Abstract
Background & Aims
The level of fecal calprotectin (FC) can predict the onset of inflammatory bowel disease (IBD) with high accuracy and precision. We evaluated the cost-effectiveness of using measurements of FC to identify adults and children who require endoscopic confirmation of IBD.
METHODS
We constructed a decision analytic tree to compare the cost-effectiveness of measuring FC before endoscopy examination with that of direct endoscopic evaluation alone. A second decision analytic tree was constructed to evaluate the cost-effectiveness of FC cut-off levels of 100 µg/g vs 50 µg/g (typically used to screen for intestinal inflammation). The primary outcome measure was the incremental cost required to avoid 1 false-negative result using FC level to diagnose new-onset IBD.
RESULTS
In adults, FC screening saved $417/patient but delayed diagnosis for 2.2/32 patients with IBD, among 100 screened patients. In children, FC screening saved $300/patient but delayed diagnosis for 4.8/61 patients with IBD, among 100 screened patients. If endoscopic biopsy analysis remained the standard for diagnosis, direct endoscopic evaluation would cost an additional $18,955 in adults and $6250 in children to avoid 1 false negative result from FC screening. Sensitivity analyses showed that cost effectiveness of FC screening varied with the sensitivity of the test and the pre-test probability of IBD in adults and children. Pre-test probabilities for IBD of ≤75% in adults and ≤65% in children made FC screening cost-effective, but cost ineffective if the probabilities were ≥85% and ≥78% in adults and children, respectively. Compared to the FC cut-off level of 100 µg/g, the cut-off level of 50 µg/g cost an additional $55 and $43 for adults and children, respectively, but yielded 2.4 and 6.1 additional accurate diagnoses of IBD per 100 screened adults and children.
CONCLUSIONS
Screening adults and children to measure fecal levels of calprotectin is effective and cost-effective in identifying those with IBD on a per-case basis when the pretest probability is ≤75% for adults and ≤65% for children. The utility of the test is greater for adults than children. Increasing the FC cut-off level to ≥50 µg/g increases diagnostic accuracy without substantially increasing total cost.
Keywords: fecal calprotectin, inflammatory bowel disease, cost-effectiveness, Crohn’s disease, ulcerative colitis, endoscopy, colonoscopy
INTRODUCTION
New-onset inflammatory bowel disease (IBD), consisting of Crohn’s disease (CD) or ulcerative colitis (UC), requires endoscopic evaluation and confirmation by histopathology. The decision to proceed with endoscopy is not always clear, particularly when classic signs are absent such as frank anemia, markedly elevated inflammatory markers, and gross hematochezia. Patients often present with non-specific symptoms, including mild abdominal pain, intermittent diarrhea, and generalized malaise. Although endoscopy is required for IBD confirmation, it potentially represents unnecessary risk among patients whose symptoms are functional in nature [1]. Evidence suggests that more than half of all patients who undergo endoscopy for non-bleeding symptoms are diagnosed with functional conditions, particularly irritable bowel syndrome (IBS) in adults and functional abdominal pain in children.
Non-invasive biomarkers in the stool represent potentially a novel and under-utilized modality to aid in IBD diagnosis. A growing body of literature has identified fecal calprotectin (FC) as a non-invasive predictive test with high sensitivity and specificity for IBD [2–6]. Calprotectin is a calcium-containing protein which makes up 60% of the cytosolic protein of neutrophil and monocytes [7], and released during acute and chronic inflammation [8].
A systematic review and meta-analysis by van Rheenen et al [9] examined the efficacy of FC in reducing unnecessary endoscopic procedures. The authors restricted the inclusion criteria to evaluate studies which contain data prior to IBD confirmation by direct endoscopic evaluation. A total of 13 studies, including 6 in adults and 7 in children and adolescents, were included. The pooled sensitivity and specificity of FC assay were 0.93 and 0.96 in adults and 0.92 and 0.76 in children.
There are 2 primary objectives of our analysis: 1) to determine the cost-effectiveness of FC screening (FCS) prior to endoscopic confirmation in suspected IBD patients versus direct endoscopic evaluation (DEE) alone, which is the current standard of care; and 2) to determine the optimal diagnostic utility of FC at either the high cut-off (100 µg/g) or the low cut-off (50 µg/g) values, which are most often used in clinical practice.
METHODS
Decision Analytic Models & Primary Outcome Measure
Two decision analytic models were built using TreeAge Pro 2011 (TreeAge software, Williamstown, WA) to evaluate the costs and accuracy associated with FCS to rule in suspected IBD patients among adults and children. Adopting a third-party payer perspective, only direct costs were considered. The base-case patient population comprised of any suspected IBD patient presenting with complaints of adverse gastrointestinal symptoms. Our primary outcome measure was the incremental cost-effectiveness ratio (ICER), designated as the incremental cost to choose one screening strategy to avert one false negative FC result.
Description of Model 1: FCS strategy vs. DEE strategy
Model 1 examines the diagnostic rationale of the FCS strategy (i.e., quantification of FC prior to an endoscopic assessment if FCS is positive) versus the DEE strategy (i.e., the current standard of care). Figure 1 shows the model schematic. In the FCS strategy, patients with suspected IBD will undergo stool testing using the FC quantification assay. Patients with a positive test result will receive the standard endoscopic evaluation (i.e., esophagogastroduodenoscopy and colonoscopy) with biopsies to detect CD or UC. The general clinical approach in practice is that any IBD patient given a false-negative FC result will return with persistent symptoms and receive the DEE confirming or ruling out potential IBD. For symptomatic patients with a true negative FC screen (e.g., IBS), the model assumes that a proportion of these patients will also require a follow-up DEE due to persistent symptoms [10]. We assumed 100% sensitivity and specificity via upper endoscopy and colonoscopy for IBD.
