Abstract
Infection with Human Immunodeficiency Virus Type 1 (HIV-1) induces defects of both cellular and humoral immune responses. Impaired CD4+ T cell help and B cell dysfunction may partially explain the low frequency of broadly neutralizing antibodies in HIV-infected individuals. To understand the extent of B cell dysfunction during HIV infection, we assessed the level of B cell activation at baseline and after stimulation with a variety of antigens. Increased levels of viremia were associated with higher baseline expression of the activation marker CD86 on B cells and with decreased ability of B cells to increase expression of CD86 after in vitro stimulation with inactivated HIV-1. In a series of cell isolation experiments B cell responses to antigen were enhanced in the presence of autologous CD4+ T cells. HIV infected individuals had a higher frequency of PD-1 expression on B cells compared to HIV- subjects and PD-1 blockade improved B cell responsiveness to HIV antigen, suggesting that inhibitory molecule expression during HIV-1 infection may contribute to some of the observed B cell defects. Our findings demonstrate that during chronic HIV infection, B cells are activated and lose full capacity to respond to antigen, but suppression of inhibitory pressures as well as a robust CD4+ T cell response may help preserve B cell function.
Introduction
Infection with HIV-1 induces defects of both cellular and humoral immune responses, inhibiting the immune system from mounting an effective response against infection. Since shortly after AIDS was identified, abnormalities of both B cell and T cell function have been described in HIV-infected individuals [1]. Persistent high level viremia is associated with increased expression of activation markers on T and B cells [2,3], hypergammaglobulinemia [1,4-6], and decreased antibody responses to in vivo vaccination [7-10]. In addition to antibody production, B cell antigen presenting function is also impaired after HIV infection [11]. While it has been suggested that B cell function may be deficient as a result of a lack of CD4+ T cell help [12], there also may be intrinsic B cell defects in HIV infection [13].
B cells in chronic viral infection have a phenotype consistent with immune exhaustion and terminal differentiation [14-16]. In HIV-infected individuals, expression of the IL-2 receptor, CD25, on B cells in response to in vitro stimulation is lower than in uninfected individuals, despite normal levels of expression of CD154 (CD40L) on CD4+ T cells. This defect persists even after the addition of supplemental IL-2 [13]. The bidirectional interaction between CD80 and CD86, ligands of the B7 family, and their receptor, CD28 on CD4+ T cells, is also critical for an effective humoral response. In HIV infection, B cells of viremic subjects not only have decreased ability to increase expression of CD80 and CD86 in response to in vitro BCR and CD40L stimulation, but they also are ineffective at stimulating CD4+ T cells, suggesting impairment in both directions of the interaction [17].
The decreased responsiveness of B cells may be due to impaired help they receive from exhausted CD4+ T helper cells in HIV infection [18-21]. Exhausted CD4 and CD8 T cells exhibit decreased responses to antigen and often express high levels of inhibitory receptors such as PD-1 and CTLA-4 on their surface. Studies have likewise termed B cells “exhausted” due to their poor proliferative capacity that is only partially restored with the addition of stimulatory cytokines and soluble CD40L [14,16]. Increased surface expression of PD-1 on T cells is sustained over the course of chronic viral infection [22,23] and may define a reversible impairment of HIV-specific T cell function [18-20,24,25]. The function of T cells from HIV-infected individuals can be partially restored by in vitro blockade of the PD-1/PD-L1 interaction [18,26,27]. After acute SIV infection, in vivo blockade of PD-1 has been shown to increase the proliferative capacity and frequency of B cells and the production of SIV-specific binding antibody [28]. B cells from HIV-infected individuals have increased expression of several inhibitory receptors, and siRNA downregulation of these receptors increases memory B cell proliferation and increases the number of antibody-secreting B cells [29]. While blocking these inhibitory pathways may provide opportunities to restore CD4+ T cell help for B cells, these interactions have not yet been directly evaluated.
We measured B cell activation markers CD25 and CD86 in the setting of chronic HIV-1 infection after in vitro culture with and without stimulation of PBMCs by a variety of antigens. We found high frequencies of CD86+ B cells in HIV-infected individuals, and their frequency correlated with the level of viremia. B cell responsiveness to inactivated HIV, however, negatively correlated with viral load. We also performed a series of co-culture experiments with purified B cells and autologous CD4+ T cells, as well as in vitro blockade of PD-1 to investigate the requirements for CD4+ T cell help and the role of inhibitory molecules for inducing B cell activation. We provide evidence that lack of HIV-specific CD4 helper responses and high PD-1 expression in the setting of HIV-1 infection both contribute to B cell dysfunction.
Materials and Methods
Study Subjects
Study subjects included seven HIV-negative controls and 21 HIV-infected individuals (Table 1). PBMCs were separated from blood samples using a Ficoll-PaqueTM Plus density gradient, cryopreserved in FBS with 10% DMSO, and stored in liquid nitrogen until thawed for immediate use. HIV-infected subjects were ART-naïve with wide ranges of CD4 T cell numbers (132 to 1374 cells/uL), viral load (50 to 76,427 copies/mL), and duration of infection (<1 to 23 years post-diagnosis). The Vanderbilt University School of Medicine IRB approved this study, and all individuals provided written informed consent. All HIV-infected individuals were recruited from the Vanderbilt Comprehensive Care Center, and HIV negative individuals were recruited from Vanderbilt University Medical Center.
