Abstract
Purpose
To evaluate the bi-directional association between urologic symptoms (urinary incontinence (UI), lower urinary tract symptoms (LUTS), and nocturia) and sleep-related variables.
Materials and Methods
Data were obtained from a prospective cohort study of 1,610 men and 2,535 women who completed baseline (2002–05) and follow-up (2006–10) phases of the Boston Area Community Health (BACH) survey, a population-based random sample survey. Sleep restriction (≤5 hours/night), restless sleep, sleep medication use, and urologic symptoms were assessed by self-report. UI was defined as weekly leakage or moderate/severe leakage, LUTS (overall, obstructive, irritative) was defined by American Urological Association Symptom Index, and nocturia was defined as urinary frequency ≥2 times/night.
Results
At the 5 year follow-up,10.0%, 8.5% and 16.0% of subjects newly reported LUTS, UI and nocturia, respectively, and 24.2%, 13.3%, 11.6% newly reported poor sleep quality, sleep restriction and use of sleep medication, respectively. Controlling for confounders, the odds of developing urologic symptoms was consistently increased for subjects who reported poor sleep quality and sleep restriction at baseline, but only baseline nocturia was positively associated with incident sleep-related problems at follow-up. Body mass index, a potential mediator, reduced selected associations between sleep and incident UI and irritative symptoms, but C-reactive protein did not.
Conclusions
These data suggest that self-reported sleep-related problems and urologic symptoms are linked bi-directionally, and BMI may be a factor in the relationship between sleep and development of urologic symptoms.
MeSH Terms: Cohort Studies, Epidemiology, Sleep, Urologic Diseases
INTRODUCTION
Urinary incontinence (UI), lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH), and nocturia are common disorders which affect quality of life (QOL).1 At the same time, sleep disorders affect 50–70 million people in the US,2 with sleep deprivation increasing in prevalence.3
It is implicitly assumed that urologic symptoms lead to sleep-related problems, and not vice versa.4, 5 However, it is plausible that sleep-related problems lead to the development of urologic symptoms, mainly through their associations with obesity,6 metabolic dysregulation,7, 8 and systemic inflammation.9 While studies have examined the bi-directional association between other health conditions and urologic outcomes,10, 11 to our knowledge, no studies have explored the bi-directional relationship between sleep and urologic symptoms.
We tested the 5-year longitudinal relations between (1) sleep-related variables at baseline and incident urologic symptoms at follow-up, (2) urologic symptoms at baseline and incident sleep-related variables at follow-up, and (3) whether body composition or inflammatory status plays a role in mediating these associations.
MATERIALS AND METHODS
Overall design and overview of data collection
Data were obtained from an observational, longitudinal cohort study of men and women, the Boston Area Community Health (BACH) Survey.12 In brief, BACH recruited a random sample of 5,502 Boston men and women aged 30–79y from three racial/ethnic groups (Black, Hispanic, White) to complete an in-person interview at baseline (2002–2005) and approximately 5 years later (2006–2010). Home visits were conducted for anthropometric measurements and interviews regarding urologic symptoms, health, and lifestyle factors. Follow-up interviews were completed on 4,144 individuals (1,610 men; 2,534 women; conditional response rate, 80.5%). All subjects provided written informed consent and the study received Institutional Review Board approval.
Measurement of sleep parameters
Sleep quality
Subjects had poor sleep quality if they endorsed “yes” in response to the following statement: “Much of the time during the last week… my sleep was restless”.
Restricted sleep
Sleep restriction was assessed by: “How much do you usually sleep?” (≤5 vs.>5 h per night). Data on sleep restriction were not collected from women at baseline, mainly because the sleep data on men were obtained in the context of a previously developed set of symptoms pertaining to male hypogonadism.
Sleep medication use
Use of prescription, over-the-counter, and supplemental medications were captured using self-report (prompts by indication) and direct observation/recording of medication labels by the interviewer, which were coded using the Slone Drug Dictionary.13 Medications within the sedative hypnotic class that are used to induce sleep were considered ‘primary sedation’ medications. See on-line material for medication coding scheme.