Figure 1. Overview of model structure.
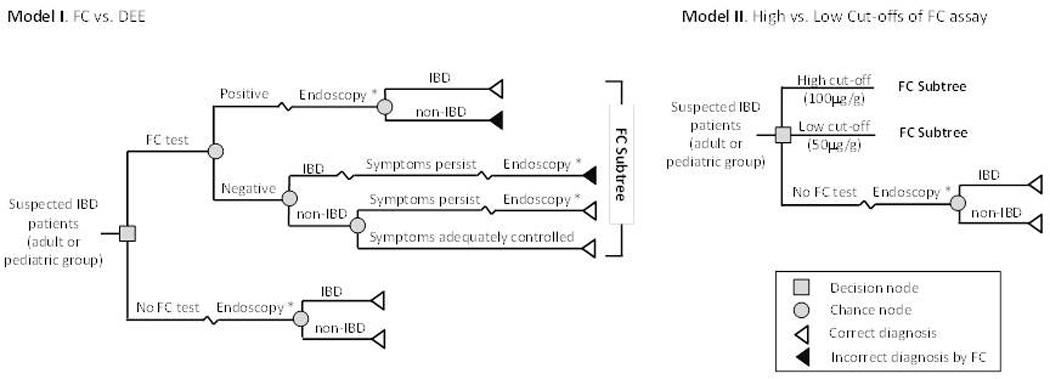
* ‘endoscopy’ = upper endoscopy and colonoscopy with histopathology
Description of Model 2: low cut-off (50 µg/g) vs. high cut-off (100 µg/g) FC values
Model 2 (Figure 1) examines the differential diagnostic accuracy of 2 FC cut-off values (50 µg/g vs. 100 µg/g), as previously reported in literature. Model 2 aims to determine the optimal FC level to maximize sensitivity and specificity. A higher cut-off value will yield higher specificity with fewer false-postive results, but will be less sensitive with more false-negative results.
Estimation of FC Sensitivity and Specificity
The overall sensitivity and specificity of FC assay for adults and children were based on the meta-analysis conducted by van Rheenen et al [9] after a separate literature investigation to determine the best input values for sensitivity and specificity of FC for both high and low cut-off values. All sensitivity and specificity values used in the models are in Table 1. Of note, after our analysis was complete, a very recent meta-analysis by Henderson et al [11] focusing only on pediatric studies reported a sensitivity of 0.978 and specificity of 0.682. These numbers had a very high correlation with our model inputs of 0.95 and 0.70 for sensitivity and specificity, respectively, for the FCS at the 50 µg/g cut-off value.
Table 1.
Model Input Parameters: Costs of Procedures and Probabilities, (95% Confidence Interval) and [Range Tested]
| Parameters | Distribution | Data Source | Reference | |||
|---|---|---|---|---|---|---|
| Cost Estimate * | CPT code | Costs, $ | ||||
| Initial Gastroenterology Office Consultation | 99205 | 199 [150 to 250] | Gamma | Medicare | [13] | |
| Follow-up Gastroenterology Office Consultation | 99215 | 140 [100 to 180] | Gamma | Medicare | [13] | |
| Upper Gastrointestinal Endoscopy and Colonoscopy, with Biopsy † | 43235 and 45380 | 1171 [700 to 2000] | Gamma | Medicare | [13] | |
| Surgical Pathology (x5) | 88305 | 529 [400 to 650] | Gamma | Medicare | [13] | |
| Fecal Calprotectin Assay | 83993 | 28 [15 to 50] | Gamma | Medicare | [13] | |
| Probability Estimate | Adult, % | Children, % | ||||
| Sensitivity | ||||||
| FC, Overall | 93 (85 to 97) | 92 (84 to 96) | Lognormal, correlated with corresponding specificity | Meta-analysis | [18] | |
| FC, Low Cut-off Point (50ug/g) | 95 (87 to 98) | 95 (83 to 99) | Meta-analysis ‡ | [18] | ||
| FC, High Cut-off Point (100ug/g) | 87 (77 to 93) | 85 (75 to 91) | Meta-analysis ‡ | [18] | ||
| Endoscopy and Colonoscopy with Histology | 100 | 100 | Not parameterized | Assumed | - | |
| Specificity | ||||||
| FC, Overall | 96 (89 to 99) | 76 (62 to 86) | Lognormal, correlated with corresponding sensitivity | Meta-analysis | [18] | |
| FC, Low Cut-off Point (50ug/g) | 85 (65 to 95) | 70 (61 to 78) | Meta-analysis ‡ | [18] | ||
| FC, High Cut-off Point (100ug/g) | 97 (94 to 99) | 80 (66 to 89) | Meta-analysis ‡ | [18] | ||
| Endoscopy and Colonoscopy with Histology | 100 | 100 | Not parameterized | Assumed | - | |
| Proportion of True Positive IBDs among Suspected | ||||||
| IBD Patients | 32 [18 to 49] | 61 [50 to 72] | Beta | Meta-analysis | [18] | |
| Non-IBD Patients with Persistent Symptoms | 60 [41 to 76] | 35 [22 to 50] | Beta | Expert opinion** | [19] | |
based on the 2012 American Medical Association Current Procedural Terminology code book and the 2012 Medicare Fee Schedule
includes facility fee for colonoscopy ($378) and for upper gastrointestinal endoscopy ($341), physician reimbursement for colonoscopy ($264) and for upper gastrointestinal endoscopy ($148) and anesthesia fee ($132); assume no bundling discount for facility fee and physician reimbursement and 30% patients receiving anesthesia
derived from the summary ROC curves in [9]. Selection of points on summary ROC curve was based on expert's judgment and consulted with eight other gastroenterologists
based on study by Dubinsky et al [19] and adjusted for poll result from 4 pediatric and 5 adult gastroenterologists
Pre-test probability of true IBD
The pre-test probability of IBD among suspected IBD patients were based on pooled estimates [9], with 32% for adults and 61% for children. Depending on provider-specific practice differences between gastroenterologists, patient demographic differences, and differences in patient preferences, the variance of the pretest probability of true IBD is likely very large and will show substantial real-life variation from these two estimates. Supporting this fact, studies included in meta-analysis reported this large variance in the pre-test probability of IBD in both groups (14% to 68% in adults and 51% to 80% in pediatrics). Our sensitivity analysis takes this variance into consideration.
