Abstract
The possibility that the mitogenic effect of fibrinogen, a major plasma protein (3 mg/ml), is mediated by specific membrane receptors was studied. Specific binding analysis showed that fibrinogen receptors are present only on hemopoietic cell lines that respond to its mitogenic effect. The mitogenic fibrinogen receptor is not recognized by antibodies specific for the platelet fibrinogen receptor or is not competitively blocked by synthetic peptides containing the Arg-Gly-Asp sequence, which is common to fibronectin, fibrinogen, vitronectin, and other cell-attachment proteins. The lymphoma-derived pre-B-cells (Raji) have 149,000 receptors, whereas the lymphoma-derived T cells (JM), which are 3 times smaller, have 54,000 receptors. These receptors have a Kd of 2 X 10(-7) M. They are inducible by stimuli specific for the cell lineage: activators of the breakdown of phosphatidylinositol phosphates, such as platelet activating factor for Raji cells, and adenylate cyclase agonists and cAMP analogues for JM cells. The stimuli have no mitogenic effect in the absence of fibrinogen; they do not change the Kd. Each stimulus increases the number of fibrinogen receptors in a dose-dependent manner, which correlates strongly (r = -0.98, n = 5) with an increased growth rate of cells in the presence of fibrinogen. This correlation concludes that the mitogenic effect of fibrinogen is controlled via receptor modulation.
Full text
PDF
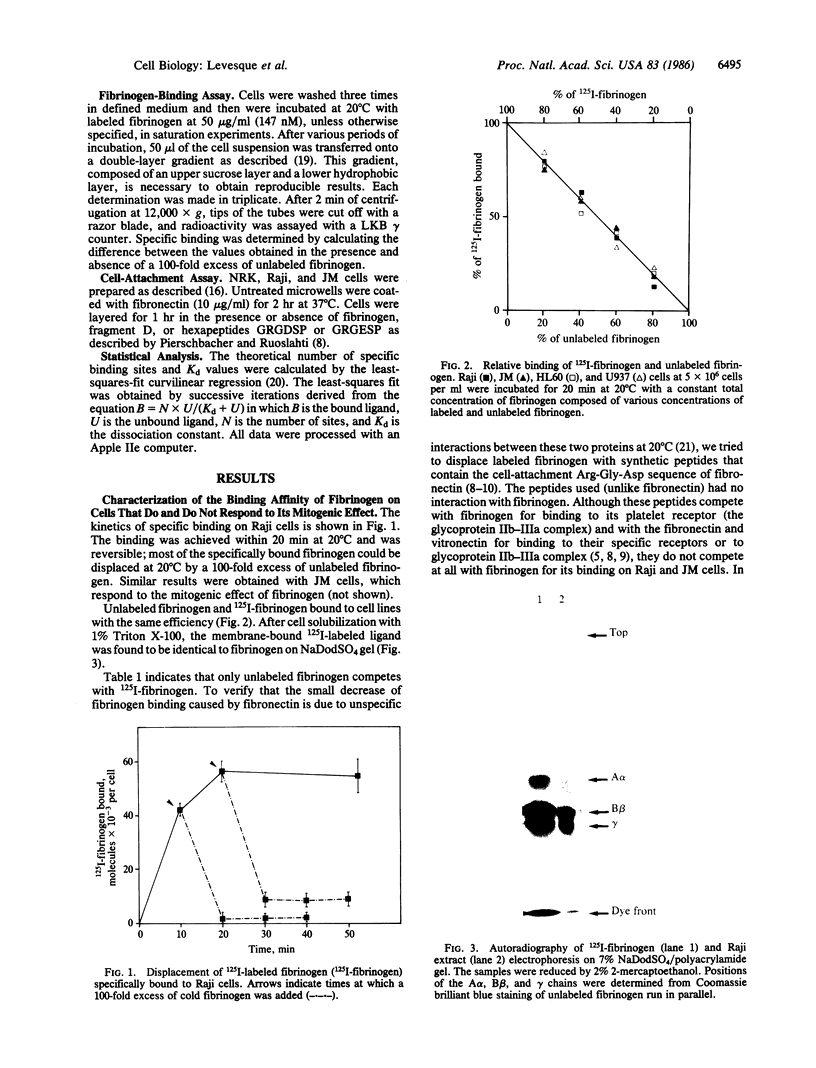
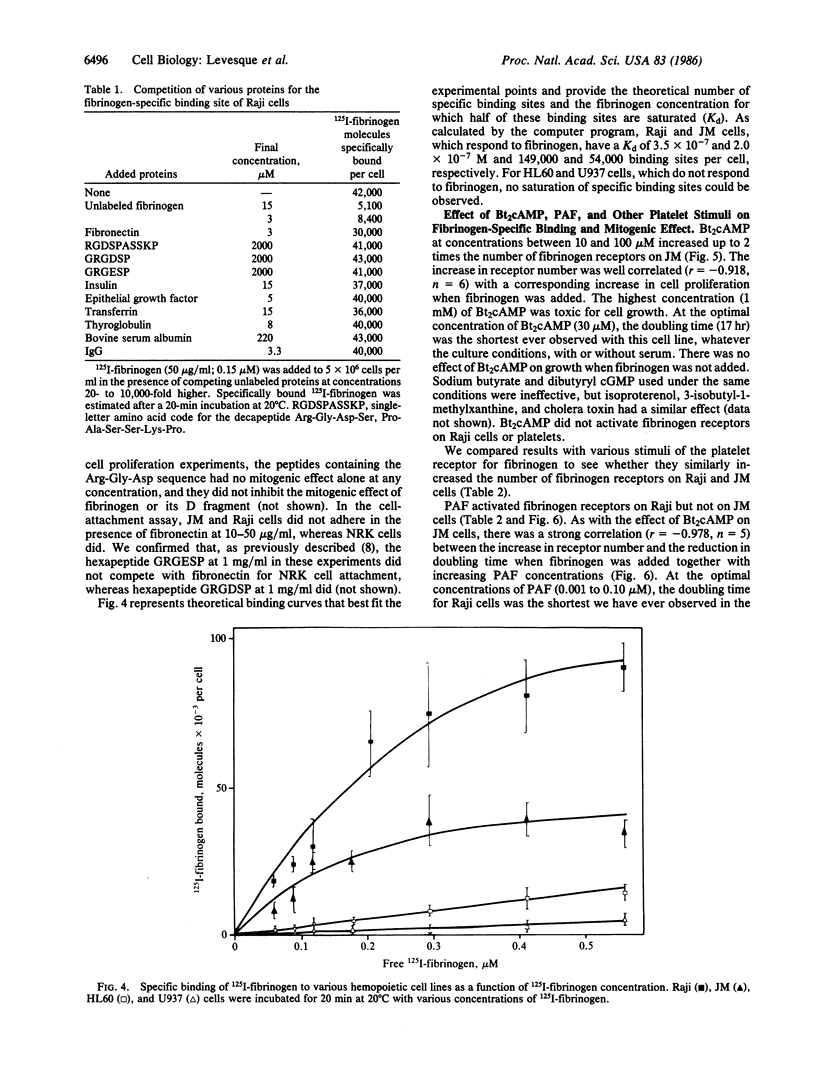
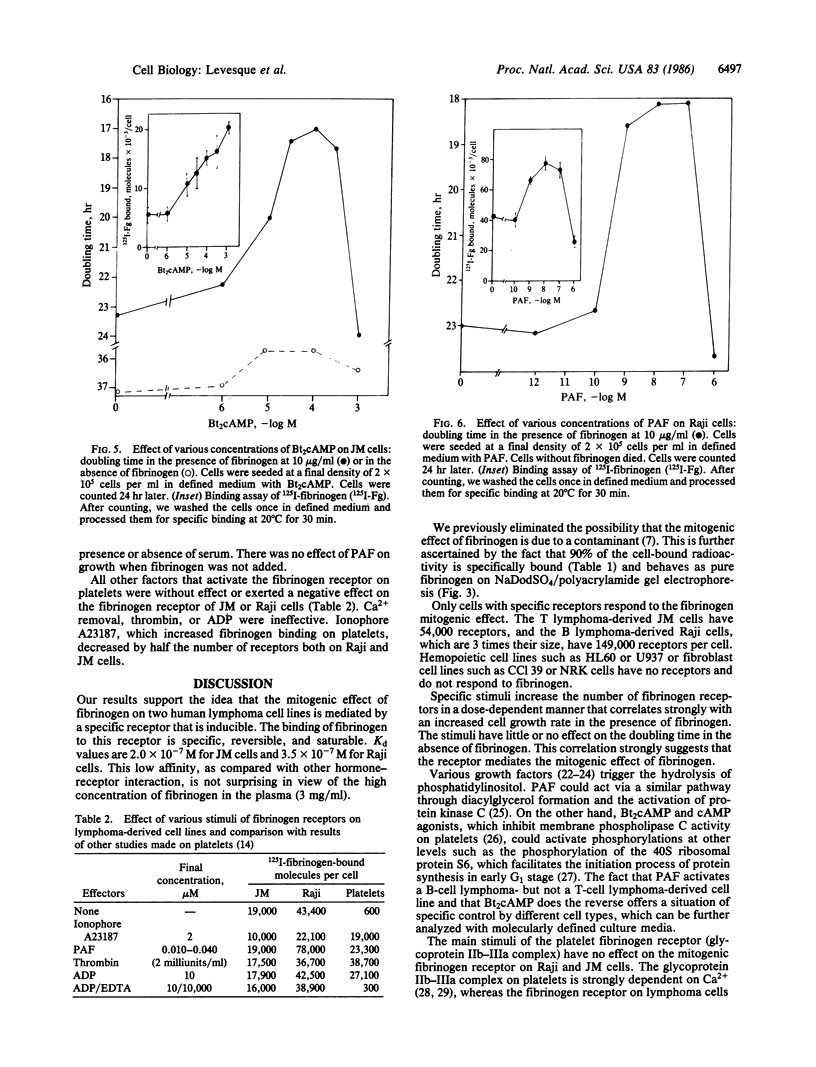

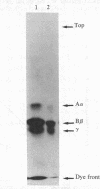
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Yamada K. M. The interaction of plasma fibronectin with fibroblastic cells in suspension. J Biol Chem. 1985 Apr 10;260(7):4492–4500. [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G., Burch J. W. A role for prostaglandins and thromboxanes in the exposure of platelet fibrinogen receptors. J Clin Invest. 