Abstract
The hepatoprotective activity of Acanthopanax koreanum Nakai extract (AE) was investigated against D-Galactosamine/Lipopolysaccharide (D-GalN/LPS)-induced liver failure rats compared with that of acanthoic acid (AA) isolated from AE. Although D-GalN/LPS (250 mg/kg body weight/10 µg/kg body weight, i.p.) induced hepatic damage, pretreatments with AE (1 and 3% AE/g day) and AA (0.037% AA, equivalent to 3% AE/g day) alleviated the hepatic damage. This effect was the result of a significant decrease in the activity of alanine transaminase. Concomitantly, both the nitric oxide and IL-6 levels in the plasma were significantly decreased by high-dose AE (AE3) treatment compared to the GalN/LPS control (AE0). This response resulted from the regulation of pro-inflammatory signaling via a decrease in TLR4 and CD14 mRNA levels in the liver. While a high degree of necrosis and hemorrhage were observed in the AE0, pretreatment with AE3 and AA reduced the extent of hepatocyte degeneration, necrosis, hemorrhage and inflammatory cell infiltrates compared to the AE0. In conclusion, these results suggest that especially high-dose AE are capable of alleviating D-GalN/LPS-induced hepatic injury by decreasing hepatic toxicity, thereby mitigating the TLR 4-dependent cytokine release. The anti-inflammatory effect of AE could be contributing to that of AA and AE is better than AA.
Keywords: Acanthopanax koreanum Nakai, acanthoic acid, toll-like receptor 4, inflammation, hepatotoxicity
Introduction
Fulminant hepatic failure (FHF) induced by bacteria, viral hepatitis, alcohol and other hepatotoxic drugs, is a severe clinical syndrome which results in massive hepatocytes necrosis. DGalactosamine (D-GalN) and lipopolysaccharide (LPS)-induced acute liver injury in mice is a promising model similar to FHF in the clinic (Galanos et al., 1979). D-GalN in combination with a small amount of LPS also leads to greater generation of reactive oxygen species (ROS) [1]. ROS stimulate tissue macrophages to produce proinflammatory cytokines, which subsequently attract inflammatory cells into the liver [2]. LPS can also trigger numerous pathological events including the production of inflammatory cytokines including tumor necrosis factorα (TNFα), interleukin-6 (IL-6), IL-12, and TNFα [3]. Toll-Like Receptor 4 (TLR4) has been identified as a receptor for LPS. Ben Ari et al. previously reported that hepatic TLR4 contributes significantly to the deleterious effects of D-GalN/LPS in inducing hepatic failure [4]. The signaling of TLR4 in response to LPS induces production of reactive oxygen species and expression of proinflammatory cytokines [5].
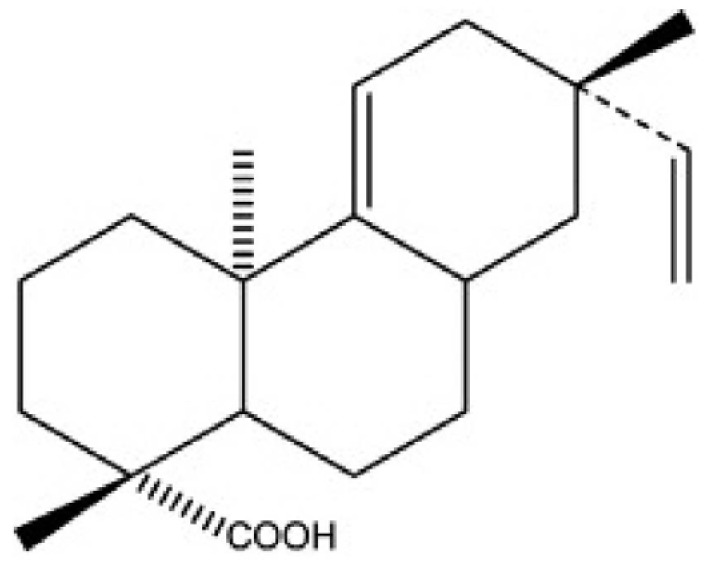
Acanthopanax species are medical plants that are well known in Korea for their adaptogenic efficacy. The roots and stems of Acanthopanax koreanum have been traditionally used in the treatment of rheumatism, diabetes, and hepatitis in Korea [6]. Concerning the botanical classification, Acanthopanax koreanum Nakai is an indigenous species from the Jeju Island of south Korea; this plant has been classified as a unique species of the Acanthopanax genus. Acanthoic acid (AA), (-)-pimara-9(11), 15-dien-19-oic acid, is a diterpene isolated from the root bark of Acanthopanax koreanum Nakai (Fig. 1). AA has been found to suppress the production of interleukin-1 (IL-1), TNFα, and interleukin-8 (IL-8) [7,8]. Previous investigations have shown that AE and AA are beneficial agents against lethality and hepatic injury in both mice and in vitro studies [8,9]. Unlike previous models of hepatitis, the current study examined the contribution of pro-inflammatory signaling to hepatic injury in a D-GalN/LPS model using not only a pharmacological but also a genetic approach.
