Abstract
The study of engineered nanomaterials for the development of technological applications, nanomedicine, and nano-enabled consumer products is an ever expanding discipline as is the concern over the impact of nanotechnology on human environmental health and safety. In this review we discuss the current state of understanding of nanomaterial skin interactions with a specific emphasis on the effects of ultra-violet radiation (UVR) skin exposure. Skin is the largest organ of the body and is typically exposed to UVR on a daily basis. This necessitates the need to understand how UVR skin exposure can influence nanomaterial skin penetration, alter nanomaterial systemic trafficking, toxicity, and skin immune function. We explore the unique dichotomy that UVR has in inducing both deleterious and therapeutic effects on skin. The subject matter covered in this review is broadly informative and will raise awareness of potential increased risks from nanomaterial skin exposure associated with specific occupational and life style choices. The UVR induced immunosuppressive response in skin raises intriguing questions that motivate future research directions in the nanotoxicology and nanomedicine fields.
Introduction
Engineered nanoscale materials (<100 nm in one dimension) made from metals, metal oxides, semiconductors, and carbon including polymers, exhibit unique optical, electrical, mechanical, biological, and physiochemical properties not present in their bulk form. These properties arise in part from an increased surface area to volume ratio where a greater % of the atoms comprising the material exist on the surface. Engineered nanomaterials (eNMs) are widely exploited in many technology fields (e.g. medicine, energy, automotive, military) promising great benefits to mankind. They are also formulated into an ever expanding consumer product market. The Project on Emerging Nanotechnology in 2011 listed 1317 nanotechnology-enabled consumer products in their inventory; an increase of 521% over 20061. Nano-enabled products use for example, carbon nanotubes to make light weight and high strength sporting equipment (racquets, bikes), nano-Ag to make antimicrobial textiles and wound care dressings, and nanoscale ZnO and TiO2 particles to formulate ultra-violet radiation (UVR) protective sunscreens and daily wear skin-care products2–4. Soft nanomaterials made from organic materials (lipids, proteins) also have wide commercial and pharmaceutical importance (liposome, solid lipid nanoparticles, dendrimers)5, 6. Currently, the FDA does not require manufacturers to label products containing eNMs and some products go to market without rigorous safety testing7–9. This has created human environmental health and safety concerns which has spurred efforts to investigate the ability of eNMs to penetrate epithelial tissue barriers and to characterize their cellular interactions10, 11. With a significant increase in nanotechnology enabled skin-care products and the corresponding increase in the potential for eNMs to contact skin, either through intentional product use or unintended environmental or occupational exposure, a significant effort has been devoted to investigate nanotechnology skin safety2, 3, 12–15.
The purpose of this review is to highlight current knowledge of eNM skin interactions with a specific focus on the effect of UVR skin exposure, which introduces unique considerations when examining the larger question of eNM skin penetration. Firstly, UVR is a ubiquitous environmental insult that can induce defects in the skin barrier function. Secondly, people frequently apply eNM containing lotions (i.e. sunscreens, daily wear cosmetics) to UVR exposed skin. Thirdly, UVR exerts an immunosuppressive effect on skin. The latter is largely linked to photo-carcinogenesis16, 17. However, UVR induced immunosuppression is also a widely exploited therapeutic modality used by dermatologists to treat many skin disorders18–22. This dichotomy of UVR having both deleterious and therapeutic effects on skin biology heightens the need to discover how UVR may modulate eNM skin interactions. In the next sections we review aspects of human skin anatomy and the effects that UVR exposure have on skin biology, emphasizing the implications for eNM skin contact. We discuss what is known from current literature about the interaction eNMs with normal and barrier impaired skin. Fascinating findings are revealed that motivate future research directions in the nanotoxicology and nanomedicine fields.
Human Skin Anatomy - Implications for eNM Skin Contact
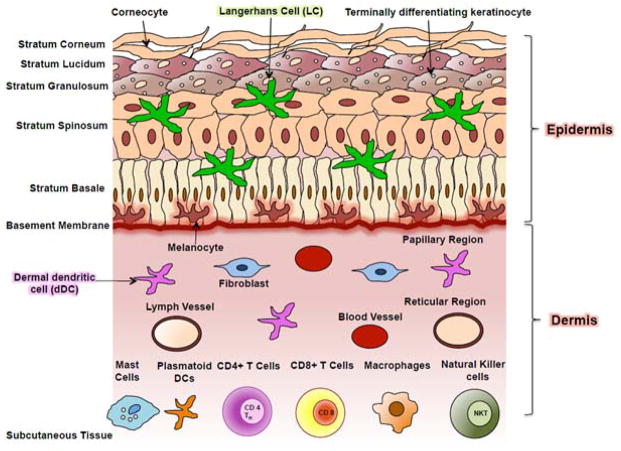
Skin is the largest organ of the body, providing key barrier functions preventing inside-out water loss and outside-in protection from environmental insults (e.g. microbes, particulates, irritants, allergens, UVR) including eNMs. Healthy human skin is divided into the epidermis (thickness: 50–100 μm) and the dermis (thickness: 300–3000 μm) which are separated by the basement membrane [Fig. 1]. Epidermis is multilayered (stratified) epithelium composed largely of keratinocytes23. The stratum basale is adjacent to the basement membrane and it contains cuboidal basal keratinocytes that have proliferative potential. The daughter cells produced differentiate to form the stratum spinosum layer that contains suprabasal transient amplifying keratinocytes. These cells further differentiate to form the stratum granulosum layer comprised of cells that contain a dense presence of keratohyalin granules and lamellar bodies. These contain the essential proteins and lipids, respectively that are needed to form the stratum corneum (thickness: 10–40 μm)24, 25, the outermost layer of the skin [Fig. 2A]. The transition from a granular cell to a corneocyte (dead keratinocyte) is characterized by the degradation of the nucleus, assembly of a cornified envelope, and a reorganization of the keratin intermediate filaments25. The keratin network in the corneocyte is held together by filaggrin, which is a highly charged, cationic protein that aids in the filament aggregation [Fig. 2B] and disulfide bonding26. The flattened corneocytes are bound by tight junctions (corneodesmosomes, Fig. 2C)27 and they are surrounded by a unique lipid lamellar bilayer matrix [Fig. 2D]28–30. The corneodesmosomes, the tortuous path between corneocytes and the lipid matrix together provide the main barrier function of skin, limiting penetration of hydrophilic and high molecular weight compounds31 including eNMs. Corneocytes are continuously sloughed off and replaced by inner keratinocytes differentiating outward. The epidermis is continuously renewed every 4–6 weeks depending on the region of the body32. Hence, the normal process of epidermal turnover can potentially hinder systemic penetration of substances that breech the stratum corneum barrier. Given the cationic nature of the filaggrin-rich stratum corneum, the lipid rich intercorneocyte space, and the overall acidity of the stratum corneum (pH ~4–5)33, the penetration of eNMs is anticipated to depend on their physiochemical properties (e.g. size, charge, composition). Understanding the factors that affect eNM stratum corneum penetration and the cellular interactions that can occur within the epidermis are critically important assessing risk from eNM skin exposure and for tailoring nanoparticle based transdermal therapeutics.
Figure 1. Schematic of human skin structure and constituent cell types.
Skin is stratified epithelial composed of the epidermis and dermis. The epidermis is mainly comprised of keratinocytes. Basal keratinocytes undergo terminal differentiation to form the stratum spinosum, stratum granulosum, and stratum corneum barrier. Stratum lucidum is an additional layer present under the stratum corneum in areas of thick skin like palms of the hands and soles of the feet. Pigment producing melanocytes and antigen presenting Langerhans cells are also present in the epidermis. The dermis is a layer rich in connective tissue and is divided into the papillary and reticular regions. The dermis contains many cell types including fibroblasts that make collagen and other extracellular matrix molecules that provide skin mechanical toughness. Adipocytes, macrophage, mast cells, plasmatoid dendritic cells (pDCs), CD4+ T cells, CD8+ T cells, Tregs, and natural killer T-cells also abundantly present in the dermis apart from other structures including pilosebaceous unit, sweat glands, nerves, blood and lymphatic vessels. Adapted from Nestle et al., 2009 [23].
Figure 2. Transmission electron micrographs illustrating unique features of skin.
(A) The stratum corneum (SC) is comprised of multiple layers of enucleated and elongated corneocytes each defined by a dense cornified envelope indicated by the black arrows. The cells in the stratum granulosum (SG) are distinguished by the presence of a nucleus (N) and a high density of keratohyalin granules (K). Adapted with permission from Madison et al., 1998 [24] (B) Electron micrograph of a corneocyte cytosol illustrating the nanoscale organization of keratin intermediate filaments. The subfilamentous molecular architecture appears as groups of electron dense spots surrounding a central dense dot. The keratin filaments are ~7.8 nm wide with a center-to-center distance of ~16 nm. (Inset box scale bar is 10 nm. Adapted with permission from Norlen and Al-Amoundi, 2004 [25] (C) Corneocytes in the stratum corneum are bound by corneodesmosome tight junctions indicated by black arrows. Racial differences exist in the density of corneodesmosomes. Scale bar is 1 μm. Adapted with permission from Gunathilake et al., 2009 [27]. (D) Electron micrograph illustrating the lipid lamellar bilayers in the intercellular space between corneocytes. Adapted with permission from Warner et al., 1999 [30].
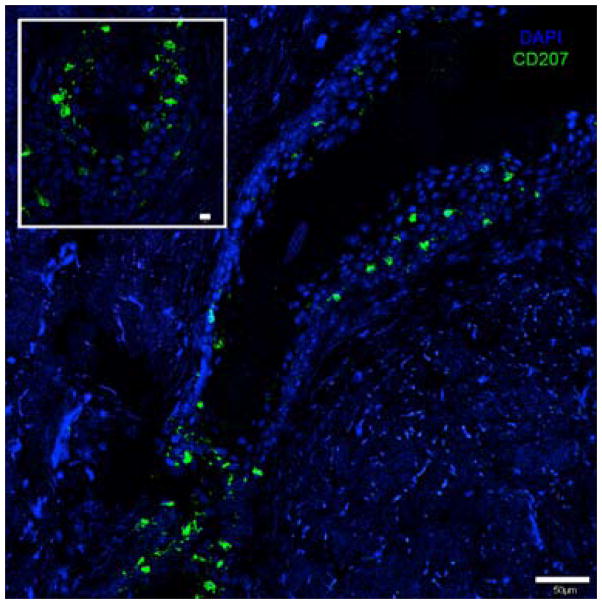
In addition, to basal and differentiated keratinocytes there are a number of other cell types present in skin that could potentiate the interaction of eNM that penetrate beyond the stratum corneum. Melanocytes, present the epidermis, produce melanin pigment which gives skin its color. They are evenly distributed along the basal layer and comprise 5% to 10% of epidermal cells34. The difference in skin color between light and dark pigmented individuals is due to the activity of melanocytes in their melanin production, not the number of melanocytes 35. Melanocytes and keratinocytes form an epidermal unit in a 1:36 ratio36. Studies investigating the sensitivity of melanocytes to eNMs are lacking. Given the critical role that melanocytes have in protecting keratinocytes from UVR exposure and their potential to transform into a deadly malignant phenotype, suggests an urgent need exists to investigate whether eNMs can alter melanocyte function. Skin also provides innate and adaptive immune functions that are readied to respond to environmental insults that breech the stratum corneum23, 37. Langerhans cells (LCs) in the epidermis and dermal dendritic cells (dDCs) in the dermis are the main antigen presenting cells (APC) in the skin38–40. LCs comprise ~2–4% of epidermal cells41–44. CD1a positive immunocytes45 and CD207 positive LCs [Fig. 3] form a tight meshwork within the epidermis and have been shown to localize around hair follicles at high densities, suggesting a need for increased immuno-surveillance surrounding these appendages. ACPs pick up antigens that breech the stratum corneum and migrate to the lymph nodes to activate the adaptive immune system. Skin contains a high density of resident T cells, ~1×106/cm2 which, for the average adult is nearly twice the number of T cells found in circulation41. Although skin lacks organized lymphoid structures, keratinocytes are able to produce a diverse repertoire of cytokines that can influence APC and T cell function46–48. Because skin comes in contact with numerous exogenous substances, the main route to allergen sensitization is consequently through skin49–53. Currently, little is understood about how eNM skin exposure may affect skin immune cell function, cytokine secretion by keratinocytes, or effect allergic skin disorders.
Figure 3. Langerhans Cell localization pattern around the hair follicle.
Immunofluorescent staining of human skin epidermis with anti-CD207-Alexa 488 (Langerin) specific for Langerhans cells showing their distribution around the hair follicle infundibulum, scale bar=50 μm. Inset shows the base of the hair follicle, scale bar=10 μm.