Measurement of urologic symptoms
Lower urinary tract symptoms
LUTS was assessed by American Urologic Association Symptom Index (AUA-SI).14 The AUA-SI was developed and validated for BPH in men,14 but has been validated to capture LUTS in women.15 Given that we were interested in separately examining the relationship between nocturia and sleep-related problems, we created a summary AUA-SI score that excluded the nocturia item. This modified score could range from 0 to 30 with higher scores indicating more (frequent) symptoms, and presence of LUTS defined as score ≥7 (moderate/severe symptoms) on this modified AUA-SI. We also examined LUTS obstructive symptom subscores (voiding symptoms) (≥5 for incomplete bladder emptying, straining, weak urinary stream, hesitancy symptoms) and irritative symptom subscores (storage symptoms) (≥4 for frequency, urgency, nocturia).
Urinary incontinence
UI was defined using Sandvik’s severity index (frequency and quantity of urine leakage),16 with presence defined as weekly or moderate/severe leakage.
Nocturia
Nocturia was defined as needing to get up to urinate more than once during the night (fairly often, usually, almost always) or ≥2 urinations at night after falling asleep.17
Potential confounding variables
Several baseline variables were considered a priori confounding variables, including age (decades), sex (male/female), race/ethnicity (Black/Hispanic/White), SES (low/medium/high) type 2 diabetes mellitus (yes/no), heart disease (yes/no), alcohol use (0/<1/1−3/3+ drinks per day), physical activity (low/medium/high), smoking (current/former/never) and anti-depressant use (yes/no). Additionally, use of sedating (e.g., antihistamines) and stimulating (e.g., amphetamines) medications not indicated for sleep disorders were considered as covariates. All variables were included in all regression models, with minor exceptions noted.
Potential mediating variables
Based on prior literature, we hypothesize the following mediational pathways: (1) body composition, assessed by weight, waist circumference, and body mass index (BMI); and (2) circulating C-reactive protein (CRP), a marker of inflammation measured with an immuno turbidimetric assay on the Hitachi 917 analyzer (Roche Diagnostics, Indianapolis, IN).
Statistical analysis
The incidence of sleep-related problems and urologic symptoms at 5 years were defined as the percentage of those with the relevant condition at follow-up, among those without the condition at baseline. The normal approximation to the binomial distribution was used to compute 95% confidence intervals (CI) on incidence. The association of baseline characteristics with incident urologic and sleep outcomes were tested with Wald-type tests.
Odds ratios (OR) and 95%CIs were estimated from multivariable logistic regression models of the association between sleep variables/urologic symptoms assessed at baseline and incidence of urologic symptoms/sleep variables at follow-up, adjusted for covariates. The multivariable models controlled for all covariates except for the primary stimulant coalition medication, which was redundant with the secondary stimulation coalition. Interactions between sex and exposures of interest as well as multicollinearity among medication coalitions were tested. Of 24 interactions with sex tested, only one was statistically significant, thus allowing us to pool data on men and women. We determined that follow-up time was similar in subjects with/without incident outcomes, thus follow-up time was not adjusted in final models.
Mediation was tested by examining whether body composition variables and CRP induced a 10% change in the exposure OR.18 Preliminary modeling identified BMI (vs. weight and waist) as more strongly related to outcomes of interest, thus we examined BMI only in mediation analyses.
Multiple imputation (15 datasets) was used to impute plausible values for missing observations using SAS 9.1.3 (SAS Institute, Cary, NC). Missing data for the variables of interest (sleep and urologic symptoms) were missing in less than 1% of subjects. Observations were weighted inversely to their probability of selection, and weights were post-stratified to the Boston population in 2000. Analyses were conducted in SUDAAN 9.0.1 (Research Triangle Institute, Research Triangle Park, NC).
Analytic samples
Analyses of outcomes were restricted to at-risk samples, i.e., those without the relevant condition at baseline. Sample sizes for incident LUTS, UI, and nocturia were 3,423, 3,724, and 2,765, respectively. Sample sizes for incident poor sleep quality, sleep medication use, and sleep restriction (men only) were 2,449, 3,529, and 1,279, respectively.