Fig. 1.
Molecular structure of Acanthoic acid (AA)
The aim of this study was to investigate the hepatoprotective effects of AE and its active component AA on the regulation of inflammatory response via their TLR4 signaling and hepatocyte necrosis against LPS/D-GalN induced hepatic damage in rats.
Materials and Methods
Plant materials
An ethanolic extract of Acanthopanax koreanum Nakai (AE) was provided by Jeju Technopark (Jeju, Korea), and acanthoic acid (AA, Fig. 1) was supplied by Natural Product Chemical Corporation (Daejeon, Korea). A voucher specimen (JBRI316) was deposited in the Herbarium of the Jejutechnopark, Biodiversity Research Institute, Korea. Stems (8 g) and roots (2 g) were ground and dried outside in the shade. This preparation of Acanthopanax koreanum Nakai was then extracted with 70% ethanol at 60℃ for 15 hours and filtered. The filtrates were concentrated, such that the percent by volume was 20%; the concentrated filtrates were then freeze-dried.
Isolation of acanthoic acid
Air-dried roots of Acanthopanax koreanum Nakai were extracted with methylene chloride/methanol (1:1) for 5 hours at 60℃; this extraction was performed twice. The extract was next fractionated using a silica gel column eluted with acetone/n-hexane (1:18). Purified AA was serial fractioneated on an octadocysilan column and a sephadex LH-20 column with 90% methanol and n-hexane/methylene chloride/methanol (4:2:1). Finally, it was fractionated on an octadecysilan column with 90% methanol. The purity of the isolated AA was 91.4% and 0.037% of the AA was equivalent to 3% of the AE (Waters Alliance HPLC systems, USA).
Animals and diets
Six-week-old male Wistar rats (Gbio Inc., Gwacheon, Korea) were housed individually at a temperature of 22 ± 2℃ with 12/12h light/dark cycles and 45 ± 5% humidity. Commercial diet (Purina Inc., Gyeonggi-Do, Korea) and tap water were supplied ad libitum prior to the experiment. The animals were divided into four groups of ten animals each. AE0 rats were fed the AIN 93G-diet (as vehicle control) for 15 days prior to the induction with d-GalN/LPS (250 mg/kg body weight and 10 µg/kg body weight). AE1, AE3 and AA rats were pretreated with the AIN 93G-diet containing 1%, 3% and 0.037% AA (the equivalent of AE3), respectively for 15 days prior to the induction of d-GalN/LPS. Twenty-two hours after injection, the rats were sacrificed, and plasma and tissue samples were prepared and preserved at -80℃. The tissues were saved and fixed in 10% phosphate-buffered formalin for the histological evaluations. The study protocol was approved by the Institutional Animal Care and Use Committee (IACUC 2011-02-040).
Plasma ALT and AST levels
Plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities were measured using a commercial kit (Asan Pharmaceutical, Seoul, Korea).
Histological analysis
Fixed liver tissues were removed from the phosphate-buffered formalin and the water in the tissues was removed with ethyl alcohol. The ethyl alcohol in the tissues was eliminated with xylene and the liver sections were embedded in paraffin for sectioning. The 5um sections were stained with hematoxylineosin (H&E).