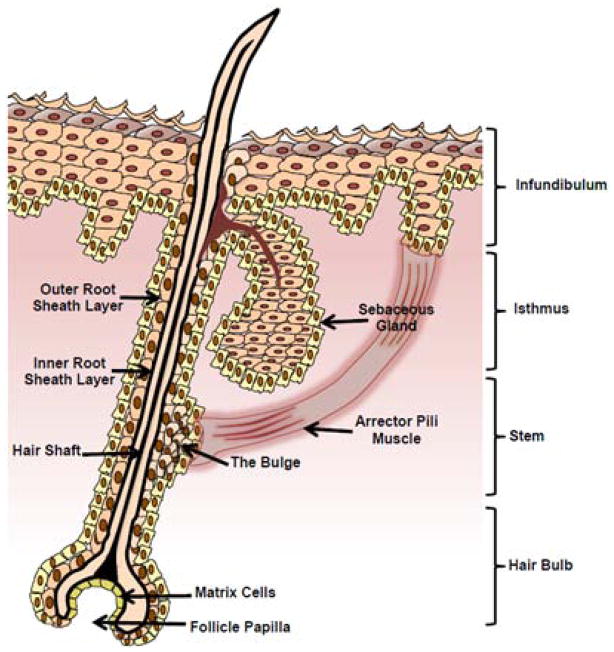
Other prominent features in the skin are hair follicles and sebaceous glands [Fig. 4]. Recent studies have solidified the important role that these structures have in mediating eNM skin penetration54–56. The pilosebaceous unit is a complex structure consisting of the hair shaft, arrector pili muscle, and the sebaceous gland. The outer root sheath (ORS) of the hair follicle is contiguous with and biochemically similar to the basal layer of the epidermis32, 57, 58. The inner layers the follicle contain hair-producing cells. Hair follicles are in a continuous cycle of anagen (hair growth phase), catagen (involution phase) and telogen (a resting phase)58, 59. Skin resident stem cells reside in a follicular niche called the hair bulge32, 60–62. This suggests the potential for eNMs to interact with and perhaps alter stem cell function if they can penetrate and accumulate in the follicle. The hair follicle infundibulum is the region extending from the opening at the skin surface down to the sebaceous gland. Because hair follicles by-pass the stratum corneum barrier and terminate in the dermis, where they gain access to vascular and lymphatic systems, the hair follicle represents an important penetration pathway (epidermal shunt) and potential reservoir for systemic delivery of topically applied substances including eNM54. Hair follicle parameters such as orifice size, density, body distribution, and racial differences have been investigated63. The highest infundibular volume and therefore largest follicular reservoirs are found on the forehead and calf regions. Whites have significantly higher follicular reservoirs compared to Asians and African-Americans64. Follicular density differences are known to contribute to racial dependences seen in transdermal drug delivery efficacy65 and this sets an expectation for racial differences to exist in how eNMs interact with skin. However, confirmatory studies have not yet been conducted nor has it been established whether eNM follicular accumulation and penetration levels depend on the hair cycle phase.
Figure 4. Schematic of the human hair follicle in late anagen phase.
The bulge, outer root sheath, hair bulb and follicle papilla are responsible for hair growth.
Effect of UVR on Skin Barrier - Implications for eNM Skin Contact
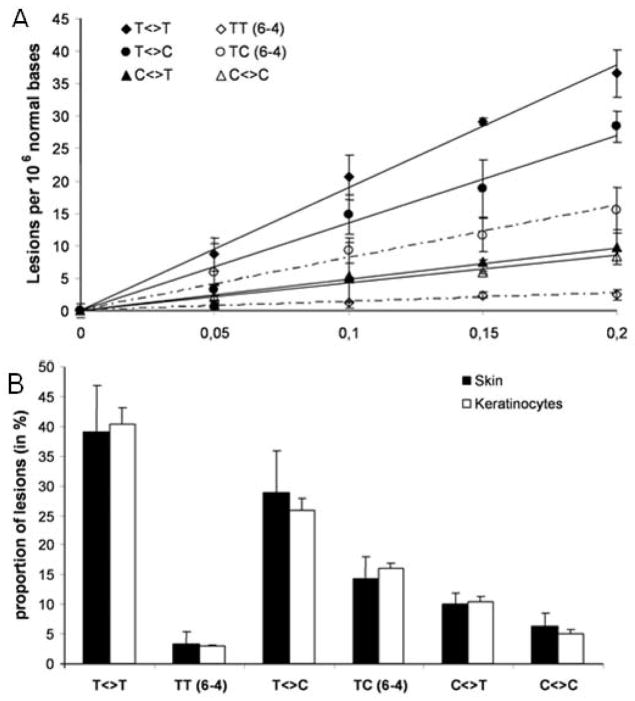
Ultra-violet solar radiation reaching the earth consists of UVA (315–400 nm), UVB (280–315 nm) and UVC (100–280nm) components. UVC is mostly absorbed in the upper atmosphere whereas UVB and UVA reach earth. UVA skin exposure penetrates deep into the dermis whereas UVB is mainly absorbed in the epidermis66. Skin exposure to UVR results in a number of biological responses including DNA damage, melanogenesis, generation of oxidative stress, vasodilation (skin erythema) and leukocyte infiltration67. Excessive and/or chronic UVR skin exposure causes sunburn, photoaging, and photocarcinogenesis68. UVB skin exposure from solar radiation or from tanning booths represent major environmental, occupational, and consumer health risks69. Unexpectedly, a growing source of UVR exposure is from compact fluorescent light bulbs, which are gaining wide acceptance as they use 75% less energy than incandescent bulbs and they produce the same lumens70. Studies suggest that defects in the phosphor coating applied to adsorb x-rays permit emission of UVA and UVC at exceedingly high levels71. Solar UVB radiation is however, the predominant concern as it is ubiquitous and a confirmed mutagen and carcinogen. It is efficiently absorbed by DNA producing cyclobutane pyrimidine dimers (CPDs); thymine to thymine (T<>T), thymine to cytosine (T<>C), and cytosine to cytosine (C<>C)72. Highly mutagenic 6,4 photoproducts are also generated but at a much lower frequency than CPDs73. Studies indicate that UVB skin exposure can generate ~519 CPD lesions per 106 normal oligonucleotide per J/cm2 [Fig. 5] 72. UVB skin exposure also induces dramatic effects on the cohesion and mechanical integrity of corneocytes in the stratum corneum74 and it induces melanogenesis. Melanocytes increase production of melanin and package it into melanosome vesicles. These are transferred to keratinocytes and positioned above the nucleus to prevent DNA damage75. Melanogenesis leads to tanning that becomes visible about 72 hours after exposure76. Defects in the mechanical integrity of the stratum corneum with the concurrent priming of keratinocytes to uptake melanosomes suggests the possibility for the increased ability of eNMs to penetrate into the viable epidermis and to be taken up by keratinocytes77.
Figure 5. Formation of bipyrimidine photoproducts within human skin exposed to UVB radiation.
(A) Photoproduct formation is linear with respect to the applied UVB dose (0–0.2 J/cm2). The results are expressed in lesions per 106 bases and are the average ± SD. (B) A similar distribution of bipyrimidine photoproducts is produced in human skin and in cultured primary keratinocytes isolated from the same donor following UVB. Reprinted with permission from Mouret et. al., 2006 [72].
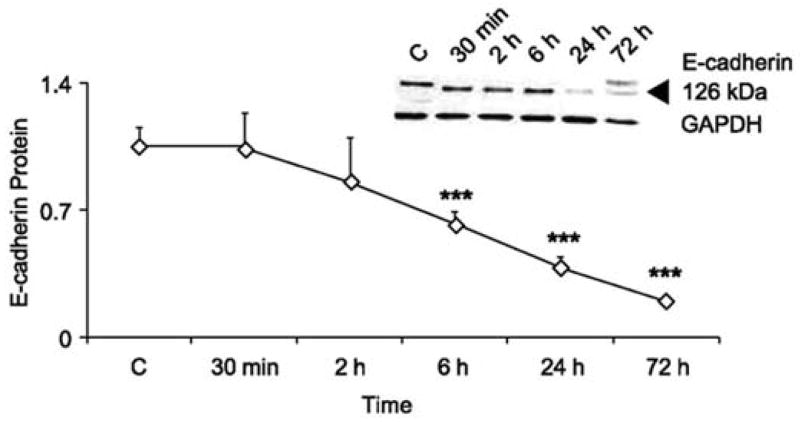
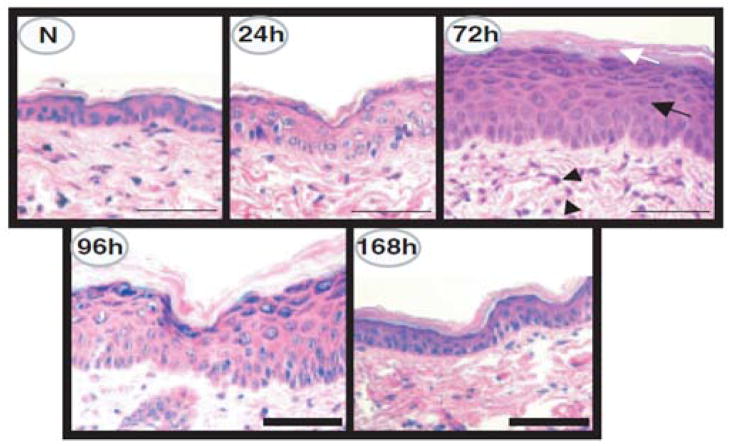
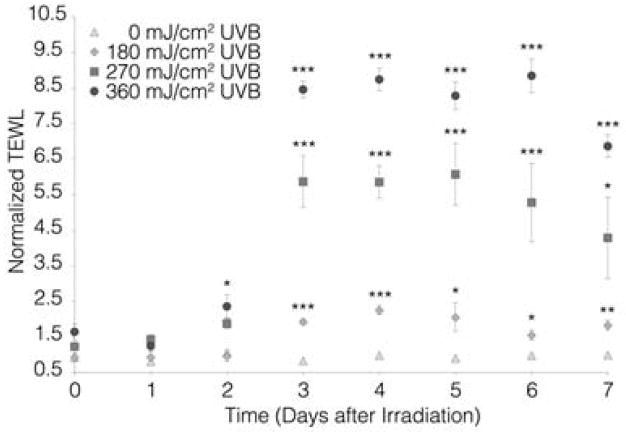
Skin has evolved elaborate defense systems to combat the mutagenic and oxidative stress effects of UVR exposure. Keratinocytes immediately respond by pausing their cell cycle to repair DNA lesions via the nucleotide excision repair process78. However, following high or chronic UVR exposure keratinocytes may not be able to repair all of the DNA damage generated which, can lead to mutagenesis and skin tumor formation. If and how eNM may alter these processes has not yet been investigated. After DNA lesions are repaired, keratinocytes down regulate E-cadherins [Fig. 6] which are important cell-cell adhesion proteins79. This facilitates a UVR-induced keratinocyte hyper-proliferation response which thickens skin to buttress the epidermal barrier function [Fig. 7]80. Hyper-proliferation results however, in a disorganized stratum corneum structure where the presence of nucleated cells in the stratum corneum can be detected 81, 82. This coincides with a measurable inside-out water loss skin barrier defect. Studies in mice (Fig. 8) indicate that the transepidermal water loss (TEWL) value is UVB dose and time dependent83. The peak water loss value occurs 3 to 4 days post UVB exposure and resolves in 7 to 10 days. Studies have not yet determined if the TEWL value is predictive of outside-in eNM stratum corneum penetration. Nor is it known whether epidermal thickening can hinder systemic transport of eNMs that do penetrate the disrupted stratum corneum or which day post UVR exposure skin is most susceptible to eNM penetration.
Figure 6. E-cadherin expression in primary mouse keratinocytes (PMK) after acute UVR exposure.
PMKs were harvested at the times indicated post UVR (40 mJ/cm2 UVB). Unirradiated PMKs were included as controls. Densitometric analysis of Western data for relative E-cadherin levels normalized to GAPDH. E-cadherin levels were significantly different from control at 6, 24, and 72 h (n = 3). ***, p < 0.001. Inset, a representative Western blot for E-cadherin from one of three separate experiments. Equal loading of protein was verified by GAPDH staining. Reprinted with permission from Brouxhon et. al. 2007 [79].
Figure 7. Characterization of UVR-induced epidermal injury in SKH-1 mice.
Hematoxylin and eosin stain photomicrographs of normal dorsal mouse skin (N) and of skin 24, 72, 96, or 168 h after UVR (180 mJ per cm2 UVB) irradiation. Note the epidermal hyperplasia (black arrow), hyperkeratosis (white arrow), and the perivascular inflammation (arrowheads) present 72 h after UVR irradiation. At 168 h post UVR irradiation, the epidermis has returned to near normal. Scale bar: 50 μm. Reprinted with permission from Tripp et. al., 2003 [80].
