Abstract
New approaches are needed to examine the diverse symptoms and comorbidities of the growing family of neurodevelopmental disorders known as autism spectrum disorder (ASD). ASD originally was thought to be a static, inheritable neurodevelopmental disorder, and our understanding of it is undergoing a major shift. It is emerging as a dynamic system of metabolic and immune anomalies involving many organ systems, including the brain, and environmental exposure. The initial detailed observation and inquiry of patients with ASD and related conditions and the histories of their caregivers and families have been invaluable. How gastrointestinal (GI) factors are related to ASD is not yet clear. Nevertheless, many patients with ASD have a history of previous antibiotic exposure or hospitalization, GI symptoms, abnormal food cravings, and unique intestinal bacterial populations, which have been proposed to relate to variable symptom severity.
In addition to traditional scientific inquiry, detailed clinical observation and recording of exacerbations, remissions, and comorbidities are needed. This article reviews the role that enteric short-chain fatty acids, particularly propionic (also called propanoic) acid, produced from ASD-associated GI bacteria, may play in the etiology of some forms of ASD. Human populations that are partial metabolizers of propionic acid are more common than previously thought. The results from pre-clinical laboratory studies show that propionic acid-treated rats display ASD-like repetitive, perseverative, and antisocial behaviors and seizure. Neurochemical changes, consistent and predictive with findings in ASD patients, including neuroinflammation, increased oxidative stress, mitochondrial dysfunction, glutathione depletion, and altered phospholipid/acylcarnitine profiles, have been observed. Propionic acid has bioactive effects on (1) neurotransmitter systems, (2) intracellular acidification and calcium release, (3) fatty acid metabolism, (4) gap junction gating, (5) immune function, and (6) alteration of gene expression that warrant further exploration. Traditional scientific experimentation is needed to verify the hypothesis that enteric short-chain fatty acids may be a potential environmental trigger in some forms of ASD. Novel collaborative developments in systems biology, particularly examining the role of the microbiome and its effects on host metabolism, immune and mitochondrial function, and gene expression, hold great promise in ASD.
Key Words: Autism spectrum disorder, propionic acid, propanoic acid, microbiome, gastrointestinal tract, mitochondria, carnitine, gap junctions, fatty acids, clostridia, neuropsychiatric disorder, animal model
Abstract
我们需要新的手段来检查不断扩大的神经发育障碍,即自闭症谱系障碍(ASD)家族各种各样的症状和并存病症。ASD 最初被认为是一种静态的、可遗传的神经发育障碍,而我们对它的理解和认识正经历着重大的转变。它最初表现为代谢和免疫动态系统的异常,其中涉及众多器官系统,包括大脑和环境暴露。针对 ASD 和相关病症患者最初阶段的详细观察和调查,以及他们的护理人员和家庭成员的历史记录情况都是非常宝贵的资料。胃肠道(GI)因素与 ASD 的关系目前尚不明确。虽然如此,许多 ASD 患者都有过抗生素暴露或住院治疗的经历、有胃肠道症状、反常的饮食冲动和独特的肠道细菌种群,这些都可能与不同的症状严重度有关。除了传统的科学调查,还需要进行详细的临床观察并做好急性发作、缓解及并存病症的记录。本文综述了与 ASD 有关的 GI 细菌所制造的肠道短链脂肪酸(尤其丙酸)在某些类型 ASD 病因学中所起的作用。 丙酸部分代谢者人群比以前想象的要常见得多。临床前实验室研究的结果显示,接受丙酸治疗的老鼠表现出和 ASD 相似的重复、持续的反社会性行为和癫痫发作。研究观察到了与 ASD 患者身上的发现一致的影响神经系统的化学物质的变化,包括神经炎症、氧化压力增加、线粒体功能障碍、谷胱甘肽缺乏症和磷脂/酰肉碱属性改变等在内的现象。丙酸对(1)神经递质系统、(2)细胞内酸化和钙释放、(3)脂肪酸代谢、(4)缝隙连接门控、(5)免疫功能,以及(6)确证需要做进一步探索的基因表达的变化都具有生物活性效 应。关于肠道短链脂肪酸可能是某 些类型 ASD 潜在的环境诱因这一 假设,尚需要传统的科学实验加以 证明。系统生物学的新的合作进 展,尤其检查微生物组在宿主代 谢、免疫和线粒体功能,以及在基 因表达中所发挥的作用及其功效, 给 ASD 研究带来了巨大的希望。
Abstract
Son necesarios nuevos enfoques que examinen los diversos síntomas y comorbilidades de la creciente familia de trastornos del desarrollo neurológico que reciben el nombre de trastorno del espectro autista (TEA). En sus comienzos se pensó que el TEA era un trastorno estático y hereditario del desarrollo neurológico, pero nuestra comprensión del mismo está cambiando sustancialmente. Se está revelando como un sistema dinámico de anomalías metabólicas e inmunitarias en el que están involucrados numerosos sistemas orgánicos, incluido el cerebro, y la exposición ambiental. La detallada observación inicial y las investigaciones sobre los pacientes con TEA y enfermedades relacionadas, así como las historias de sus cuidadores y familiares, han sido inestimables. Aún no está clara la forma en que los factores gastrointestinales (GI) se relacionan con el TEA. Sin embargo, hay muchos pacientes con TEA que tienen antecedentes de exposición previa a antibióticos o de hospitalización, síntomas gastrointestinales, antojos anormales de comida y poblaciones bacterianas intestinales únicas, que se ha sugerido están relacionados con la gravedad variable de los síntomas.
