Abstract
Taurine is an endogenous ligand acting on glycine receptors in many brain regions, including the hippocampus, prefrontal cortex, and nucleus accumbens (nAcc). These areas also contain low concentrations of zinc, which is known to potentiate glycine receptor responses. Despite an increasing awareness of the role of the glycine receptor in the rewarding properties of drugs of abuse, the possible interactions of these compounds with zinc has not been thoroughly addressed. Two-electrode voltage-clamp electrophysiological experiments were performed on α1, α2 α1β and a2β glycine receptors expressed in Xenopus laevis oocytes. The effects of zinc alone, and zinc in combination with other positive modulators on the glycine receptor, were investigated when activated by the full agonist glycine versus the partial agonist taurine. Low concentrations of zinc enhanced responses of maximally-effective concentrations of taurine but not glycine. Likewise, chelation of zinc from buffers decreased responses of taurine- but not glycine-mediated currents. Potentiating concentrations of zinc decreased ethanol, isoflurane, and toluene enhancement of maximal taurine currents with no effects on maximal glycine currents. Our findings suggest that the concurrence of high concentrations of taurine and low concentrations of zinc attenuate the effects of additional modulators on the glycine receptor, and that these conditions are more representative of in vivo functioning than effects seen when these modulators are applied in isolation.
Keywords: Ethanol, Isoflurane, Toluene, Zinc, Allosteric modulation, Glycine receptor
1. Introduction
The glycine receptor (GlyR) is a member of the cys-loop receptor superfamily of ligand-gated ion channels. It has a pentameric structure composed of either α (homomeric) or α & β (heteromeric) subunits arranged around a central anion-conducting pore. GlyR are responsible for the majority of fast inhibitory neurotransmission in the brainstem and spinal cord, with γ–aminobutyric acid type A (GABAA) receptors primarily fulfilling this role in the brain. However, GlyR are also found in many brain regions including the hippocampus, nAcc and prefrontal cortex (Baer et al., 2009; Jonsson et al., 2012, 2009; Lu and Ye, 2011; Lynch, 2004; Malosio et al., 1991; van den Pol and Gorcs, 1988; Waldvogel et al., 2007). Because GlyR are found in brain regions associated with hypnosis, memory and the rewarding properties of drugs of abuse, it is reasonable to hypothesize that these receptors may play a role in the effects of these agents in vivo.
A wide variety of compounds act as allosteric modulators of the GlyR, including alcohols, inhaled drugs of abuse and anesthetics, neurosteroids, tropeines, divalent cations, and many others (Beckstead et al., 2000; Cheng and Kendig, 2002; Downie et al., 1996; Harvey et al., 1999; Laube et al., 1995; Molander et al., 2007, 2005; Yamashita et al., 2001; Yevenes and Zeilhofer, 2011). Thus, the GlyR has emerged as a logical target for the possible development of pharmacological agents to treat substance abuse (Tipps et al., 2010). Allosteric modulators exert their greatest enhancing effects on low concentrations of glycine that are unlikely to be seen at GlyR in vivo except at the initiation or tail-end of synaptic events or perhaps at extrasynaptic receptors (Scimemi and Beato, 2009). Indeed these compounds have negligible effects when tested with saturating concentrations of glycine (Kirson et al., 2012; McCracken et al., 2010).
The sulfonic acid taurine acts as a partial agonist at the GlyR, possessing approximately 50% of the efficacy of glycine (Lape et al., 2008). Taurine is the second most abundant amino acid in the brain, and has been implicated as an endogenous ligand of the GlyR in multiple brain regions (Albrecht and Schousboe, 2005; Dahchour et al., 1996; Ericson et al., 2006; Mori et al., 2002; Rodríguez-Navarro et al., 2009). Although glycine receptors are found synaptically in a variety of brain stem nuclei (Ferragamo et al., 1998; Lim et al., 2000) and in cerebellum (Dieudonne et al., 1995) they are also found extrasynaptically, where taurine and β-alanine may be acting as the endogenous agonists (Mori et al., 2002). Taurine may reach concentrations as high as 20 mM in astrocytes, from which it is released by osmoregulatory mechanisms (Albrecht and Schousboe, 2005). Extracellular taurine concentrations measured by microdialysis range from 1–100 μM but, by their nature, likely underestimate concentrations found locally around astrocytes.
Taurine plays a role in the effects of ethanol in the nAcc (Ericson et al., 2011). Although allosteric modulators have no effects when tested using saturating concentrations of full agonists at the GlyR, this is not true when saturating concentrations of partial agonists are tested (Albrecht and Schousboe, 2005; Kirson et al., 2012; Scimemi and Beato, 2009). In this paper we examined the possible interactions of these allosteric modulators with the ubiquitous GlyR modulator, zinc, on glycine- and taurine-activated GlyR.
Zinc modulation of the GlyR is biphasic in nature, with concentrations below 10 μM enhancing responses, while higher concentrations inhibit GlyR functioning (Harvey et al., 1999; Laube et al., 2000, 1995; McCracken et al., 2010). Zinc is present at low and variable levels in standard buffer solutions (Kay, 2004; McCracken et al., 2010), and is also present throughout the brain at low nM concentrations known to potentiate GlyR responses (Frederickson et al., 2006a, b; McCracken et al., 2010). Although zinc is packaged into some synaptic vesicles, even upon release concentrations remain below 10 μM (Frederickson et al., 2006a). Zinc modulation of the glycine-activated GlyR has been extensively studied but the interactions between zinc and the taurine-activated GlyR have not been characterized to the same extent, especially in conjunction with ethanol or other modulators. Previous studies of zinc modulation of taurine-activated GlyR responses focused on adding zinc at both enhancing and inhibiting concentrations, without controlling for the background zinc likely to be found at biologically-relevant concentrations (Laube et al., 2000). In this study we further characterize zinc modulation, as well as compare zinc’s interactions with other allosteric modulators on the glycine- vs. taurine-activated GlyR.
2. Materials and Methods
2.1 - Reagents
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) except isoflurane which was obtained from Anaquest (New Providence, NJ).
2.2 - Oocyte isolation and cDNA injection
Xenopus laevis were obtained from Nasco (Fort Atkinson, WI) and housed at 19°C on a 12-h light/dark cycle. During surgery, performed in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care regulations, portions of ovaries were removed and placed in isolation media containing 108 mM NaCl, 1 mM EDTA, 2 mM KCl, and 10 mM HEPES. Forceps were used to manually remove the thecal and epithelial layers from stage V and VI oocytes. The oocyte follicular layer was removed using a 10 min incubation in 0.5 mg/ml type 1A collagenase in buffer containing 83 mM NaCl, 2 mM MgCl2, and 5 mM HEPES. Animal poles of oocytes were injected with human glycine α1, α2 α1β, or α2β receptor subunit cDNAs (1.5 ng/30 nl) in a modified pBK-cytomegalovirus vector (Mihic et al., 1997), using a micropipette (10–15 μm tip size) attached to an electronically-activated microdispenser. When heteromeric receptors were to be studied, α and β subunit cDNAs were injected in a 1:20 α:β concentration ratio to ensure incorporation of the β subunits into receptors. Oocytes were stored in the dark at 19°C in 96-well plates containing modified Barth’s saline (MBS) [88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 10 mM HEPES, 0.82 mM MgSO4•7H2O, 0.33 mM Ca(NO3)2, 0.91 mM CaCl2 at pH 7.5] supplemented with 2 mM sodium pyruvate, 0.5 mM theophylline, 10 U/ml penicillin, 10 mg/l streptomycin and 50 mg/l gentamicin, and sterilized by passage through a 0.22 μm filter.