Figure 8. The effect of UVR on SKH mouse skin barrier function as measured by transepidermal water loss (TEWL).
UVR exposure increases the barrier defect in a UVB dose (0 to 360 mJ/cm2 UVB) and time dependent manner. A statistically significant increase in TEWL is observed for all exposures with the peak defect ranging from days 3 to 6 post-UVR exposure. Each value is reported as the mean ± SEM (n=4, *p < 0.05, **p < 0.01, ***p < 0.001). Reprinted from Mortensen et. al., 2013 [83].
UVB induced Immunosuppression - Implications for eNM Skin Contact
UVB skin exposure is immunosuppressive which has a consequential role in skin photocarcinogenesis84. It is known that UVB exposure induces skin resident APCs to exit skin and migrate to the lymph nodes85–87. Migration initiates immediately post UVB exposure and the number of APC in skin remains low for 4–14 days. This phenomenon has long been studied using an in vivo mouse model of contact hypersensitivity (CHS)43, 88–91. Here, topically applied contact allergens or haptens, such as dinitroflourobenzene (DNFB) and oxazolone (OXA), interact with biomolecules in skin to create antigenic substances that induces T cell mediated sensitization. Subsequent skin exposure to the same haptan days later (challenge phase) induces severe skin inflammation that is quantifiable91. However, if during sensitization the hapten is applied to skin pre-exposed to low doses of UVB radiation, the CHS response after the challenge is significantly inhibited. The cellular mechanisms of the UVB-induced immunosuppression response are generally understood. LCs normally uptake UVB-damaged skin cell debris and migrate to local lymph nodes where they induce T regulatory cells (Tregs) against self-antigen92. This is important for the induction of immunologic self-tolerance and protection from developing photosensitivity disorders91–95. When hapten is topically applied to UVB exposed skin, LCs mediate generation of Tregs against the hapten. Consequently, upon challenge the mice exhibit a significantly reduced CHS response in an antigen-specific fashion91, 92, 96. Antigen specificity distinguishes UVB-Tregs from systemic drug-induced immunosuppression91. Because UVB skin exposure has the potential to impair skin barrier function and to induce a skin immunosuppressive response, it can also potentially influence eNM skin penetration, alter systemic trafficking, toxicity, and skin immune function. What is known about these will be revealed in the next sections.
Engineered Nanomaterial Skin Interactions
Over the past 10 years increasing research has focused on the fundamental questions of whether or not eNMs can penetrate the stratum corneum barrier and what factors can impact penetration. Factors include for example, the eNM physiochemical properties (size, shape, composition, charge, surface energy)97–101, vehicle affects102, 103 and the skin barrier status (healthy, injured, or diseased)83, 97, 104–106. In surveying the literature it has become clear that analytical instrument detection sensitivity and sample analysis volume, combined with the wide ranging use of skin models, eNM types and coatings have created challenges to generating both qualitative and quantitative conclusions regarding eNM skin penetration107. Tissue histology and transmission electron microscopy (TEM) analysis are common methods used to investigate eNM skin penetration but these techniques suffer from limitations with tissue sampling and the inability to distinguish particles from intrinsic tissue features and background signal. Reliance on mass spectrometry techniques to track eNM persistence in skin, systemic transport, or organ distribution patterns limits the ability to determine if penetration occurred as intact particles or as dissociated ions83, 104, 108, 109. Some eNMs including quantum dots (QD) and ZnO nanoparticles do exhibit unique fluorescent and/or nonlinear optical signatures that can help distinguish penetration of intact particles from ions, but the detection sensitivity can be limited by tissue autofluorescence107, 110–114. Nonetheless, despite these complexities, progress is being made toward advancing our understanding of eNM skin interactions and our ability to draw conclusions about nanotechnology skin safety, as can be gleaned from many recent reviews2, 3, 12, 14, 15, 115–117.
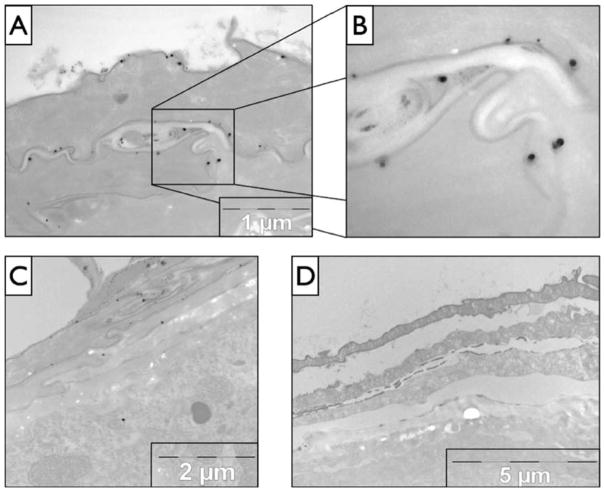
In general, substances that contact skin have three basic mechanisms to penetrate the stratum corneum barrier - transcellular through corneocytes, intercellular between corneocytes, and penetration via skin appendages (e.g. sweat glands, the follicular infundibidum)118. The transcelluar route is considered to be of little importance because the corneocyte cell membrane (cornified envelop) is highly impermeable although this is somewhat still debated119, 120. From the transdermal drug delivery field it is largely accepted that substances ~500 Da (~2.5 nm diameter) cannot penetrate the healthy SC barrier31. Vehicles in which substances are applied to skin can however enhance the penetration of higher molecular weight substances into deep skin layers, including eNM121, 122. Penetration enhancers generally work by increasing intercellular fluidity of the lipid lamella in the stratum corneum or by extracting non-covalently bound amphiphilic lipids from the stratum corneum123. From existing literature it can confidently be said that the healthy skin stratum corneum presents a formidable barrier to outside-in eNM penetration. However, in life it is quite common for skin to have barrier defective regions resulting from mechanical damage (cuts or scrapes), UVB exposure, use of harsh soaps or cosmetic products containing certain ingredients (depilatory agents, sodium lauryl sulphate, alpha-hydroxy acids) or from dermatologic disorders. Cutaneous defects can facilitate penetration of exogenous substances including large protein antigens (dust mite and plant allergens) and virus particles (20 to 100 nm dia.)124, 125. Studies also show that barrier defective skin is more susceptible to penetration of eNMs and that eNMs penetrate the stratum corneum intercellularly through the lipid lamellae [Fig. 9]83, 97, 105, 106, 126–129.
Figure 9. TEM imaging of mouse skin sections suggesting quantum dot nanoparticles penetrate intercellular between corneocytes.
(A) The penetration pathway through the stratum corneum is between corneocytes which is shown in more detail in (B) where the large dark spots are quantum dots. (C) Another skin section demonstrating the penetration pathway showing quantum dot present in the stratum granulosum. (D) A negative control (no quantum dots). Reprinted with permission from Mortensen et. al., 2008 [159].
Until recently the follicular penetration pathway had received little attention because hair follicles comprise ~0.1% of the total skin area56. Recent work has however, revealed that the follicular infundibulum, including sebaceous glands, comprise efficient and long-term reservoirs suited for accumulation of eNM54, 55. The high density of antigen presenting cells localized around the hair follicle [Fig. 3] and the presence of stem cells in the hair bulge have led researchers to target the follicular pathway for vaccine and drug delivery using eNM100, 130–134. Efficient access to the infundibulum reservoir requires however, that the sebaceous deposits and other debris in the follicular orifice be cleared. Opening the follicles using a cyanoacrylate-tape stripping processes has been shown to enhance deeper follicular penetration of particles45, 132. Particle accumulation, the depth of penetration, and retention in the infundibulum depend on particle size and these can be further enhanced by mechanical massaging of skin45, 135–140. Repetitive body motions such as walking or wrist flexing can also stress skin in ways that could potentially enhance follicular accumulation and eNM skin penetration.
The ability of eNM to accumulate in hair follicles and exhibit enhanced penetration through barrier defective skin heightens concerns as the prevalence of common skin disorders with known barrier defects (e.g. atopic dermatitis, contact dermatitis, psoriasis) are on the rise49, 141, 142. Few studies have however, been conducted on diseased skin making it difficult to draw general conclusions on whether skin disorders predispose individuals to increased risk of eNM penetration112. Studies are also needed to determine if hyperkeratotic disorders (e.g. psoriasis, epidermolytic hyperkeratosis) hinder penetration due to the thickened epidermis or enhance penetration due to malformations in the epidermal structure and barrier function. Similarly, little known about the effect of eNM skin exposure on allergic skin disorders143, 144. Studies are needed to determine if eNMs can induce or exacerbate allergic contact sensitization which is a leading occupational health concern69. The observation that UVR exposure can induce a skin barrier defect81, 83, 103 is a unique concern considering the associated immunosuppressive effect. What is known about these will be examined in next.
Interactions of Engineered Nanomaterials with UVR Exposed Skin
Nanomaterials can contact UVR exposed skin through the intentional use of UVR protective lotions, nanoparticle-containing therapeutic treatments, or through unintentional exposure from environmental sources. By far the most important eNM to be studied in the context of UVR skin exposure is that of ZnO and TiO2103, 110. Other eNM types that have been studied on UVR exposed skin include fluorescent QDs which facilitate tissue tracking83, 105; polymer nanoparticles145, polymer coated platinum nanoparticles146 and iron oxide nanoparticles147 for improved UVR protection. The interest in nanoparticle based UVR protective lotions stems from the fact that many common organic UVR filters such as, 2-ethylhexyl 4-methoxy cinnamate (OMC) and 3-benzylidene camphor (3-BC), can penetrate through skin and they have been detected in urine and breast milk148, 149. Organic filters can also exacerbate generation of UVR-induced reactive oxygen species (ROS) in skin150. The purported advantage of particle-based UVR filters is that their large size would hinder stratum corneum penetration and skin retention (follicular reservoir effect) to improve photoprotection151.
Metal oxide particle filters work by reflecting, scattering and absorbing UVR. The relative contribution of these mechanisms depends on particle size, crystal structure, and wavelength152, 153. The high surface energy the TiO2 and ZnO oxides causes primary particles of size 5 to 20 nm to aggregate (irreversibly bound) and these can further form agglomerates (loosely bound aggregates). Agglomerates that exceed 1 μm efficiently scatter visible light and thus apply to skin as an unappealing white gritty film154. Dispersants are used to maintain particle aggregates in the size range of 30–150 nm which is optimal for UVB and UVA absorption and for producing lotions that apply clear to skin [Fig. 10]. A consequence of increasing the UVR absorption capacity of nanoscale metal oxides is the increased photogeneration of radicals and ROS which can cause DNA lesions and lipid peroxidation155. Generally, the anatase form of TiO2 displays higher photoactivity, ROS production and cytotoxicity than the rutile form which is preferred for use in UVR protective cosmetics155, 156. To hinder agglomeration and to help protect skin from contacting the potential phototoxic TiO2 and ZnO metal oxide core, the particles are usually coated with for example silica, aluminum hydroxide, or methicone145, 157, 158. While much is understood about the formulation of UVR protective cosmetics and how to control the photoactive properties of eNM, very little is understood quantitatively or mechanistically about the penetration of nanoscale TiO2 and ZnO, or other eNM through UVR exposed skin. This has spurred increasing efforts over the past 5 years to examine this specific question. Because the biological effects of UVR on skin exposure evolve over time and involve the immune system, the most useful data will be generated using in vivo skin models. Here we review what has been gleaned from use of in vivo mouse83, 159, pig103, and human skin models108, 109.
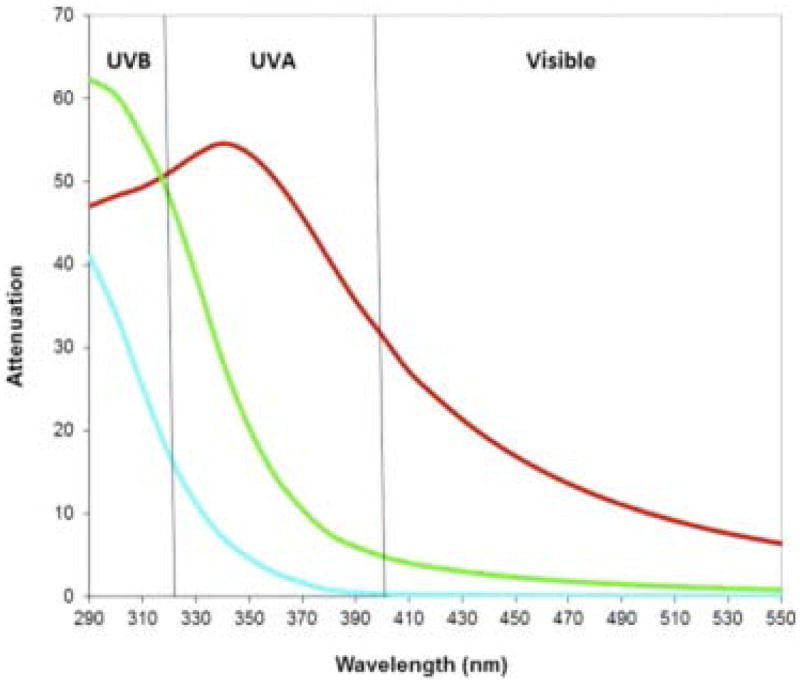
Figure 10. Dependence of UV-visible light attenuation on TiO2 nanoparticle aggregate size.