Además de la investigación científica tradicional, es necesaria una detallada observación clínica y el registro de exacerbaciones, remisiones y comorbilidades. En este artículo se revisa el papel que pueden desempeñar en la etiología de algunas formas de TEA los ácidos grasos de cadena corta intestinales, en particular el ácido propiónico (también denominado propanoico), que se produce por la acción de bacterias GI asociadas al TEA. Las poblaciones humanas que son metabolizadoras parciales del ácido propiónico son más frecuentes de lo que se pensaba anteriormente. Los resultados de estudios preclínicos de laboratorio revelan que las ratas tratadas con ácido propiónico muestran unos comportamientos repetitivos, perseverativos y antisociales, así como convulsiones, similares a los del TEA. Se han observado cambios neuroquímicos, de valor pronóstico y coherentes con los hallazgos en pacientes con TEA, que incluyen la neuroinflamación, el aumento del estrés oxidativo, la disfunción mitocondrial, la disminución de glutatión y la alteración de los perfiles de fosfolípidos y acilcarnitina. El ácido propiónico tiene efectos bioactivos sobre (1) los sistemas neurotransmisores, (2) la acidificación intracelular y la liberación de calcio, (3) el metabolismo de los ácidos grasos, (4) la activación/desactivación de las uniones intercelulares comunicantes, (5) la función inmunitaria y (6) la alteración de la expresión génica, los cuales justifican una exploración con mayor detalle. Es necesaria la experimentación científica tradicional para verificar la hipótesis de que los ácidos grasos de cadena corta intestinales puedan potencialmente representar un desencadenante ambiental en algunas formas de TEA. Los desarrollos colectivos novedosos en biología de sistemas, en particular los que examinan el papel del microbioma y sus efectos sobre el metabolismo anfitrión, la función inmunitaria y mitocondrial, y la expresión génica, constituyen una gran esperanza en el TEA.
INTRODUCTION
I am often asked how I came to consider the possibility that propionic acid, a seemingly innocuous enteric short-chain fatty acid fermentation product of many gastrointestinal (GI) bacteria and a common food preservative, may play a key role in linking the seemingly disparate behaviors, comorbid symptoms, proposed etiologies, and treatments of autism spectrum disorder (ASD).1 The hypothesis occurred through a long series of seemingly unrelated exposures to the stories of children and adults, many with medical conditions seemingly unrelated to ASD, before, during, and after my medical and scientific training. Through this journey, a series of events occurred verging on Jungian synchronicity, and even outright errors in my attempt to understand the complexity and potential causes of dietary, gut, and infective links of neuropsychiatric disease.
These twists and turns suggest there might be something as far-fetched as a simple GI bacteria that could secrete a bioactive compound—namely propionic acid—that could travel through the bloodstream like a hormone to the central nervous system (CNS). This molecule could tinker with the host brain's biochemistry and “hijack” the brain to crave the foods the bacteria uses as fuel and through diarrhea or fecal smearing, elicit behaviors in the human host to ensure the bacteria's survival. Two key questions:
Could a substance, through co-evolution of the bacteria and host via biochemical processes, be capable of creating repetitive movements, restrictive interests, social impairment, and in some cases, paradoxical isolated brilliance—all behaviors associated with ASD? These biochemical processes include neuroplasticity, learning and memory, neurotransmitters, mitochondria energy production, immune function, intra- and inter-cellular interactions, and gene expression.
