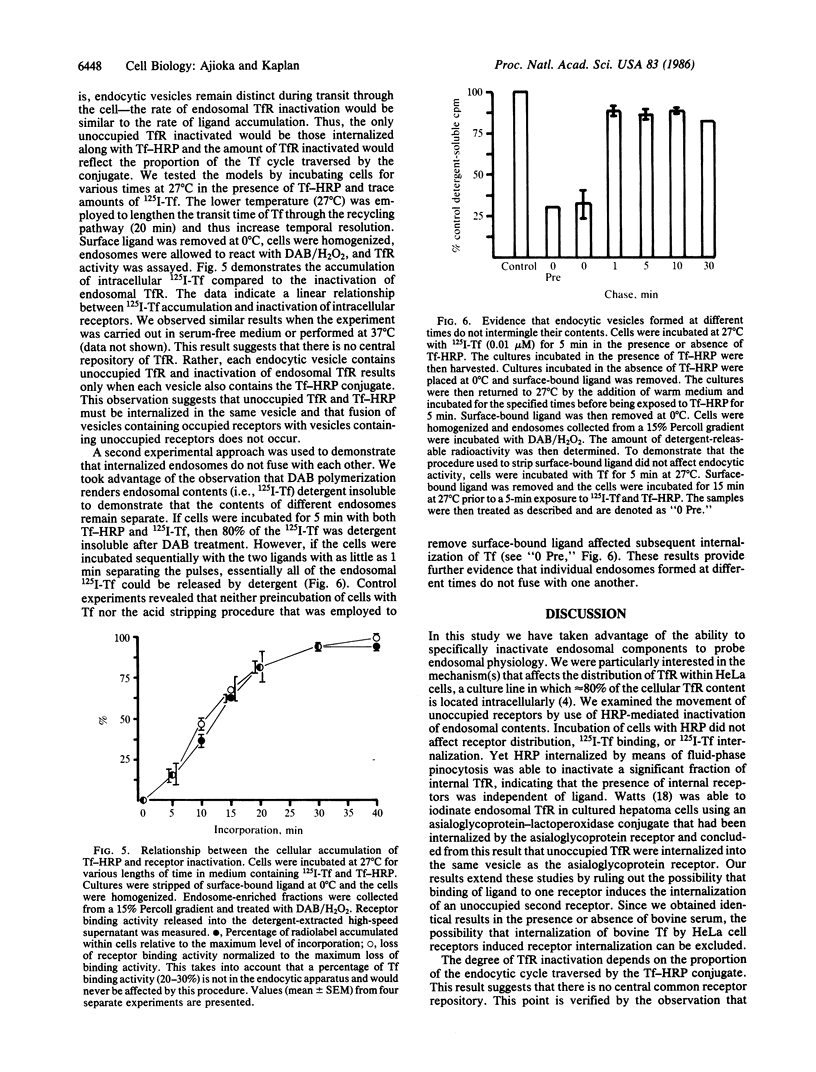
In HeLa cells the majority of transferrin (Tf) receptors are found within the endocytic apparatus, with only 20% of receptors exposed at the cell surface. Receptor distribution is unaltered by the presence or absence of Tf. The mechanism responsible for the cellular distribution of receptors was explored by selectively inactivating receptors within the endocytic apparatus. This was accomplished by employing Tf-horseradish peroxidase conjugates. Peroxidase-catalyzed oxidation of diaminobenzidine within an endosome destroys Tf receptor activity. Using such conjugates, we have demonstrated that the majority of internal Tf receptors could be inactivated when less than 6.0% of receptors were occupied by the conjugate at steady state. This result indicates that occupied and unoccupied receptors are in the same compartment. Furthermore, horseradish peroxidase that was internalized by fluid-phase pinocytosis inactivated intracellular Tf receptors in the absence of Tf; this indicates that the presence of internal receptors is ligand independent. Following exposure of cells to the conjugate, receptor inactivation was proportional to the percentage of the endocytic cycle traversed by the conjugate--that is, the rate of ligand accumulation was the same as the rate of endosomal Tf receptor inactivation. When the Tf-horseradish peroxidase conjugate and 125I-labeled Tf were internalized simultaneously, both ligands were found in the same compartment. However, if the two ligands were administered as separate pulses and the period between pulses was as short as 1 min, the ligands remained separate within the cell. Together these results demonstrate that the intracellular pool of Tf receptors reflects the constitutive internalization of unoccupied Tf receptors, which, once internalized, remain segregated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleil J. D., Bretscher M. S. Transferrin receptor and its recycling in HeLa cells. EMBO J. 1982;1(3):351–355. doi: 10.1002/j.1460-2075.1982.tb01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buys S. S., Keogh E. A., Kaplan J. Fusion of intracellular membrane pools with cell surfaces of macrophages stimulated by phorbol esters and calcium ionophores. Cell. 1984 Sep;38(2):569–576. doi: 10.1016/0092-8674(84)90511-7. [DOI] [PubMed] [Google Scholar]
- Courtoy P. J., Quintart J., Baudhuin P. Shift of equilibrium density induced by 3,3'-diaminobenzidine cytochemistry: a new procedure for the analysis and purification of peroxidase-containing organelles. J Cell Biol. 1984 Mar;98(3):870–876. doi: 10.1083/jcb.98.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch P. J., Rosen O. M., Rubin C. S. Identification and characterization of a latent pool of insulin receptors in 3T3-L1 adipocytes. J Biol Chem. 1982 May 25;257(10):5350–5358. [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Lodish H. F., Schwartz A. L. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983 Jan;32(1):277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- Hopkins C. R. Intracellular routing of transferrin and transferrin receptors in epidermoid carcinoma A431 cells. Cell. 1983 Nov;35(1):321–330. doi: 10.1016/0092-8674(83)90235-0. [DOI] [PubMed] [Google Scholar]