With decreasing particle size UV protection shifts to shorter wavelengths. Blue line, 20 nm; green line, 50 nm; and red line, 100 nm. Particles with average aggregate size of ~ 50 nm offer high UVB attenuation and lower visible light scattering but less UVA absorption. Reprinted with permission from Wang and Tooley, 2011 [154].
The impact of UVR on nanoparticle skin penetration was first investigated by DeLouise and coworkers using the SKH hairless mouse model159. Carboxylated (dihydrolipoic acid coated) QDs were topically applied to mice in a 30% glycerol vehicle immediately following UVR exposure (270 mJ/cm2 UVB). The mice wore Elizabethan collars to prevent grooming. After 8 and 24 hr, skin samples were qualitatively examined for the presence of QD using tissue histology, confocal microscopy, and silver enhanced TEM with EDAX analysis. Low levels of penetration were seen in both the non-UVR and the UVR exposed mice but qualitatively much higher levels of penetration were observed in the UVR exposed mice. TEM images suggested that QDs penetrated the stratum corneum barrier along the intercellular space between corneocytes [Fig. 9]. Interestingly, despite the high UVB dose employed, the penetration levels observed were estimated to be extremely small compared to the dose applied but a quantitative organ analysis study was not performed. Because mouse skin is much thinner than human skin and the QDs physiochemical and dispersive properties differ from the metal oxides formulated in UVR protective lotions, other groups were motivated to investigate the penetration of TiO2 and ZnO lotions applied to UVR exposed pig and human skin.
Gulson and coworkers108 recruited human subject volunteers (n = 20) to investigate the penetration of ZnO particles through UVR exposed skin. Subjects applied an oil-water based sunscreen containing isotopically labeled ZnO particles (68Zn) to their backs twice a day for 5 consecutive days. Two different sunscreen formulations were tested: a nano-formulation containing of particles with primary size ~19 nm and a bulk-formulation containing particles >100 nm. After each sunscreen application, subjects laid in the sun on their bellies for a minimum of 30 min. The minimum average UVB dose was estimated to be 180 mJ/cm2 per 30 min exposure. Venous blood and urine samples were collected. Results found small increases in tracer 68Zn levels in the blood and urine from all subjects however, the overwhelming majority of applied 68Zn was not absorbed (<0.001% absorbed in blood). Tracer levels in the blood and urine from female subjects who received the nano-formulation appeared to be higher than males receiving the same treatment and higher than all subjects receiving the bulk-formulation. Authors conclude that small amounts of Zn from ZnO particles in the sunscreen could be absorbed through the skin and detected in blood and urine of healthy subjects exposed to sunlight. A similar conclusion was reached in a follow-up study using different a sunscreen formulation and a different UVB exposure109. However, neither study was able to distinguish if the 68Zn detected had been absorbed as particles or as soluble Zn ion or both. In fact, a recent study reported that ZnO in commercial sunscreens is dissociated to Zn ion under UVB irradiation 160. Also, the Gulson studies did not control for total UVB dose or sunscreen usage on other body parts, so it is unclear if sunscreen was applied to barrier defective skin. It is important to note that the ability to distinguish Zn ions from ZnO particles in skin with sufficient sensitivity to overcome skin background signal has recently been demonstrated using sophisticated NIR multi-photon and second harmonic generation microscopy techniques110, 161, 162. Studies of sunscreen applied to human skin with and without barrier impairment, including patients with psoriasis and atopic dermatitis all find little evidence that ZnO nanoparticles can penetration beyond the SC110, 112, 114, 128. These techniques have, however, not yet been utilized to investigate the potential for ZnO particles to penetrate barrier impaired skin induced by UVR exposure.
While studies on human skin are ideal, conducting human subjects research is expensive and limiting. Animal models are less expensive and offer improved control over UVB dose and topical administration. Medical literature has long recognized that pig skin is anatomically and physiologically the most similar to human skin163. Monteiro-Riviere and co-workers103 used white Yorkshire pigs to investigate the effect of UVR exposure on nanoscale TiO2 and ZnO skin penetration. The pigs received a UVB dose of 100 to 120 mJ/cm2 that induced a pale red erythema. One day after UVR exposure the sunscreens were topically applied to a controlled area. Metal oxides particles were incorporated into oil/water (o/w) and water/oil (w/o) sunscreen formulations. The application area was occluded and a second sunscreen dose was applied on day 2. On day 3 the skin was harvested for analysis. TEM results found that both nanoparticle types primarily localized as large agglomerates on the skin surface. On UVR exposed skin TiO2 (not ZnO) particles penetrated into layers deeper of the stratum corneum. A similar result was observed on explant pig ears164. The superficial penetration observed was modulated to some extent by the nature of the topical formulation; with the w/o formulation permitting deeper penetration of TiO2 into stratum corneum layers. This finding corroborates an early study that reported microfine TiO2 penetrated deeper into human skin from an oily dispersion than from an aqueous one165. The authors further conclude from TEM and histology analysis that UVB exposed pig skin showed slightly enhance penetration and that TiO2 penetrated deeper into the SC in both normal and UVB-exposed skin compared with ZnO. However, using the more sensitive technique of time-of-flight secondary ion mass spectrometry (TOF-SIMS), elemental Ti was detected in the epidermis and superficial dermis and elemental Zn also was detected in the upper epidermis. Again, it is not known whether the elements were absorbed as soluble ion or as particles, but the results emphasize the importance of considering the analytical technique detection sensitivity in drawing definitive conclusions about eNM skin penetration.
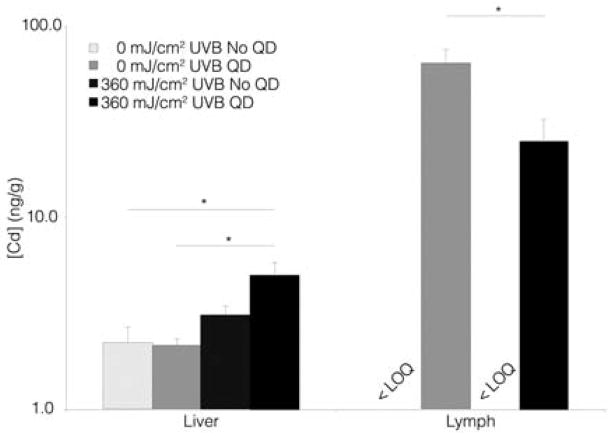
While tissue histology, optical and electron microscopy techniques can provide important insight into the localization of eNM in the skin and can yield some understanding of cellular penetration mechanisms, these techniques do not yield quantitative information. To more fully understand the extent of eNM skin penetration and the systemic translocation, the techniques of inductively coupled plasma mass spectrometry (ICP-MS) and atomic absorption spectroscopy (AAS) are often used to evaluate eNM elemental organ collection patterns. Guided by results of an earlier study, that found the liver and lymph nodes were the major collection sites of Cd following dermal injection of (CdSe/ZnS core/shell) QDs using ICP-MS 111, our lab quantitatively investigated the effect of UVR skin exposure on the penetration of QDs topically applied to mice using AAS [Fig. 11]. SHK hairless mice we exposed to UVR (360 mJ/cm2 UVB) and negatively charge QDs (DLHA-coated CdSe/ZnS core/shell) were topically applied 3 to 4 days post UVR exposure at the peak of the TEWL defect [Fig. 8]. After 24 hr the Cd concentration in the lymph nodes and livers of UVR exposed mice were quantified relative to controls (no UVR)83. Results detected a baseline Cd level in the livers of control mice (vehicle treatment only); the likely source of this Cd was from their food. Application of QDs to UVR exposed skin found a statistically significant increase in liver Cd but the increase as a % of the applied does was very low. The baseline levels (vehicle treatment only) of Cd in the lymph nodes (with and without UVR) were below the limit of quantification (LOQ). Application of QDs to control mice (no UVR) produced an unexpected result in that Cd was detected in the lymph nodes; suggesting QDs penetrated barrier intact skin. This result contrasted histologic and TEM analysis of tissues sections, but due to the limitations of sampling and detection sensitivity mentioned earlier and the increased sensitivity of AAS, it is plausible that QDs can penetrate mouse skin. Interestingly, application of QDs to UVR exposed (barrier defective) skin produced a ~45% lower level of Cd the lymph nodes. This result was also unexpected and suggested an effect of UVR on the mechanism of systemic transport of QDs to the lymph nodes and implicated the role of skin resident APCs. Previous work established that LCs can uptake and traffic polymer NPs topically applied to skin to the lymph nodes45, 166. Due to the immunosuppressive effect that UVR has on skin we quantified LC density before and days 3–4 post UVR exposure83. Results found that the LC density in skin was depleted by ~80% at the time QDs were topically applied. Hence, the lower Cd level in UVR treated mice is consistent with the lesser availability of LCs to uptake and traffic QDs to the lymph node.
Figure 11. Cadmium tissue level in distal organs following 24 hr QD nanoparticle application to SHK mice as a function of UVR.
Liver results show that QD exposure to control mice (no UVR) does not significantly increase Cd level. Application of QDs to UVR exposed mice did statistically increase liver Cd relative to controls (no UVR with or without QD). Lymph node results show that the background Cd level was below the limit of quantification (<LOQ) in vehicle-treated animals with and without UVR exposure. Unexpectedly, application of QDs to control mice (no UVB) produced a high Cd level in the lymph nodes suggesting QDs penetrated intact mouse skin. QD application to UVR exposed mice produced lower Cd level suggesting a UVR dependent cellular transport QD mechanism to lymph node Each value is reported as the mean ± SEM (n=5, *p < 0.05). Reprinted from Mortensen et al., 2013 [83].
Based on the above, intriguing questions arise regarding the potential for eNM that contact UVR exposed skin to either alter or induce skin immune responses. As discussed above topical application of antigen to UVB exposed skin results in the generation of antigen-specific Treg cells. Since UVB exposure has been shown to increases slightly the skin penetration of eNMs and that LCs have been shown capable of up-taking eNM and transporting them to lymph nodes, it is curious to question whether humans can developed tolerance to topically applied nanoscale materials. It is also intriguing to question the affect that eNM may have on LC function and their ability to present antigen. These specific questions have not yet been investigated. However, the potential for eNMs to modulate skin immune function is a rapidly growing field. A few recent examples are briefly discussed below.
Modulating the Skin Immune Responses with Nanoparticles
Some studies have investigated the potential to develop contact dermatitis following eNM skin exposure and to examine how topical application of eNMs may alter symptoms of allergic skin disorders. One recent study reported that subcutaneous injection of different size TiO2 nanoparticles (15, 50, 100 nm) (not topically applied) exacerbated development of atopic dermatitis (AD) symptoms (ear thickening, protein expression of inflammatory molecules) in mice induced by mite allergen, but no effect of particle size was observed167. In a companion paper a similar study was done using different size polystyrene (PS) nanoparticles (25, 50, 100 nm)101. Results found that injected PS nanoparticles aggravated AD-like symptoms even without co-exposure to mite allergen. In contrast to TiO2, a size effect was observed with the smaller PS particles producing greater symptoms. These findings were corroborated by another group that injected TiO2 nanoparticles (20, 230 nm) prior to topical sensitization with dinitrocholorobenzene (DNCB) and results showed TiO2 exacerbated AD symptoms in mice168. Contrasting results have also been reported where topical application of both PS and TiO2 nanoparticles to barrier intact skin models did not induce acute cutaneous irritation or exacerbate a skin sensitization response169. Topical application of mesoporous silica particles (100 nm spheres) also did not induce an ear swelling response in mice or exacerbate allergic contact dermatitis symptoms even when co-administered with dinitrofluorobenzene (DNFB)170. The likely differences observed between injection and topical application is the magnitude of the eNMs in the epidermis.