Could scientific testing of an animal model or a population overexposed to this substance, either from enhanced production or reduced breakdown, lead to an understanding of how nontraditional treatments for ASD might work in some patients and lead to novel ways to identify, treat, or prevent this family of disorders?
PECULIAR KIDS, PERSEVERATION, AND PILLOWS
When I was in high school and early university, I worked with special needs children. We saw children with very strange symptoms interspersed with what appeared to be normal intelligence. They seemed to understand what was going on, but they didn't speak, or they said the same thing over and over again. In addition, they had very restrictive interests, strange movements, and bizarre cravings for food. I was told they suffered from a condition known as autism. Many had digestive issues, and I remember waiting (and waiting!) for some of them to have a bowel movement. There was a dichotomy in these people who appeared cognitively impaired but also were gifted. I recall meeting a little girl who was blind and did not say anything; she sat all night in her bunk singing song after song with incredible accuracy. In retrospect, this would have been labeled classic autism. I also recall a nice elderly British lady who came in with her son who was in his 40s, congenitally blind, nonverbal, and hemiplegic. She asked if I would like to listen to her son play the piano. My jaw dropped when with one hand he played Rhapsody in Blue by George Gershwin. Another patient in one of the camps where I worked came to me late one night saying he did not feel so good and confessed that he had completely eaten his pillow!
During my undergraduate education in physiology at the University of Western Ontario, I was fortunate to work with a group of visionary neuroscientists looking at the biology of behavior in very different ways. They were investigating the specific electro-physiology, neuroanatomy, and neurochemistry of different classes of behavior rather than developing theories about why these classes of behavior occurred. The basic science work was broad and indirectly related to conditions such as Alzheimer's, Parkinson's, epilepsy, obsessive-compulsive disorder, and schizophrenia.2–4 Donald Peter Cain, PhD, was interested in learning and memory, while Martin Kavaliers, PhD, studied social behavior. Klaus-Peter Ossenkopp, PhD, was interested in the biology of “sickness behavior,” the sensory, mood, feeding, cognitive, and social alterations that occur with infectious disease, immune disorders, environmental toxins, and malignancy.5 The neuroscientists taught me how to think and not to be afraid to think outside of conventional approaches. They were not dismissive of novel ideas, and they made me appreciate that hypotheses, although essential, are not enough. They must be tested. I thought all medicine and medical research into the neurobiology of behavior used this model. I was incorrect.
MEDICAL RE-EDUCATION
In graduate school in neurophysiology at the University of Toronto, medical school at McMaster's University in Hamilton, Ontario, and residency at Western University, London, Ontario, I concentrated on electives in clinical neuroscience, neurology, neurosurgery, and psychiatry. I learned that in addition to mechanisms of disease, the understanding of human behavior and the importance of the medical history—allowing people to tell their story—were central to medicine. I was interested in the brain and the neurology of schisms—having some aspect of the brain impaired and then discovering that the person was completely “in there”; it's just that their basal ganglia did not work well. If someone were drooling and barely moving, you might assume that they would not be cognizant; that is not true. I remember a girl with severe choreo-athetosis cerebral palsy who wrote poetry with a symbol device that allows nonverbal people to express themselves. She had an enormous supplement book attached to her chair that she would laboriously leaf through to select the appropriate word for her compositions. The people I worked with saw these special-needs people as individuals with conditions a lot of us have—good and not so good. The clinicians and scientists were not afraid to ask tough or unpopular questions, but they seldom interacted with each other.
During my residency, I also worked in gastroenterology, seemingly far away from the clinical neurosciences. Some patients had a GI blockage—something structural, inflammatory, or irritable bowel syndrome—without any observable cause. They were often anxious and obsessional and were sometimes called “neurotic” or “crazy” and considered a matter for psychiatry. But some of these patients with GI problems, like the patients I had seen in neurology, were smart, albeit obsessional, vigilant, and meticulous—qualities that in some cases held them in good stead with their jobs. I wondered if something else was going on.
Like most residents working in internal medicine in the middle of the night, I saw many patients with alcohol-related disorders. There were many chronic patients engaging in bizarre repetitive behaviors and psychoses, induced not only by alcohol but also a high-protein meal. These behaviors could often reverse through diet and “cleaning out the gut” with lactulose or metronidazole. We were told, “You are removing ‘middle-molecules’6 made from the GI bacteria that can't be cleared by the damaged liver.” It was not clear what these metabolic products were.