2.3 - Two-electrode voltage-clamp electrophysiology
Oocytes expressed GlyR within 24 h, and all electrophysiological measurements were made within 5 days of cDNA injection. Before electrophysiological recording, oocytes were placed in a 100 μl bath with the animal poles facing upwards and impaled with two high-resistance (0.5–10 MΩ) glass electrodes filled with 3 M KCl. Cells were voltage-clamped at −70 mV using an OC-725C oocyte clamp (Warner Instruments, Hamden, CT) and perfused with MBS at a rate of 2 ml/min using a Masterflex USA peristaltic pump (Cole Parmer Instrument Co., Vernon Hills, IL) through 18-gauge polyethylene tubing. All drug solutions were prepared in MBS, MBS + 2.5 mM tricine, or MBS + 100 nM ZnCl. When saturating concentrations of agonists were applied, applications lasted for 15 s for the short-application experiments and for 26 min for the continuous application experiments. For experiments using submaximal concentrations of taurine, concentrations that yielded 5 percent of the maximally-effective taurine response (EC5) were applied for 30 s. For short-application experiments, modulators were co-applied with agonists following a 30 s preincubation of modulator alone. For continuous application experiments, modulators were co-applied with agonist for 2 minutes following agonist application alone. All drug applications during short-application experiments were followed by six to ten min washout periods to allow for complete receptor resensitization. Loss of volatile compounds through tubing and evaporation from bath was previously measured (Beckstead et al., 2002, 2000; Mihic et al., 1994; Yamakura et al., 1999). All concentrations reported are the bath concentrations to which the oocytes were exposed. Data were acquired at a rate of 1 kHz using a Powerlab 4/30 digitizer with LabChart version 7 software (ADInstruments, Bella Vista, NSW, Australia).
2.4 - Data Analysis
Peak currents were measured and used in data analysis. Currents observed in the presence of agonist plus modulators were compared with currents generated by agonist without the modulator of interest present. Experimental values are listed as the mean ± S.E.M. Significant differences between experimental conditions were determined using ANOVA or repeated measures ANOVA and post hoc tests, as indicated. SigmaPlot version 11.0 (Systat Software, San Jose, CA) was used for statistical testing.
3. Results
3.1 - Chelation of endogenous zinc decreases responses to taurine
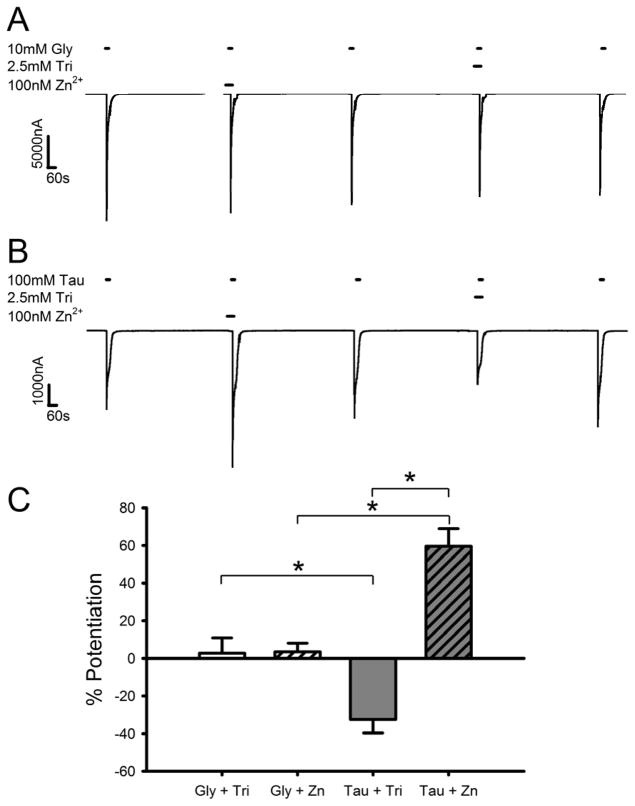
We previously showed that various allosteric modulators of GlyR, such as ethanol and some anesthetics, have different effects on currents generated by saturating concentrations of glycine versus taurine (Kirson et al., 2012). In order to compare zinc modulation of GlyR currents generated by maximally-effective concentrations of glycine and taurine, we compared currents generated by co-applications of agonist and either 100 nM zinc or 2.5 mM of the zinc chelator tricine with currents generated by agonist alone. Fig. 1A shows a sample tracing of successive 15 s applications of 10 mM glycine in the presence of a background concentration of zinc, 2.5 mM tricine, or 100 nM zinc. Fig. 1B shows the same experimental protocol as in 1A but with 100 mM taurine instead employed as the agonist. Addition of 100 nM zinc enhanced saturating taurine currents almost 60% (Fig. 1C) while leaving saturating glycine-mediated currents unchanged. Elimination of background levels of zinc in the perfusion buffer by co-application of tricine decreased saturating taurine currents while having no effects on saturating glycine currents. A two-way ANOVA showed a significant interaction effect between the concentration of zinc in the buffer and the agonist tested [F(1,37) = 34.4, p < 0.001]. A Student-Newman-Keuls (SNK) multiple comparison post-hoc test showed significant differences between glycine and taurine both in the presence of 2.5 mM tricine [q = 4.6, p < 0.01] and 100 nM zinc [q = 7.1, p < 0.001], as well as a significant effect of zinc concentration when taurine was the agonist [q = 12.3, p < 0.001].
Fig. 1.
Zn2+ affects currents elicited by maximally-effective concentrations of taurine but not glycine. A) Sample tracings showing the effect of a maximally-effective concentration of glycine applied in the presence or absence of Zn2+. The tracing shows 15 s co-applications of 10 mM glycine with either 2.5 mM tricine or 100 nM Zn2+ following a 30 s preincubation with tricine or Zn2+. Each of these applications was preceded and followed by applications of 10 mM glycine alone in buffer containing background levels of Zn2+. Horizontal bars over tracings indicate time of exposure to glycine, tricine, or Zn2+. Washouts 10 min in duration separated agonist applications. B) Sample tracing showing the effect of a maximally-effective concentration of taurine applied in the presence or absence of Zn2+. The tracing follows the same protocol as in panel A. Horizontal bars over tracing indicate time of exposure to taurine, tricine, or Zn2+. C) Summary of the effects of Zn2+ on brief applications of maximally-effective concentrations of glycine or taurine. The y-axis represents the percent current potentiation observed in the presence or absence of Zn2+ compared with that produced by glycine or taurine in background levels of Zn2+. Data are shown as mean ± S.E.M. of 9–11 oocytes. *, p < 0.05.