Inflammatory cytokine production
Hepatic tumor necrosis factor α (TNFα) levels were examined using a DuoSet ELISA kit (R&D systems, Minneapolis, USA). Plasma interleukin 6 (IL-6) levels were determined with a Quantikine ELISA kit (R&D systems, Minneapolis, USA). NO production in the plasma and liver was measured using a commercial kit (Invitrogen Co., Carlsbad, USA) according to the manufacturer's instructions.
Quantitative TaqMan reverse transcription polymerase chain reaction analysis
Total RNA was extracted from the liver and AT samples using TRIZOL (Invitrogen Co.). RNA concentration and quality were determined with a BioSpec-nano spectrophotometer (Shimadzu Corp., Kyoto, Japan). cDNA was constructed using the High Capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA, USA). Quantitative RT-PCR was performed using the TaqMan method in the Step-One-Plus RT-PCR System (Applied Biosystems). The primer sets for the target genes were toll-like receptor 4 (TLR4) mRNA (Rn00569848_m1), CD14 (Rn00572656_m1), and β-actin (Rn00667869_m1). Amplifications were performed starting with a 10 min template denaturation step at 95℃, followed by 40 cycles at 95℃ for 15s and 60℃ for 1 min. The relative amounts of these mRNAs were normalized to the amount of β-actin and the relative amounts of the RNAs were calculated using the comparative CT Method.
Statistical analysis
Results were presented as mean ± standard error. Statistical analyses were performed using the Statistical Analysis Systems package, version 9.2 (SAS Institute, Cary, NY, USA). The differences between the treated groups were analyzed by one-way analysis of variance (ANOVA) with post hoc Duncan's multiple range tests. Results were considered statistically significant at P < 0.05.
Results
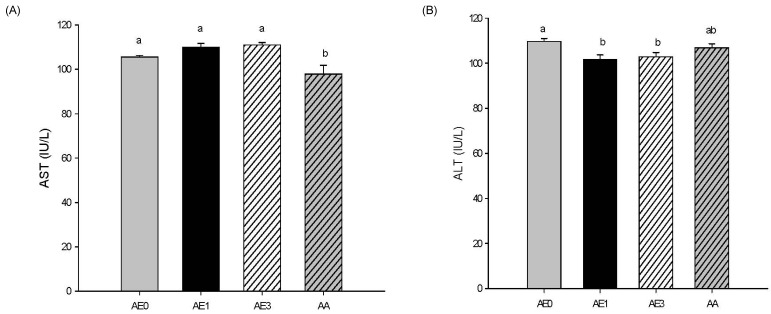
Effects of AE and AA on plasma ALT/AST levels in D-GalN/LPS induced hepatic injury rats
Plasma AST activity was not significantly different in the AE1 and AE3 groups compared to the AE0 group. However, AA pretreatment led to a significant decrease in plasma AST levels compared to those of the AE0 group (Fig. 2A). Plasma ALT activity was significantly decreased in the AE1 and AE3 groups compared to the AE0 group (Fig. 2B), but there was no difference between the AA and AE0 groups.
Fig. 2.
