Abstract
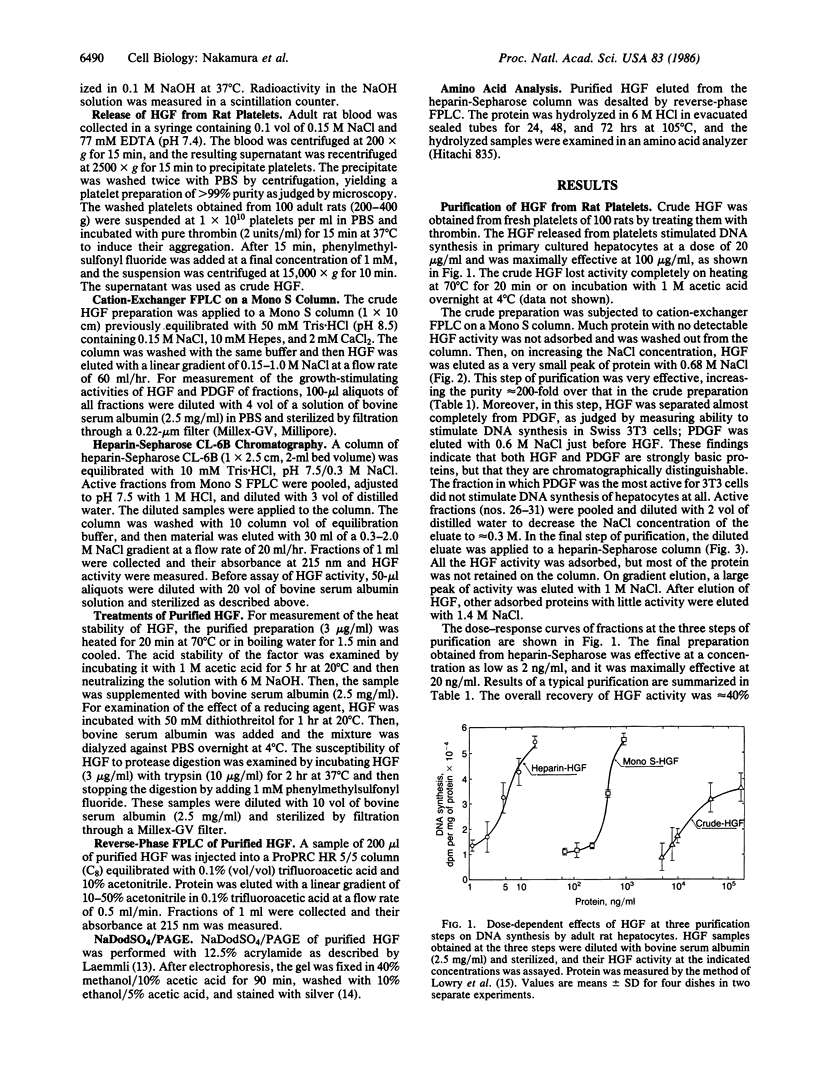
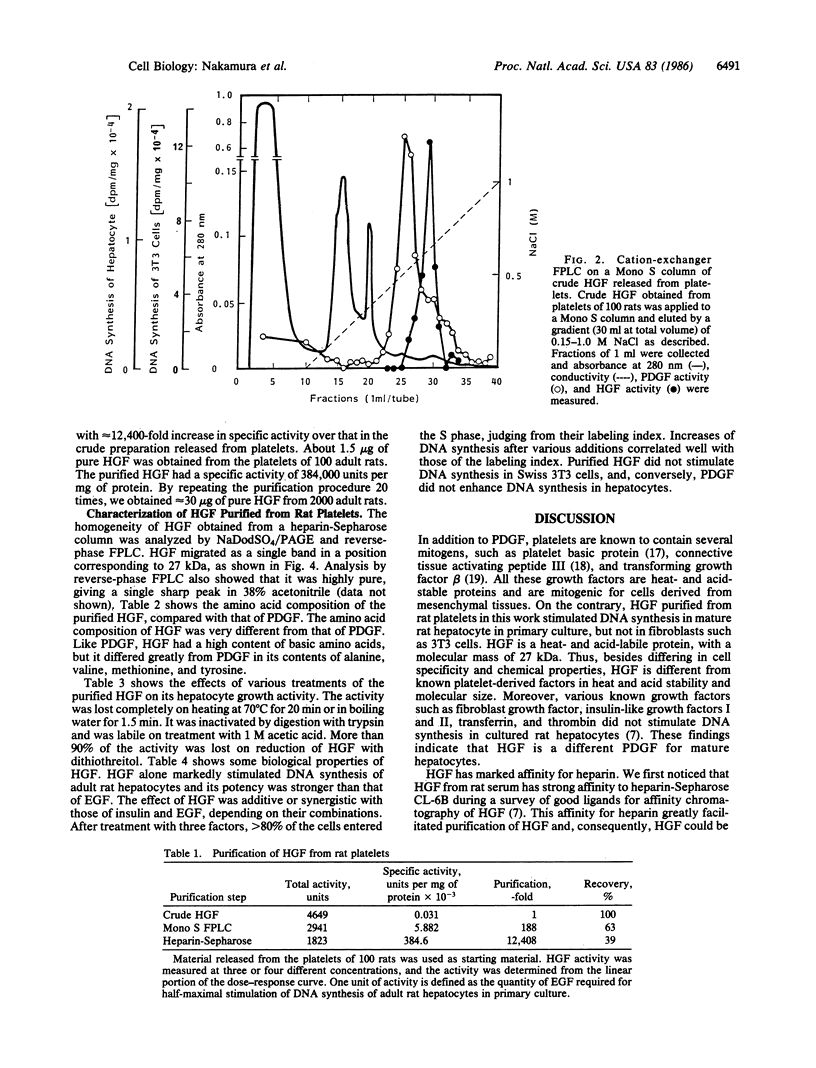
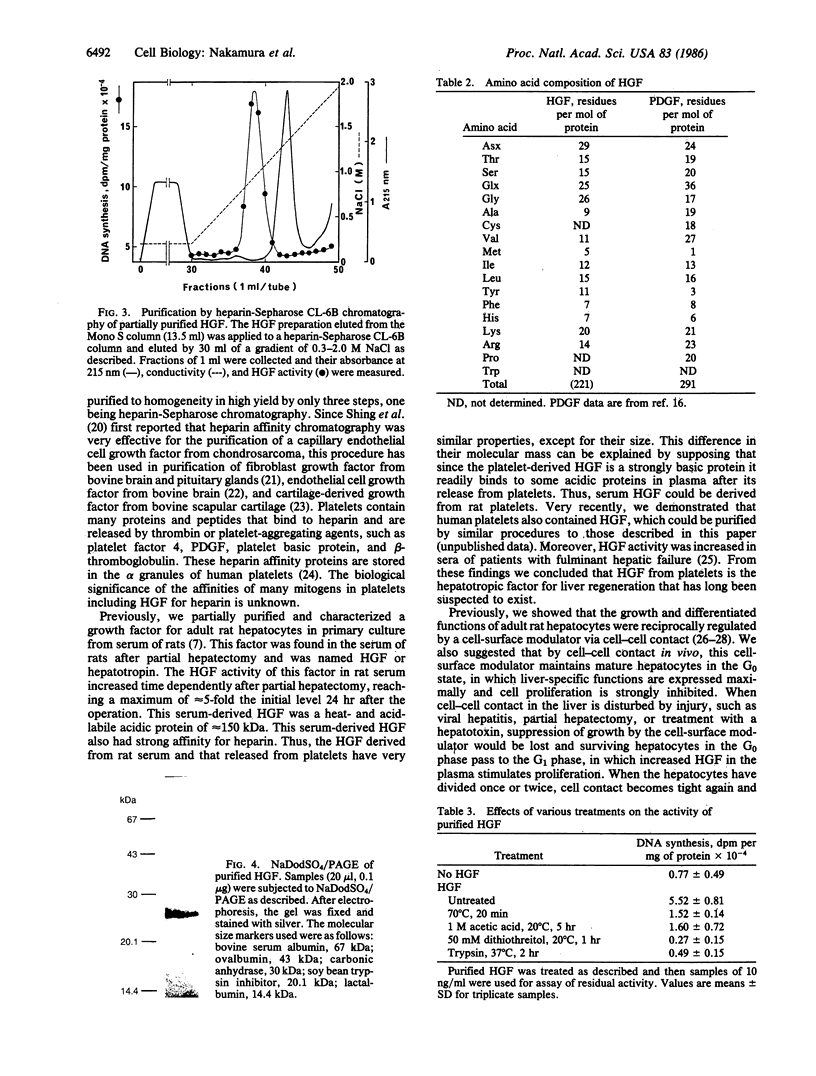
A growth factor (HGF) stimulating DNA synthesis of adult rat hepatocytes in primary culture was found in rat platelets. HGF was purified from rat platelets to homogeneity by a three-step procedure: stimulation of its release from platelets by thrombin, cation-exchanger fast protein liquid chromatography on a Mono S column, and heparin-Sepharose chromatography. HGF was clearly distinguishable from the platelet-derived growth factor (PDGF) by fast protein liquid chromatography. HGF was a heat- and acid-labile cationic protein that was inactivated by reduction with dithiothreitol. Its molecular mass was estimated to be 27 kDa by NaDodSO4/PAGE and its amino acid composition was very different from that of PDGF. The purified HGF stimulated DNA synthesis in adult rat hepatocytes at 2 ng/ml and was maximally effective at 20 ng/ml; its effect was additive or synergistic with those of insulin and EGF, depending on their combinations. HGF did not stimulate DNA synthesis of Swiss 3T3 cells, while PDGF did not stimulate that of hepatocytes. Thus, HGF showed clearly different cell specificity from PDGF in its growth-promoting activities. These findings indicate that HGF is a growth factor in platelets for mature hepatocytes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Castor C. W., Miller J. W., Walz D. A. Structural and biological characteristics of connective tissue activating peptide (CTAP-III), a major human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1983 Feb;80(3):765–769. doi: 10.1073/pnas.80.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D. M., Dean D. Sexual maturity and migratory behaviour of the tropical eel, Ahlia egmontis. Nature. 1970 Jul 11;227(5254):189–190. doi: 10.1038/227189a0. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Proffitt R. T., Baenziger J. U., Chang D., Kennedy B. B. Human platelet-derived growth factor. Purification and resolution into two active protein fractions. J Biol Chem. 1981 Sep 10;256(17):8896–8899. [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K., Watanabe K., Koga M. Induction of mitosis in primary cultures of adult rat hepatocytes under serum-free conditions. Biochem Biophys Res Commun. 1982 Jan 15;104(1):259–265. doi: 10.1016/0006-291x(82)91968-4. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Chao F. C., Stiles C. D., Antoniades H. N., Scher C. D. Platelet alpha granules contain a growth factor for fibroblasts. Blood. 1979 Jun;53(6):1043–1052. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maciag T., Mehlman T., Friesel R., Schreiber A. B. Heparin binds endothelial cell growth factor, the principal endothelial cell mitogen in bovine brain. Science. 1984 Aug 31;225(4665):932–935. doi: 10.1126/science.6382607. [DOI] [PubMed] [Google Scholar]
- McGowan J. A., Bucher N. L. Pyruvate promotion of DNA synthesis in serum-free primary cultures of adult rat hepatocytes. In Vitro. 1983 Mar;19(3 Pt 1):159–166. doi: 10.1007/BF02618054. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G., Cianciulli H. D., Novotny A. R., Kligerman A. D., Strom S. C., Jirtle R. L. Liver regeneration studies with rat hepatocytes in primary culture. Cancer Res. 1982 Nov;42(11):4673–4682. [PubMed] [Google Scholar]
- Nakamura T., Ichihara A. Control of growth and expression of differentiated functions of mature hepatocytes in primary culture. Cell Struct Funct. 1985 Mar;10(1):1–16. doi: 10.1247/csf.10.1. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nakayama Y., Ichihara A. Reciprocal modulation of growth and liver functions of mature rat hepatocytes in primary culture by an extract of hepatic plasma membranes. J Biol Chem. 1984 Jul 10;259(13):8056–8058. [PubMed] [Google Scholar]