Proportion of non-IBD patients undergoing DEE
A prior investigation by Dubinsky et al [19] estimated a 50% probability for this input value based on 5 gastroenterology clinicians’ experience. We took this estimate into consideration, and also polled 4 pediatric and 5 adult faculty gastroenterologists at Lucile Packard Children’s Hospital and Stanford University Medical Center. The probability estimate for pediatric patients ranged from 20% to 50%. Our model used 35% as the base case value in children. The probability estimate for adult patients ranged from 60% to 90%, but was potentially left-skewed by a general consensus that second-opinion referrals from community-based gastroenterologists to academic practices are generally common, thereby potentially increasing this probability. To maintain consistency with literature, our model used the lower bound, 60%, as the base case value in adults.
Costs and Outcome Measures
Cost variables included initial and follow-up gastroenterology outpatient consultation visits, the standard FC assay, and DEE with biopsies and histological assessment by pathology. Cost for the DEE with biopsies includes institutional facility fee, physician reimbursement, and anesthesia fee. Cost for histological assessment includes five standard analyses of biopsy specimens from each anatomic segment. The corresponding CPT code for each item was identified from the 2012 American Medical Association Current Procedural Terminology Code Book [12]. Cost estimation of each code was based on 2012 Medicare reimbursement rates [13].
Decision Analysis
Base case analysis
The ICER of each strategy was calculated, which compares the incremental costs to incremental health outcomes between competing strategies. In our analysis in Model 1, the ICER is the additional cost required to choose either the FCS or DEE strategy in order to avert one false negative result in IBD (i.e., to yield one additional true IBD diagnosis). Similarly, in Model 2, the ICER is the additional cost required to choose either the low or high FC cut-off value in order to avert one false negative result.
Sensitivity analysis
One-way sensitivity analysis was performed on each parameter for both models and both patient groups, varying each parameter within 95% confidence intervals. Parameters affecting ICER were analyzed in tornado diagrams (Figure 2). A probabilistic sensitivity analysis (PSA) was performed using the first-order Monte Carlo simulation with 3,000 iterations to assess the impact of composite model parameter uncertainties. A key analytic difference of our PSA from other PSAs for diagnostic tests – where sensitivity and specificity are often modeled as uncorrelated beta distributions – was that logit-transformed bivariate normal distributions were used to optimally depict sensitivity and specificity, as this approach most consistently aligns with the bivariate random effects model used in the meta-analysis by van Rheenan et al. Results were presented as cost-effectiveness acceptability curves, showing the likelihood for a strategy to be cost-effective in a range of willingness-to-pay (WTP) thresholds to avert undiagnosed IBD. To understand variance in the pre-test probability for true IBD, a three-way sensitivity analysis was conducted to evaluate the impact of the diagnostic accuracy of FCS at different pre-test probabilities.
Figure 2. One-Way Sensitivity Analysis.
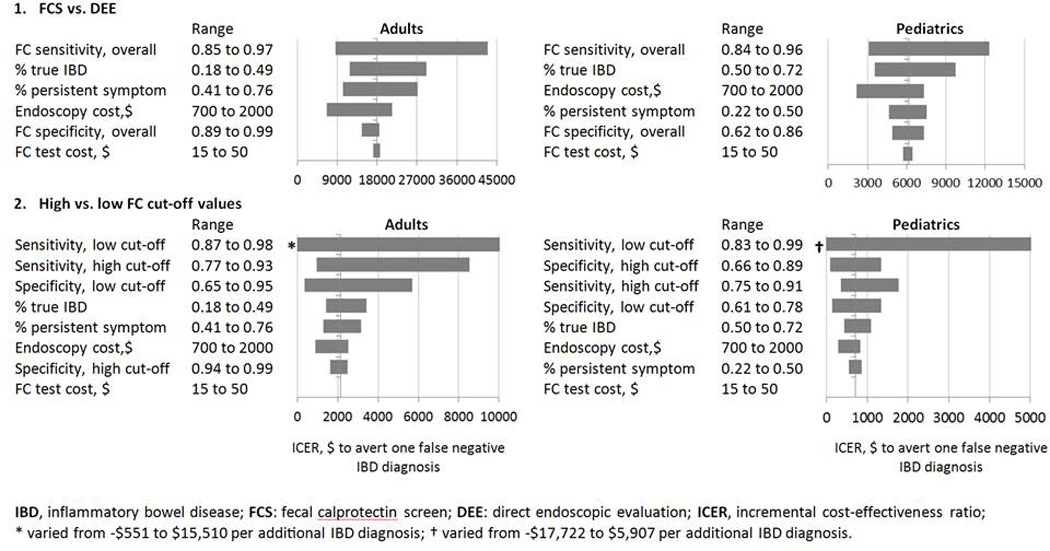
* varied from -$551 to $15,510 per additional IBD diagnosis; † varied from -$17,722 to $5,907 per additional IBD diagnosis.