1981 Oct;68(4):981–987. doi: 10.1172/JCI110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Platelet-activating factor stimulates metabolism of phosphoinositides in horse platelets: possible relationship to Ca2+ mobilization during stimulation. Proc Natl Acad Sci U S A. 1983 Feb;80(4):965–968. doi: 10.1073/pnas.80.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. S., Peerschke E. I., Scudder L. E., Sullivan C. A. A murine monoclonal antibody that completely blocks the binding of fibrinogen to platelets produces a thrombasthenic-like state in normal platelets and binds to glycoproteins IIb and/or IIIa. J Clin Invest. 1983 Jul;72(1):325–338. doi: 10.1172/JCI110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F. Fibrinogen and fibrin. Annu Rev Biochem. 1984;53:195–229. doi: 10.1146/annurev.bi.53.070184.001211. [DOI] [PubMed] [Google Scholar]
- Graber S. E., Hawiger J. Evidence that changes in platelet cyclic AMP levels regulate the fibrinogen receptor on human platelets. J Biol Chem. 1982 Dec 25;257(24):14606–14609. [PubMed] [Google Scholar]
- Hatzfeld J. A., Hatzfeld A., Maigne J. Fibrinogen and its fragment D stimulate proliferation of human hemopoietic cells in vitro. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6280–6284. doi: 10.1073/pnas.79.20.6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Specific receptors for platelet-derived growth factor on cells derived from connective tissue and glia. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3664–3668. doi: 10.1073/pnas.78.6.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEKWICK R. A., MACKAY M. E., NANCE M. H., RECORD B. R. The purification of human fibrinogen. Biochem J. 1955 Aug;60(4):671–683. doi: 10.1042/bj0600671b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunicki T. J., Pidard D., Rosa J. P., Nurden A. T. The formation of Ca++-dependent complexes of platelet membrane glycoproteins IIb and IIIa in solution as determined by crossed immunoelectrophoresis. Blood. 