The above studies reveal the importance of particle composition on exacerbating AD-like symptoms and suggest a potential concern if these nanoscale materials were to contact severely barrier impaired skin. Clearly, for eNM to exert an immunomodulatory affect they must be able to penetrate skin to an appreciable extent. Penetration can be modulated by physical means (injection or stratum corneum depletion) or perhaps by varying both the eNM core and surface composition. Studies with both nanoscale gold and silver particles suggest these metals have a greater propensity to penetrate intact skin than metal oxides. For example, an in vivo study showed that topical application of 200 nm Ag particles formulated in a nanolipid carrier o/w cream exhibited a high capacity to reduce AD-like symptoms171. Similarly, topical application siRNA coated gold nanoparticles (~50 nm) designed to down regulate epidermal growth factor receptor (EGFR) freely penetrated the mouse epidermis and a human skin equivalent model within hours of application172. Following a 3 week application protocol of the siRNA Au particles to hairless mice nearly abolished EGFR expression and reduced the thickness of the epidermis by almost 40%. Whether the differences observed in skin penetration between nanoscale metal and metal oxide particles is driven by surface coating, electronic properties, or the tendency of metal oxide particles aggregate to large sizes that hinder skin penetration is not yet fully understood. The role that nanoparticle conductivity has in modulating skin biology is also poorly understood. Studies have shown that bimetallic nanoparticles (Cu/Zn 20 nm) applied to skin can induce a galvanic couple and that the electrical stimulus generated can reduced the skin inflammatory response to sensitizing agents173. Citrate coated Au nanoparticles were also reported to interfere with downstream IL-1β signaling inhibiting the production of PI3 kinases and proinflammatory TNF-α release in a size dependent manner with 5 nm particles, but not 20 nm, exhibiting the greatest neutralizing effect174. Highly conducting fullerene nanoparticles were reported to exhibit a potent anti-oxidant free radical scavenger activity and inhibit allergic anaphylaxis response in vivo175. Hence, the above examples clearly illustrate the rich potential for developing novel immunomodulatory therapeutics for treating skin disorders using eNMs but the role of particle conductivity has not yet been elucidated.
Conclusions
In this review we discussed the current state of understanding of the interactions of eNMs with skin. We explored the unique dichotomy that UVR exposure has on skin, raising awareness of the challenges to developing a generalized view of eNM skin penetration and translocation. While barrier impaired skin is seen as more susceptible for eNM penetration, the biological and immunologic responses of skin to the impairment means (chemical, physical, disease) will be differ and thus can influence the extent and mechanisms of eNM skin penetration and translocation. Existing literature currently suggests that UVR skin exposure can slightly enhance the penetration of eNM. Significant health issues from eNM occupational exposures or from use of nano-enabled products have not yet emerged. Nonetheless, it is curious to consider if eNM immune-tolerance via UVR-Treg generation exists or if it tolerance to haptans can be modulated by eNM contact with UVR exposed skin (i.e. TiO2 and ZnO). In vivo models will be essential moving forward for examining these questions and elucidating the central role that eNM composition (core/coating) has on dictating skin interaction so that the deleterious and therapeutic benefits of eNMs can be minimized and maximized, respectively. A further concern with the current state of understanding is the limitations imposed by the models and instrumentation utilized. It is challenging to relate acute high dose studies to real world human exposures. Similarly, it is difficult to extrapolate the significance of results that find intradermal injections of eNM can exacerbate AD-like symptoms to realistic topical human exposure conditions. The ability to attain a definitive consensus on the ability of eNMs to penetrate beyond stratum corneum is limited by instrumentation detection sensitivity. Clearly, there is an urgent need for nonbiased means to amplify the detection of eNM presence in tissues107. Simple and widely assessable methods that can distinguish soluble ion from particle penetration are also needed to advance the fields of nanotoxicology and nanomedicine.
Acknowledgments
The authors would like to thank the NIH/NIEHS (R01 ES021492), the CDC (OH009970) and the NSF (CBET-083789) for financial support.
References
- 1.Nanotechnologies PoE. on-line inventory of nanotechnology-based consumer products. 2013 Available at: http://www.nanotechproject.org/inventories/consumer/analysis_draft/
- 2.Wiechers JW, Musee N. Engineered inorganic nanoparticles and cosmetics: facts, issues, knowledge gaps and challenges. J Biomed Nanotechnol. 2010;6:408–431. doi: 10.1166/jbn.2010.1143. [DOI] [PubMed] [Google Scholar]
- 3.Nohynek GJ, Antignac E, Re T, Toutain H. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol Appl Pharmacol. 2010;243:239–259. doi: 10.1016/j.taap.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 4.De Volder MF, Tawfick SH, Baughman RH, Hart AJ. Carbon nanotubes: present and future commercial applications. Science. 2013;339:535–539. doi: 10.1126/science.1222453. [DOI] [PubMed] [Google Scholar]
- 5.Iqbal MA, Md S, Sahni JK, Baboota S, Dang S, Ali J. Nanostructured lipid carriers system: recent advances in drug delivery. J Drug Target. 2012;20:813–830. doi: 10.3109/1061186X.2012.716845. [DOI] [PubMed] [Google Scholar]
- 6.Raj S, Jose S, Sumod US, Sabitha M. Nanotechnology in cosmetics: Opportunities and challenges. J Pharm Bioallied Sci. 2012;4:186–193. doi: 10.4103/0975-7406.99016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtis N. Require labels on cosmentic products containing nanoparticles. 2013. [Google Scholar]
- 8.Gruère GP. Labeling nano-enabled consumer products. Nano Today. 2011;6:117–121. [Google Scholar]
- 9.Kessler R. Engineered nanoparticles in consumer products: understanding a new ingredient. Environ Health Perspect. 2011;119:a120–125. doi: 10.1289/ehp.119-a120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLouise L, Mortensen L, Elder A. Breeching Epithelial Barriers – Physiochemical Factors Impacting Nanomaterial Translocation and Toxicity. In: Webster TJ, editor. Safety of Nanoparticles. Springer; New York: 2009. pp. 33–62. [Google Scholar]
- 11.Elder A, Vidyasagar S, DeLouise L. Physicochemical factors that affect metal and metal oxide nanoparticle passage across epithelial barriers. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:434–450. doi: 10.1002/wnan.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baroli B. Skin absorption and potential toxicity of nanoparticulate nanomaterials. J Biomed Nanotechnol. 2010;6:485–496. doi: 10.1166/jbn.2010.1147. [DOI] [PubMed] [Google Scholar]
- 13.Brandt O, Mildner M, Egger AE, Groessl M, Rix U, Posch M, Keppler BK, Strupp C, Mueller B, Stingl G. Nanoscalic silver possesses broad-spectrum antimicrobial activities and exhibits fewer toxicological side effects than silver sulfadiazine. Nanomedicine. 2012;8:478–488. doi: 10.1016/j.nano.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Robertson TA, Sanchez WY, Roberts MS. Are commercially available nanoparticles safe when applied to the skin? J Biomed Nanotechnol. 2010;6:452–468. doi: 10.1166/jbn.2010.1145. [DOI] [PubMed] [Google Scholar]
- 15.Papakostas D, Rancan F, Sterry W, Blume-Peytavi U, Vogt A. Nanoparticles in dermatology. Arch Dermatol Res. 2011;303:533–550. doi: 10.1007/s00403-011-1163-7. [DOI] [PubMed] [Google Scholar]
- 16.Gruber F, Zamolo G, Kastelan M, Massari LP, Cabrijan L, Peharda V, Batinac T. Photocarcinogenesis--molecular mechanisms. Coll Antropol. 2007;31 (Suppl 1):101–106. [PubMed] [Google Scholar]
- 17.Sreevidya CS, Fukunaga A, Khaskhely NM, Masaki T, Ono R, Nishigori C, Ullrich SE. Agents that reverse UV-Induced immune suppression and photocarcinogenesis affect DNA repair. J Invest Dermatol. 2010;130:1428–1437. doi: 10.1038/jid.2009.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green C, Diffey BL, Hawk JL. Ultraviolet radiation in the treatment of skin disease. Phys Med Biol. 1992;37:1–20. doi: 10.1088/0031-9155/37/1/001. [DOI] [PubMed] [Google Scholar]
- 19.Honigsmann H. History of phototherapy in dermatology. Photochem Photobiol Sci. 2013;12:16–21. doi: 10.1039/c2pp25120e. [DOI] [PubMed] [Google Scholar]
- 20.Sapam R, Agrawal S, Dhali TK. Systemic PUVA vs. narrowband UVB in the treatment of vitiligo: a randomized controlled study. Int J Dermatol. 2012;51:1107–1115. doi: 10.1111/j.1365-4632.2011.05454.x. [DOI] [PubMed] [Google Scholar]
- 21.Almutawa F, Alnomair N, Wang Y, Hamzavi I, Lim HW. Systematic Review of UV-Based Therapy for Psoriasis. Am J Clin Dermatol. 2013;14:87–109. doi: 10.1007/s40257-013-0015-y. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes S, Marques Pinto G, Cardoso J. Development of new lesions: a complication of psoralen plus ultraviolet A light therapy for mycosis fungoides. Photodermatol Photoimmunol Photomed. 2012;28:278–279. doi: 10.1111/j.1600-0781.2012.00679.x. [DOI] [PubMed] [Google Scholar]
- 23.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madison KC, Swartzendruber DC, Wertz PW, Downing DT. Lamellar granule extrusion and stratum corneum intercellular lamellae in murine keratinocyte cultures. J Invest Dermatol. 1988;90:110–116. doi: 10.1111/1523-1747.ep12462065. [DOI] [PubMed] [Google Scholar]
- 25.Norlen L, Al-Amoudi A. Stratum corneum keratin structure, function, and formation: the cubic rod-packing and membrane templating model. J Invest Dermatol. 2004;123:715–732. doi: 10.1111/j.0022-202X.2004.23213.x. [DOI] [PubMed] [Google Scholar]
- 26.Kuechle MK, Presland RB, Lewis SP, Fleckman P, Dale BA. Inducible expression of filaggrin increases keratinocyte susceptibility to apoptotic cell death. Cell Death Differ. 2000;7:566–573. doi: 10.1038/sj.cdd.4400687. [DOI] [PubMed] [Google Scholar]
- 27.Gunathilake R, Schurer NY, Shoo BA, Celli A, Hachem JP, Crumrine D, Sirimanna G, Feingold KR, Mauro TM, Elias PM. pH-regulated mechanisms account for pigment-type differences in epidermal barrier function. J Invest Dermatol. 2009;129:1719–1729. doi: 10.1038/jid.2008.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouwstra JA, Gooris GS, van der Spek JA, Bras W. Structural investigations of human stratum corneum by small-angle X-ray scattering. J Invest Dermatol. 1991;97:1005–1012. doi: 10.1111/1523-1747.ep12492217. [DOI] [PubMed] [Google Scholar]
- 29.Groen D, Gooris GS, Bouwstra JA. New insights into the stratum corneum lipid organization by X-ray diffraction analysis. Biophys J. 2009;97:2242–2249. doi: 10.1016/j.bpj.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warner RR, Boissy YL, Lilly NA, Spears MJ, McKillop K, Marshall JL, Stone KJ. Water disrupts stratum corneum lipid lamellae: damage is similar to surfactants. J Invest Dermatol. 1999;113:960–966. doi: 10.1046/j.1523-1747.1999.00774.x. [DOI] [PubMed] [Google Scholar]
- 31.Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9:165–169. doi: 10.1034/j.1600-0625.2000.009003165.x. [DOI] [PubMed] [Google Scholar]
- 32.Yu BD, Mukhopadhyay A, Wong C. Skin and hair: models for exploring organ regeneration. Hum Mol Genet. 2008;17:R54–59. doi: 10.1093/hmg/ddn086. [DOI] [PubMed] [Google Scholar]