RESULTS
Model 1: FCS strategy vs. DEE strategy
Table 2 shows the results for adult and pediatric groups for Model 1 (FCS vs. DEE). In every diagnostic scenario, choosing the FCS strategy reduces direct costs, but incurs lower immediate accuracy than the DEE strategy. The FCS strategy at low or high cut-offs has the potential to delay the diagnosis in a small number of patients if the decision to perform the DEE hinges on whether the FC quantification met designated cut-offs, tested at 50 µg/g and 100 µg/g levels.
Table 2.
Base-Case Summary of Cost-Effectiveness (CE) Analysis for 100 suspected IBD patients, by Model and Age Group
| Strategy | Cost per patient, $ |
Increment al cost per patient*, $ |
IBD diagnosis †, n (%) |
Incremental false negative IBD, n |
Incremental CE ratio ‡ |
|---|---|---|---|---|---|
| FCS vs. DEE, Adults | |||||
| DEE | 1,899 | - | 32.0 (100.0%) | - | 18,955 |
| FCS | 1,482 | −417 | 29.8 (93.1%) | −2.2 | |
| FCS vs. DEE, Pediatrics | |||||
| DEE | 1,899 | - | 61.0 (100.0%) | - | 6,250 |
| FCS | 1,599 | −300 | 56.2 (92.1%) | −4.8 | |
| High vs. Low Cut-offs, Adults | |||||
| DEE | 1,899 | - | 32.0 (100.0%) | - | 21,353 |
| FCS, low cut-off | 1,536 | −363 | 30.3 (94.7%) | −1.7 | 2,292 |
| FCS, high cut-off | 1,481 | −55 | 27.9 (87.2%) | −2.4 | |
| High vs. Low Cut-offs, Pediatrics | |||||
| DEE | 1,899 | - | 61.0 (100.0%) | - | 9,133 |
| FCS, low cut-off | 1,625 | −274 | 58.0 (95.1%) | −3.0 | 705 |
| FCS, high cut-off | 1,582 | −43 | 51.9 (85.1%) | −6.1 | |
negative number indicates cost saving, as compared to the next more costly strategy
exclude delayed diagnoses. The percentage indicates the proportion of correct diagnosis of IBD among true IBD patients
cost to avert a false negative result in IBD diagnosis, as compared with the next less costly strategy
Adults (FCS vs. DEE)
In adults, the FCS strategy saves $417/patient, but delays the diagnosis in 2.2/32 IBD patients per 100 suspected IBD patients. If the FCS strategy is the reference strategy, choosing the DEE over the FCS results in an additional $18,955 to avert one false negative FCS. In other words, if DEE remains to be the current standard of care, around $19,000 extra is required to forgo the delayed diagnosis generated by 1 false negative FCS.
Children (FCS vs. DEE)
In children, the FCS strategy saves $300/patient, but delays the diagnosis in 4.8/61 IBD patients per 100 suspected IBD patients. Choosing to forgo any FCS prior to DEE, the DEE strategy would incur an additional $6,250 to avert one false negative FCS. Compared to adults, the FCS strategy in children is less cost-effective (i.e., less additional cost to avert 1 delayed diagnosis), but the FCS strategy in children with suspected IBD is still considerably cost-saving. Of note, the model cannot account for short and long-term health consequences (e.g., nutrition, growth) in children with IBD who had a false negative FCS and experienced delayed diagnosis (see Discussion).
Model 2: low cut-off (50 µg/g) vs. high cut-off (100 µg/g) FC values
Table 2 shows the results for adult and pediatric groups for Model 2, which aims to determine the optimal sensitivity at low (50 µg/g) and high (100 µg/g) FC cut-off values. Currently, despite the growing FC data in literature, there is no standardization on which level should be considered a “positive” FCS. Therefore, the goal of this model is to enhance diagnostic accuracy – minimizing false negatives – to maximize the proportion of screened patients who truly need endoscopic confirmation for IBD. Our results show that choosing the FCS strategy at the low cut-off value of 50 µg/g is slightly more expensive than the high cut-off of 100 µg/g, but the low cut-off strategy had a substantially higher degree of accuracy.
Adults (FC cut-off at 50 µg/g vs. 100 µg/g)
When considering FCS at the low FC cut-off value of 50 µg/g for adults, the FCS strategy accurately detected an additional 2.4 true positives per 100 screened patients for an additional cost of $41/screened patient. If the high FC cut-off value of 100 µg/g were the reference strategy, choosing the low cut-off FCS strategy incurs an additional $2,292 to avert one false negative FCS (i.e., to detect 1 additional true positive IBD) compared to the high FC cut-off strategy. If any FCS strategy is bypassed altogether, choosing to undergo the DEE strategy alone is equivalent to expending an additional $21,353 to detect one additional IBD diagnosis.
Pediatrics (FC cut-off at 50 µg/g vs. 100 µg/g)
Similarly, when considering the low FC cut-off value in children, the FCS strategy accurately detected an additional 6.1 true positives per 100 screened patients for an additional cost of $43/screened patient. If the high cut-off value of 100 µg/g were the reference strategy, choosing the low cut-off FCS strategy incurs an additional $705 to detect 1 additional true positive IBD patient.