Effects of AE and AA pretreatment on (A) AST and (B) ALT levels in Wistar rats injected with D-GalN/LPS. abValues with different letters are significantly different at P < 0.05 level, as indicated by Duncan's multiple range test. AE0, D-GalN/LPS; AE1, D-GalN/LPS + 1% AE; AE3, D-GalN/LPS + 3% AE; AA, D-GalN/LPS + AA
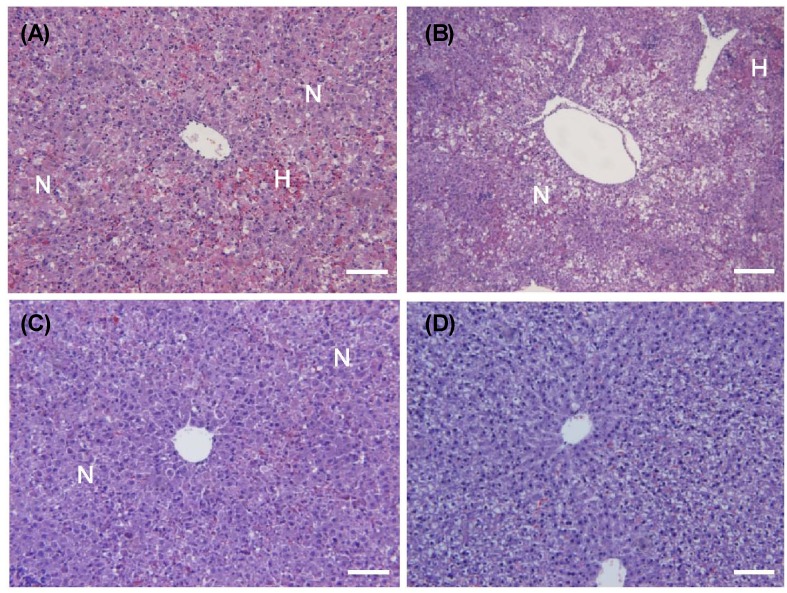
Effects of AE and AA on hepatic morphology in D-GalN/LPS induced hepatic injury rats
In the histological analyses, D-GalN/LPS induced focal necrosis (N) with hemorrhage (H) (Fig. 3A). Necrotic lesions presented primarily with a periportal distribution pattern. The necrotic regions contained hepatocytes with eosinophilic cytoplasms, the appearance of pyknotic or absent nuclei, and the loss of normal cellular architecture. There were no histological differences between the AE1 and AE0 groups (Fig. 3B). However, the AE3 and AA groups showed remarkable reductions in hepatocyte degeneration, necrosis, hemorrhage, eosinophilia of hepatocytes and inflammatory cell infiltrates as compared to the AE0 group (Fig. 3C, 3D).
Fig. 3.
Effects of AE and AA pretreatment on histopathologic changes in Wistar rats injected with D-GalN/LPS. Representative H&E stained rat liver samples from (A) AE0, D-GalN/LPS; (B) AE1, D-GalN/LPS + 1% AE; (C) AE3, D-GalN/LPS + 3% AE; (D) AA, D-GalN/LPS + AA. Bar, 100 mm; magnification, 200x; N, Necrosis; H, hemorrhage.
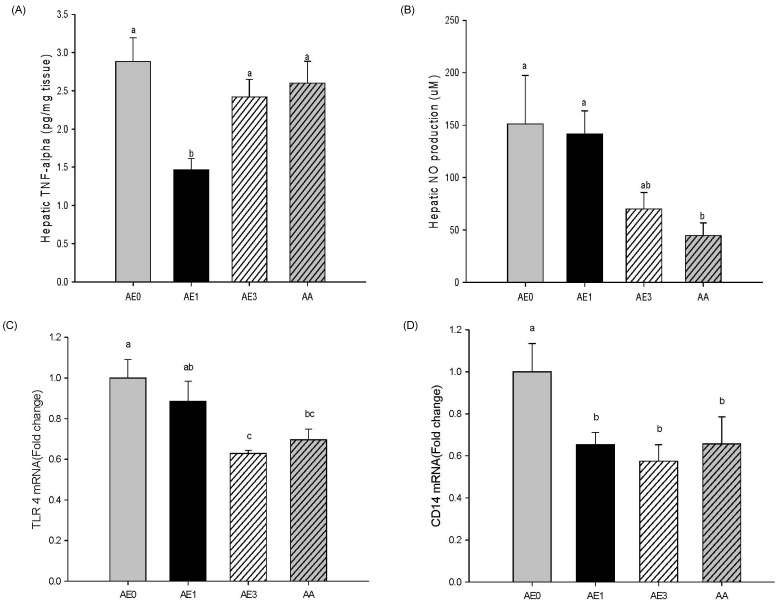
Effects of AE and AA on the hepatic inflammatory response in D-GalN/LPS induced hepatic injury rats
As shown Fig. 4A, hepatic TNFα levels were significantly reduced in the AE1 group compared to the AE0 group; however, the TNFα levels in the AE3 group tended to be lower than the AE0 group. Hepatic NO production in the AE3 group was remarkably decreased in comparison with the AE0 group. Furthermore, the AA group had significantly reduced hepatic NO levels compared with the AE0 group (Fig. 4B). Accordingly, the effects of AE or AA on the pro-inflammatory signaling were examined by the expression of TLR4 and CD14 mRNA in the livers (Fig. 4C and 4D). Hepatic TLR4 mRNA levels in the AE3 and AA groups were significantly reduced compared to the AE0 group. TLR4 mRNA levels in the AE1 group showed a decreasing tendency compared to levels in the AE0 group. Hepatic CD14 mRNA levels were significantly reduced in the AE1, AE3 and AA groups compared to the AE0 group. TLR4 and CD14 mRNA expression showed a dose-dependent relationship with AE, yet there were no significant differences between the AE3 and AA groups.