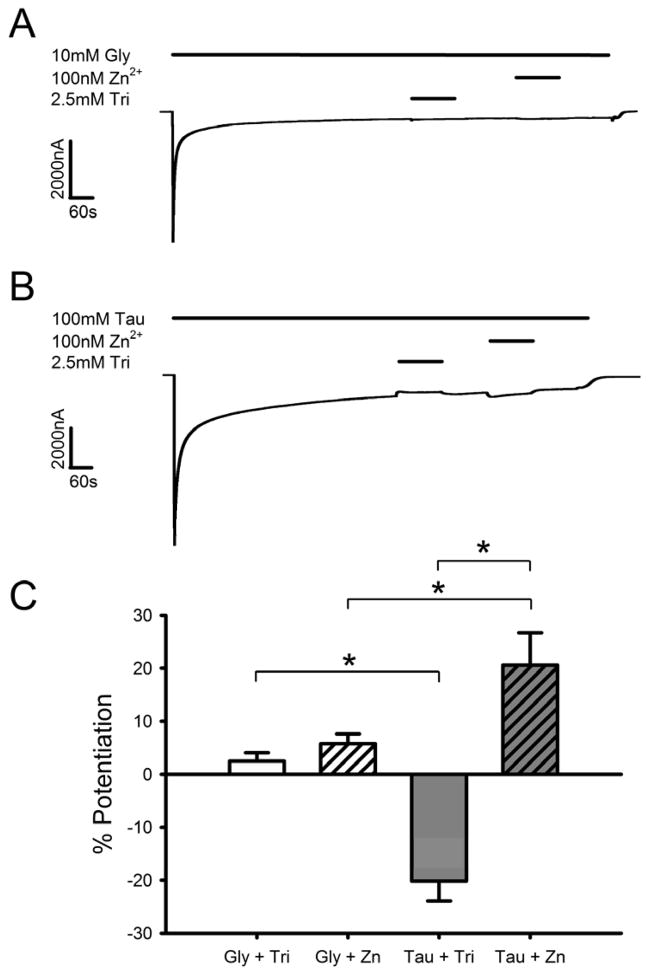
We next looked at the effects of zinc modulation using saturating agonist concentrations that were applied continuously for 10 min, followed by 2 min co-applications of agonist and either 2.5 mM tricine or 100 nM zinc. This continuous agonist application approach allows for channels to equilibrate between the open and desensitized states (Figs. 2A, B). Under these conditions all receptors have bound agonist and are either activated (opening) or desensitized. In this experimental paradigm the effects of modulators can thus only be due to their effects on channel opening/closing kinetics (Popen) or desensitization/resensitization rates and not on possible effects on agonist affinity. As seen in Fig. 2C, the effects exhibited by zinc trended in the same directions as those seen in Fig. 1C, but to a smaller degree. Co-application of 100 nM zinc produced greater potentiation of saturating taurine currents compared to saturating glycine currents. Co-application of tricine showed a decrease in saturating taurine responses compared to a negligible increase in saturating glycine. Similar to the short application experiments, a two-way ANOVA showed a significant interaction effect between the concentration of zinc in the buffer and the agonist tested [F(1,16) = 34.3, p < 0.001]. A SNK multiple comparison post-hoc test revealed significant differences between glycine and taurine both in the presence of 2.5 mM tricine [q = 7.1, p < 0.001] and 100 nM zinc [q = 4.6, p < 0.01], as well as a significant effect of zinc concentration when taurine was the agonist [q = 11.6, p < 0.001].
Fig. 2.
Zn2+ affects currents elicited by long exposures to maximally-effective concentrations of taurine but not glycine. A) Sample tracing showing the effects of 2.5 mM tricine or 100 nM Zn2+ co-applied with saturating concentrations of glycine after 10 min of continuous glycine application. Two min applications of 10 mM glycine in either 2.5 mM tricine or 100 nM Zn2+ were preceded and followed by 10 mM glycine in buffer containing background levels of Zn2+. Horizontal bars over the tracing indicate time of exposure to glycine, tricine, or Zn2+. B) Sample tracing showing the effects of 2.5 mM tricine or 100 nm Zn2+ co-applied with saturating concentrations of taurine, after 10 min of continuous taurine application. The tracing follows the same protocol as in panel A. Horizontal bars over the tracing indicate time of exposure to taurine, tricine, or Zn2+. C) Summary of the effects of maximally-effective concentrations of glycine or taurine in the presence or absence of Zn2+ during continuous agonist exposures. The y-axis represents the percent current enhancement observed in the presence or absence of Zn2+ compared with the glycine or taurine current level immediately preceding tricine or zinc co-application. Data are shown as mean ± S.E.M. of 4–6 oocytes. *, p < 0.05.
3.2 - Zinc and ethanol interactions
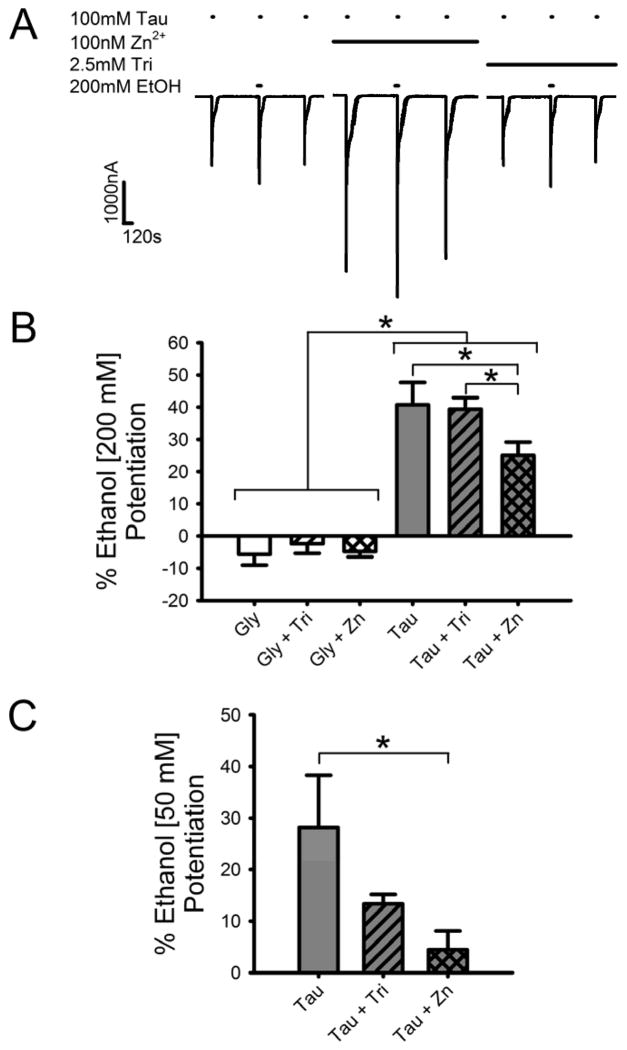
Physiologically-relevant low nM concentrations of zinc enhance ethanol modulation of GlyR currents generated by submaximal but not maximally-effective concentrations of glycine (McCracken et al., 2010). As these two modulators are likely to be present concurrently at GlyR in vivo, we compared the effects of enhancing concentrations of zinc on ethanol modulation of GlyR activated by maximally-effective glycine or taurine concentrations. We first looked at the effects of 15 s co-applications of maximally-effective concentrations of agonist and 200 mM ethanol in the presence of background levels of zinc and also in the presence of 2.5 mM tricine or 100 nM zinc. Fig. 3A shows that ethanol modulation of the maximally-effective taurine response is present in all three different zinc concentrations. However, 100 nM zinc significantly decreased [Two-way Repeated Measures (RM) ANOVA with SNK multiple comparison procedure; q = 5.0, p < 0.01; q = 4.6, p < 0.01, respectively] the degree of ethanol percent potentiation of a saturating taurine concentration compared to the potentiation seen in either the background zinc level or after zinc chelation. In background levels of zinc, or in the presence of 2.5 mM tricine, or 100 nM zinc, 200 mM ethanol produced significantly greater enhancement of responses in saturating taurine than glycine [q = 12.9, p < 0.001; q = 11.6, p < 0.001; q = 8.3, p < 0.001, respectively], as there was negligible inhibition of glycine currents observed instead (Fig. 3B). The same trends were observed using 50 mM ethanol, just with a lower degree of ethanol potentiation (Fig. 3C) [q = 3.85, p < 0.05, comparing taurine alone with taurine plus zinc].
Fig. 3.
Zn2+ affects ethanol potentiation of currents elicited by maximally-effective concentrations of taurine but not glycine. A) Sample tracings showing the effect of Zn2+ on brief applications of maximally-effective concentrations of taurine. Taurine (100 mM) was co-applied with 200 mM ethanol for 15 s following a 30 s preincubation with 200 mM ethanol. Ethanol applications were flanked by 15 s applications of maximally-effective taurine applied alone. Series of applications were carried out in buffer containing background levels of Zn2+, 2.5 mM tricine or 100 nM Zn2+. Horizontal bars over tracings indicate time of exposure to taurine, tricine, Zn2+ or ethanol. B) Summary of the effects of 200 mM ethanol on brief applications of maximally-effective concentrations of glycine or taurine in the presence of a background level of Zn2+, the absence of Zn2+ produced by tricine, or the addition of 100 nM Zn2+. The y-axis represents the percent current potentiation observed with ethanol co-application, in background Zn2+, 2.5 mM tricine or 100 nM Zn2+. Data are shown as mean ± S.E.M. of 6 oocytes. C) Summary of the effects of 50 mM ethanol on brief applications of maximally-effective concentrations of taurine in the presence of a background level of Zn2+, the absence of Zn2+ produced by tricine, or the addition of 100 nM Zn2+. The y-axis represents the percent current potentiation observed with ethanol co-application, in background Zn2+, 2.5 mM tricine or 100 nM Zn2+. Data are shown as mean ± S.E.M. of 8 oocytes. *, p < 0.05.