RESULTS
Demographic profile of the population
The mean baseline age of the BACH Survey population who completed follow-up (N=4,144) was 49y. A total of 1,610 men and 2,534 women completed the follow-up assessment, with women slightly older than men at baseline (50y vs. 48y).
Incident urologic symptoms
At the 5-year follow-up, LUTS, UI and nocturia, respectively, were reported by 10.0%, 8.5%and 16.0% of subjects who did not have these symptoms at baseline (Supplemental Table 1). Incidence of obstructive symptoms was 7.0% (95% CI: 5.6, 8.6%) and irritative symptoms 20.7% (95% CI: 18.2, 23.6%) (data not shown). Crude incidence of urologic symptoms was related to increasing age (P<0.001) and several other factors.
Incident sleep outcomes
Newly reported poor sleep quality, sleep restriction and use of sleep medication observed in 24.2% (95% CI: 21.3, 27.3%), 13.3% (95% CI: 10.6, 16.4%), 11.6% (95% CI: 10.0, 13.4%) of subjects at the 5-year follow-up (Supplemental Table 2). Compared with urologic symptoms, crude incidence of sleep variables exhibited fewer associations with confounding variables of interest. Selected results showed that incident poor sleep occurred most commonly in subjects with low SES [32.2% (95% CI: 26.7, 38.2%)] and incident sleep restriction occurred most commonly in Blacks[20.9% (95% CI: 16.3, 26.3%)].
Association between sleep and incidenturologic symptoms
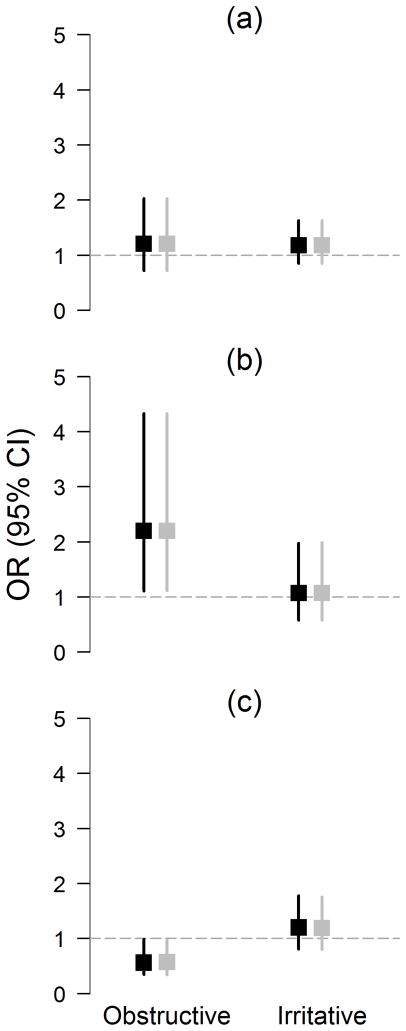
Controlling for confounders, poor sleep quality was associated with incident LUTS (OR=1.73, 95% CI: 1.20, 2.51), including both irritative (OR=1.55,95% CI: 1.06, 2.27) and obstructive (OR=2.15, 95% CI: 1.39, 3.32) LUTS (Figure 1), as well as nocturia (OR=1.42, 95% CI: 1.02, 1.97) (Table 2). Sleep restriction was associated with incident LUTS (OR=1.98, 95% CI: 1.03, 3.78), including incident irritative symptoms (OR=2.58, 95% CI: 1.41, 4.73) but not obstructive symptoms. Findings from models that included the nocturia item in the total LUTS score were comparable. Sleep restriction was also associated with UI (OR=2.42, 95% CI: 1.07, 5.48) but not with nocturia. Sleep medication use was unrelated to incident urologic symptoms. BMI reduced the relationship between sleep restriction and incident UI and irritative symptoms, with 19% and 12% reductions in the ORs, respectively. Closer examination of BMI with sleep restriction and incident UI and irritative symptoms (data not shown) revealed that sleep restriction and incident outcomes were more commonly observed in obese subjects. CRP did not mediate any associations.