Fig. 4.
Effects of AE and AA pretreatment on hepatic (A) TNF-α, (B) nitric oxide, (C) Toll-like-receptor 4 mRNA, and (D) CD14 mRNA levels in Wistar rats injected with D-GalN/LPS. abValues with different letters are significantly different at P < 0.05 level, as indicated by Duncan's multiple range test. AE0, D-GalN/LPS; AE1, D-GalN/LPS + 1% AE; AE3, D-GalN/LPS + 3% AE; AA, D-GalN/LPS + AA
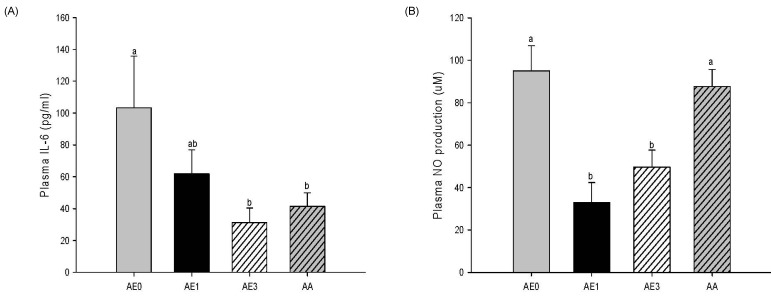
Effects of AE and AA on the plasma cytokine levels in D-GalN/LPS induced hepatic injury rats
Plasma IL-6 level also showed a remarkable decrease in the AE1 group compared to the AE0 group. In addition, the AE3 group and the AA group had significantly reduced plasma levels of IL-6 compared to the AE0 group (Fig. 5A). Plasma NO production was significantly decreased in the AE1 and AE3 groups compared to the AE0 group. Nonetheless, there was no significant difference in NO production between the AA and AE0 groups (Fig. 5B).
Fig. 5.
Effects of AE and AA pretreatment on plasma (A) IL-6 and (B) nitric oxide levels in Wistar rats injected with D-GalN/LPS. abcValues with different letters are significantly different at P < 0.05 level, as indicated by Duncan's multiple range test. AE0, D-GalN/LPS; AE1, D-GalN/LPS + 1% AE; AE3, D-GalN/LPS + 3% AE; AA, D-GalN/LPS + AA
Discussion
In this study, we used acute liver damage rats injected with D-GalN/LPS to investigate the hepatoprotective effects of AE and AA on the regulation of the inflammatory response through the inhibition of both TLR 4-dependent cytokine production. In the D-GalN model, the bacterial cell wall component LPS is used to initiate the inflammatory response. Recognition of many microbial toxins occurs through the activation of Toll-like receptors (TLR) that cause the induction of an innate immune response [10,11]. TLR 2 mediates responses in a wide variety of bacterial products including lipoproteins derived from gram- negative and - positive bacteria. In contrast, TLR4 is specific for LPS derived from gram negative bacteria [10,11]. TLR4 prompted cytokines with the stimulated Kupffer cells, exist in macrophage and recognize LPS or the LPS-CD14 complex [12]. It mediate macrophage activation and pro-inflammatory cytokine release [13,14]. TLR4 activation first engages a set of adaptor family members that link TLR4 to the serine/threonine kinases; this interaction results in the activation of the transcriptional factor NF-κB, which regulates the expression of several immunomodulatory cytokines [15]. The TLR4 mRNA expression level, in the liver of rats with D-GalN-induced acute hepatic failure, is increased compared to control rats [12]. In contrast to, in this study AE or AA downregulated the levels of CD14 and TLR4 mRNA against D-GalN/LPS induced hepatic damage. In addition, the