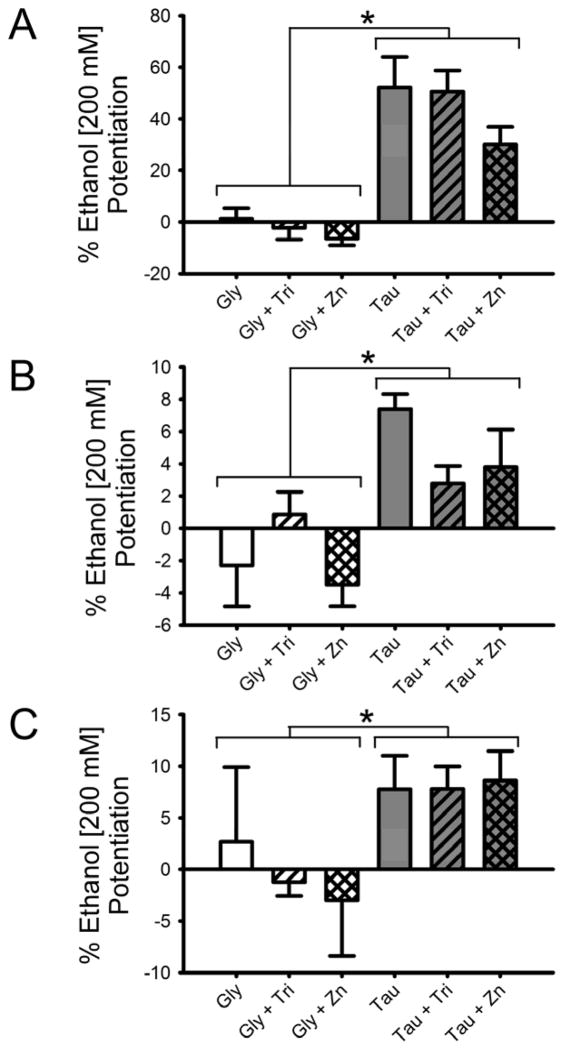
We next looked at the combined effects of zinc and ethanol on receptors comprised of different subunits. The heteromeric α1β GlyR is the predominant adult form found in brainstem and spinal cord of mammals and is likely to be found synaptically, as the β subunit is involved in anchoring the GlyR via its interactions with gephyrin (Kirsch and Betz, 1995). As shown in Fig. 4A, the effects of combined ethanol and zinc on the heteromeric channel exhibit the same trends as those seen on the homomeric channel but with slightly increased enhancement of taurine currents for the heteromeric channel. A Two-way RM ANOVA with SNK multiple comparison procedure showed ethanol potentiation of currents in 100 nM zinc significantly decreased from both background zinc [q = 3.8, p <0.05] and 2.5 mM tricine [q = 3.1, p < 0.05], as well as significantly greater ethanol percent potentiation of taurine vs glycine currents [q = 7.5, p = 0.001]. We also performed equivalent experiments on α2 homomeric (Fig. 4B) and α2β (Fig. 4C) heteromeric receptors. Two-way RM ANOVAs with SNK multiple comparison procedures showed significantly greater ethanol percent potentiation of taurine vs glycine currents [α2, q = 7.14, p < 0.01; α2β, q = 4.6, p < 0.05]. We found that taurine had very low efficacy on GlyR containing α2 subunits: 13.2 ± 5.4% in α2 homomers and 6.1 ± 1.4% in α2β heteromeric GlyR, compared to the effects produced by a saturating concentration of glycine.
Fig. 4.
Zinc/ethanol interactions on GlyR composed of a variety of different subunits. Summaries of the effects of ethanol on brief applications of maximally-effective concentrations of glycine or taurine in the presence of a background level of Zn2+, the absence of Zn2+ produced by tricine, or the addition of 100 nM Zn2+ on the α1β GlyR (A) α2 GlyR (B) or α2β GlyR (C). The y-axes represent the percent current potentiation observed with ethanol co-application, in background Zn2+, 2.5 mM tricine or 100 nM Zn2+. Data are shown as mean ± S.E.M. of 5–8 oocytes. *, p < 0.05.
3.3 - Zinc and isoflurane interactions
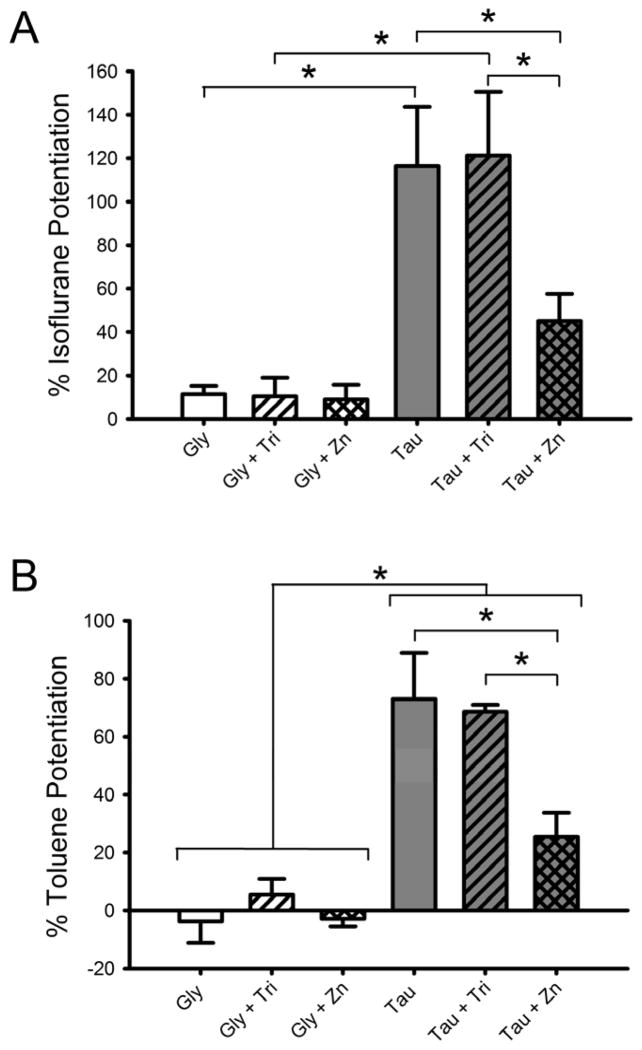
Because biologically-relevant concentrations of zinc affect ethanol potentiation of saturating taurine currents, we investigated other allosteric modulators of the GlyR for zinc-modulator interactions. The inhaled volatile anesthetic isoflurane was tested using the same experimental protocols as those used for ethanol. Fig. 5A shows the results of 15s co-applications of 0.55 mM isoflurane with either 10 mM glycine or 100 mM taurine in the presence of background levels of zinc, 2.5 mM tricine or 100 nM zinc. As for ethanol, isoflurane potentiation of saturating taurine currents in either background zinc or 2.5 mM tricine were very similar, while the addition of 100 nM zinc significantly decreased the isoflurane percent potentiation from those levels [Two-way RM ANOVA with SNK multiple comparison procedure; q = 4.1, p < 0.02; q = 4.4, p < 0.05, respectively]. Saturating glycine-mediated currents were minimally affected by isoflurane whether in background levels of zinc, 2.5 mM tricine, or 100 nM zinc. Isoflurane enhancement was significantly different between glycine and taurine in the presence of a background level of zinc [q = 7.4, p < 0.001] and in 2.5 mM tricine [q = 7.8, p < 0.001].