Table 1. HIV+ subject characteristics.
| Subject ID | Time post-infection (years) | Viral Load (copies/mL) | CD4+ T cells (per mm3 blood) | |
|---|---|---|---|---|
| 1 | 10031 | 11 | 50 | 856 |
| 2 | 10071 | 14 | 50 | 950 |
| 3 | 10040 | 14 | 50 | 1,161 |
| 4 | 10067 | 17 | 199 | 814 |
| 5 | 10004 | 19 | 253 | 360 |
| 6 | 10060 | 4 | 722 | 688 |
| 7 | 10070 | 12 | 750 | 915 |
| 8 | 20018 | 2 | 1,886 | 1,374 |
| 9 | 20011 | <1 | 2,655 | 512 |
| 10 | 10023 | 6 | 7,178 | 390 |
| 11 | 10027 | 14 | 7,340 | 378 |
| 12 | 10054 | 3 | 7,522 | 350 |
| 13 | 10035 | 20 | 7,750 | 464 |
| 14 | 10026 | 6 | 8,865 | 414 |
| 15 | 10108 | 3 | 9,698 | 960 |
| 16 | 20028 | <1 | 15,800 | 515 |
| 17 | 10042 | 23 | 20,211 | 331 |
| 18 | 10114 | 17 | 20,574 | 966 |
| 19 | 10076 | 6 | 21,339 | 700 |
| 20 | 20017 | 1 | 23,317 | 435 |
| 21 | 10086 | 17 | 76,427 | 132 |
Organized by increasing viral load.
In vitro cell stimulation
Peripheral blood mononuclear cells (PBMCs) from each subject were cultured at 10 x106 cells/mL in 48-well plates (2x106 cells/well) in R10 medium (RPMI 1640 containing 10% heat inactivated FCS, 2 mM L-glutamine, 50 ug/mL penicillin, 50 ug/mL streptomycin, and 10mM Hepes) and co-stimulated with anti-CD28 (1ug/mL, BD Biosciences) and anti-CD49d (1ug/mL, BD). Cells were non-specifically stimulated with plate-bound OKT3 (1 ug/ml, ATCC) or Staphylococcal Enterotoxin B (SEB) (1ug/mL, Sigma). HIV-specific stimulation was performed with AT-2 inactivated HIV-1 MN particles (0.53ug/mL p24, Lot# P3964, generously provided by Dr. Jeff Lifson) [30,31], or HIV-1 p24 core protein (1ug/mL, Protein Sciences). As controls, PBMCs were incubated with media alone, or HIV-1 MN control, containing AT-2 treated microvesicles prepared from matched uninfected cultures, used at a comparable total protein concentration (Lot# P3914) [30,31]. At 24 hours post-stimulation, PBMCs were recovered, washed, and stained with the appropriate surface marker antibodies. For experiments evaluating PD-1 blockade, PBMCs were incubated overnight with 10ug/ml anti-PD-1 (EH12.2H7, BioLegend).
Flow cytometric evaluation of lymphocyte surface molecules
B and T cell surface markers were analyzed by flow cytometry after 24 hours of stimulation using a combination of an amine-reactive viability dye (LIVE/DEAD Aqua, Invitrogen), CD3-AF700 (UCHT1, BD), CD4-PETR (S3.5, Invitrogen), CD8-V450 (RPA-T8, BD), CD19-PECy-7 (SJ25C1, BD), CD25-FITC (2A3, BD), CD86-PE (2331, BD) and PD-1-PE (EH12.2H7, BioLegend). In prior studies, we used an indirect staining method for detection of PD-1 with purified anti-PD-1 (Mouse IgG1, clone EH12.2H7, BioLegend) followed by goat-anti-mouse IgG-Pacific Blue (Molecular Probes) [32,33]. However, this lead to very high background staining of CD19+ B cells (i.e. the goat anti-mouse antibody labeled B cells even in the absence of a primary antibody) that could not be overcome by incubation of cells with human serum. The anti-PD-1-PE directly conjugated antibody yielded frequencies of PD-1 expression on CD4+ T cells similar to the indirect method. CD4v4-PE (L120, BD) was used to stain CD4 cells after they had been purified using methods described below. Lymphocytes were discriminated based on cell size and granularity using forward and side-scatter properties. Non-viable cells were gated out of further analysis. CD19+ B cells were evaluated for surface expression of activation (CD25 and CD86) markers. CD4+ T cells were selected from their parent CD3+ T cell population and evaluated for CD25 expression.
In vitro stimulation after B cell and CD4+ T cell isolation
CD19+ B cells were purified using EasySep Human CD19 Positive Selection Kit and Robosep (StemCell Technologies). CD4+ T cells were purified from the CD19-depleted fraction using EasySep Human CD4 Positive Selection Kit and Robosep (StemCell Technologies). Purity was assessed after each selection step by flow cytometry: B cell purity ranged from 92-99% of live cells and CD4+ cell purity ranged from 70-90% (depending on the subject’s monocyte population). Total PBMCs, positively selected B cells, or positively selected B cells combined with positively selected CD4+ T cells were cultured overnight in a 48-well plate. Cells were left unstimulated or were stimulated with p24 antigen or SEB. As a positive control for B cell stimulation, a subset of cells from each condition was stimulated with soluble CD40L (10ng/mL, GIBCO) and purified anti-human IgM (10ug/mL, BioLegend). After 24 hours cells were washed and stained with the appropriate surface marker antibodies.
Statistical Analysis
All statistical analysis was done using GraphPad Software (Prism 6). Correlations were performed using the Spearman rank method. All paired comparisons were analyzed with the Wilcoxon matched pairs t-test while unpaired comparisons were analyzed using the Mann Whitney t-test.