- 33.Wagner H, Kostka KH, Lehr CM, Schaefer UF. pH profiles in human skin: influence of two in vitro test systems for drug delivery testing. Eur J Pharm Biopharm. 2003;55:57–65. doi: 10.1016/s0939-6411(02)00125-x. [DOI] [PubMed] [Google Scholar]
- 34.Holbrook KA, Underwood RA, Vogel AM, Gown AM, Kimball H. The appearance, density and distribution of melanocytes in human embryonic and fetal skin revealed by the anti-melanoma monoclonal antibody, HMB-45. Anat Embryol (Berl) 1989;180:443–455. doi: 10.1007/BF00305119. [DOI] [PubMed] [Google Scholar]
- 35.Alaluf S, Atkins D, Barrett K, Blount M, Carter N, Heath A. Ethnic variation in melanin content and composition in photoexposed and photoprotected human skin. Pigment Cell Res. 2002;15:112–118. doi: 10.1034/j.1600-0749.2002.1o071.x. [DOI] [PubMed] [Google Scholar]
- 36.Hoath SB, Leahy DG. The organization of human epidermis: functional epidermal units and phi proportionality. J Invest Dermatol. 2003;121:1440–1446. doi: 10.1046/j.1523-1747.2003.12606.x. [DOI] [PubMed] [Google Scholar]
- 37.Di Meglio P, Perera GK, Nestle FO. The multitasking organ: recent insights into skin immune function. Immunity. 2011;35:857–869. doi: 10.1016/j.immuni.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 39.Clausen BE, Kel JM. Langerhans cells: critical regulators of skin immunity? Immunol Cell Biol. 2010;88:351–360. doi: 10.1038/icb.2010.40. [DOI] [PubMed] [Google Scholar]
- 40.Ochoa MT, Loncaric A, Krutzik SR, Becker TC, Modlin RL. “Dermal dendritic cells” comprise two distinct populations: CD1+ dendritic cells and CD209+ macrophages. J Invest Dermatol. 2008;128:2225–2231. doi: 10.1038/jid.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, Kupper TS. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 42.Maurer DSG. Langerhans cells. In: AWLMaT, editor. Dendritic Cells: Biology and Clinical Applications. 2. San Diego CA USA: Academic Press; 2001. pp. 35–50. [Google Scholar]
- 43.Honda T, Egawa G, Grabbe S, Kabashima K. Update of immune events in the murine contact hypersensitivity model: toward the understanding of allergic contact dermatitis. J Invest Dermatol. 2013;133:303–315. doi: 10.1038/jid.2012.284. [DOI] [PubMed] [Google Scholar]
- 44.van der Aar AM, Picavet DI, Muller FJ, de Boer L, van Capel TM, Zaat SA, Bos JD, Janssen H, George TC, Kapsenberg ML, et al. Langerhans Cells Favor Skin Flora Tolerance through Limited Presentation of Bacterial Antigens and Induction of Regulatory T Cells. J Invest Dermatol. 2013;133:1240–1249. doi: 10.1038/jid.2012.500. [DOI] [PubMed] [Google Scholar]
- 45.Vogt A, Combadiere B, Hadam S, Stieler KM, Lademann J, Schaefer H, Autran B, Sterry W, Blume-Peytavi U. 40 nm, but not 750 or 1,500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. J Invest Dermatol. 2006;126:1316–1322. doi: 10.1038/sj.jid.5700226. [DOI] [PubMed] [Google Scholar]
- 46.Albanesi C, De Pita O, Girolomoni G. Resident skin cells in psoriasis: a special look at the pathogenetic functions of keratinocytes. Clin Dermatol. 2007;25:581–588. doi: 10.1016/j.clindermatol.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 47.Ansel J, Perry P, Brown J, Damm D, Phan T, Hart C, Luger T, Hefeneider S. Cytokine modulation of keratinocyte cytokines. J Invest Dermatol. 1990;94:101S–107S. doi: 10.1111/1523-1747.ep12876053. [DOI] [PubMed] [Google Scholar]
- 48.Shirakata Y. Regulation of epidermal keratinocytes by growth factors. J Dermatol Sci. 2010;59:73–80. doi: 10.1016/j.jdermsci.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Arts JH, Bloksma N, Leusink-Muis T, Kuper CF. Respiratory allergy and pulmonary irritation to trimellitic anhydride in Brown Norway rats. Toxicol Appl Pharmacol. 2003;187:38–49. doi: 10.1016/s0041-008x(02)00023-6. [DOI] [PubMed] [Google Scholar]
- 50.Beck LA, Leung DY. Allergen sensitization through the skin induces systemic allergic responses. J Allergy Clin Immunol. 2000;106:S258–263. doi: 10.1067/mai.2000.110159. [DOI] [PubMed] [Google Scholar]
- 51.De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949–963. doi: 10.1038/jid.2011.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karol MH. Respiratory effects of inhaled isocyanates. Crit Rev Toxicol. 1986;16:349–379. doi: 10.3109/10408448609037467. [DOI] [PubMed] [Google Scholar]
- 53.Warbrick EV, Dearman RJ, Kimber I. Induced changes in total serum IgE concentration in the Brown Norway rat: potential for identification of chemical respiratory allergens. J Appl Toxicol. 2002;22:1–11. doi: 10.1002/jat.830. [DOI] [PubMed] [Google Scholar]
- 54.Chourasia R, Jain SK. Drug targeting through pilosebaceous route. Curr Drug Targets. 2009;10:950–967. doi: 10.2174/138945009789577918. [DOI] [PubMed] [Google Scholar]
- 55.Lademann J, Richter H, Schanzer S, Knorr F, Meinke M, Sterry W, Patzelt A. Penetration and storage of particles in human skin: perspectives and safety aspects. Eur J Pharm Biopharm. 2011;77:465–468. doi: 10.1016/j.ejpb.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 56.Schaefer H, Lademann J. The role of follicular penetration. A differential view. Skin Pharmacol Appl Skin Physiol. 2001;14 (Suppl 1):23–27. doi: 10.1159/000056386. [DOI] [PubMed] [Google Scholar]
- 57.Alonso L, Fuchs E. Stem cells of the skin epithelium. Proc Natl Acad Sci U S A. 2003;100 (Suppl 1):11830–11835. doi: 10.1073/pnas.1734203100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimomura Y, Christiano AM. Biology and genetics of hair. Annu Rev Genomics Hum Genet. 2010;11:109–132. doi: 10.1146/annurev-genom-021610-131501. [DOI] [PubMed] [Google Scholar]
- 59.Ebling FJ. Hair. J Invest Dermatol. 1976;67:98–105. doi: 10.1111/1523-1747.ep12512509. [DOI] [PubMed] [Google Scholar]
- 60.Ohyama M. Hair follicle bulge: a fascinating reservoir of epithelial stem cells. J Dermatol Sci. 2007;46:81–89. doi: 10.1016/j.jdermsci.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 61.Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 62.Lin KK, Andersen B. Have hair follicle stem cells shed their tranquil image? Cell Stem Cell. 2008;3:581–582. doi: 10.1016/j.stem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 63.Otberg N, Richter H, Schaefer H, Blume-Peytavi U, Sterry W, Lademann J. Variations of hair follicle size and distribution in different body sites. J Invest Dermatol. 2004;122:14–19. doi: 10.1046/j.0022-202X.2003.22110.x. [DOI] [PubMed] [Google Scholar]
- 64.Mangelsdorf S, Otberg N, Maibach HI, Sinkgraven R, Sterry W, Lademann J. Ethnic variation in vellus hair follicle size and distribution. Skin Pharmacol Physiol. 2006;19:159–167. doi: 10.1159/000093050. [DOI] [PubMed] [Google Scholar]
- 65.Singh I, Morris AP. Performance of transdermal therapeutic systems: Effects of biological factors. Int J Pharm Investig. 2011;1:4–9. doi: 10.4103/2230-973X.76721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bruls WA, Slaper H, van der Leun JC, Berrens L. Transmission of human epidermis and stratum corneum as a function of thickness in the ultraviolet and visible wavelengths. Photochem Photobiol. 1984;40:485–494. doi: 10.1111/j.1751-1097.1984.tb04622.x. [DOI] [PubMed] [Google Scholar]
- 67.Tran TT, Schulman J, Fisher DE. UV and pigmentation: molecular mechanisms and social controversies. Pigment Cell Melanoma Res. 2008;21:509–516. doi: 10.1111/j.1755-148X.2008.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Polefka TG, Meyer TA, Agin PP, Bianchini RJ. Effects of solar radiation on the skin. J Cosmet Dermatol. 2012;11:134–143. doi: 10.1111/j.1473-2165.2012.00614.x. [DOI] [PubMed] [Google Scholar]
- 69.Birmingham D. Overview: Occupational Skin Diseases. Skin Diseases. 2011 Vol. Encyclopedia of Occupational Health and Safety. [Google Scholar]
- 70.Energy UDo. Fluorescent Lighting. Available at: http://www.eere.energy.gov/basics/buildings/fluorescent.html.
- 71.Mironava T, Hadjiargyrou M, Simon M, Rafailovich MH. The effects of UV emission from compact fluorescent light exposure on human dermal fibroblasts and keratinocytes in vitro. Photochem Photobiol. 2012;88:1497–1506. doi: 10.1111/j.1751-1097.2012.01192.x. [DOI] [PubMed] [Google Scholar]
- 72.Mouret S, Baudouin C, Charveron M, Favier A, Cadet J, Douki T. Cyclobutane pyrimidine dimers are predominant DNA lesions in whole human skin exposed to UVA radiation. Proc Natl Acad Sci U S A. 2006;103:13765–13770. doi: 10.1073/pnas.0604213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vella F. In: Biochemistry. Mathews CK, van Holde KE, editors. Benjamin/Cummings Publishing Co; Redwood City, CA, USA: 1990. p. 1299. £24.95. [Google Scholar]; Biochemical Education. 1990;18:154–154. [Google Scholar]
- 74.Biniek K, Levi K, Dauskardt RH. Solar UV radiation reduces the barrier function of human skin. Proc Natl Acad Sci U S A. 2012;109:17111–17116. doi: 10.1073/pnas.1206851109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu XS, Masedunskas A, Weigert R, Copeland NG, Jenkins NA, Hammer JA. Melanoregulin regulates a shedding mechanism that drives melanosome transfer from melanocytes to keratinocytes. Proc Natl Acad Sci U S A. 2012;109:E2101–2109. doi: 10.1073/pnas.1209397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tadokoro T, Yamaguchi Y, Batzer J, Coelho SG, Zmudzka BZ, Miller SA, Wolber R, Beer JZ, Hearing VJ. Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J Invest Dermatol. 2005;124:1326–1332. doi: 10.1111/j.0022-202X.2005.23760.x. [DOI] [PubMed] [Google Scholar]
- 77.Mortensen LJ, Ravichandran S, Delouise LA. The impact of UVB exposure and differentiation state of primary keratinocytes on their interaction with quantum dots. Nanotoxicology. 2012 doi: 10.3109/17435390.2012.733437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marionnet C, Pierrard C, Lejeune F, Sok J, Thomas M, Bernerd F. Different oxidative stress response in keratinocytes and fibroblasts of reconstructed skin exposed to non extreme daily-ultraviolet radiation. PLoS One. 2010;5:e12059. doi: 10.1371/journal.pone.0012059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brouxhon S, Kyrkanides S, O’Banion MK, Johnson R, Pearce DA, Centola GM, Miller JN, McGrath KH, Erdle B, Scott G, et al. Sequential down-regulation of E-cadherin with squamous cell carcinoma progression: loss of E-cadherin via a prostaglandin E2-EP2 dependent posttranslational mechanism. Cancer Res. 2007;67:7654–7664. doi: 10.1158/0008-5472.CAN-06-4415. [DOI] [PubMed] [Google Scholar]
- 80.Tripp CS, Blomme EA, Chinn KS, Hardy MM, LaCelle P, Pentland AP. Epidermal COX-2 induction following ultraviolet irradiation: suggested mechanism for the role of COX-2 inhibition in photoprotection. J Invest Dermatol. 2003;121:853–861. doi: 10.1046/j.1523-1747.2003.12495.x. [DOI] [PubMed] [Google Scholar]
- 81.Holleran WM, Uchida Y, Halkier-Sorensen L, Haratake A, Hara M, Epstein JH, Elias PM. Structural and biochemical basis for the UVB-induced alterations in epidermal barrier function. Photodermatol Photoimmunol Photomed. 1997;13:117–128. doi: 10.1111/j.1600-0781.1997.tb00214.x. [DOI] [PubMed] [Google Scholar]
- 82.Del Bino S, Vioux C, Rossio-Pasquier P, Jomard A, Demarchez M, Asselineau D, Bernerd F. Ultraviolet B induces hyperproliferation and modification of epidermal differentiation in normal human skin grafted on to nude mice. Br J Dermatol. 2004;150:658–667. doi: 10.1111/j.0007-0963.2004.05886.x. [DOI] [PubMed] [Google Scholar]