1981 Aug;58(2):268–278. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levesque J. P., Hatzfeld A., Hatzfeld J. A method to measure receptor binding of ligands with low affinity. Application to plasma proteins binding assay with hemopoietic cells. Exp Cell Res. 1985 Feb;156(2):558–562. doi: 10.1016/0014-4827(85)90563-4. [DOI] [PubMed] [Google Scholar]
- Marguerie G. A., Edgington T. S., Plow E. F. Interaction of fibrinogen with its platelet receptor as part of a multistep reaction in ADP-induced platelet aggregation. J Biol Chem. 1980 Jan 10;255(1):154–161. [PubMed] [Google Scholar]
- Marguerie G. A., Plow E. F., Edgington T. S. Human platelets possess an inducible and saturable receptor specific for fibrinogen. J Biol Chem. 1979 Jun 25;254(12):5357–5363. [PubMed] [Google Scholar]
- Marguerie G. A., Plow E. F. The fibrinogen-dependent pathway of platelet aggregation. Ann N Y Acad Sci. 1983 Jun 27;408:556–566. doi: 10.1111/j.1749-6632.1983.tb23272.x. [DOI] [PubMed] [Google Scholar]
- Martin-Pérez J., Siegmann M., Thomas G. EGF, PGF2 alpha and insulin induce the phosphorylation of identical S6 peptides in swiss mouse 3T3 cells: effect of cAMP on early sites of phosphorylation. Cell. 1984 Feb;36(2):287–294. doi: 10.1016/0092-8674(84)90222-8. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- McGregor J. L., Brochier J., Wild F., Follea G., Trzeciak M. C., James E., Dechavanne M., McGregor L., Clemetson K. J. Monoclonal antibodies against platelet membrane glycoproteins. Characterization and effect on platelet function. Eur J Biochem. 1983 Mar 15;131(2):427–436. doi: 10.1111/j.1432-1033.1983.tb07281.x. [DOI] [PubMed] [Google Scholar]