Results
B cell responsiveness correlates with HIV viremia
We first measured the baseline expression of the activation markers CD86 and CD25 on B cells from a cohort of HIV-infected individuals with a wide range of viremia (Table 1) and seven HIV-negative controls. We found that the baseline frequency of CD86-expressing B cells in HIV-infected individuals was higher than that of uninfected control subjects (medians of 38% compared to 26%, p=0.03) and positively correlated with viral load (Figure 1; r=0.63, p=0.003). Baseline expression of CD25 on B cells (p=0.48) or CD4+ T cells (p=0.97) did not correlate with viral load.
Figure 1. CD86+ B cells are more frequent in HIV+ than HIV- subjects and correlate with viremia.

PBMCs from HIV- (open circles) or HIV+ (closed circles) subjects were incubated overnight without stimulation and evaluated for surface level CD86 expression on B cells. HIV+ subjects had a higher frequency of CD86+ B cells compared to HIV- subjects (unpaired t-test not shown on graph, p=0.03). The frequency of CD19+CD86+ B cells in HIV+ individuals correlates with the level of viremia (r=.63; p=.003). Correlation statistics shown are derived from HIV+ subject data only and do not include data from HIV- subjects.
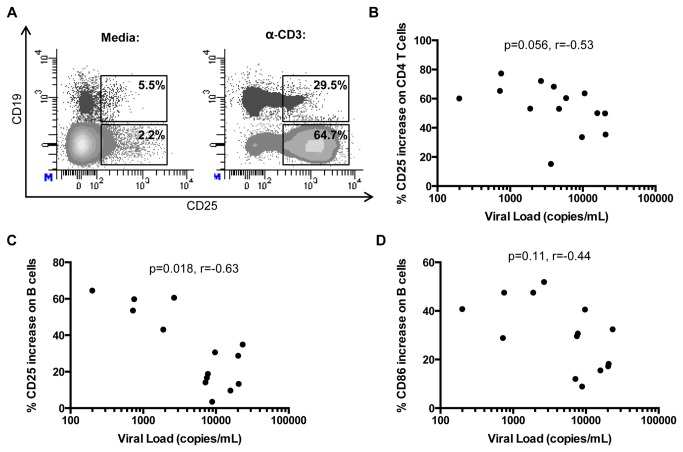
It has previously been shown that B cells from HIV-infected individuals have a diminished ability to express CD25 after in vitro stimulation, and this contributes to low proliferative capacity [13]. We measured the expression of CD25 on both CD4+ T cells and B cells after direct stimulation of PBMCs with anti-CD3 antibody in 14 HIV-infected individuals off anti-retroviral therapy with a range of viral loads (Figure 2A). CD25 expression on CD4+ T cells increased after anti-CD3 stimulation, and there was a weak negative correlation between the change in expression of CD25 and viral load (Figure 2B, r=-0.53, p=0.056). There was a strong inverse correlation between the change in expression of CD25 on B cells (Figure 2C, r=-0.63, p=0.02) and viral load. The change in expression of CD86 on B cells in response to anti-CD3 did not correlate with viral load (Figure 2D, r=-0.44, p=0.11).
Figure 2. Change in CD25 expression on B and CD4+ T cells negatively correlates with viral load.
PBMCs were cultured overnight with or without anti-CD3 stimulation. Change in CD25 or CD86 expression was determined by subtracting the frequency of expression before stimulation from the frequency of expression after stimulation. (A) Representative plots of CD25 expression on CD4+ T cells and B cells with (bottom) and without (top) anti-CD3 stimulation. CD4+ T cell population shown is CD3+CD4+CD19- and B cell population shown is CD3-CD4-CD19+. (B-D) Change in expression of CD25 on CD4+ T cells (r= -.53; p= .056) (B), CD25 on B cells (r= -.63; p= .018) (C), and CD86 on B cells (r= -.44; p= .11) (D) correlates negatively with viral load.
To measure antigen-specific responses, we next evaluated the ability of B cells to respond to in vitro stimulation with aldrithiol-2 (AT-2) inactivated HIV-1 MN in the presence of CD4+ T cells. Bidirectional activation of B cells and CD4+ T cells requires co-stimulatory interactions between CD80/CD86 on B cells and CD28 on CD4+ T cells. We stimulated PBMC with AT-2 inactivated HIV-1 MN or HIV-1 MN control (Figure 3A). Responses to treatment by HIV-1 MN control were not above baseline levels. In HIV-negative control subjects, we observed minor differences between expression of CD86 on B cells by inactivated HIV antigen compared to the control (median of -0.3%). However, HIV-infected individuals responded to HIV antigen, and the degree of CD86 expression was inversely correlated with the level of viremia (median of 4.95%, r=-0.60, p=0.006) (Figure 3B). Responsiveness of CD4+ T Cells to HIV-1 MN as measured by increased expression of CD25 was significantly higher in HIV infected individuals compared to negative controls (p=.02), but the degree of responsiveness to HIV antigen did not correlate with viral load (p=0.4, data not shown). These results demonstrate that in chronic HIV-1 infection, the activation state and responsiveness of B cells to both HIV and non-HIV antigens are much better correlates of control of viremia than similar parameters on CD4+ T cells.
Figure 3. Magnitude of B cell responses to inactivated HIV-1 inversely correlates with viral load.