- 83.Mortensen LJ, Jatana S, Gelein R, De Benedetto A, De Mesy Bentley KL, Beck LA, Elder A, Delouise LA. Quantification of quantum dot murine skin penetration with UVR barrier impairment. Nanotoxicology. 2013 doi: 10.3109/17435390.2012.741726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beissert S, Schwarz T. Ultraviolet-induced immunosuppression: implications for photocarcinogenesis. Cancer Treat Res. 2009;146:109–121. doi: 10.1007/978-0-387-78574-5_10. [DOI] [PubMed] [Google Scholar]
- 85.Aberer W, Schuler G, Stingl G, Honigsmann H, Wolff K. Ultraviolet light depletes surface markers of Langerhans cells. J Invest Dermatol. 1981;76:202–210. doi: 10.1111/1523-1747.ep12525745. [DOI] [PubMed] [Google Scholar]
- 86.Stingl G, Gazze-Stingl LA, Aberer W, Wolff K. Antigen presentation by murine epidermal langerhans cells and its alteration by ultraviolet B light. J Immunol. 1981;127:1707–1713. [PubMed] [Google Scholar]
- 87.Toews GB, Bergstresser PR, Streilein JW. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980;124:445–453. [PubMed] [Google Scholar]
- 88.Schwarz A, Maeda A, Schwarz T. Alteration of the migratory behavior of UV-induced regulatory T cells by tissue-specific dendritic cells. J Immunol. 2007;178:877–886. doi: 10.4049/jimmunol.178.2.877. [DOI] [PubMed] [Google Scholar]
- 89.Schwarz A, Maeda A, Wild MK, Kernebeck K, Gross N, Aragane Y, Beissert S, Vestweber D, Schwarz T. Ultraviolet radiation-induced regulatory T cells not only inhibit the induction but can suppress the effector phase of contact hypersensitivity. J Immunol. 2004;172:1036–1043. doi: 10.4049/jimmunol.172.2.1036. [DOI] [PubMed] [Google Scholar]
- 90.Schwarz A, Navid F, Sparwasser T, Clausen BE, Schwarz T. In vivo reprogramming of UV radiation-induced regulatory T-cell migration to inhibit the elicitation of contact hypersensitivity. J Allergy Clin Immunol. 2011;128:826–833. doi: 10.1016/j.jaci.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 91.Schwarz T. The dark and the sunny sides of UVR-induced immunosuppression: photoimmunology revisited. J Invest Dermatol. 2010;130:49–54. doi: 10.1038/jid.2009.217. [DOI] [PubMed] [Google Scholar]
- 92.Schwarz T, Schwarz A. Molecular mechanisms of ultraviolet radiation-induced immunosuppression. Eur J Cell Biol. 2011;90:560–564. doi: 10.1016/j.ejcb.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 93.Beissert S, Schwarz A, Schwarz T. Regulatory T cells. J Invest Dermatol. 2006;126:15–24. doi: 10.1038/sj.jid.5700004. [DOI] [PubMed] [Google Scholar]
- 94.Schwarz A, Noordegraaf M, Maeda A, Torii K, Clausen BE, Schwarz T. Langerhans cells are required for UVR-induced immunosuppression. J Invest Dermatol. 2010;130:1419–1427. doi: 10.1038/jid.2009.429. [DOI] [PubMed] [Google Scholar]
- 95.Kiss F, Anstey AV. A review of UVB-mediated photosensitivity disorders. Photochem Photobiol Sci. 2013;12:37–46. doi: 10.1039/c2pp25275a. [DOI] [PubMed] [Google Scholar]
- 96.Schwarz T. Regulatory T cells induced by ultraviolet radiation. Int Arch Allergy Immunol. 2005;137:187–193. doi: 10.1159/000086330. [DOI] [PubMed] [Google Scholar]
- 97.Abdel-Mottaleb MM, Moulari B, Beduneau A, Pellequer Y, Lamprecht A. Surface-charge-dependent nanoparticles accumulation in inflamed skin. J Pharm Sci. 2012;101:4231–4239. doi: 10.1002/jps.23282. [DOI] [PubMed] [Google Scholar]
- 98.Hirai T, Yoshikawa T, Nabeshi H, Yoshida T, Tochigi S, Ichihashi K, Uji M, Akase T, Nagano K, Abe Y, et al. Amorphous silica nanoparticles size-dependently aggravate atopic dermatitis-like skin lesions following an intradermal injection. Part Fibre Toxicol. 2012;9:3. doi: 10.1186/1743-8977-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim ST, Saha K, Kim C, Rotello VM. The Role of Surface Functionality in Determining Nanoparticle Cytotoxicity. Acc Chem Res. 2013 doi: 10.1021/ar3000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rancan F, Gao Q, Graf C, Troppens S, Hadam S, Hackbarth S, Kembuan C, Blume-Peytavi U, Ruhl E, Lademann J, et al. Skin penetration and cellular uptake of amorphous silica nanoparticles with variable size, surface functionalization, and colloidal stability. ACS Nano. 2012;6:6829–6842. doi: 10.1021/nn301622h. [DOI] [PubMed] [Google Scholar]
- 101.Yanagisawa R, Takano H, Inoue KI, Koike E, Sadakane K, Ichinose T. Size effects of polystyrene nanoparticles on atopic dermatitislike skin lesions in NC/NGA mice. Int J Immunopathol Pharmacol. 2010;23:131–141. doi: 10.1177/039463201002300112. [DOI] [PubMed] [Google Scholar]
- 102.Hagar I, Labouta LKE-K, Kraus Tobias, Schneider M. Mechanism and determinants of nanoparticle penetration through human skin. Nanoscale Research Letters. 2011;3:10. doi: 10.1039/c1nr11109d. [DOI] [PubMed] [Google Scholar]
- 103.Monteiro-Riviere NA, Wiench K, Landsiedel R, Schulte S, Inman AO, Riviere JE. Safety evaluation of sunscreen formulations containing titanium dioxide and zinc oxide nanoparticles in UVB sunburned skin: an in vitro and in vivo study. Toxicol Sci. 2011;123:264–280. doi: 10.1093/toxsci/kfr148. [DOI] [PubMed] [Google Scholar]
- 104.Gopee NV, Roberts DW, Webb P, Cozart CR, Siitonen PH, Latendresse JR, Warbitton AR, Yu WW, Colvin VL, Walker NJ, et al. Quantitative determination of skin penetration of PEG-coated CdSe quantum dots in dermabraded but not intact SKH-1 hairless mouse skin. Toxicol Sci. 2009;111:37–48. doi: 10.1093/toxsci/kfp139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mortensen LJ, Oberdorster G, Pentland AP, Delouise LA. In vivo skin penetration of quantum dot nanoparticles in the murine model: the effect of UVR. Nano Lett. 2008;8:2779–2787. doi: 10.1021/nl801323y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang LW, Monteiro-Riviere NA. Assessment of quantum dot penetration into intact, tape-stripped, abraded and flexed rat skin. Skin Pharmacol Physiol. 2008;21:166–180. doi: 10.1159/000131080. [DOI] [PubMed] [Google Scholar]
- 107.Mortensen LJ, Ravichandran S, Zheng H, DeLouise LA. Progress and challenges in quantifying skin permeability to nanoparticles using a quantum dot model. J Biomed Nanotechnol. 2010;6:596–604. doi: 10.1166/jbn.2010.1156. [DOI] [PubMed] [Google Scholar]
- 108.Gulson B, McCall M, Korsch M, Gomez L, Casey P, Oytam Y, Taylor A, McCulloch M, Trotter J, Kinsley L, et al. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicol Sci. 2010;118:140–149. doi: 10.1093/toxsci/kfq243. [DOI] [PubMed] [Google Scholar]
- 109.Gulson B, Wong H, Korsch M, Gomez L, Casey P, McCall M, McCulloch M, Trotter J, Stauber J, Greenoak G. Comparison of dermal absorption of zinc from different sunscreen formulations and differing UV exposure based on stable isotope tracing. Sci Total Environ. 2012;420:313–318. doi: 10.1016/j.scitotenv.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 110.Darvin ME, Konig K, Kellner-Hoefer M, Breunig HG, Werncke W, Meinke MC, Patzelt A, Sterry W, Lademann J. Safety assessment by multiphoton fluorescence/second harmonic generation/hyper-Rayleigh scattering tomography of ZnO nanoparticles used in cosmetic products. Skin Pharmacol Physiol. 2012;25:219–226. doi: 10.1159/000338976. [DOI] [PubMed] [Google Scholar]
- 111.Gopee NV, Roberts DW, Webb P, Cozart CR, Siitonen PH, Warbritton AR, Yu WW, Colvin VL, Walker NJ, Howard PC. Migration of intradermally injected quantum dots to sentinel organs in mice. Toxicol Sci. 2007;98:249–257. doi: 10.1093/toxsci/kfm074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin LL, Grice JE, Butler MK, Zvyagin AV, Becker W, Robertson TA, Soyer HP, Roberts MS, Prow TW. Time-correlated single photon counting for simultaneous monitoring of zinc oxide nanoparticles and NAD(P)H in intact and barrier-disrupted volunteer skin. Pharm Res. 2011;28:2920–2930. doi: 10.1007/s11095-011-0515-5. [DOI] [PubMed] [Google Scholar]
- 113.Prow TW. Multiphoton microscopy applications in nanodermatology. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:680–690. doi: 10.1002/wnan.1189. [DOI] [PubMed] [Google Scholar]
- 114.Raphael AP, Sundh D, Grice JE, Roberts MS, Soyer HP, Prow TW. Zinc oxide nanoparticle removal from wounded human skin. Nanomedicine (Lond) 2013 doi: 10.2217/nnm.12.196. [DOI] [PubMed] [Google Scholar]
- 115.Baroli B. Penetration of nanoparticles and nanomaterials in the skin: fiction or reality? J Pharm Sci. 2010;99:21–50. doi: 10.1002/jps.21817. [DOI] [PubMed] [Google Scholar]
- 116.Nohynek GJ, Dufour EK. Nano-sized cosmetic formulations or solid nanoparticles in sunscreens: a risk to human health? Arch Toxicol. 2012;86:1063–1075. doi: 10.1007/s00204-012-0831-5. [DOI] [PubMed] [Google Scholar]
- 117.Smijs TGPS. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnology, Science and Applications. 2011;4:17. doi: 10.2147/NSA.S19419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Subedi RK, Oh SY, Chun MK, Choi HK. Recent advances in transdermal drug delivery. Arch Pharm Res. 2010;33:339–351. doi: 10.1007/s12272-010-0301-7. [DOI] [PubMed] [Google Scholar]
- 119.Benson HA. Transdermal drug delivery: penetration enhancement techniques. Curr Drug Deliv. 2005;2:23–33. doi: 10.2174/1567201052772915. [DOI] [PubMed] [Google Scholar]
- 120.Barbero AM, Frasch HF. Transcellular route of diffusion through stratum corneum: results from finite element models. J Pharm Sci. 2006;95:2186–2194. doi: 10.1002/jps.20695. [DOI] [PubMed] [Google Scholar]
- 121.Kuo TR, Wu CL, Hsu CT, Lo W, Chiang SJ, Lin SJ, Dong CY, Chen CC. Chemical enhancer induced changes in the mechanisms of transdermal delivery of zinc oxide nanoparticles. Biomaterials. 2009;30:3002–3008. doi: 10.1016/j.biomaterials.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 122.Labouta HI, El-Khordagui LK, Schneider M. Could chemical enhancement of gold nanoparticle penetration be extrapolated from established approaches for drug permeation? Skin Pharmacol Physiol. 2012;25:208–218. doi: 10.1159/000338688. [DOI] [PubMed] [Google Scholar]
- 123.Karande P, Jain A, Ergun K, Kispersky V, Mitragotri S. Design principles of chemical penetration enhancers for transdermal drug delivery. Proc Natl Acad Sci U S A. 2005;102:4688–4693. doi: 10.1073/pnas.0501176102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu I-LWL-F. Epicutaneous sensitization with protein antigen. Dermatologica Sinica. 2012;30:5. [Google Scholar]
- 125.McLean W, Shepherd JP, Brann CR, Westmoreland D. Risks associated with occupational glass injury in bar staff with special consideration of hepatitis B infection. Occup Med (Lond) 1997;47:147–150. doi: 10.1093/occmed/47.3.147. [DOI] [PubMed] [Google Scholar]
- 126.Jakasa I, Verberk MM, Bunge AL, Kruse J, Kezic S. Increased permeability for polyethylene glycols through skin compromised by sodium lauryl sulphate. Exp Dermatol. 2006;15:801–807. doi: 10.1111/j.1600-0625.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- 127.Larese Filon F, Crosera M, Timeus E, Adami G, Bovenzi M, Ponti J, Maina G. Human skin penetration of cobalt nanoparticles through intact and damaged skin. Toxicol In Vitro. 2013;27:121–127. doi: 10.1016/j.tiv.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 128.Prow TW, Monteiro-Riviere NA, Inman AO, Grice JE, Chen X, Zhao X, Sanchez WH, Gierden A, Kendall MA, Zvyagin AV, et al. Quantum dot penetration into viable human skin. Nanotoxicology. 2012;6:173–185. doi: 10.3109/17435390.2011.569092. [DOI] [PubMed] [Google Scholar]
- 129.Ravichandran SML, DeLouise LA. Quantification of human skin barrier function and susceptibility to quantum dot skin penetration. Nanotoxicology. 2011;5:11. doi: 10.3109/17435390.2010.537381. [DOI] [PubMed] [Google Scholar]
- 130.Blume-Peytavi U, Vogt A. Human hair follicle: reservoir function and selective targeting. Br J Dermatol. 2011;165 (Suppl 2):13–17. doi: 10.1111/j.1365-2133.2011.10572.x. [DOI] [PubMed] [Google Scholar]