- Melchers F., Erdei A., Schulz T., Dierich M. P. Growth control of activated, synchronized murine B cells by the C3d fragment of human complement. Nature. 1985 Sep 19;317(6034):264–267. doi: 10.1038/317264a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Orly J., Sato G. Fibronectin mediates cytokinesis and growth of rat follicular cells in serum-free medium. Cell. 1979 Jun;17(2):295–305. doi: 10.1016/0092-8674(79)90155-7. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ginsberg M. H., Plow E. F., Ruoslahti E. Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp--specific adhesion receptors. Science. 1986 Mar 28;231(4745):1559–1562. doi: 10.1126/science.2420006. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5766–5770. doi: 10.1073/pnas.82.17.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Rees A. R., Adamson E. D., Graham C. F. Epidermal growth factor receptors increase during the differentiation of embryonal carcinoma cells. Nature. 1979 Sep 27;281(5729):309–311. doi: 10.1038/281309a0. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Hayman E. G., Pierschbacher M., Engvall E. Fibronectin: purification, immunochemical properties, and biological activities. Methods Enzymol. 1982;82(Pt A):803–831. doi: 10.1016/0076-6879(82)82103-4. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Vaheri A. Interaction of soluble fibroblast surface antigen with fribrinogen and fibrin. J Exp Med. 1975 Feb 1;141(2):497–501. doi: 10.1084/jem.141.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. V., Metcalfe J. C., Hesketh T. R., Smith G. A., Moore J. P. Mitogens increase phosphorylation of phosphoinositides in thymocytes. 1984 Nov 29-Dec 5Nature. 312(5993):462–465. doi: 10.1038/312462a0. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanishi J., Takai Y., Kaibuchi K., Sano K., Castagna M., Nishizuka Y. Synergistic functions of phorbol ester and calcium in serotonin release from human platelets. Biochem Biophys Res Commun. 1983 Apr 29;112(2):778–786. doi: 10.1016/0006-291x(83)91529-2. [DOI] [PubMed] [Google Scholar]