down-regulation of TLR4 and CD14 mRNA by AE led to a decrease in the cytokines IL-6 and TNFα. The hepatic TLR4 mRNA levels increased in parallel to those of TNFα and CD14 mRNA as compared to the levels in the control animals [12]. The proinflammatory cytokine cascade, including TNFα or IL-6, is considered to play an important role in the pathophysiology outcome of liver injury. Especially, TNFα is the terminal mediator of hepatic apoptosis and organ failure. Massive hepatocyte apoptosis induced by TNFα from macrophages is the dominant mechanism of liver injury model [16]. In the histological analyses, D-GalN/LPS induced focal necrosis with hemorrhage. However, the high-dose of AE or AA equivalent to the high-dose of AE showed alleviation of liver pathological injury as compared to the AE0 and reduced IL-6 levels in the plasma via TLR4 signaling. Notably, these effects alleviated the hepatic pathology. AA has been found to suppress the production of inflammatory cytokines [7,8]. The effects of AE3 and AA were not different. Therefore the anti-inflammatory effect of AE could be contributing to that of AA. On the other hand, Plasma NO production was significantly decreased in the AE1 and AE3 groups compared to the AE0 group. The change in TLR 4 mRNA between AE0 and AE1 was not significant, but plasma NO was significant. The measurement of NO itself is difficult because of its very short half-life, and the stable metabolites of NO, have been frequently used as a reliable plasma measurement of NO production. Specifically, the association between basal circulating NO levels and genotypic or phenotypic in vivo variations is not understood [17].
In conclusion, AE exerted hepatoprotective effects against liver damage by inhibiting the D-GalN/LPS-induced up-regulation of the expression of CD14 and TLR4. AE and AA are capable of improvement of liver function, alleviation of liver pathological injury and mitigation of the release of inflammatory cytokines via TLR4 signaling against D-GalN/LPS-induced hepatic damage. These results suggest that anti-inflammatory action of AE is the possible hepatoprotective mechanism. Further study is needed to investigate whether AE intake may help to maintain a healthy hepatic function in liver failure subjects.
Acknowledgements
We thank Jeju Technopark (Jeju, Korea) for the gift of the Acanthopanax koreanum Nakai extract and the acanthoic acid.
Footnotes
This project was supported by the National Platform Technology Project (10033818) from Ministry of Knowledge Economy in Korea, the MKE (Ministry of Knowledge Economy), Korea, under the RIS (Regional Innovation System) support program (B0012328) supervised by the KIAT (Korea Institute for Advancement of Technology) and Korean Ministry of Education, Science and Technology (Brain Korea 21).