- 131.Hansen S, Lehr CM. Nanoparticles for transcutaneous vaccination. Microb Biotechnol. 2012;5:156–167. doi: 10.1111/j.1751-7915.2011.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lademann J, Richter H, Schaefer UF, Blume-Peytavi U, Teichmann A, Otberg N, Sterry W. Hair follicles - a long-term reservoir for drug delivery. Skin Pharmacol Physiol. 2006;19:232–236. doi: 10.1159/000093119. [DOI] [PubMed] [Google Scholar]
- 133.Patzelt A, Lademann J. Drug delivery to hair follicles. Expert Opin Drug Deliv. 2013;10:787–797. doi: 10.1517/17425247.2013.776038. [DOI] [PubMed] [Google Scholar]
- 134.Vogt A, Mandt N, Lademann J, Schaefer H, Blume-Peytavi U. Follicular targeting--a promising tool in selective dermatotherapy. J Investig Dermatol Symp Proc. 2005;10:252–255. doi: 10.1111/j.1087-0024.2005.10124.x. [DOI] [PubMed] [Google Scholar]
- 135.Gratieri T, Schaefer UF, Jing L, Gao M, Kostka KH, Lopez RF, Schneider M. Penetration of quantum dot particles through human skin. J Biomed Nanotechnol. 2010;6:586–595. doi: 10.1166/jbn.2010.1155. [DOI] [PubMed] [Google Scholar]
- 136.Patzelt A, Richter H, Knorr F, Schafer U, Lehr CM, Dahne L, Sterry W, Lademann J. Selective follicular targeting by modification of the particle sizes. J Control Release. 2011;150:45–48. doi: 10.1016/j.jconrel.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 137.Rouse JG, Yang J, Ryman-Rasmussen JP, Barron AR, Monteiro-Riviere NA. Effects of mechanical flexion on the penetration of fullerene amino acid-derivatized peptide nanoparticles through skin. Nano Lett. 2007;7:155–160. doi: 10.1021/nl062464m. [DOI] [PubMed] [Google Scholar]
- 138.Schneider M, Stracke F, Hansen S, Schaefer UF. Nanoparticles and their interactions with the dermal barrier. Dermatoendocrinol. 2009;1:197–206. doi: 10.4161/derm.1.4.9501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tinkle SS, Antonini JM, Rich BA, Roberts JR, Salmen R, DePree K, Adkins EJ. Skin as a route of exposure and sensitization in chronic beryllium disease. Environ Health Perspect. 2003;111:1202–1208. doi: 10.1289/ehp.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Toll R, Jacobi U, Richter H, Lademann J, Schaefer H, Blume-Peytavi U. Penetration profile of microspheres in follicular targeting of terminal hair follicles. J Invest Dermatol. 2004;123:168–176. doi: 10.1111/j.0022-202X.2004.22717.x. [DOI] [PubMed] [Google Scholar]
- 141.Leung DY. Infection in atopic dermatitis. Curr Opin Pediatr. 2003;15:399–404. doi: 10.1097/00008480-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 142.Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649–655. doi: 10.1067/mjd.2000.107773. [DOI] [PubMed] [Google Scholar]
- 143.Jang J, Lim DH, Choi IH. The impact of nanomaterials in immune system. Immune Netw. 2010;10:85–91. doi: 10.4110/in.2010.10.3.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zolnik BS, Gonzalez-Fernandez A, Sadrieh N, Dobrovolskaia MA. Nanoparticles and the immune system. Endocrinology. 2010;151:458–465. doi: 10.1210/en.2009-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.do Nascimento DF, Silva AC, Mansur CR, de Presgrave RF, Alves EN, Silva RS, Ricci-Junior E, de Freitas ZM, dos Santos EP. Characterization and evaluation of poly(epsilon-caprolactone) nanoparticles containing 2-ethylhexyl-p-methoxycinnamate, octocrylene, and benzophenone-3 in anti-solar preparations. J Nanosci Nanotechnol. 2012;12:7155–7166. doi: 10.1166/jnn.2012.5832. [DOI] [PubMed] [Google Scholar]
- 146.Yoshihisa Y, Honda A, Zhao QL, Makino T, Abe R, Matsui K, Shimizu H, Miyamoto Y, Kondo T, Shimizu T. Protective effects of platinum nanoparticles against UV-light-induced epidermal inflammation. Exp Dermatol. 2010;19:1000–1006. doi: 10.1111/j.1600-0625.2010.01128.x. [DOI] [PubMed] [Google Scholar]
- 147.Murray AR, Kisin E, Inman A, Young SH, Muhammed M, Burks T, Uheida A, Tkach A, Waltz M, Castranova V, et al. Oxidative Stress and Dermal Toxicity of Iron Oxide Nanoparticles In Vitro. Cell Biochem Biophys. 2012 doi: 10.1007/s12013-012-9367-9. [DOI] [PubMed] [Google Scholar]
- 148.Krause M, Klit A, Blomberg Jensen M, Soeborg T, Frederiksen H, Schlumpf M, Lichtensteiger W, Skakkebaek NE, Drzewiecki KT. Sunscreens: are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. Int J Androl. 2012;35:424–436. doi: 10.1111/j.1365-2605.2012.01280.x. [DOI] [PubMed] [Google Scholar]
- 149.Schlumpf M, Kypke K, Wittassek M, Angerer J, Mascher H, Mascher D, Vokt C, Birchler M, Lichtensteiger W. Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: correlation of UV filters with use of cosmetics. Chemosphere. 2010;81:1171–1183. doi: 10.1016/j.chemosphere.2010.09.079. [DOI] [PubMed] [Google Scholar]
- 150.Hanson KM, Gratton E, Bardeen CJ. Sunscreen enhancement of UV-induced reactive oxygen species in the skin. Free Radic Biol Med. 2006;41:1205–1212. doi: 10.1016/j.freeradbiomed.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 151.Newman MD, Stotland M, Ellis JI. The safety of nanosized particles in titanium dioxide- and zinc oxide-based sunscreens. J Am Acad Dermatol. 2009;61:685–692. doi: 10.1016/j.jaad.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 152.Stamatakis PPB, Salzman GC, Bohren CF, Allen TB. Optimum particle size of titanium dioxide and zinc oxide for attenuation of ultraviolet radiation. The Journal of Coatings Technology. 1990;62:3. [Google Scholar]
- 153.ANB. The design, fabrication, and photocatalytic utility of nanostructured semiconductors: focus on TiO2-based nanostructures. Nanotchnol Sci Appl. 2011;4:30. doi: 10.2147/NSA.S9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang SQ, Tooley IR. Photoprotection in the era of nanotechnology. Semin Cutan Med Surg. 2011;30:210–213. doi: 10.1016/j.sder.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 155.Tyner KM, Wokovich AM, Godar DE, Doub WH, Sadrieh N. The state of nano-sized titanium dioxide (TiO2) may affect sunscreen performance. International Journal of Cosmetic Science. 2011;33:234–244. doi: 10.1111/j.1468-2494.2010.00622.x. [DOI] [PubMed] [Google Scholar]
- 156.Sayes CM, Wahi R, Kurian PA, Liu Y, West JL, Ausman KD, Warheit DB, Colvin VL. Correlating nanoscale titania structure with toxicity: a cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol Sci. 2006;92:174–185. doi: 10.1093/toxsci/kfj197. [DOI] [PubMed] [Google Scholar]
- 157.Carlotti M, Ugazio E, Sapino S, Fenoglio I, Greco G, Fubini B. Role of particle coating in controlling skin damage photoinduced by titania nanoparticles. Free Radic Res. 2009;43:312– 322. doi: 10.1080/10715760802716633. [DOI] [PubMed] [Google Scholar]
- 158.Fisichella M, Berenguer F, Steinmetz G, Auffan M, Rose J, Prat O. Intestinal toxicity evaluation of TiO2 degraded surface-treated nanoparticles: a combined physico-chemical and toxicogenomics approach in caco-2 cells. Particle and Fibre Toxicology. 2012;9:18. doi: 10.1186/1743-8977-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mortensen L, Oberdorster G, Pentland A, Delouise L. In vivo skin penetration of quantum dot nanoparticles in the murine model: the effect of UVR. Nano letters Nano Letters. 2008;8:8. doi: 10.1021/nl801323y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Martorano LM, Stork CJ, Li YV. UV irradiation-induced zinc dissociation from commercial zinc oxide sunscreen and its action in human epidermal keratinocytes. Journal of Cosmetic Dermatology. 2010;9:276–286. doi: 10.1111/j.1473-2165.2010.00521.x. [DOI] [PubMed] [Google Scholar]
- 161.Konig K, Riemann I. High-resolution multiphoton tomography of human skin with subcellular spatial resolution and picosecond time resolution. J Biomed Opt. 2003;8:432–439. doi: 10.1117/1.1577349. [DOI] [PubMed] [Google Scholar]
- 162.Dai DC, Xu SJ, Shi SL, Xie MH, Che CM. Efficient multiphoton-absorption-induced luminescence in single-crystalline ZnO at room temperature. Opt Lett. 2005;30:3377–3379. doi: 10.1364/ol.30.003377. [DOI] [PubMed] [Google Scholar]
- 163.Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001;9:66–76. doi: 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 164.Miquel-Jeanjean C, Crépel F, Raufast V, Payre B, Datas L, Bessou-Touya S, Duplan H. Penetration Study of Formulated Nanosized Titanium Dioxide in Models of Damaged and Sun-Irradiated Skins. Photochemistry and Photobiology. 2012;88:1513–1521. doi: 10.1111/j.1751-1097.2012.01181.x. [DOI] [PubMed] [Google Scholar]
- 165.Bennat C, Muller-Goymann CC. Skin penetration and stabilization of formulations containing microfine titanium dioxide as physical UV filter. Int J Cosmet Sci. 2000;22:271–283. doi: 10.1046/j.1467-2494.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 166.Lee PW, Hsu SH, Tsai JS, Chen FR, Huang PJ, Ke CJ, Liao ZX, Hsiao CW, Lin HJ, Sung HW. Multifunctional core-shell polymeric nanoparticles for transdermal DNA delivery and epidermal Langerhans cells tracking. Biomaterials. 2010;31:2425–2434. doi: 10.1016/j.biomaterials.2009.11.100. [DOI] [PubMed] [Google Scholar]
- 167.Yanagisawa R, Takano H, Inoue K, Koike E, Kamachi T, Sadakane K, Ichinose T. Titanium dioxide nanoparticles aggravate atopic dermatitis-like skin lesions in NC/Nga mice. Exp Biol Med (Maywood) 2009;234:314–322. doi: 10.3181/0810-RM-304. [DOI] [PubMed] [Google Scholar]
- 168.Hussain S, Smulders S, De Vooght V, Ectors B, Boland S, Marano F, Van Landuyt KL, Nemery B, Hoet PH, Vanoirbeek JA. Nano-titanium dioxide modulates the dermal sensitization potency of DNCB. Part Fibre Toxicol. 2012;9:15. doi: 10.1186/1743-8977-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Park YH, Jeong SH, Yi SM, Choi BH, Kim YR, Kim IK, Kim MK, Son SW. Analysis for the potential of polystyrene and TiO2 nanoparticles to induce skin irritation, phototoxicity, and sensitization. Toxicol In Vitro. 2011;25:1863–1869. doi: 10.1016/j.tiv.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 170.Lee S, Yun HS, Kim SH. The comparative effects of mesoporous silica nanoparticles and colloidal silica on inflammation and apoptosis. Biomaterials. 2011;32:9434–9443. doi: 10.1016/j.biomaterials.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 171.Keck CM, Schwabe K. Silver-nanolipid complex for application to atopic dermatitis skin: rheological characterization, in vivo efficiency and theory of action. J Biomed Nanotechnol. 2009;5:428–436. doi: 10.1166/jbn.2009.1053. [DOI] [PubMed] [Google Scholar]
- 172.Zheng D, Giljohann DA, Chen DL, Massich MD, Wang XQ, Iordanov H, Mirkin CA, Paller AS. Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proc Natl Acad Sci U S A. 2012;109:11975–11980. doi: 10.1073/pnas.1118425109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kaur S, Lyte P, Garay M, Liebel F, Sun Y, Liu JC, Southall MD. Galvanic zinc-copper microparticles produce electrical stimulation that reduces the inflammatory and immune responses in skin. Arch Dermatol Res. 2011;303:551–562. doi: 10.1007/s00403-011-1145-9. [DOI] [PubMed] [Google Scholar]
- 174.Sumbayev VV, Yasinska IM, Garcia CP, Gilliland D, Lall GS, Gibbs BF, Bonsall DR, Varani L, Rossi F, Calzolai L. Gold nanoparticles downregulate interleukin-1beta-induced pro-inflammatory responses. Small. 2013;9:472–477. doi: 10.1002/smll.201201528. [DOI] [PubMed] [Google Scholar]
- 175.Ryan JJ, Bateman HR, Stover A, Gomez G, Norton SK, Zhao W, Schwartz LB, Lenk R, Kepley CL. Fullerene Nanomaterials Inhibit the Allergic Response. The Journal of Immunology. 2007;179:665–672. doi: 10.4049/jimmunol.179.1.665. [DOI] [PubMed] [Google Scholar]