A young baker who had a rapid onset of psychosis, initially thought to be schizophrenia, amazed me. He was brought in due to an acute febrile event initially thought to be a neuroleptic malignant syndrome and then an as-yet undetermined GI illness. His behavior was extremely perseverative; he spent most of the day on the ward repeating my name—“Dr MacFabe! Dr MacFabe! Dr MacFabe!”—over and over again. The attending physician diagnosed him with CNS Whipple's disease,7 caused by Tropheryma whipplei, a bacteria ubiquitous in soil. A course of antibiotics cured him within a week. As he recovered, I listened to him recall meeting with me earlier; he said it felt to him like I was someone from a dream who had become real.
Another woman was thought to be a “naive, clumsy, and a slow learner” in a family of high achievers. She could barely write but could paint and draw beautifully. She was a selfless parent and spouse. She had been diagnosed as learning disabled with anxiety and a depressive disorder and finally postmenopausal, until the progressive onset of Parkinsonian-like tremors that led to a re-evaluation of her condition when she was in her late 50s. The presence of Kayser-Fleisher rings on ophthalmic examination led to a diagnosis of Wilson's disease, a disorder of copper metabolism8 that countless other physicians had missed. Despite her age, she markedly improved with penacillamine chelation. She reported, “I can move and speak much better. Now I can pronounce words like ‘reiterate.’ Words don't skip off the page when I read. I am not as tearful as before, and I can evaluate people's intents better.” The fact that in one case diet, in another a GI bacteria, and in another a defect in metal-related brain metabolism could induce such bizarre behaviors in patients—and that these conditions are potentially reversible—was fascinating to me.
PROPIONIC ACID AND THE INTERNET
My postdoctoral work was focused in understanding the mechanisms responsible for acute neuronal injury in hypoxia, seizure, stroke, and neurotrauma. We also developed compounds for neuroprotection.9 Our lab was looking at the biochemistry of oxygen-deprived cells. Chris Naus, PhD, was using compounds to change pH or close gap junctions, intercellular protein channels composed of connexins, which affected intracellular connectivity. In addition to effects on cardiac, GI, and embryonic physiology, gap junctions were found to have effects on nervous system development and neural and glial electrotonic coupling. I learned that propionic acid closes gap junctions. If it is placed on cells, the pH lowers9–11; normally when pH changes, even a little, neurons die, but remarkably, the pH was producing an acidic intracellular environment and these cells seemed to recover. No one could tell me why. The compound seemed to concentrate inside of cells.10,12
When the Internet era was beginning, I went online to purchase propionic acid and discovered that it is used everywhere in the food industry, particularly in wheat and dairy products both naturally and as a preservative against mold.13 Furthermore, many bacteria in the human and ruminant guts produce it as a fermentation product after digesting sugars, starches, and some proteins. Sidney Finegold, MD, of the University of California, Los Angeles (UCLA), had shown that some children with ASD, particularly those with regression and GI symptoms, are infected with subspecies of Clostridia14–16 that also make propionic acid.17 Furthermore, there were anectodal reports that some ASD patients markedly, albeit transiently, improved with oral vancomycin, which would eradicate most gut bacteria, including Clostridia.
In the late 1980s and early 1990s, autism diagnoses became more frequent. ASD was first reported as 1 in 10 000 in the 1950s; recently, it was 1 in 88. It was originally rare, and now most people know someone who has this disorder or had it in their families. Many parents are adamant that their children started out normally. They show videos of early development, first birthday parties, and children speaking and interacting normally and then watch helplessly as their children regress, with major GI symptoms and foul-smelling stools. Traditional genetic studies account for only a small subset of cases (5% to 25%),18 and there are cases of identical twins with ASD with discordant severities of the disorder19 and some families in which one twin is completely normal. Historically, when disorders run in families, an inherited disorder may turn out to have an environmental cause; in the case of tuberculosis, it is an infective cause. Twins usually, but not always, have similar early environments—they shared the same womb, were born in the same hospital, and usually shared similar postnatal environments. So could an environmental cause be the case in ASD?
There were divergent suggestions of environmental causes of ASD in the 1990s—vaccinations, environmental metals, pesticides, childhood infections, and immune interactions.20 Propionic acid affects many of these processes related to cell metabolism and gap junctions, in both the brain and the GI tract. If propionic acid is doing this to brain cells in a Petri dish, what happens when we eat it? It is in the food these children crave13 and produced by bacteria associated with opportunistic infections associated with early use of antibiotics.16,17 And if certain antibiotic-resistant bacteria in our GI tracts are producing propionic acid after we ingest carbohydrate-rich food, particularly those bacteria associated with ASD, what is it doing to our brains and GI tracts?