Fig. 5.
Zn2+ decreases isoflurane and toluene potentiation of currents elicited by maximally-effective concentrations of taurine but not glycine. These experiments were carried out in the same manner as the ethanol experiments described in Fig. 3. A) Effect of isoflurane on currents elicited by brief applications of maximally-effective glycine (10 mM) or taurine (100 mM) concentrations in the presence or absence of Zn2+. Agonist was co-applied with 0.55 mM isoflurane for 15 s following a 30 s preincubation with 0.55 mM isoflurane. Data are shown as mean + S.E.M. of 6 oocytes. B) Effect of toluene on currents elicited by brief applications of maximally-effective concentrations of glycine (10 mM) or taurine (100 mM) in the presence or absence of Zn2+. Agonist was co-applied with 0.42 mM toluene for 15 s following a 30 s preincubation with 0.42 mM toluene. Data are shown as mean ± S.E.M. of 4 oocytes. *, p < 0.05.
3.4 - Zinc and toluene interactions
The next allosteric modulator tested was the inhaled drug of abuse toluene. Fig. 5B shows the results of the brief co-applications of 0.42 mM toluene with either 10 mM glycine or 100 mM taurine in the presence of background levels of zinc, 2.5 mM tricine, or 100 nM zinc. Toluene had negligible effects on 10 mM glycine currents and its effects on 100 mM taurine currents were similar to effects seen with ethanol and isoflurane. Again, similar to ethanol and isoflurane, toluene percent potentiation of saturating taurine-mediated currents was significantly reduced in the presence of 100 nM zinc, compared to the other two zinc conditions [Two-way RM ANOVA with SNK multiple comparison procedure; q = 6.3, p < 0.002; q = 5.7, p < 0.002, respectively]. Toluene enhancement of saturating taurine was significantly greater than effects on saturating glycine in background levels of zinc [q = 8.8, p < 0.001], 2.5 mM tricine [q = 7.2, p < 0.001], and 100 nM zinc [q = 3.3, p < 0.05].
3.5 – Zinc & ethanol interactions at a low taurine concentration
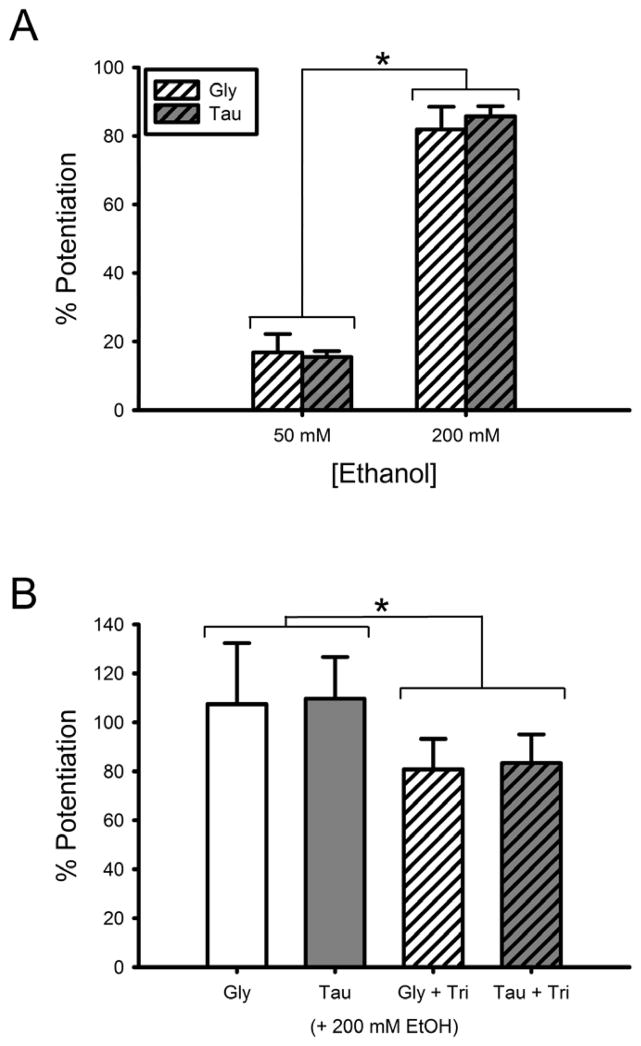
We previously found that zinc enhances ethanol potentiation of low concentrations of glycine, and that the degree of ethanol enhancement is reduced when zinc is chelated by tricine (McCracken et al., 2010). Although we saw minor ethanol effects with changing zinc levels at saturating concentrations of glycine, we did see changes in ethanol potentiation of saturating concentrations of taurine based on the level of zinc present (Fig. 2B). Thus we extended the observations made in the McCracken et al. (2010) paper, this time using EC5 concentrations of taurine. We first tested whether ethanol potentiation of EC5 taurine currents in α1 GlyR depends on the concentration of zinc. Fig. 6A shows that when zinc is chelated with tricine, the degree of ethanol potentiation of EC5 taurine-mediated currents is the same as EC5 glycine-mediated currents for both 50 mM and 200 mM ethanol. We next tested if chelation of zinc significantly reduces ethanol potentiation of EC5 taurine-mediated currents as it does for glycine-mediated currents. Fig 6B shows the results of 200 mM ethanol effects on EC5 glycine and taurine currents, with and without the addition of 2.5 mM tricine. With no differences between agonists, ethanol potentiation was significantly lower in 2.5 mM tricine compared to background levels of zinc [Two-way ANOVA with SNK multiple comparison procedure; q = 3.7, p < 0.05].
Fig. 6.
Zn2+ affects ethanol potentiation of low concentrations of full and partial agonists. A) Ethanol potentiation of the effects of 5% maximal (EC5) glycine- or taurine-mediated currents in the presence of 2.5 mM tricine (i.e., in the absence of Zn2+). EC5 glycine and taurine were co-applied with either 50 mM or 200 mM ethanol following a 30 s preincubation with ethanol. Data are shown as mean + S.E.M. of 5 oocytes. B) Ethanol potentiation of EC5 glycine and taurine is enhanced by Zn2+. EC5 glycine or taurine were co-applied with 200 mM ethanol following preincubation with ethanol, in the presence of either background Zn2+ (left two bars) or 2.5 mM tricine (right two bars). Data are shown as mean + S.E.M. of 9 oocytes. *, p < 0.05.
3.6 – Zinc/modulator interaction effects on desensitization rates
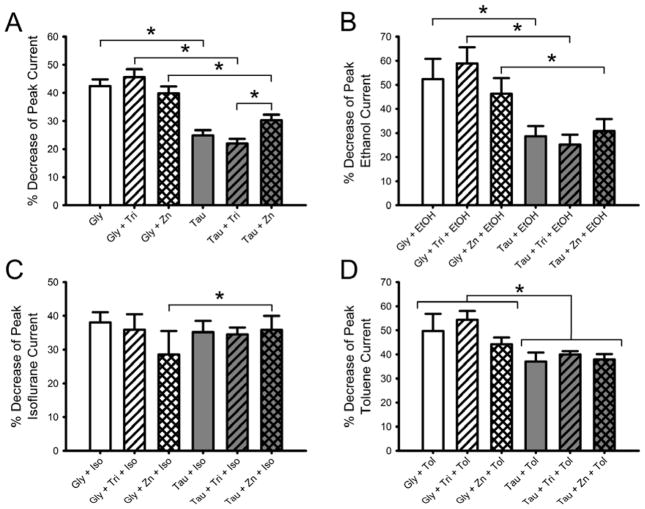
We next looked at the effects of changing zinc concentration on desensitization rates. Desensitization rates were determined by comparing the peak current to that measured five seconds post-peak for saturating glycine and taurine currents, in the presence of background levels of zinc or in the presence of 2.5 mM tricine or 100 nM zinc. Changing the zinc concentration did not affect the desensitization rates of glycine-activated currents in α1 GlyR (Fig. 7A). Taurine-mediated desensitization was significantly slower than for glycine-mediated currents in background zinc [Two-way ANOVA with SNK multiple comparison procedure; q = 8.5, p < 0.001], as well as in 2.5 mM tricine [q = 10.4, p < 0.001], and in 100 nM zinc [q = 4.2, p < 0.01]. Additionally, desensitization of taurine-mediated currents in 2.5 mM tricine and 100 nM zinc were significantly different [q = 3.7, p < 0.05].