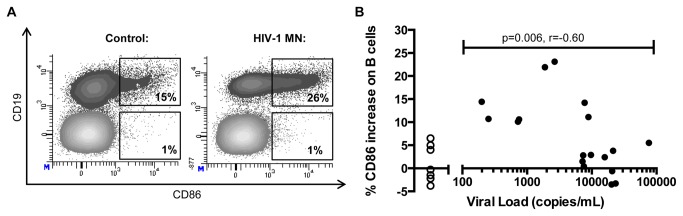
(A) Expression of CD86 on B cells after stimulation of PBMC with HIV-1 MN control (left column) or HIV-1 MN (right column). Shown are representative plots from one individual (subject 10071).. (B) PBMCs from 21 HIV-infected individuals (closed circles) and 7 HIV-negative control subjects (open circles) were incubated with inactivated HIV-1 MN, and changes in CD86 expression on B cells were measured. Change was calculated by subtracting the frequency of CD86 expression from stimulation with the HIV-1 MN control (containing no HIV proteins) from stimulation with HIV-1 MN. CD86 expression on B cells in response to HIV-antigen in HIV infected individuals is negatively correlated with viral load (r= -.6; p= .006). Correlation statistics are only applied to HIV-infected individuals and do not include data from uninfected subjects.
Autologous CD4+ T cells enhance B cell responses to HIV antigen
We next evaluated the contribution of CD4+ T cells for B cell responses to antigenic stimulation. We purified B cells from 7 HIV-infected individuals who demonstrated responses to HIV antigen and who had sufficient numbers of PBMCs available for analysis, and measured CD86 expression after in vitro stimulation in the presence or absence of CD4+ T cells. Previous studies have demonstrated that HIV-specific CD4+ helper responses are primarily Gag-specific [26,34]. In our lab we observed that CD4+ T cells respond more robustly to p24 protein stimulation compared to inactivated HIV-1 MN stimulation (data not shown). Since we sought to measure the CD4+ T cell contribution to B cell activation, in this series of cell isolation experiments we stimulated cultures with HIV p24 antigen. We found that in the absence of CD4+ T cells, purified B cell populations had a markedly reduced ability to respond to the superantigen SEB (Figure 4A; p= .02) or HIV p24 antigen (Figure 4B; p= .02). The ability of B cells to respond to antigen was restored after addition of autologous CD4+ T cells back to the cultures. As a positive control, B cells were able to directly respond to anti-IgM and soluble CD40L to a similar extent whether CD4+ T cells were present or not (data not shown). These data suggest that in this system, B cells have an enhanced response to HIV antigen when they receive help from antigen-specific CD4+ T cells.
Figure 4. Purified B cell responses to p24 are enhanced by addition of autologous CD4+ T cells.
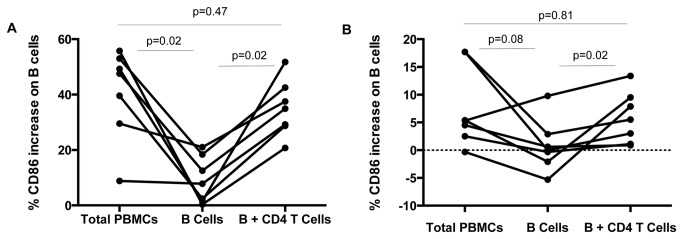
Change in the frequency of CD86+ B cells in response to SEB (A) or HIV p24 antigen (B) was evaluated in total PBMC culture, purified B cell culture, or purified B cells co-cultured with autologous purified CD4+ T cells.
PD-1 blockade improves B cell responses to HIV antigen
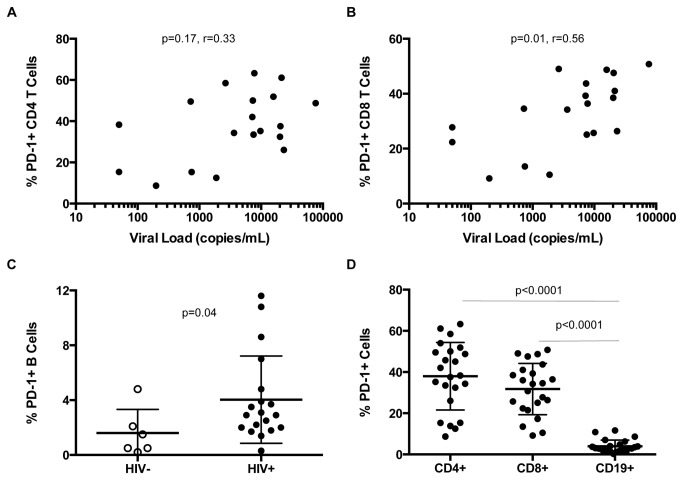
We next investigated whether PD-1 expression on B or T cells was related to the ability of B cells to express CD86 in response to in vitro stimulation. We measured surface PD-1 expression on T cells and B cells in our cohort; there was no correlation between frequencies of PD-1-expressing CD4+ T cells and viremia (Figure 5A; r=.33, p=.17), but higher frequencies of PD-1-expressing CD8+ T cells were associated with increasing levels of viremia (Figure 5B; r=0.56, p=0.01). While the frequency of PD-1+ CD4+ T cells (p = .06) and B cells (Figure 5C; p=0.04) was higher in HIV+ compared to HIV- individuals, PD-1 expression on these cell subsets did not correlate with viral load. The PD-1 expression on CD19+ B cells was significantly lower than that of CD8+ T cells and CD4+ T cells for all individuals studied (Figure 5D; p<0.0001).
Figure 5. Frequency of PD-1 surface expression on lymphocytes is elevated during HIV infection.
Expression of PD-1 on B cells, CD4+ T cells, and CD8+ cells was measured directly ex vivo. (A) In HIV-infected individuals, PD-1 expression on CD4+ is not correlated with viral load (r=.33; p=.17). (B) In HIV-infected individuals PD-1 expression on CD8+ T cells correlated positively with viral load (r= .56; p=.01). (C) PD-1 expression is higher on B cells from HIV+ (closed circles) compared to HIV- (open circles) subjects (p=.04). (D) The frequency of PD-1 surface expression is significantly lower on B cells compared to CD4 (p< .0001) and CD8 T cells (p<.0001) in HIV infection.