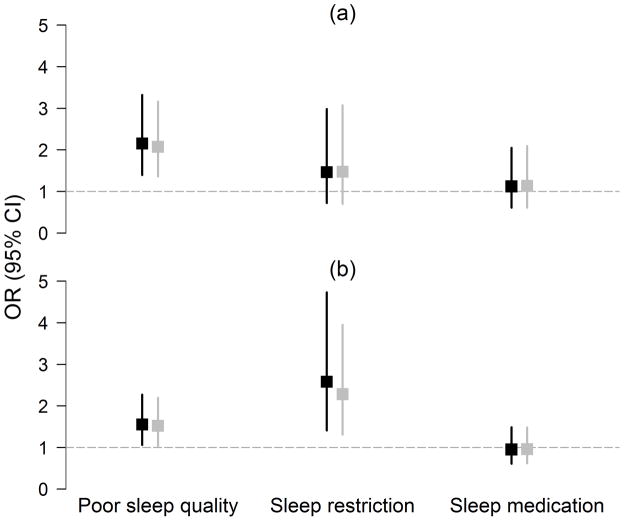
Figure 1. Multivariable Regression Models of Sleep Variables Predicting Incident Obstructive and Irritative LUTS Symptoms, BACH Survey (2002–2010).
Exposures (sleep) were assessed at baseline. Black boxes represent ORs obtained from multivariable logistic regression models with incident urologic symptoms[(a) obstructive symptoms; (b) irritative symptoms] predicted by the sleep exposure variable and controlling for age, sex, race, SES, diabetes, heart disease, alcohol use, physical activity, smoking, anti-depressant use, secondary sedation, and secondary stimulation. Grey boxes represent ORs obtained with further adjustment for BMI. Note: Multivariable logistic regression models for sleep restriction does not control for sex as this measure is collected only in men.
Table 2.
Multivariable Associations between Baseline Urologic Symptoms and Incident Sleep outcomes at Follow-Up, BACH Survey (2002–2010)
| Incident sleep outcomes | ||||||
|---|---|---|---|---|---|---|
| Poor sleep quality | Sleep restriction4 | Sleep medications | ||||
| Exposurea/Model | OR (95% CI) | Pb | OR (95% CI) | Pb | OR (95% CI) | Pb |
| LUTS | ||||||
| Multivariable-adjustedc | 0.90 (0.61, 1.32) | 0.58 | 1.29 (0.68, 2.45) | 0.43 | 0.87 (0.53, 1.42) | 0.57 |
| Multivariable-adjusted plus BMI | 0.90 (0.61, 1.32) | 0.58 | 1.30 (0.68, 2.47) | 0.43 | 0.85 (0.52, 1.39) | 0.51 |
| UI | ||||||
| Multivariable-adjustedc | 0.70 (0.40, 1.25) | 0.23 | 0.31 (0.11, 0.87) | 0.03 | 1.25 (0.69, 2.27) | 0.46 |
| Multivariable-adjusted plus BMI | 0.71 (0.40, 1.25) | 0.23 | 0.31 (0.11, 0.87) | 0.03 | 1.29 (0.71, 2.35) | 0.40 |
| Nocturia | ||||||
| Multivariable-adjustedc | 1.98 (1.38, 2.85) | <.001 | 1.12 (0.66, 1.89) | 0.67 | 1.27 (0.82, 1.97) | 0.29 |
| Multivariable-adjusted plus BMI | 1.98 (1.38, 2.85) | <.001 | 1.12 (0.67, 1.89) | 0.67 | 1.32 (0.85, 2.04) | 0.21 |
Abbreviations: BACH, Boston Area Community Health; BMI, body mass index; CI, Confidence interval; LUTS, lower urinary tract symptoms; OR, Odds ratio; UI, urinary incontinence;
Exposures assessed at baseline.
P-value testing the null hypothesis that the OR=1.00.
Multivariable logistic regression model with incident sleep disorder predicted by urologic symptom exposure variable and controlling for age, sex, race, SES, diabetes, heart disease, alcohol use, physical activity, smoking, anti-depressant use, secondary sedation, and secondary stimulation.
Multivariable logistic regression models for sleep restriction do not control for sex as this measure is collected only in men.