- Nakamura T., Nakayama Y., Teramoto H., Nawa K., Ichihara A. Loss of reciprocal modulations of growth and liver function of hepatoma cells in culture by contact with cells or cell membranes. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6398–6402. doi: 10.1073/pnas.81.20.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Nawa K., Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1450–1459. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Teramoto H., Tomita Y., Ichihara A. Two types of growth inhibitor in rat platelets for primary cultured rat hepatocytes. Biochem Biophys Res Commun. 1986 Jan 29;134(2):755–763. doi: 10.1016/s0006-291x(86)80485-5. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Tomita Y., Hirai R., Yamaoka K., Kaji K., Ichihara A. Inhibitory effect of transforming growth factor-beta on DNA synthesis of adult rat hepatocytes in primary culture. Biochem Biophys Res Commun. 1985 Dec 31;133(3):1042–1050. doi: 10.1016/0006-291x(85)91241-0. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Tomita Y., Ichihara A. Density-dependent growth control of adult rat hepatocytes in primary culture. J Biochem. 1983 Oct;94(4):1029–1035. doi: 10.1093/oxfordjournals.jbchem.a134444. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Yoshimoto K., Nakayama Y., Tomita Y., Ichihara A. Reciprocal modulation of growth and differentiated functions of mature rat hepatocytes in primary culture by cell--cell contact and cell membranes. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7229–7233. doi: 10.1073/pnas.80.23.7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Paul D., Niewiarowski S., Varma K. G., Rucinski B., Rucker S., Lange E. Human platelet basic protein associated with antiheparin and mitogenic activities: purification and partial characterization. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5914–5918. doi: 10.1073/pnas.77.10.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D., Piasecki A. Rat platelets contain growth factor(s) distinct from PDGF which stimulate DNA synthesis in primary adult rat hepatocyte cultures. Exp Cell Res. 1984 Sep;154(1):95–100. doi: 10.1016/0014-4827(84)90670-0. [DOI] [PubMed] [Google Scholar]
- Russell W. E., McGowan J. A., Bucher N. L. Biological properties of a hepatocyte growth factor from rat platelets. J Cell Physiol. 1984 May;119(2):193–197. doi: 10.1002/jcp.1041190208. [DOI] [PubMed] [Google Scholar]
- Russell W. E., McGowan J. A., Bucher N. L. Partial characterization of a hepatocyte growth factor from rat platelets. J Cell Physiol. 1984 May;119(2):183–192. doi: 10.1002/jcp.1041190207. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Shing Y., Folkman J., Sullivan R., Butterfield C., Murray J., Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984 Mar 23;223(4642):1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- Sullivan R., Klagsbrun M. Purification of cartilage-derived growth factor by heparin affinity chromatography. J Biol Chem. 1985 Feb 25;260(4):2399–2403. [PubMed] [Google Scholar]
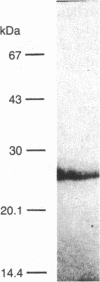
- Tanaka K., Sato M., Tomita Y., Ichihara A. Biochemical studies on liver functions in primary cultured hepatocytes of adult rats. I. Hormonal effects on cell viability and protein synthesis. J Biochem. 1978 Oct;84(4):937–946. doi: 10.1093/oxfordjournals.jbchem.a132207. [DOI] [PubMed] [Google Scholar]
- Tomita Y., Nakamura T., Ichihara A. Control of DNA synthesis and ornithine decarboxylase activity by hormones and amino acids in primary cultures of adult rat hepatocytes. Exp Cell Res. 1981 Oct;135(2):363–371. doi: 10.1016/0014-4827(81)90172-5. [DOI] [PubMed] [Google Scholar]