Fig. 7.
Zn2+ levels do not significantly affect desensitization of glycine- or taurine-activated glycine receptors. A) Zn2+ does not affect the rate of desensitization produced by maximally-effective concentrations of glycine or taurine. Percent decrease in peak current observed 5 s post-peak is graphed for each agonist/Zn2+ level combination. Data are shown as mean + S.E.M. of 26–35 oocytes. B) Zn2+ and ethanol do not affect the rate of desensitization produced by maximally-effective concentrations of glycine or taurine. Percent decrease in peak current seen 5 s post-peak in the presence of 200 mM ethanol is graphed for each agonist/Zn2+ level combination. Data are shown as mean + S.E.M. of 6 oocytes. C) Zn2+ levels have no effect on isoflurane changes in desensitization rates of taurine-activated glycine receptor currents. Percent decrease in peak current observed 5 s post peak in the presence of 0.55 mM isoflurane is graphed for each agonist/Zn2+ level combination. Data are shown as mean + S.E.M. of 6 oocytes. D) Zn2+ and toluene do not interact to affect desensitization rates of maximally-effective concentrations of glycine and taurine. Percent decrease in peak current seen 5 s post-peak in the presence of 0.42 mM toluene is graphed for each agonist/Zn2+ level combination. Data are shown as mean + S.E.M. of 5 oocytes. *, p < 0.05.
Because drugs of abuse and zinc are likely to be found concomitantly at receptors in vivo, we examined the effects of different allosteric modulators on desensitization rates in the presence of background zinc, 2.5 mM tricine, or 100 nM zinc. Fig. 7B shows the desensitization rates produced by 10 mM glycine and 100 mM taurine applied with 200 mM ethanol in the three different zinc concentrations. Taurine + ethanol current desensitization was significantly slower than that seen in glycine + ethanol currents in background zinc [Two-way RM ANOVA with SNK multiple comparison procedure; q = 7.7, p < 0.001], 2.5 mM tricine [q = 11.0, p < 0.001], and 100 nM zinc [q = 5.1, p < 0.01]. Additionally, desensitization of glycine + ethanol currents in 2.5 mM tricine and 100 nM zinc were significantly different [q = 4.3, p < 0.05].
Fig. 7C shows the results for desensitization experiments involving isoflurane. Desensitization was similar for 10 mM glycine with 0.55 mM isoflurane currents and 100 mM taurine with 0.55 mM isoflurane currents, with the only significant difference found between glycine + isoflurane and taurine + isoflurane in 100 nM zinc buffer [Two-way RM ANOVA with SNK multiple comparison procedure; q = 3.5, p < 0.05]. Fig. 7D shows the effects of toluene on desensitization mediated by either glycine or taurine. There was a significant effect of agonist [Two-way RM ANOVA with SNK multiple comparison procedure; q = 4.2, p < 0.05].
4. Discussion
The dopaminergic projection from the ventral tegmental area (VTA) to the nAcc is critical to the perception of the rewarding properties of drugs of abuse, and ethanol’s actions on the GlyR may be particularly important in these brain regions. For example, extracellular dopamine levels increase in the nAcc following ethanol (Imperato and Di Chiara, 1986) or glycine (Molander et al., 2005) administration and glycine perfusion into the nAcc of rats decreases alcohol consumption (Molander et al., 2005). Many volatile agents such as isoflurane and toluene also have abuse liability (Johnston et al., 2012; Wilson et al., 2008). Toluene also increases extracellular dopamine levels in the VTA and nAcc when perfused into the former (Riegel et al., 2007). Most studies examining the effects of these agents on GlyR functioning do so by determining the effects of single modulators in isolation. However, since zinc is ubiquitous in cerebrospinal and interstitial fluids at GlyR-potentiating levels, the effects of drugs of abuse on GlyR in vivo will always be seen in combination with zinc effects. In fact one could go so far as to say that studying these compounds in the absence of zinc would represent an artificial situation unlikely to be found in vivo. Since taurine and glycine may act as GlyR agonists in brain regions such as the nAcc, we examined the effects of combinations of allosteric modulators on GlyR activated by these agonists. Maximally-effective concentrations of taurine but not glycine were affected by zinc chelation (Figs. 1 & 2). This is likely the result of glycine producing a very high probability of channel opening (intra-cluster Po ~ 1), with maximally-effective concentrations keeping the channel open almost 100% of the time before it desensitizes, regardless of the presence of any allosteric modulator. As a partial agonist, taurine has a much lower Po (~ 0.5) at maximally-effective concentrations (Lape et al., 2008), allowing for zinc to exhibit its enhancing effects. The zinc potentiation seen after 10 min of continuous taurine perfusion could be attributable to zinc: (1) increasing Po; (2) enhancing conductance; (3) decreasing the desensitization rate or; (4) increasing the rate of GlyR resensitization. Previous studies performed using low agonist concentrations show that zinc does increase Po but it does not affect conductance (Laube et al., 2000). Our data (Fig 7A) shows that zinc can also increase the desensitization rate in taurine-activated GlyR, which would tend to counteract its Po enhancing effects. In this way, zinc acts in a very similar fashion to other GlyR allosteric modulators (Kirson et al., 2012). The decrease in responses seen when zinc is chelated by tricine suggests that submaximal glycine responses and all taurine responses are overestimated when not controlling for background zinc concentrations in buffer solutions.
Because zinc is ubiquitous in the CNS, the effects of any drugs of abuse or other allosteric modulators of interest on GlyR functioning require comparison in the presence and absence of potentiating concentrations of zinc. In the presence of a maximally-effective concentration of glycine, changing the zinc concentration had no effect on responses for any of the modulators tested, since 10 mM glycine has already produced a maximal response. However, effects on maximally-effective concentrations of taurine were expected and seen. If zinc and other positive modulators were acting in an additive or synergistic manner, we would expect the 100 nM zinc condition to give a higher degree of potentiation than our standard buffers, with chelation of zinc by 2.5 mM tricine giving a lower degree of potentiation than both other conditions. However we did not find this to be the case for maximally-effective concentrations of taurine with alcohol, isoflurane, or toluene.
Chelation of zinc with tricine did not change the alcohol, isoflurane, or toluene potentiation of a maximally-effective taurine concentration when compared to the effects seen in the presence of background levels of zinc (Figs. 3B, 5A, & 5B). However, at submaximal concentrations of glycine or taurine, chelation of background zinc does decrease ethanol potentiation of GlyR responses (Fig. 6B). The most parsimonious explanation for the lack of change seen at higher levels of taurine would be that background levels of zinc during those particular experiments were low. However, the addition of 100 nM zinc to buffers, ensuring that the concentration of zinc is in the potentiating range, led to a significant reduction in the ethanol, isoflurane, and toluene potentiation of taurine-activated GlyR responses (Figs. 3B, 5A & 5B). This finding cannot be explained by simple competition between modulators as zinc is not believed to bind the receptor at the same locations as alcohols, anesthetics, and inhalants (Beckstead et al., 2000; Laube et al., 2000; Lynch et al., 1998; Mihic et al., 1997; Yamakura et al., 1999). However, zinc does increase the Po of the taurine-activated GlyR (Laube et al., 2000), in effect mimicking the glycine-bound GlyR. When this occurs and the GlyR Po approaches 1 any other positive modulator present will as a result produce decreased percent enhancement compared to when less zinc is present.