Association between urologic symptoms and incident sleep outcomes
Overall LUTS were not associated with incident sleep-related problems. However, obstructive symptoms were inversely associated with incident sleep medication use (OR=0.59, 95% CI: 0.35, 0.99) and positively associated with incident sleep restriction (OR=2.20, 95% CI: 1.11, 4.33) (Figure 2), with no mediation by BMI or CRP. Nocturia was associated with poor sleep quality (OR=1.98, 95% CI: 1.38, 2.85). (Table 3). Unexpectedly, UI was associated with reduced risk of incident sleep restriction (OR=0.31, 95% CI: 0.11, 0.87).
Figure 2. Multivariable Regression Models of Irritative and Obstructive LUTS Symptoms Predicting Incident Sleep Variables, BACH Survey (2002–2010).
Exposures (LUTS) were assessed at baseline. Black boxes represent ORs obtained from multivariable logistic regression models with incident sleep disorders[(a) poor sleep quality, (b) sleep restriction, (c) sleep medication] predicted by the urologic symptom exposure variable and controlling for age, sex, race, SES, diabetes, heart disease, alcohol use, physical activity, smoking, anti-depressant use, secondary sedation, and secondary stimulation. Grey boxes represent ORs obtained with further adjustment for BMI. Note: Multivariable logistic regression models for sleep restriction does not control for sex as this measure is collected only in men.
DISCUSSION
In this population-based, longitudinal study of over 4,000 men and women, there was a bi-directional relationship between self-reported sleep-related variables and urologic symptoms, with BMI, but not CRP, possibly mediating the association between restricted sleep and urologic symptoms. Although these observational data do not demonstrate causality, they suggest that improving sleep health may be a novel means of improving urologic symptoms. It is known that improving urologic function improves sleep outcomes.
The literature is replete with reports showing that urologic symptoms, particularly nocturia, have profound effects on sleep. However, to our knowledge, few if any studies have shown that sleep-related variables predict incident urologic symptoms, despite the known influence of sleep disturbance on factors which tend to cluster with urologic symptoms, including obesity,19 systemic inflammation, 20 type 2 diabetes,21 and other comorbidities.22 In this study, sleep restriction (measured only among men) was associated with incident LUTS and UI, and poor sleep quality among men and women was associated with incident LUTS and nocturia. Poor sleep quality increased the risk of obstructive and irritative LUTS symptoms, while sleep restriction was associated with more than a 2-fold risk of developing irritative symptoms. The latter is of interest in light of previous studies showing impaired cardio metabolic function of sleep-deprived individuals,7, 8 and fits conceptually with storage/irritative LUTS resulting from altered sensory innervation of the bladder or autonomic hyperactivity, which is noted in both those with LUTS and sleep disturbances.
Our results are consistent with cross-sectional studies showing that urologic symptoms are prevalent in patients with sleep disorders [e.g., obstructive sleep apnea (OSA) 23] and with a longitudinal study that showed improvements in LUTS lead to improvements in sleep disturbance.5 Voiding is cited as the main reason for waking at night24 and sleep disturbance mediates the effect of nocturia on QOL.25 Nocturia and urgency correlate negatively with direct measures of sleep efficiency26 and sleep quality.27 What has been debatedis whether patients are awakened because of the urge to void, or whether they are awakened for some other reason, and then sense the need to void.28 The current study was not designed to address this issue.
One straightforward explanation of our findings is the commonly observed phenomenon of patients with sleep disorders awakening from sleep and realizing that they have to void, quite apart from any particular urinary dysfunction. 28 However, it is not likely that this fully accounts for our observations, since other LUTS (not specifically nocturia) were also significantly related to poor sleep. So even if repeated awakenings among subjects with sleep-related problems constitutes a ‘risk’ for a nocturnal void (which may be reported by the subject as nocturia), it should not constitute a risk for non-nocturia LUTS including incomplete emptying, weak stream, and other symptoms.