The concept that ASD could be a closed gap junction state seems to fit. Gap junctions are gated by a number of factors including dopamine, calcium, and cytokines; all influenced by propionic acid.21 Gap junctions play a major role in cellular differentiation, and in particular, peripheral nerve, cardiac, uterine, and GI function. However, in the CNS, gap junction coupling is vital for the synchronization of neural electrical activity within discrete functional cell groups, and it is more extensive during early brain development and neuronal migration. Astrocytes are electrotonically connected by gap junctions, forming a syncytium to spatially buffer calcium, glutamate, and potassium.22 Apoptotic factors are capable of passing through these glial gap junctions.9,11 Therefore, closed glial gap junctions may render neurons hyperexcitable to rising extracellular potassium and glutamate,11 while closed neuronal gap junctions would be neuroprotective.9 In turn, this decrease in gap junction coupling may lead to inhibited cortical pruning in development, consistent with the larger brain size found in ASD.21 Gap junction communication is involved in neurotransmission in the basal ganglia, prefrontal cortex, nucleus accumbens, and hippocampus: all areas that are implicated in seizure and movement disorders. Intrastriatal injections of gap junction blockers produce stereotypical movements, hyperlocomotion, and disruption of motor sequencing in rodents.23,24 Furthermore, gap junction knockout mice show abnormal brain development, exaggerated responses to neurotoxic insults, seizure disorder, and abnormal behaviors.25 Interestingly, gap junction blockers also inhibit tight junctions in many cellular systems,26 thereby possibly contributing to altered barrier function in vascular endothelia and GI tract in ASD.27 Given these findings, it seemed possible that propionic acid-induced alterations to gap junction function and in neural development, as well as systemic effects (ie, GI motility), may play a role in ASD. These potential mechanisms were intriguing, but they had to be tested.
SELF-CENTERED GASTROINTESTINAL BACTERIA
How do people act when they're sick? Sometimes their behavior changes, but not necessarily in a bad way. There is a gestalt of the things I had seen—special-needs children, my patients, findings from the laboratory, and patients with digestive system complaints who were told they were crazy. And now infectious disease experts are finding unique populations of GI bacteria, which on closer evaluation actually produce bioactive fermentation products, particularly following ingestion of the foods many of these patients crave. Are these behaviors coming from the top, or are they coming from the bottom up—from the GI tract to the brain? Perhaps humans are a bit like cows, which after ingestion of largely indigestible plant material, use the fermentation products of their own GI bacteria (notably propionibacteria) as their primary energy source.28
As I was considering these concepts, interesting work was emerging in the field of ASD that was radically different from the “structural brain disorder” point of view. Work on methylation and glutathione chemistry by Jill James, PhD, at the University of Arkansas29,30 and Richard Deth, PhD, of Northeastern University31; Drs Abha and Ved Chauhan in Staten Island were studying oxidative stress,32 and Martha Herbert, MD, PhD, at Harvard was studying very interesting postnatal brain changes in autistic brain.20
I was knowledgeable about gap junctions from my work in neuroprotection.9,11 I read about propionic acid and other short-chain fatty acids (i.e. butyrate, acetate) in the gastroenterology literature and was fascinated by what these simple molecules could do. In addition to propionic acid's effects on gap junctions,33 it modulated diverse physiological processes including cell signaling,34 neurotransmitter function,35 free radical production and mitochondrial function,36 lipid metabolism,37 immune function,38 and modulation of gene expression.39
As a weak acid, propionic acid becomes more lipid-soluble in acidotic conditions (ie, during “sickness”) and concentrated within cells.12,33 It is actively taken up in the GI tract and brain via specific propionic acid transporters and activates specific G protein–coupled receptors in the immune system, gut, and brain.40–42 It affects the regularity of GI tract motility.43 There are groups of patients with a variety of inherited metabolic disorders, such as propionic acidemia who are unable to metabolize propionic acid.36 These populations are more common than previously thought44,45 and often present either earlier or later with regressive cognitive impairment, seizure, and movement disorder, often in the context of GI symptoms. Propionic acid's habit of “hiding” inside cells makes it difficult to detect, even in patients with a metabolic crisis. Other similar compounds like ethanol and valproic acid, both prenatal risk factors for ASD, also impair propionic acid metabolism.46,47 Now it seems these GI tract short-chain fatty acids are linked to other theories for ASD. Are these bacteria acting by “controlling” the host? Can GI tract bacterial metabolites affect brain function and behavior?