Since the α2 subunit appears to be expressed at higher levels than α1 in higher brain regions (Jonsson et al., 2012) we studied ethanol/zinc interactions on a variety of different GlyR subtypes activated by taurine vs glycine (Figs. 3B, 4A–C). The α2-containing receptors were previously shown to be less sensitive to ethanol (Mascia et al., 1996), and zinc (Miller et al., 2005), than those that contain α1 subunits and this was also reflected in our experiments using a maximally-effective concentration of taurine. We performed these experiments in the absence and presence of the β subunit which anchors the GlyR to gephyrin in the synapse (Kirsch and Betz, 1995). Badanich et al (2013) found that in the orbitofrontal cortex, where α2-containing GlyR are thought to predominate (Jonsson et al., 2012), there was no effect of the glycine receptor antagonist strychnine on neuronal holding current, but strychnine did antagonize ethanol-induced changes in holding current and spike firing. The most parsimonious explanation for these findings is that there are insufficient extracellular glycine or taurine concentrations to significantly affect holding current, unless ethanol is present. It is reasonable to hypothesize that ethanol promotes the release of glycine or taurine and it may also then enhance the effects of these compounds on the GlyR, although the Badanich et al. (2013) study does not address the relative importance of each phenomenon. If taurine is the responsible agonist then our findings raise two possible complicating issues. First, like Shmieden et al. (1992), we found that, relative to glycine, taurine has very low efficacy on α2-containing GlyR, much lower than its efficacy on α1-containing GlyR. Even at saturating concentrations, taurine could at best have only modest effects on α2 GlyR function. The next issue is that ethanol weakly enhances taurine-mediated currents in α2 and α2β GlyR. These findings are difficult to reconcile with the possibility that the effects seen in the Badanich et al. (2013) study are mediated by taurine on α2-containing receptors in the orbitofrontal cortex. Further studies addressing these issues are warranted.
5. Conclusions
In summary, low concentrations of zinc potentiated GlyR responses from maximally-effective concentrations of taurine but not glycine. Chelation of zinc reduced GlyR responses produced by maximally-effective concentration of taurine but not glycine, and thus any experiments conducted in the absence of zinc chelation will tend to overestimate taurine efficacy. This would also be true when submaximal glycine concentrations are used. At the low concentrations tested, zinc decreased the enhancing effect of taurine-mediated responses for all drugs of abuse tested when applied in combination with them. As taurine is likely to be an endogenous GlyR ligand in brain regions affected by these compounds, the presence of low concentrations of zinc in these regions results in GlyR responses that are not the result of a simple summation of responses to single modulators, and this needs to be taken into consideration when investigating the role of the GlyR in vivo. It is highly likely that previously published studies of this receptor have in reality been studying the zinc-modulated GlyR. To truly understand how allosteric modulators act at the GlyR one must examine their actions in the context of the physiological milieu at the receptor, reflecting in vivo conditions, as well as studying each modulator’s contribution in isolation.
Highlights.
The zinc-chelating agent tricine decreases the effects of a maximally-effective concentration of taurine, but not glycine, on glycine receptors.
Ethanol, isoflurane and the inhalant toluene enhance the effects of a maximally-effective concentration of taurine but not glycine.
Addition of 100 nM zinc decreases the magnitude of ethanol, isoflurane and toluene potentiation of taurine-activated glycine receptor responses.
Acknowledgments
This research was supported by National Institute on Alcohol Abuse & Alcoholism grant 5P01AA020683.
Abbreviations
- EC5
effective concentration producing 5% of maximal effect
- GlyR
glycine receptor
- MBS
Modified Barth’s Saline
- nAcc
nucleus accumbens
- SNK
Student-Newman-Keuls
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albrecht J, Schousboe A. Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem Res. 2005;30:1615–1621. doi: 10.1007/s11064-005-8986-6. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Mulholland PJ, Beckley JT, Trantham-Davidson H, Woodward JJ. Ethanol reduces neuronal excitability of lateral orbitofrontal cortex neurons via a glycine receptor dependent mechanism. Neuropsychopharmacol. 2013;38:1176–1188. doi: 10.1038/npp.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer K, Waldvogel HJ, Faull RLM, Rees MI. Localization of glycine receptors in the human forebrain, brainstem, and cervical spinal cord: an immunohistochemical review. Front Mol Neurosci. 2009;2:25. doi: 10.3389/neuro.02.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Phelan R, Trudell JR, Bianchini MJ, Mihic SJ. Anesthetic and ethanol effects on spontaneously opening glycine receptor channels. J Neurochem. 2002;82:1343–1351. doi: 10.1046/j.1471-4159.2002.01086.x. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Weiner JL, Eger EI, Gong DH, Mihic SJ. Glycine and gamma-aminobutyric acid(A) receptor function is enhanced by inhaled drugs of abuse. Mol Pharmacol. 2000;57:1199–1205. [PubMed] [Google Scholar]
- Cheng G, Kendig JJ. Pre- and postsynaptic volatile anaesthetic actions on glycinergic transmission to spinal cord motor neurons. Br J Pharmacol. 2002;136:673–684. doi: 10.1038/sj.bjp.0704760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, Quertemont E, De Witte P. Taurine increases in the nucleus accumbens microdialysate after acute ethanol administration to naive and chronically alcoholised rats. Brain Res. 1996;735:9–19. doi: 10.1016/0006-8993(96)00537-9. [DOI] [PubMed] [Google Scholar]
- Dieudonne S. Glycinergic synaptic currents in Golgi cells of the rat cerebellum. Proc Natl Acad Sci USA. 1995;92:1441–1445. doi: 10.1073/pnas.92.5.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie DL, Hall AC, Lieb WR, Franks NP. Effects of inhalational general anaesthetics on native glycine receptors in rat medullary neurones and recombinant glycine receptors in Xenopus oocytes. Br J Pharmacol. 1996;118:493–502. doi: 10.1111/j.1476-5381.1996.tb15430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M, Chau P, Clarke RB, Adermark L, Söderpalm B. Rising taurine and ethanol concentrations in nucleus accumbens interact to produce dopamine release after ethanol administration. Addict Biol. 2011;16:377–385. doi: 10.1111/j.1369-1600.2010.00245.x. [DOI] [PubMed] [Google Scholar]
- Ericson M, Molander A, Stomberg R, Söderpalm B. Taurine elevates dopamine levels in the rat nucleus accumbens; antagonism by strychnine. Eur J Neurosci. 2006;23:3225–3229. doi: 10.1111/j.1460-9568.2006.04868.x. [DOI] [PubMed] [Google Scholar]
- Ferragamo MJ, Golding NL, Oertel D. Synaptic inputs to stellate cells in the ventral cochlear nucleus. J Neurophysiol. 1998;79:51–63. doi: 10.1152/jn.1998.79.1.51. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Giblin LJ, Balaji RV, Masalha R, Ferederickson CJ, Zeng Y, Lopez EV, Koh JY, Chorin U, Besser L, Hershfinkel M, Li Y, Thompson RB, Krezel A. Synaptic release of zinc from brain slices: factors governing release, imaging, and accurate calculation of concentration. J Neurosci Methods. 2006a;154:19–29. doi: 10.1016/j.jneumeth.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Giblin LJ, Krezel A, McAdoo DJ, Mueller RN, Zeng Y, Balaji RV, Masalha R, Thompson RB, Fierke CA, Sarvey JM, de Valdenebro M, Prough DS, Zornow MH. Concentrations of extracellular free zinc (pZn)e in the central nervous system during simple anesthetization, ischemia and reperfusion. Exp Neurol. 2006b;198:285–293. doi: 10.1016/j.expneurol.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Harvey RJ, Thomas P, James CH, Wilderspin A, Smart TG. Identification of an inhibitory Zn2+ binding site on the human glycine receptor alpha1 subunit. J Physiol. 1999;520:53–64. doi: 10.1111/j.1469-7793.1999.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future national survey results on drug use, 1975–2011: Volume I, secondary school students. Ann Arbor: Institute for Social Research, The University of Michigan; 2012. [Google Scholar]