To determine whether lymphocyte PD-1 expression affects B cell stimulation, we evaluated the effect of PD-1 blockade on in vitro B cell activation in response to HIV antigen. We observed that increased CD86 expression on B cells in response to inactivated HIV-1 MN was enhanced by in vitro PD-1 blockade in HIV-infected individuals (Figure 6; p=0.003). However, while statistically significant, the median increase in CD86 expression in the presence of anti-PD-1 was modest (median absolute increase of 1.4%; median relative increase of 36%). We found no correlations between the effect of PD-1 blockade and viral load, baseline expression of CD86 on B cells, or baseline expression of PD-1 on CD4+ T cells or B cells. In summary, these data suggest that the elevated PD-1 expression on B cells or CD4+ T cells in chronic HIV infection may contribute to impaired B cell responsiveness.
Figure 6. PD-1 blockade improves B cell responses to stimulation with inactivated HIV-1.
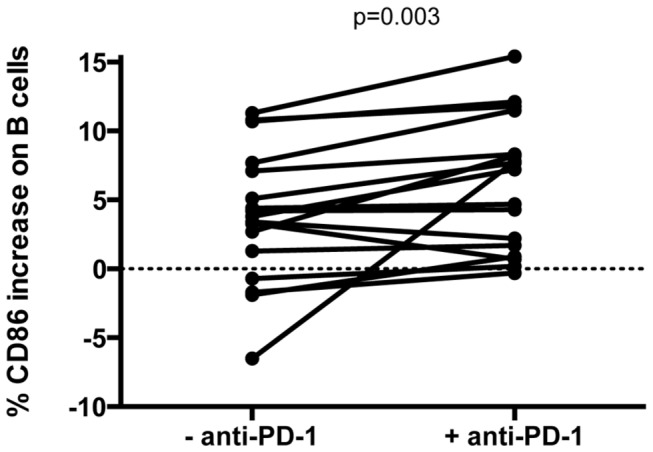
PBMCs were cultured overnight with or without anti-PD-1 and stimulated with inactivated HIV-1 MN protein. Change in response to MN stimulation was calculated by subtracting stimulation with MN control protein from stimulation with HIV-1 MN protein (p= .003).
Discussion
HIV replication causes B cell hyperactivation, susceptibility to apoptosis, and poor proliferative capacity. This dysfunction in HIV-infected individuals is characterized by hypergammaglobulinemia and deficient antibody responses to both neo and recall antigens [7-10,35-38]. Here we confirm prior studies that the degree of B cell activation, as measured by the frequency of CD86-expressing B cells, is not only higher in HIV+ individuals [11,39], but also correlates with the level of viremia. We also extend these studies by demonstrating that the ability of B cells to respond to HIV antigen is a strong correlate of control of viremia.
Few studies have evaluated the relationship between B cell responses to antigen and viral load. The most consistent correlates of control of viremia are the ability of CD4+ T cells [34,40] and CD8+ T cells [41] to proliferate for several days in culture in response to HIV antigen. The frequency of HIV-specific interferon gamma-producing T cells has not been shown to correlate with control of viral replication [42], but the frequency of T cells able to secrete multiple cytokines and exert effector function is associated with proliferation following antigen exposure, and thus may be a better correlate of immune control of viremia. However, significant frequencies of polyfunctional T cells are typically observed in subjects with viral loads <1,000 copies/mL [43,44]. While we did not perform assays for cytokine responses to HIV stimulation in this study, we measured changes in CD25 expression on CD4+ T cells after stimulation with inactivated HIV-1 and the ability of B cells to respond to HIV antigen was a better correlate of viremia
B cell activation has been linked to decreased B cell responsiveness to stimulation and decreased proliferative capacity [11,45]. Moir et. al. found that after T cell stimulation with anti-CD3, the ability of B cells to express CD25 was diminished with increasing viral load, but a strict correlation was not observed [13]. We confirmed this finding in our cohort, but also found CD86 expression on B cells in response to inactivated HIV-1 to be a strong negative correlate of control of viremia. Our study was not designed to determine which regions of the virus were targeted by these responses. AT-2 inactivated HIV-1 MN contains structurally intact viral proteins, and while we suspect these B cell responses are directed against Env, we can’t rule out recognition of other structural proteins (e.g. Gag) that are exposed during the inactivation process. Nevertheless, B cell responsiveness to HIV antigen is potentially an even stronger negative correlate of control of viremia than the frequency of virus-specific T cell responses.
We next evaluated whether the lack of CD4+ T cell help contributes to decreased B cell responsiveness. Initially, we performed stimulations after depleting CD4+ T cells by negative selection, but found considerable B cell responses to HIV antigen. This raised the possibility that either B cells were directly responding to antigen, or antigen presenting cells remaining in culture were providing significant help (data not shown). Repeat experiments were performed with positively selected B cells with or without the addition of autologous CD4+ T cells. B cells alone had a limited ability to respond to the T cell stimulant SEB, and this ability to respond to antigen was reconstituted with the addition of CD4+ T cells. Isolated B cells also had a diminished response to p24 stimulation, which likewise was restored by the addition of CD4+ T cells to the culture. This isolation procedure had no effect on the ability of B cells to respond directly to stimulation with anti-IgM and CD40L. We also ensured that our isolation methods were not altering the activation state of the B cells by comparing the difference in CD86 expression on B cells before and after isolation. While we cannot rule out the possibility that some of the B cell activation we observed was due to the direct recognition of HIV antigen by B cells, the results of our B and T cell isolation experiments suggest that antigen-specific CD4+ T cell help enhances B cell activation.