A common underlying mechanism(s) may link these two disorders. Obesity and metabolic dysfunction are emerging areas of interest in both urology and sleep medicine. There are strong epidemiologic data showing linkages between LUTS/BPH and metabolic syndrome,21 likely mediated via pathways not assessed in the current study, including altered estrogen to androgen ratios, increased autonomic nervous system activation, vasopressin secretion, and impaired smooth muscle functioning as a result of impaired nitric oxide synthase, cyclic guanosine monophosphate, and RhoA/ROCK signaling. Furthermore, metabolic disturbances accompany acute7 and chronic8 sleep deprivation. These pathways impact the regulation of smooth muscle tone and are thus, fundamental to healthy urologic function given the requirements for coordinated relaxation/contraction of the detrusor and bladder musculature. These factors may interact in an interrelated manner, e.g., sleep disturbance is known to result in increased autonomic nervous system activation, which has been shown to be related to LUTS secondary to BPH,29 which may feedback to influence sleep and so on. If obesity or metabolic dysfunction are involved in this relationship, as suggested by our data showing BMI as a potentially relevant mediating variable, this would indicate that a weight loss or exercise could be an effective means of improving sleep problems and urinary symptoms simultaneously.
Limitations and strengths of the current study merit consideration. The BACH Survey focused on symptoms of urinary function, not diagnosed conditions, and certain measurements of interest (e.g., urine flow volume, prostate volume or prostate-specific antigen levels in men) were not available. The focus on symptoms is appropriate given the primacy of symptoms in the LUTS/BPH complex. The measures of sleep were by self-report and we have no specific questions regarding OSA at baseline. Furthermore, the current design does not address the dynamic nature of urologic symptoms or sleep-related problems, due to the fact that interim measurements were not available for either. Presumably, this would have the effect of increasing measurement error, thus, reducing the chance of rejecting null hypotheses. Finally, to our knowledge, the Sandvik index has not been formally validated in men, but our prior publications30 showing differences in prevalence of UI between ‘known groups’ (e.g., men vs. women, young vs. old, healthy vs. unhealthy) support the concept that UI as measured by the Sandvik severity index possesses construct validity. Significant strengths of the study include its population-based longitudinal design, its large sample size, and a relatively low 20% loss-to-follow-up rate. The breadth of questions on urologic symptoms included allows probing for different symptom relationships. Importantly, the loss-to-follow-up rate was unrelated to urologic symptoms, so our results are not subject to attrition bias.
CONCLUSIONS
In conclusion, in this population-based longitudinal study of men and women, self-reported sleep-related problems and urologic symptoms are linked bi-directionally, and BMI may play a role in the relationship between sleep restriction and development of urologic symptoms. Thus, sleep may be a novel risk factor for urologic symptoms, and as such, patients with sleep disorders should be screened and appropriately treated for urologic symptoms, which may positively benefit QOL outcomes in patients with sleep-related problems.
Supplementary Material
Table 1.
Multivariable Associations Between Baseline Sleep Variables and Incidence of Urologic Symptoms at Follow-Up, BACH Survey (2002–2010)
| Incident urologic symptoms | ||||||
|---|---|---|---|---|---|---|
| LUTS | UI | Nocturia | ||||
| Exposurea/Model | OR (95% CI) | Pb | OR (95% CI) | Pb | OR (95% CI) | Pb |
| Poor sleep quality | ||||||
| Multivariable-adjustedc | 1.73 (1.20, 2.51) | 0.004 | 1.50 (0.98, 2.31) | 0.06 | 1.42 (1.02, 1.97) | 0.04 |
| Multivariable-adjusted plus BMI | 1.64 (1.14, 2.35) | 0.008 | 1.39 (0.94, 2.05) | 0.10 | 1.42 (1.02, 1.97) | 0.04 |
| Sleep restrictiond | ||||||
| Multivariable-adjustedc | 1.98 (1.03, 3.78) | 0.04 | 2.42 (1.07, 5.48) | 0.03 | 1.03 (0.61, 1.77) | 0.90 |
| Multivariable-adjusted plus BMI | 1.89 (0.98, 3.65) | 0.06 | 1.96 (0.95, 4.04) | 0.07 | 1.02 (0.59, 1.76) | 0.93 |
| Sleep medications | ||||||
| Multivariable-adjustedc | 1.21 (0.70, 2.07) | 0.50 | 0.68 (0.37, 1.24) | 0.20 | 1.05 (0.69, 1.58) | 0.83 |
| Multivariable-adjusted plus BMI | 1.21 (0.69, 2.13) | 0.50 | 0.68 (0.38, 1.22) | 0.20 | 1.05 (0.69, 1.59) | 0.83 |
Abbreviations: BACH, Boston Area Community Health; BMI, body mass index; CI, Confidence interval; LUTS, lower urinary tract symptoms; OR, Odds ratio; UI, urinary incontinence
Exposures assessed at baseline.