- Jonsson S, Kerekes N, Hyytiä P, Ericson M, Söderpalm B. Glycine receptor expression in the forebrain of male AA/ANA rats. Brain Res. 2009;1305:S27–S36. doi: 10.1016/j.brainres.2009.09.053. [DOI] [PubMed] [Google Scholar]
- Jonsson S, Morud J, Pickering C, Adermark L, Ericson M, Söderpalm B. Changes in glycine receptor subunit expression in forebrain regions of the Wistar rat over development. Brain Res. 2012;1446:12–21. doi: 10.1016/j.brainres.2012.01.050. [DOI] [PubMed] [Google Scholar]
- Kay AR. Detecting and minimizing zinc contamination in physiological solutions. BMC Physiol. 2004;4:4. doi: 10.1186/1472-6793-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch J, Betz H. The postsynaptic localization of the glycine receptor-associated protein gephyrin is regulated by the cytoskeleton. J Neurosci. 1995;15:4148–4156. doi: 10.1523/JNEUROSCI.15-06-04148.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirson D, Todorovic J, Mihic SJ. Positive allosteric modulators differentially affect full versus partial agonist activation of the glycine receptor. J Pharmacol Exp Ther. 2012;342:61–70. doi: 10.1124/jpet.112.191486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lape R, Colquhoun D, Sivilotti LG. On the nature of partial agonism in the nicotinic receptor superfamily. Nature. 2008;454:722–727. doi: 10.1038/nature07139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H. Kinetic and mutational analysis of Zn2+ modulation of recombinant human inhibitory glycine receptors. J Physiol. 2000;522(Pt 2):215–230. doi: 10.1111/j.1469-7793.2000.t01-1-00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Rundström N, Kirsch J, Schmieden V, Betz H. Modulation by zinc ions of native rat and recombinant human inhibitory glycine receptors. J Physiol. 1995;483:613–619. doi: 10.1113/jphysiol.1995.sp020610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Alvarez FJ, Walmsley B. GABA mediates presynaptic inhibition at glycinergic synapses in a rat auditory brainstem nucleus. J Physiol. 2000;525:447–459. doi: 10.1111/j.1469-7793.2000.t01-1-00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ye JH. Glycine-activated chloride currents of neurons freshly isolated from the prefrontal cortex of young rats. Brain Res. 2011;1393:17–22. doi: 10.1016/j.brainres.2011.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Jacques P, Pierce KD, Schofield PR. Zinc potentiation of the glycine receptor chloride channel is mediated by allosteric pathways. J Neurochem. 1998;71:2159–2168. doi: 10.1046/j.1471-4159.1998.71052159.x. [DOI] [PubMed] [Google Scholar]
- Malosio ML, Marquèze-Pouey B, Kuhse J, Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 1991;10:2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia MP, Mihic SJ, Valenzuela CF, Schofield PR, Harris RA. A single amino acid determines differences in ethanol actions on strychnine-sensitive glycine receptors. Mol Pharmacol. 1996;50:402–406. [PubMed] [Google Scholar]
- McCracken LM, Trudell JR, Goldstein BE, Harris RA, Mihic SJ. Zinc enhances ethanol modulation of the alpha1 glycine receptor. Neuropharmacology. 2010;58:676–681. doi: 10.1016/j.neuropharm.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ, McQuilkin SJ, Eger EI, 2nd, Ionescu P, Harris RA. Potentiation of gamma-aminobutyric acid type A receptor-mediated chloride currents by novel halogenated compounds correlates with their abilities to induce general anesthesia. Mol Pharmacol. 1994;46:851–857. [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Miller PS, Da Silva HM, Smart TG. Molecular basis for zinc potentiation at strychnine-sensitive glycine receptors. J Biol Chem. 2005;280:37877–37884. doi: 10.1074/jbc.M508303200. [DOI] [PubMed] [Google Scholar]
- Molander A, Lidö HH, Löf E, Ericson M, Söderpalm B. The glycine reuptake inhibitor Org 25935 decreases ethanol intake and preference in male wistar rats. Alcohol Alcohol. 2007;42:11–18. doi: 10.1093/alcalc/agl085. [DOI] [PubMed] [Google Scholar]
- Molander A, Löf E, Stomberg R, Ericson M, Söderpalm B. Involvement of accumbal glycine receptors in the regulation of voluntary ethanol intake in the rat. Alcohol Clin Exp Res. 2005;29:38–45. doi: 10.1097/01.alc.0000150009.78622.e0. [DOI] [PubMed] [Google Scholar]
- Mori M, Gahwiler BH, Gerber U. Beta-alanine and taurine as endogenous agonists at glycine receptors in rat hippocampus in vitro. J Physiol. 2002;539:191–200. doi: 10.1113/jphysiol.2001.013147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegel AC, Zapata A, Shippenberg TS, French ED. The abused inhalant toluene increases dopamine release in the nucleus accumbens by directly stimulating ventral tegmental area neurons. Neuropsychopharmacology. 2007;32:1558–1569. doi: 10.1038/sj.npp.1301273. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Navarro JA, Gonzalo-Gobernado R, Herranz AS, GonŸlez-Vigueras JM, Solís JM. High potassium induces taurine release by osmosensitive and osmoresistant mechanisms in the rat hippocampus in vivo. J Neurosci Res. 2009;87:208–217. doi: 10.1002/jnr.21818. [DOI] [PubMed] [Google Scholar]
- Schmieden V, Kuhse J, Betz H. Agonist pharmacology of neonatal and adult glycine receptor alpha subunits: identification of amino acid residues involved in taurine activation. EMBO J. 1992;11:2025–2032. doi: 10.1002/j.1460-2075.1992.tb05259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimemi A, Beato M. Determining the neurotransmitter concentration profile at active synapses. Mol Neurobiol. 2009;40:289–306. doi: 10.1007/s12035-009-8087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipps ME, Lawshe JE, Ellington AD, Mihic SJ. Identification of novel specific allosteric modulators of the glycine receptor using phage display. J Biol Chem. 2010;285:22840–22845. doi: 10.1074/jbc.M110.130815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Gorcs T. Glycine and glycine receptor immunoreactivity in brain and spinal cord. J Neurosci. 1988;8:472–492. doi: 10.1523/JNEUROSCI.08-02-00472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldvogel HJ, Baer K, Allen KL, Rees MI, Faull RLM. Glycine receptors in the striatum, globus pallidus, and substantia nigra of the human brain: an immunohistochemical study. J Comp Neurol. 2007;502:1012–1029. doi: 10.1002/cne.21349. [DOI] [PubMed] [Google Scholar]
- Wilson JE, Kiselanova N, Stevens Q, Lutz R, Mandler T, Tran ZV, Wischmeyer PE. A survey of inhalational anaesthetic abuse in anaesthesia training programmes. Anaesthesia. 2008;63:616–620. doi: 10.1111/j.1365-2044.2008.05444.x. [DOI] [PubMed] [Google Scholar]
- Yamakura T, Mihic SJ, Harris RA. Amino acid volume and hydropathy of a transmembrane site determine glycine and anesthetic sensitivity of glycine receptors. J Biol Chem. 1999;274:23006–23012. doi: 10.1074/jbc.274.33.23006. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Ueno T, Akaike N, Ikemoto Y. Modulation of miniature inhibitory postsynaptic currents by isoflurane in rat dissociated neurons with glycinergic synaptic boutons. Eur J Pharmacol. 2001;431:269–276. doi: 10.1016/s0014-2999(01)01421-2. [DOI] [PubMed] [Google Scholar]
- Yevenes GE, Zeilhofer HU. Allosteric modulation of glycine receptors. Br J Pharmacol. 2011;164:224–236. doi: 10.1111/j.1476-5381.2011.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]