To investigate a potential mechanism for poor responses of B and T cells in HIV infection, we evaluated the role of immune exhaustion through expression of PD-1 on these cell populations. In accordance with prior studies, we found that while PD-1 expression on CD8+ T cells correlated with the level of viremia, there was a weaker relationship between PD-1 expression of CD4+ T cells and the level of viremia [18-20]. PD-1 expression on B cells was much lower than that of CD4+ and CD8+ T cells, but higher on B cells from HIV-infected compared to HIV-uninfected individuals. Boliar et. al. recently reported a correlation between the frequency of PD-1+ B cells and viral load (7), but this relationship was driven by individuals with CD4+ T cell counts <200/mm3. We did not see this correlation in our study cohort, likely because all but one of our study subjects had CD4+ T cell counts >300/mm3.
In vitro PD-1 blockade of PBMC from HIV-infected individuals has been shown to augment HIV-specific CD4+ and CD8+ T cell function (23). We found in vitro PD-1 blockade to modestly increase the ability of B cells to respond to HIV antigen. These experiments do not explain whether decreased B cell responsiveness to stimulation reflects increased PD-1 expression on B cells, CD4+ T cells, or both. However, since these interactions are bi-directional it is reasonable to assume increased PD-1 expression on both subsets of cells play a role in reducing B cell activation. Furthermore, we did not find a direct relationship between the frequency of PD-1 high CD4+ T cells or B cells, and the ability of PD-1 blockade to increase the magnitude of B cell responses. We did not investigate other pathways, such as CTLA-4, Tim-3 or LAG-3 [46-48] that may also contribute to immune exhaustion, but our results demonstrate reversal of immune exhaustion may be one of many modalities that could serve as a potential immunotherapy to help restore CD4-mediated B cell help.
We simultaneously evaluated surface expression markers of activation on T and B cells, and did not evaluate specific subsets of B cells that may be preferentially impaired [49]. Furthermore, some studies have demonstrated decreased ability of HIV-infected individuals B cells to differentiate toward plasmablasts in long-term culture. This has been attributed to decreased ability of T follicular helper cells (CD4+CXCR5+) to secrete IL-21 [50,51]. Our study consisted of overnight assays not designed to evaluate B cell differentiation or antibody secretion. Future studies will be designed to evaluate the specific subsets of antigen-specific T cells responsible for the activation and differentiation of B cells.
Here we show B cell responsiveness to HIV antigen is a sensitive correlate of control of viremia. We also show that B cell responsiveness to non-specific and HIV-specific stimulation can be enhanced by the presence of CD4+ T cells and blockade of the inhibitory receptor PD-1. Understanding the interactions between CD4+ T helper cells and B cells will increase our understanding of the humoral immune response over the course of HIV infection, as well as factors which contribute to its preservation or dysfunction. A successful HIV vaccine will likely need to generate both robust cellular immune responses and broadly neutralizing antibodies [52], therefore understanding the precise interactions between CD4+ T cells and B cells will be of paramount importance. Our ongoing experiments will more specifically characterize subsets of CD4+ T cells able to efficiently stimulate B cells to differentiate into antibody-producing plasmablasts, and will help evaluate vaccines designed to elicit helper responses and neutralizing antibodies.
Acknowledgments
We would like to acknowledge David K. Flaherty, the laboratory manager at the Vanderbilt Flow Cytometry Shared Resource, for his critical input and help with the design of our flow cytometry experiments. HIV MN and control proteins were kindly provided by Jeff Lifson, AIDS and Cancer Virus Program, SAIC Frederick, Inc., Frederick National Laboratory, Frederick, MD.
Funding Statement
This work was supported by the National Institutes of Health (SAK P01 AI78064 and KJN T32 AI1089554). Flow cytometric cell acquisition or sorting was performed by the Vanderbilt-Meharry Center for AIDS Research Immunopathogenesis Core, an NIH funded program (P30 AI54999). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lane HC, Masur H, Edgar LC, Whalen G, Rook AH et al. (1983) Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med 309: 453-458. doi: 10.1056/NEJM198308253090803. PubMed: 6224088. [DOI] [PubMed] [Google Scholar]
- 2. Förster R, Schweigard G, Johann S, Emrich T, Kremmer E et al. (1997) Abnormal expression of the B-cell homing chemokine receptor BLR1 during the progression of acquired immunodeficiency syndrome. Blood 90: 520–525. PubMed: 9226150. [PubMed] [Google Scholar]
- 3. Martínez-Maza O, Crabb E, Mitsuyasu RT, Fahey JL, Giorgi JV (1987) Infection with the human immunodeficiency virus (HIV) is associated with an in vivo increase in B lymphocyte activation and immaturity. Journal of Immunology 138: 3720–3724. PubMed: 2953790. [PubMed] [Google Scholar]
- 4. Pahwa R, Good R, Pahwa S (1987) Prematurity, hypogammaglobulinemia, and neuropathology with human immunodeficiency virus (HIV). Infection - Proceedings of the National Academy of Sciences of the United States of America 84: 3826. doi: 10.1073/pnas.84.11.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morris L, Binley JM, Clas BA, Bonhoeffer S, Astill TP et al. (1998) HIV-1 antigen–specific and–nonspecific B cell responses are sensitive to combination antiretroviral therapy. J Exp Med 188: 233–245. doi: 10.1084/jem.188.2.233. PubMed: 9670036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shirai A, Cosentino M, Leitman-Klinman SF, Klinman DM (1992) Human immunodeficiency virus infection induces both polyclonal and virus-specific B cell activation. J Clin Invest 89: 561–566. doi: 10.1172/JCI115621. PubMed: 1737846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lange CG, Lederman MM, Medvik K, Asaad R, Wild M et al. (2003) Nadir CD4+ T-cell count and numbers of CD28+ CD4+ T-cells predict functional responses to immunizations in chronic HIV-1 infection. AIDS 17: 2015-2023. doi: 10.1097/00002030-200309260-00002. PubMed: 14502004. [DOI] [PubMed] [Google Scholar]