Sensitivity analysis
Deterministic Sensitivity Analysis (Model 1)
For adults, Model 1 shows that choosing DEE results in a considerable ICER of $18,955 to avert one false negative FCS. The tornado diagram for Model 1 (upper left panel of Figure 2) supports the notion that despite realistic variations in the model parameters, choosing DEE over FCS strategy resulted an ICER above $7,000 – $40,000 to avert one false negative FCS. For children, Model 1 reveals that choosing DEE results in a smaller ICER of $6,250 to avert one false negative FCS. The tornado diagram for Model 1 (upper right panel in Figure 2) supports this finding as the ICER generally stayed within $2,000 – $12,000 to avert one false negative FCS. In both groups, changes in FC sensitivity induces the largest impact to the cost-effectiveness compared to other important variables. Although not as sensitive of a variable as FC assay sensitivity, the proportion of true IBD among screened patients and the proportion of persistent symptoms among screened patients are 2 variables that could conceivably impact cost-effectiveness. In contrast, variations in specificity and cost of the FC assay do not impact overall ICER for both adults and children.
Deterministic Sensitivity Analysis (Model 2)
Tornado diagrams for both adults and children in Model 2 (lower panel of Figure 2) indicate that the cost-effectiveness of either high or low FC cut-off strategies is sensitive to changes in the sensitivity and specificity of the FC assay, especially the sensitivity at the low cut-off value. However, it should be noted that changing the sensitivity (specificity) at one cut-off value while holding sensitivity (specificity) at the other cut-off value constant is statistically not realistic. When other key parameters were varied, the ICER generally stays below $3,500 and $2,000 per 1 additional true IBD diagnosis for adults and children, respectively. Testing the model parameters by inducing variable changes over a wide spectrum of scenarios – where patients’ age, probability of true IBD, likelihood of receving DEE in non-IBD, and costs associated with procedures and services – show that the ICER is robust to model variations. In other words, despite real-life changes to individual variables, the FCS strategy at the low cut-off value for both adults and children is a cost-effective alternative to the current standard of care.
Probabilistic Sensitivity Analysis
The results of our PSA come from 3,000 independent simulations of both Model 1 and Model 2 with individual model parameters varying within their respective 95% confidence intervals. Cost-effectiveness acceptability curves for both models in adults and children are shown in Figure 3. In Model 1, the FCS strategy is more likely to be cost-effective in adult patients up to a willingness-to-pay (WTP) threshold of $18,712 to avert one false negative FCS. Above this WTP threshold, the DEE is more likely to be cost-effective. In children, the WTP threshold is $5,913 to avert one false negative FCS. In Model 2, the low cutoff FCS strategy shows a wider WTP range for cost-effectiveness (i.e., $2,914 to $16,137 and $312 to $8,874 to avoid one false negative FCS in adults and children, respectively) than the high cut-off FCS strategy ($0 to $2,914 and $0 to $312, correspondingly). This is consistent with the one-way sensitivity analysis showing that the low cut-off FCS strategy is more cost-effective in various settings.
Figure 3. Probabilistic Sensitivity Analysis.
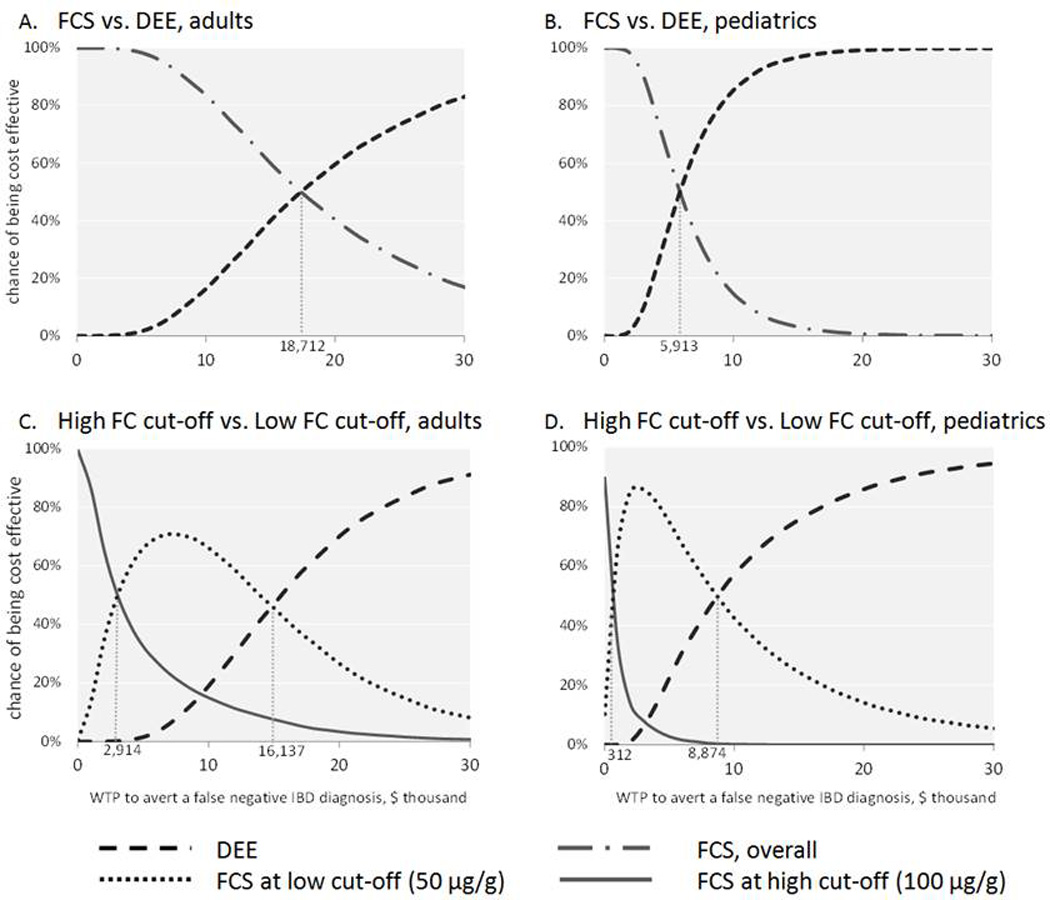
Acceptability curves from the 3,000 independent simulations. Figure A and B show FCS strategy decreases in cost-effectiveness at higher WTP. Figure C and D show that FCS at low FC cut-off values have a wider range of WTP (between 2 vertical hurdles) in which FCS at 50µg/g is the more likely cost-effective than FCS at 100µg/g (between the left hurdle and the origin).