P-value testing the null hypothesis that the OR=1.00.
Multivariable logistic regression model of incident urologic symptom as a function of baseline presence of sleep parameter controlling for age, sex, race, SES, diabetes, heart disease, alcohol use, physical activity, smoking, anti-depressant use, secondary sedation medication usage, and secondary stimulation medication usage.
Multivariable logistic regression models do not control for sex as sleep restriction was collected only in men at baseline.
Acknowledgments
Research or Project Support/Funding: This study was supported by the following awards: R21MD006769 from the National Institute on Minority Health and Health Disparities; U01DK056842 from the National Institute of Diabetes and Digestive and Kidney Disorders; CDTA 150S829 from the VA Clinical Science Research and Development Career Development Program; SIU Urology Endowment Fund, Havana Day Dreamers Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIMHD, NIDDK, NIH, VA, SIU Urology Endowment Fund, or Havana Day Dreamers Foundation.
Abbreviations
- AUA-SI
American Urologic Association Symptom Index
- BACH
Boston Area Community Health
- BMI
body mass index
- BPH
benign prostatic hyperplasia
- CI
Confidence interval
- CRP
C-reactive protein
- LUTS
lower urinary tract symptoms
- OR
Odds ratio
- OSA
obstructive sleep apnea
- QOL
quality of life
- SAS
Statistical Analysis System
- SES
socioeconomic status
- UI
urinary incontinence
Footnotes
Conflicts of Interest: None
References
- 1.Kupelian V, Wei JT, O’Leary MP, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Intern Med. 2006;166:2381. doi: 10.1001/archinte.166.21.2381. [DOI] [PubMed] [Google Scholar]
- 2.Colten H, Altevogt B, editors. Institute of Medicine Report: Sleep Disorders and Sleep Deprivation, An Unmet Public Health Problem. Washington D. C: The National Academies press; 2006. [PubMed] [Google Scholar]
- 3.Knutson KL, Van Cauter E, Rathouz PJ, et al. Trends in the prevalence of short sleepers in the USA: 1975–2006. Sleep. 33:37. doi: 10.1093/sleep/33.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helfand BT, McVary KT, Meleth S, et al. The relationship between lower urinary tract symptom severity and sleep disturbance in the CAMUS trial. J Urol. 2011;185:2223. doi: 10.1016/j.juro.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Helfand BT, Lee JY, Sharp V, et al. Associations Between Improvements in Lower Urinary Tract Symptoms and Sleep Disturbance Over Time in the CAMUS Trial. J Urol. 2012 doi: 10.1016/j.juro.2012.07.104. [DOI] [PubMed] [Google Scholar]
- 6.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 8.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 9.Simpson N, Dinges DF. Sleep and inflammation. Nutr Rev. 2007;65:S244. doi: 10.1111/j.1753-4887.2007.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 10.Hakkinen JT, Shiri R, Koskimaki J, et al. Depressive symptoms increase the incidence of nocturia: Tampere Aging Male Urologic Study (TAMUS) J Urol. 2008;179:1897. doi: 10.1016/j.juro.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 11.Shiri R, Koskimaki J, Tammela TL, et al. Bidirectional relationship between depression and erectile dysfunction. J Urol. 2007;177:669. doi: 10.1016/j.juro.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 12.McKinlay JB, Link CL. Measuring the urologic iceberg: design and implementation of the Boston Area Community Health (BACH) Survey. Eur Urol. 2007;52:389. doi: 10.1016/j.eururo.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelley KE, Kelley TP, Kaufman DW, et al. The Slone Drug Dictionary: A research driven pharmacoepidemiology tool. Pharmacoepidemiol Drug Safety. 2003;12:168. [Google Scholar]