- 8. Steinhoff MC, Auerbach BS, Nelson KE, Vlahov D, Becker RL et al. (1991) Antibody responses to Haemophilus influenzae type B vaccines in men with human immunodeficiency virus infection. N Engl J Med 325: 1837-1842. doi: 10.1056/NEJM199112263252603. PubMed: 1683682. [DOI] [PubMed] [Google Scholar]
- 9. Valdez H, Smith KY, Landay A, Connick E, Kuritzkes DR, et al. (2000) Response to immunization with recall and neoantigens after prolonged administration of an HIV-1 protease inhibitor-containing regimen. ACTG 375 team. AIDS Clinical Trials Group. AIDS 14: 11-21 [DOI] [PubMed] [Google Scholar]
- 10. Klatt NR, Vinton CL, Lynch RM, Canary LA, Ho J et al. (2011) SIV infection of rhesus macaques results in dysfunctional T- and B-cell responses to neo and recall Leishmania major vaccination. Blood 118: 5803-5812. doi: 10.1182/blood-2011-07-365874. PubMed: 21960586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malaspina A, Moir S, Kottilil S, Hallahan CW, Ehler LA et al. (2003) Deleterious effect of HIV-1 plasma viremia on B cell costimulatory function. J Immunol 170: 5965-5972. PubMed: 12794123. [DOI] [PubMed] [Google Scholar]
- 12. Bussmann BM, Reiche S, Bieniek B, Krznaric I, Ackermann F et al. (2010) Loss of HIV-specific memory B-cells as a potential mechanism for the dysfunction of the humoral immune response against HIV. Virology 397: 7-13. doi: 10.1016/j.virol.2009.11.003. PubMed: 19962720. [DOI] [PubMed] [Google Scholar]
- 13. Moir S, Ogwaro KM, Malaspina A, Vasquez J, Donoghue ET et al. (2003) Perturbations in B cell responsiveness to CD4+ T cell help in HIV-infected individuals. Proc Natl Acad Sci U S A 100: 6057–6062. doi: 10.1073/pnas.0730819100. PubMed: 12730375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moir S, Ho J, Malaspina A, Wang W, DiPoto AC et al. (2008) Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med 205: 1797–1805. doi: 10.1084/jem.20072683. PubMed: 18625747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moir S, Malaspina A, Ogwaro KM, Donoghue ET, Hallahan CW et al. (2001) HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc Natl Acad Sci U S A 98: 10362–10367. doi: 10.1073/pnas.181347898. PubMed: 11504927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moir S, Malaspina A, Pickeral OK, Donoghue ET, Vasquez J et al. (2004) Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. Journal of Experimental Medicine 200: 587. doi: 10.1084/jem.20032236. PubMed: 1550818415353552. [DOI] [PubMed] [Google Scholar]
- 17. Malaspina A, Moir S, Kottilil S, Hallahan CW, Ehler LA et al. (2003) Deleterious effect of HIV-1 plasma viremia on B cell costimulatory function. Journal of Immunology 170: 5965–5972. PubMed: 12794123. [DOI] [PubMed] [Google Scholar]
- 18. Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES et al. (2006) PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443: 350-354. doi: 10.1038/nature05115. PubMed: 16921384. [DOI] [PubMed] [Google Scholar]
- 19. Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E et al. (2006) PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med 203: 2281-2292. doi: 10.1084/jem.20061496. PubMed: 16954372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S et al. (2006) Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 12: 1198-1202. doi: 10.1038/nm1482. PubMed: 16917489. [DOI] [PubMed] [Google Scholar]
- 21. Velu V, Kannanganat S, Ibegbu C, Chennareddi L, Villinger F et al. (2007) Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol 81: 5819-5828. doi: 10.1128/JVI.00024-07. PubMed: 17376899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP et al. (2006) Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439: 682-687. doi: 10.1038/nature04444. PubMed: 16382236. [DOI] [PubMed] [Google Scholar]
- 23. Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH (2006) Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med 203: 2223-2227. doi: 10.1084/jem.20061800. PubMed: 17000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bennett F, Luxenberg D, Ling V, Wang IM, Marquette K et al. (2003) Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol 170: 711-718. PubMed: 12517932. [DOI] [PubMed] [Google Scholar]
- 25. Hokey DA, Johnson FB, Smith J, Weber JL, Yan J et al. (2008) Activation drives PD-1 expression during vaccine-specific proliferation and following lentiviral infection in macaques. Eur J Immunol 38: 1435-1445. doi: 10.1002/eji.200737857. PubMed: 18389475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW et al. (2007) Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol 8: 1246-1254. doi: 10.1038/ni1515. PubMed: 17906628. [DOI] [PubMed] [Google Scholar]