Three-way Sensitivity Analysis
Due to the variability of the pre-test probability for IBD in children, we performed a three-way sensitivity analysis to further understand the utility of the FCS strategy in pediatrics, as shown in Figure 4 at 4 different pre-test probabilities of 35%, 50%, 65%, and 80%. The same analysis for adults is only shown in Appendix A. The adult group shows a smaller variation in the pre-test probability of true IBD between studies included in meta-analysis than in the pediatric literature [9]. The results of our three-way sensitivity analysis show that the FCS strategy becomes less cost-effective when there is a higher probability of true IBD. In general, the FCS strategy for children is highly cost-effective when the pre-test probability of true IBD is ≤65%, as it requires at least $5,000 to avert one false negative FCS by DEE. For comparison, in adults, the FCS strategy is highly cost-effective when the pre-test probability of true IBD is ≤75%. In adults and children, the FCS strategy is generally cost-ineffective at high pre-test probability for IBD (≥85% in adults and ≥78% in children).
Figure 4. 3-Way Sensitivity Analysis for Using FC Assay in Pediatric Group.
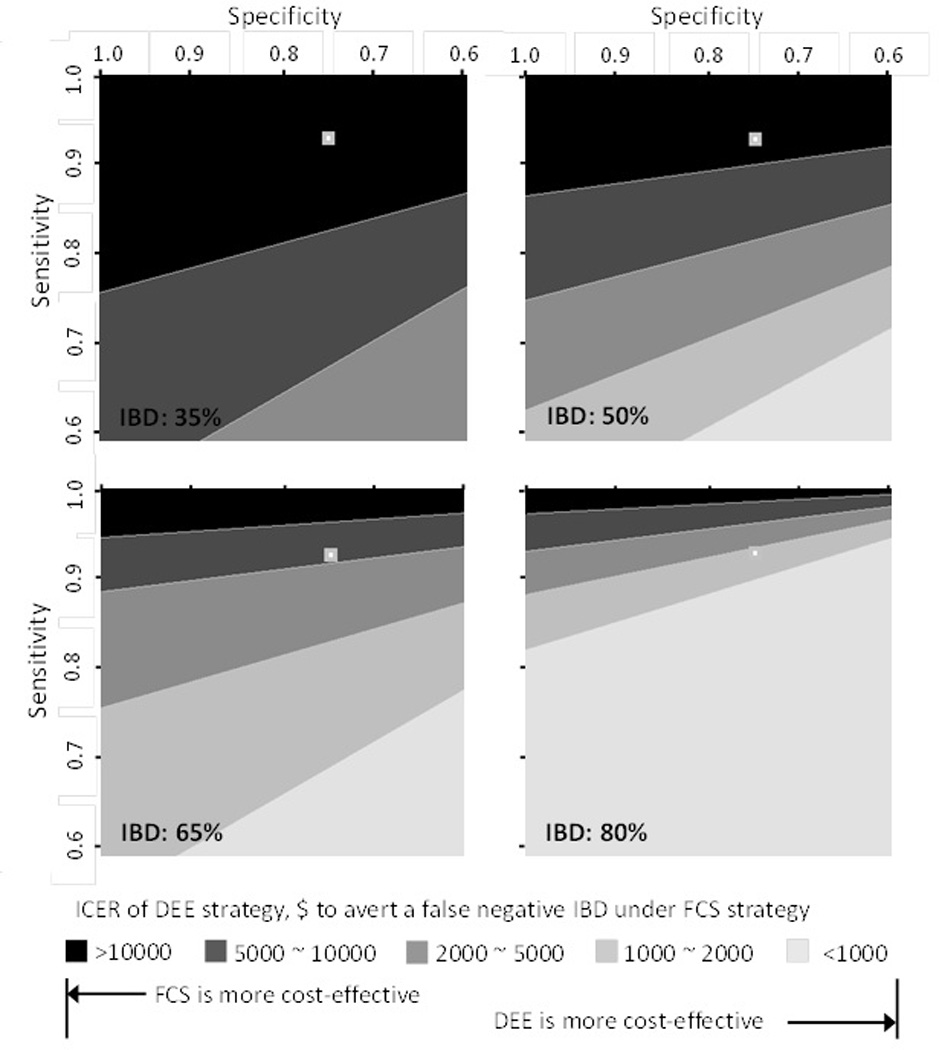
ICER of DEE strategy at different pre-test probabilities of true IBD, as compared with FCS strategy.
DISCUSSION
We present the first cost-effectiveness analysis on FC as a diagnostic tool for IBD.