- 14.Barry MJ, Fowler FJ, Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 15.Okamura K, Nojiri Y, Osuga Y, et al. Psychometric analysis of international prostate symptom score for female lower urinary tract symptoms. Urology. 2009;73:1199. doi: 10.1016/j.urology.2009.01.054. [DOI] [PubMed] [Google Scholar]
- 16.Sandvik H, Hunskaar S, Seim A, et al. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health. 1993;47:497. doi: 10.1136/jech.47.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tikkinen KA, Johnson TM, Tammela TL, 2nd, et al. Nocturia frequency bother, and quality of life: how often is too often? A population-based study in Finland. Eur Urol. 2010;57:488. doi: 10.1016/j.eururo.2009.03.080. [DOI] [PubMed] [Google Scholar]
- 18.Rothman KJ, Greenland S, editors. Modern Epidemiology. 2. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 19.Link CL, Steers WD, Kusek JW, et al. The Association of Adiposity and Overactive Bladder Appears to Differ by Gender: Results from the Boston Area Community Health Survey. J Urol. 2011 doi: 10.1016/j.juro.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kupelian V, McVary KT, Barry MJ, et al. Association of C-reactive protein and lower urinary tract symptoms in men and women: results from Boston Area Community Health survey. Urology. 2009;73:950. doi: 10.1016/j.urology.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kupelian V, McVary KT, Kaplan SA, et al. Association of lower urinary tract symptoms and the metabolic syndrome: results from the Boston Area Community Health Survey. J Urol. 2009;182:616. doi: 10.1016/j.juro.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgerald MP, Link CL, Litman HJ, et al. Beyond the lower urinary tract: the association of urologic and sexual symptoms with common illnesses. Eur Urol. 2007;52:407. doi: 10.1016/j.eururo.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parthasarathy S, Fitzgerald M, Goodwin JL, et al. Nocturia, sleep-disordered breathing, and cardiovascular morbidity in a community-based cohort. PLoS One. 2012;7:e30969. doi: 10.1371/journal.pone.0030969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bing MH, Moller LA, Jennum P, et al. Prevalence and bother of nocturia, and causes of sleep interruption in a Danish population of men and women aged 60–80 years. BJU Int. 2006;98:599. doi: 10.1111/j.1464-410X.2006.06390.x. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura K, Oka Y, Kamoto T, et al. Night-time frequency, sleep disturbance and general health-related quality of life: is there a relation? Int J Urol. 2009;16:96. doi: 10.1111/j.1442-2042.2008.02185.x. [DOI] [PubMed] [Google Scholar]
- 26.Tsujimura A, Takao T, Miyagawa Y, et al. Urgency is an independent factor for sleep disturbance in men with obstructive sleep apnea. Urology. 2010;76:967. doi: 10.1016/j.urology.2010.01.070. [DOI] [PubMed] [Google Scholar]
- 27.Asplund R. Nocturia in relation to sleep, somatic diseases and medical treatment in the elderly. BJU Int. 2003;91:302. [PubMed] [Google Scholar]
- 28.Pressman MR, Figueroa WG, Kendrick-Mohamed J, et al. Nocturia. A rarely recognized symptom of sleep apnea and other occult sleep disorders. Arch Intern Med. 1996;156:545. doi: 10.1001/archinte.156.5.545. [DOI] [PubMed] [Google Scholar]
- 29.McVary KT, Rademaker A, Lloyd GL, et al. Autonomic nervous system over activity in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2005;174:1327. doi: 10.1097/01.ju.0000173072.73702.64. [DOI] [PubMed] [Google Scholar]
- 30.Tennstedt SL, Link CL, Steers WD, et al. Prevalence of and risk factors for urine leakage in a racially and ethnically diverse population of adults: the Boston Area Community Health (BACH) Survey. Am J Epidemiol. 2008;167:390. doi: 10.1093/aje/kwm356. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.