- Kaplan J. Cell contact induces an increase in pinocytotic rate in cultured epithelial cells. Nature. 1976 Oct 14;263(5578):596–597. doi: 10.1038/263596a0. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Harford J., van Renswoude J. Rapid internalization of the transferrin receptor in K562 cells is triggered by ligand binding or treatment with a phorbol ester. Proc Natl Acad Sci U S A. 1984 May;81(10):3005–3009. doi: 10.1073/pnas.81.10.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. E., Ray F., Ward J. H., Kushner J. P., Kaplan J. Internalization and subcellular localization of transferrin and transferrin receptors in HeLa cells. J Biol Chem. 1983 Jul 25;258(14):8751–8758. [PubMed] [Google Scholar]
- May W. S., Jacobs S., Cuatrecasas P. Association of phorbol ester-induced hyperphosphorylation and reversible regulation of transferrin membrane receptors in HL60 cells. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2016–2020. doi: 10.1073/pnas.81.7.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Pickart C. M., Jencks W. P. Energetics of the calcium-transporting ATPase. J Biol Chem. 1984 Feb 10;259(3):1629–1643. [PubMed] [Google Scholar]
- Snider M. D., Rogers O. C. Intracellular movement of cell surface receptors after endocytosis: resialylation of asialo-transferrin receptor in human erythroleukemia cells. J Cell Biol. 1985 Mar;100(3):826–834. doi: 10.1083/jcb.100.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P., Schlesinger P. H., Sigardson E., Rodman J. S., Lee Y. C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980 Jan;19(1):207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Pricer W. E., Jr, Ashwell G. Subcellular membrane topology and turnover of a rat hepatic binding protein specific for asialoglycoproteins. J Biol Chem. 1979 Feb 25;254(4):1038–1043. [PubMed] [Google Scholar]
- Vincent R., Nadeau D. A micromethod for the quantitation of cellular proteins in Percoll with the Coomassie brilliant blue dye-binding assay. Anal Biochem. 1983 Dec;135(2):355–362. doi: 10.1016/0003-2697(83)90696-6. [DOI] [PubMed] [Google Scholar]
- Wall D. A., Wilson G., Hubbard A. L. The galactose-specific recognition system of mammalian liver: the route of ligand internalization in rat hepatocytes. Cell. 1980 Aug;21(1):79–93. doi: 10.1016/0092-8674(80)90116-6. [DOI] [PubMed] [Google Scholar]
- Ward J. H., Kushner J. P., Kaplan J. Regulation of HeLa cell transferrin receptors. J Biol Chem. 1982 Sep 10;257(17):10317–10323. [PubMed] [Google Scholar]
- Watts C. In situ 125I-labelling of endosome proteins with lactoperoxidase conjugates. EMBO J. 1984 Sep;3(9):1965–1970. doi: 10.1002/j.1460-2075.1984.tb02077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C. Rapid endocytosis of the transferrin receptor in the absence of bound transferrin. J Cell Biol. 1985 Feb;100(2):633–637. doi: 10.1083/jcb.100.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel P. H., Oka J. A. The surface content of asialoglycoprotein receptors on isolated hepatocytes is reversibly modulated by changes in temperature. J Biol Chem. 1983 Apr 25;258(8):5089–5094. [PubMed] [Google Scholar]
- Wiley H. S., Cunningham D. D. The endocytotic rate constant. A cellular parameter for quantitating receptor-mediated endocytosis. J Biol Chem. 1982 Apr 25;257(8):4222–4229. [PubMed] [Google Scholar]
- Wiley H. S., Kaplan J. Epidermal growth factor rapidly induces a redistribution of transferrin receptor pools in human fibroblasts. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7456–7460. doi: 10.1073/pnas.81.23.7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Hanover J. A., Dickson R. B., Pastan I. Morphologic characterization of the pathway of transferrin endocytosis and recycling in human KB cells. Proc Natl Acad Sci U S A. 1984 Jan;81(1):175–179. doi: 10.1073/pnas.81.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]