We report 2 key summary findings from our analysis:
Compared to DEE, the FCS strategy is cost-saving for both adults and children on a percase basis when the pretest probability is ≤75% in adults and ≤65% in children – with FCS representing a more cost-effective strategy in adults than in children. Continuing to perform DEE as the standard of care would cost around $19,000 in adults and $6,000 in children to avert one delayed IBD diagnosis from a false negative FCS. The FCS strategy becomes more cost-effective when the pre-test probability for true IBD is low (≤75% and ≤65% in adults and children, respectively), but is less cost-effective when the pretest probability for true IBD is high (≥78% and ≥85% in adults and children, respectively). In general, the FCS strategy is particularly cost-effective when baseline clinical suspicion for IBD is low to moderate.
Compared to the high FC cut-off value of 100 µg/g, the low FC cut-off value of 50 µg/g would substantially reduce the likelihood of false negative FCS – minimizing delayed diagnosis of true IBD. Standardizing “positive” FC results to be any FC value ≥50 µg/g expends approximately $2,292 in adults and $705 in children to avert one delayed IBD. In general, the FCS strategy set at a positive result of FC levels ≥50 µg/g is cost-effective and enhances the utility of FC as a screening test for IBD.
Despite analytical attempts to describe the utility of FC in real-life clinical settings by generating specific pre-test probability cut-offs for cost-effectiveness, individual patient correlation remains invaluable. One example is in moderate-to-severe UC presentation, where the patient may have on-going hematochezia and possibly other constitutional clinical signs (e.g., fevers, anemia, elevated inflammatory markers). In such a scenario – in keeping with our cost-ineffective pre-test probability cut-offs of ≥78% and ≥85% in adults and children, respectively – expeditious diagnostic workup and DEE should take clinical priority. FCS at 50 µg/g could be clinically relevant among patients who are considered to possibly have IBD, IBS, or an infectious/post-infectious process prior to DEE.
Supporting the growing evidence consistently indicating the clinical effectiveness of FC as a non-invasive biomarker to detect gut inflammation and limit unnecessary referrals for endoscopy [14, 15], our study contributes to this established knowledge by specifically quantifying the specific tradeoffs in direct costs and diagnostic accuracy between the FCS and the DEE strategies. Direct societal costs and unnecessary risks associated with endoscopies and colonoscopies among patients with non-IBD diagnoses should be considered from an individual case-by-case basis. However, the results from our study provide quantitative parameters to guide health policy recommendations on the optimal use FCS in adults and children undergoing IBD screening.
Among screened adults, both model 1 and model 2 are robust to realistic changes to input variations. For adults, the policy for IBD screening with FCS at 50 µg/g prior to endoscopic biopsies would save direct costs from unnecessary procedures and minimize patient risk, without compromising diagnostic accuracy. However, focus specifically on pediatrics, a clear policy using FCS for screening is difficult. Similar to the adult models, both pediatric Model 1 and Model 2 indicate cost-savings and high diagnostic accuracy with FCS at 50 µg/g, but the pediatric ICER was $6,250 to avert one false negative FCS – approximately 3 times less than in the adult cohort. It is important to note that while DEE represents an invasive procedure, children undergoing screening for IBD who show a distinct change or borderline trends in biometric measurements (e.g., weight loss or poor weight gain, failure-to-thrive, short stature relative to peers or expected growth) meet criteria for formal IBD screening with standard DEE [16]. The classic example is a child with CD without frank alterations in serologic disease indicators, but has suboptimal biometric measurements. Conceivably, in this scenario, a consideration for IBD is necessary to forgo long-term derangements of health [17, 18]. From a modeling standpoint, the major contributor in the observed cost-effectiveness difference between adult and pediatric FCS strategies is attributed to the significant specificity difference of FCS in adults (96.6%) and children (74.0%).
Although the best available data were used produce the highest degree of granularity in model outputs, we acknowledge that the idea of “delayed IBD diagnosis” after a false negative FCS as an outcome measure potentially introduces ambiguity in the time-to-diagnosis of true IBD. It is not precisely known – although clinical experience would estimate a 1- to 3-month period based on common outpatient clinic visit intervals – as to the actual timeframe a delayed IBD diagnosis really represents. A loss to follow-up or missed clinic appointments could cause substantially prolonged time to the accurate IBD diagnosis – especially in sub-acute or minimally-active baseline disease. Furthermore, it is difficult to assume some quantifiable health consequence as a direct result of a false negative FCS. Therefore, in order to exhaustively assess the effect of this more uncertain parameter, we converted this outcome measure (i.e., delayed diagnosis) into the conventional utility-driven QALYs to gain perspective and comparison (Appendix B). Based on the literature, we assumed an undiagnosed IBD patient to have a utility of 0.65 and an IBD patient with disease control to have a utility of 0.85 [19, 20]. (Note: The utility of 0.65 is likely an over-estimate of the quality-of-life decrement in minimally active IBD – patients who are most likely to delay DEE.) Even if a baseline 3-month delay is assumed after a false negative FCS, the ICER for the DEE strategy would be $379,100/QALY for adults and $125,000/QALY for children – both above the conventional cost-effective threshold of <$50,000/QALY in the US [21], making FCS the preferred strategy in both adults and children. In our analysis, we aimed to precisely estimate the direct costs related to 1 additional true or delayed diagnosis of IBD based on screening strategies since the use of QALYs can also lead to ambiguous interpretation of results, more often than concrete outcome measures based on the idea of “true” vs. “false” and “positives” vs. “negatives.”