References
- 1.Neihörster M, Inoue M, Wendel A. A link between extracellular reactive oxygen and endotoxin-induced release of tumour necrosis factor α in vivo. Biochem Pharmacol. 1992;43:1151–1154. doi: 10.1016/0006-2952(92)90626-t. [DOI] [PubMed] [Google Scholar]
- 2.Mayer AM, Spitzer JA. Modulation of superoxide anion generation by manoalide, arachidonic acid and staurosporine in liver infiltrated neutrophils in a rat model of endotoxemia. J Pharmacol Exp Ther. 1993;267:400–409. [PubMed] [Google Scholar]
- 3.Fiuza C, Suffredini AF. Human models of innate immunity: local and systemic inflammatory responses. J Endotoxin Res. 2001;7:385–388. [PubMed] [Google Scholar]
- 4.Ben Ari Z, Avlas O, Pappo O, Zilbermints V, Cheporko Y, Bachmetov L, Zemel R, Shainberg A, Sharon E, Grief F, Hochhauser E. Reduced hepatic injury in Toll-like receptor 4-deficient mice following D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure. Cell Physiol Biochem. 2012;29:41–50. doi: 10.1159/000337585. [DOI] [PubMed] [Google Scholar]
- 5.Medvedev AE, Kopydlowski KM, Vogel SN. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J Immunol. 2000;164:5564–5574. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- 6.Kang HS, Kim YH, Lee CS, Lee JJ, Choi I, Pyun KH. Suppression of interleukin-1 and tumor necrosis factor-α production by acanthoic acid, (-)-pimara-9(11),15-dien-19-oic acid, and it antifibrotic effects in vivo. Cell Immunol. 1996;170:212–221. doi: 10.1006/cimm.1996.0154. [DOI] [PubMed] [Google Scholar]
- 7.Park EJ, Zhao YZ, Kim YH, Lee JJ, Sohn DH. Acanthoic acid from Acanthopanax koreanum protects against liver injury induced by tert-butyl hydroperoxide or carbon tetrachloride in vitro and in vivo. Planta Med. 2004;70:321–327. doi: 10.1055/s-2004-818943. [DOI] [PubMed] [Google Scholar]
- 8.Nan JX, Jin XJ, Lian LH, Cai XF, Jiang YZ, Jin HR, Lee JJ. A diterpenoid acanthoic acid from Acanthopanax koreanum protects against D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure in mice. Biol Pharm Bull. 2008;31:738–742. doi: 10.1248/bpb.31.738. [DOI] [PubMed] [Google Scholar]
- 9.Wu YL, Jiang YZ, Jin XJ, Lian LH, Piao JY, Wan Y, Jin HR, Joon Lee J, Nan JX. Acanthoic acid, a diterpene in Acanthopanax koreanum, protects acetaminophen-induced hepatic toxicity in mice. Phytomedicine. 2010;17:475–479. doi: 10.1016/j.phymed.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Seki E, De Minicis S, Gwak GY, Kluwe J, Inokuchi S, Bursill CA, Llovet JM, Brenner DA, Schwabe RF. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119:1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qureshi ST, Gros P, Malo D. Host resistance to infection: genetic control of lipopolysaccharide responsiveness by TOLL-like receptor genes. Trends Genet. 1999;15:291–294. doi: 10.1016/s0168-9525(99)01782-5. [DOI] [PubMed] [Google Scholar]
- 12.Kitazawa T, Tsujimoto T, Kawaratani H, Fujimoto M, Fukui H. Expression of Toll-like receptor 4 in various organs in rats with D-galactosamine-induced acute hepatic failure. J Gastroenterol Hepatol. 2008;23:e494–e498. doi: 10.1111/j.1440-1746.2007.05246.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee WM. Acute liver failure. N Engl J Med. 1993;329:1862–1872. doi: 10.1056/NEJM199312163292508. [DOI] [PubMed] [Google Scholar]
- 14.Nakama T, Hirono S, Moriuchi A, Hasuike S, Nagata K, Hori T, Ido A, Hayashi K, Tsubouchi H. Etoposide prevents apoptosis in mouse liver with D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure resulting in reduction of lethality. Hepatology. 2001;33:1441–1450. doi: 10.1053/jhep.2001.24561. [DOI] [PubMed] [Google Scholar]
- 15.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 16.Kawaguchi K, Kikuchi S, Hasegawa H, Maruyama H, Morita H, Kumazawa Y. Suppression of lipopolysaccharide-induced tumor necrosis factor-release and liver injury in mice by naringin. Eur J Pharmacol. 1999;368:245–250. doi: 10.1016/s0014-2999(98)00867-x. [DOI] [PubMed] [Google Scholar]
- 17.Wang XL, Mahaney MC, Sim AS, Wang J, Wang J, Blangero J, Almasy L, Badenhop RB, Wilcken DE. Genetic contribution of the endothelial constitutive nitric oxide synthase gene to plasma nitric oxide levels. Arterioscler Thromb Vasc Biol. 1997;17:3147–3153. doi: 10.1161/01.atv.17.11.3147. [DOI] [PubMed] [Google Scholar]