- 27. Porichis F, Kwon DS, Zupkosky J, Tighe DP, McMullen A et al. (2011) Responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood 118: 965-974. doi: 10.1182/blood-2010-12-328070. PubMed: 21652684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Velu V, Titanji K, Zhu B, Husain S, Pladevega A et al. (2009) Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458: 206-210. PubMed: 19078956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kardava L, Moir S, Wang W, Ho J, Buckner CM et al. (2011) Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J Clin Invest 121: 2614-2624. doi: 10.1172/JCI45685. PubMed: 21633172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rossio JL, Esser MT, Suryanarayana K, Schneider DK, Bess JW Jr et al. (1998) Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol 72: 7992-8001. PubMed: 9733838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rutebemberwa A, Bess JW Jr, Brown B, Arroyo M, Eller M et al. (2007) Evaluation of aldrithiol-2-inactivated preparations of HIV type 1 subtypes A, B, and D as reagents to monitor T cell responses. AIDS Res Hum Retroviruses 23: 532-542. doi: 10.1089/aid.2006.0136. PubMed: 17506610. [DOI] [PubMed] [Google Scholar]
- 32. Conrad JA, Ramalingam RK, Duncan CB, Smith RM, Wei J et al. (2012) Antiretroviral therapy reduces the magnitude and T cell receptor repertoire diversity of HIV-specific T cell responses without changing T cell clonotype dominance. J Virol 86: 4213-4221. doi: 10.1128/JVI.06000-11. PubMed: 22258246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Conrad JA, Ramalingam RK, Smith RM, Barnett L, Lorey SL et al. (2011) Dominant clonotypes within HIV-specific T cell responses are programmed death-1high and CD127low and display reduced variant cross-reactivity. J Immunol 186: 6871-6885. doi: 10.4049/jimmunol.1004234. PubMed: 21562156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE et al. (1997) Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278: 1447-1450. doi: 10.1126/science.278.5342.1447. PubMed: 9367954. [DOI] [PubMed] [Google Scholar]
- 35. Lane HC, Masur H, Edgar LC, Whalen G, Rook AH et al. (1983) Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med 309: 453-458. doi: 10.1056/NEJM198308253090803. PubMed: 6224088. [DOI] [PubMed] [Google Scholar]
- 36. Morris L, Binley JM, Clas BA, Bonhoeffer S, Astill TP et al. (1998) HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J Exp Med 188: 233-245. doi: 10.1084/jem.188.2.233. PubMed: 9670036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pahwa R, Good RA, Pahwa S (1987) Prematurity, hypogammaglobulinemia, and neuropathology with human immunodeficiency virus (HIV). Infection - Proc Natl Acad Sci U S A 84: 3826-3830. doi: 10.1073/pnas.84.11.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shirai A, Cosentino M, Leitman-Klinman SF, Klinman DM (1992) Human immunodeficiency virus infection induces both polyclonal and virus-specific B cell activation. J Clin Invest 89: 561-566. doi: 10.1172/JCI115621. PubMed: 1737846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moir S, Malaspina A, Pickeral OK, Donoghue ET, Vasquez J et al. (2004) Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med 200: 587-599. doi: 10.1084/jem.20032236. PubMed: 1550818415353552. [DOI] [PubMed] [Google Scholar]
- 40. Kalams SA, Buchbinder SP, Rosenberg ES, Billingsley JM, Colbert DS et al. (1999) Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J Virol 73: 6715-6720. PubMed: 10400769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP et al. (2008) Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29: 1009-1021. doi: 10.1016/j.immuni.2008.10.010. PubMed: 19062316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Betts MR, Ambrozak DR, Douek DC, Bonhoeffer S, Brenchley JM et al. (2001) Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J Virol 75: 11983-11991. doi: 10.1128/JVI.75.24.11983-11991.2001. PubMed: 11711588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA et al. (2006) HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107: 4781-4789. doi: 10.1182/blood-2005-12-4818. PubMed: 16467198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Akinsiku OT, Bansal A, Sabbaj S, Heath SL, Goepfert PA (2011) Interleukin-2 production by polyfunctional HIV-1-specific CD8 T cells is associated with enhanced viral suppression. J Acquir Immune Defic Syndr 58: 132-140. doi: 10.1097/QAI.0b013e318224d2e9. PubMed: 21637109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moir S, Ogwaro KM, Malaspina A, Vasquez J, Donoghue ET et al. (2003) Perturbations in B cell responsiveness to CD4+ T cell help in HIV-infected individuals. Proc Natl Acad Sci U S A 100: 6057-6062. doi: 10.1073/pnas.0730819100. PubMed: 12730375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Antonelli LR, Mahnke Y, Hodge JN, Porter BO, Barber DL et al. (2010) Elevated frequencies of highly activated CD4+ T cells in HIV+ patients developing immune reconstitution inflammatory syndrome. Blood 116: 3818-3827. doi: 10.1182/blood-2010-05-285080. PubMed: 20660788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Khaitan A, Unutmaz D (2011) Revisiting immune exhaustion during HIV infection. Curr HIV/AIDS Rep 8: 4-11. doi: 10.1007/s11904-010-0066-0. PubMed: 21188556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR et al. (2008) Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med 205: 2763-2779. doi: 10.1084/jem.20081398. PubMed: 19001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moir S, Ho J, Malaspina A, Wang W, DiPoto AC et al. (2008) Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med 205: 1797-1805. doi: 10.1084/jem.20072683. PubMed: 18625747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA et al. (2013) Human Circulating PD-1(+)CXCR3(-)CXCR5(+) Memory Tfh Cells Are Highly Functional and Correlate with Broadly Neutralizing HIV Antibody Responses. Immunity 39: 758-769. doi: 10.1016/j.immuni.2013.08.031. PubMed: 24035365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J et al. (2013) Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med 19: 494-499. doi: 10.1038/nm.3109. PubMed: 23475201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Walker BD, Ahmed R, Plotkin S (2011) Moving ahead an HIV vaccine: use both arms to beat HIV. Nat Med 17: 1194-1195. doi: 10.1038/nm.2529. PubMed: 21988996. [DOI] [PubMed] [Google Scholar]