In summary, our investigation of using FC as a screening modality for new-onset IBD prior to endoscopic biopsies is a cost-effective strategy in adults and children with suspected IBD, on a per-case basis. Patient-specific IBD screening practices are advised since a lower pretest probability for IBD enhances the cost-effectiveness of the FCS strategy. Additionally, using the lower cut-off FC value of 50 µg/g reduces false negatives without adding substantial costs.
Acknowledgements
KP is supported by the National Institute for Health DK094868A. The manuscript contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations
- CD
Crohn’s disease
- DEE
direct endoscopic evaluation
- FC
fecal calprotectin
- FCS
fecal calprotectin screening
- IBD
inflammatory bowel disease
- ICER
incremental cost-effectiveness ratio
- QALY
quality adjusted life year
- ROC
receiver operating characteristic curve
- UC
ulcerative colitis
- WTP
willingness to pay
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: conception and design by ZY and KP; original data collection and review by NC and ZY; data analysis and interpretation by ZY and KP; manuscript writing by KP, ZY, and NC; final approval of manuscript by KP, ZY, and NC
Disclosures: The authors disclose no conflicts of interest.
Bibliography
- 1.Ing C, et al. Long-term Differences in Language and Cognitive Function After Childhood Exposure to Anesthesia. Pediatrics. 2012;130(3):e476–e485. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 2.Limburg PJ, et al. Prospective evaluation of fecal calprotectin as a screening biomarker for colorectal neoplasia. Am J Gastroenterol. 2003;98(10):2299–2305. doi: 10.1111/j.1572-0241.2003.07630.x. [DOI] [PubMed] [Google Scholar]
- 3.Carroccio A, et al. Diagnostic accuracy of fecal calprotectin assay in distinguishing organic causes of chronic diarrhea from irritable bowel syndrome: a prospective study in adults and children. Clin Chem. 2003;49(6 Pt 1):861–867. doi: 10.1373/49.6.861. [DOI] [PubMed] [Google Scholar]
- 4.Diamanti A, et al. Diagnostic work-up of inflammatory bowel disease in children: the role of calprotectin assay. Inflamm Bowel Dis. 2010;16(11):1926–1930. doi: 10.1002/ibd.21257. [DOI] [PubMed] [Google Scholar]
- 5.Schoepfer AM, et al. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009;15(12):1851–1858. doi: 10.1002/ibd.20986. [DOI] [PubMed] [Google Scholar]
- 6.Quail MA, et al. Fecal calprotectin complements routine laboratory investigations in diagnosing childhood inflammatory bowel disease. Inflamm Bowel Dis. 2009;15(5):756–759. doi: 10.1002/ibd.20820. [DOI] [PubMed] [Google Scholar]
- 7.Steinbakk M, et al. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990;336(8718):763–765. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- 8.Roseth AG, et al. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992;27(9):793–798. doi: 10.3109/00365529209011186. [DOI] [PubMed] [Google Scholar]
- 9.van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341:c3369. doi: 10.1136/bmj.c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubinsky MC, et al. Suspected inflammatory bowel disease--the clinical and economic impact of competing diagnostic strategies. Am J Gastroenterol. 2002;97(9):2333–2342. doi: 10.1111/j.1572-0241.2002.05988.x. [DOI] [PubMed] [Google Scholar]
- 11.Henderson P AN, Wilson DC. The Diagnostic Accuracy of Fecal Calprotectin During the Investigation of Suspected Pediatric Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2013 doi: 10.1038/ajg.2013.131. [DOI] [PubMed] [Google Scholar]
- 12.Association AM. CPT 2012: Standard Edition. Chicago: IL: American Medical Association; 2011. [Google Scholar]
- 13.Centers for Medicare & Medicaid Services Medicare Fee-for-Service Payment. [[cited 2012 September 1st]];2012 Available from: http://www.cms.gov/Medicare/Medicare.html. [Google Scholar]
- 14.Kok L ES, Witteman BJ, Goedhard JG, Muris JW, Moons KG, de Wit NJ. Diagnostic accuracy of point-of-care fecal calprotectin and immunochemical occult blood tests for diagnosis of organic bowel disease in primary care: the Cost-Effectiveness of a Decision Rule for Abdominal Complaints in Primary Care (CEDAR) study. Clin Chem. 2013;58(6):989–998. doi: 10.1373/clinchem.2011.177980. [DOI] [PubMed] [Google Scholar]
- 15.Mindemark M LA. Ruling out IBD: estimation of the possible economic effects of pre-endoscopic screening with F-calprotectin. Clin Biochem. 2012;45(7–8):552–562. doi: 10.1016/j.clinbiochem.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Rufo PA, et al. Health supervision in the management of children and adolescents with IBD: NASPGHAN recommendations. J Pediatr Gastroenterol Nutr. 2012;55(1):93–108. doi: 10.1097/MPG.0b013e31825959b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heuschkel R, et al. Guidelines for the management of growth failure in childhood inflammatory bowel disease. Inflamm Bowel Dis. 2008;14(6):839–849. doi: 10.1002/ibd.20378. [DOI] [PubMed] [Google Scholar]
- 18.American Gastroenterological Association medical position statement: guidelines on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124(3):791–794. doi: 10.1053/gast.2003.50107. [DOI] [PubMed] [Google Scholar]
- 19.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38(6):583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Allen E, Nicolaidis C, Helfand M. The evaluation of rectal bleeding in adults. A cost-effectiveness analysis comparing four diagnostic strategies. J Gen Intern Med. 2005;20(1):81–90. doi: 10.1111/j.1525-1497.2005.40077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8(2):165–178. doi: 10.1586/14737167.8.2.165. [DOI] [PubMed] [Google Scholar]