THE MICROBIOME: AN INNER RAINFOREST
About 2 decades ago, the detailed study of the microbiome48 in health and disease began, thanks to the development of new techniques including high throughput 16S ribosomal RNA microarrays and sequencing and metabolomics. This is because most bacteria cannot be cultured. Initially, bacteria were thought to be “bad” and to cause disease. The goal was identification and annihilation with antibiotics—something that many ASD children had been exposed to prenatally and in the first few years of life. Others were exposed to abnormal bacterial populations, either from the mother if she had GI disease, via Caesarean section delivery, or exposure to antibiotic-resistant bacterial populations in the obstetrical ward.14,49,50 The concept of microbial systems, which outnumber host cells 100:1, acting like a complex “inner rainforest” of organisms was new. The idea of microbiome as a functional colony that interacts symbiotically with itself and us21,48,51–54 was in its infancy. The concept has grown exponentially since then.55 Could antibiotics act like “clear cutting” of this inner rainforest, causing harm to a rapidly developing ecosystem in a child's first 18 months of life?
The microbiome produces an array of bioactive metabolic products capable of entering systemic circulation.52 It is important to note that the enteric micro-biome and its metabolic products are not static and can be altered throughout an individual's life cycle, particularly during the first 18 months of life.55 The metabolic products from the GI tract microbiome can have profound and dynamic effects on host metabolism, immune function, and gene expression. This happens in many organ systems including the CNS.21,48,51–54 It also is important to consider the effects of infant formula vs breastfeeding, a high-calorie Western diet and exposure to antibiotics and disinfectants in human beings, animals, and plants on the alteration of the human microbiome and its metabolites. These should be considered a possible source of environmental triggers of many diseases of increasing incidence,48 including ASD. This is particularly evident from human populations who have migrated to Western societies, such as the Somali diaspora, who appear to have a much higher incidence of ASD than exists in their country of origin.56
Could GI tract bacteria control behavior? There are examples of this in biology. Rabies and Bornavirus infect the CNS in animals and induce aggression that spreads the virus in the saliva from one animal to another through biting behavior. Cordyceps (Ophiocordyceps unilateralis) is a fungal infection that affects the behavior of ants, causing them to climb to the top of plants before they die. The fruiting bodies of the fungus then sprout out of the dead insect to spread spores. Toxoplasmosis causes rodents to act without an appropriate fear response, leading to transmission of the infectious agent through cats via predation and ultimately on to humans.1,57,58 Mundane acts such as sneezing with a common cold or increased gastric motility leading to nausea and vomiting in viral gastroenteritis are in the best “interest” of the infectious agent. Could similar things be happening with carbohydrate craving, diarrhea, and fecal smearing in ASD to feed and spread bacteria? The concept seemed heretical but plausible.
Families of ASD children often become more alienated when told they “imagined” their child's regression and that there is little that can be done. They are often encouraged to use medications to partially reduce aggressive behavior and wait their turn for the under-funded behavioral intervention programs that might take years to enter and years to complete. The strain of dealing with these children can destroy families and end productive careers, leading desperate and vulnerable parents to turn to unproven controversial treatments that can be costly, potentially dangerous, and without confirmed effectiveness. I was perplexed and intrigued by the seemingly disparate observations of bizarre food cravings, GI symptoms, epilepsy, infectious processes, and metabolic disturbances in this growing number of affected children. However, there were reports that some children appeared to improve, either spontaneously, after certain broad spectrum antibiotics, or possibly by altering their diet. I wondered if there might be a common digestive system link to these findings even if current understanding in conventional Western medicine could do little for these children.
KILEE PATCHELL-EVANS AUTISM RESEARCH GROUP
I once had a vivid dream that I was walking along a beach with my late mother, who passed away when I was 11 years old. We were looking at everyone fishing in the surf—monotonously, uncreatively, and repetitively casting their lines in unison into the waves and catching nothing. My mother pointed to a tidal pond teeming with life, remarked how beautiful it was, and said that every creature was working together and interacting with each other. “Nobody is looking at that” she said to me. “You should look at that.”
My first meeting with David Patchell-Evans, or “Patch” as he prefers, was remarkable. In 2003, I contacted him on the advice of the caregiver of his daughter Kilee, who was afflicted with severe regressive autism. The caregiver said, “I'd like you to meet the father of this child.” When I met him, he told me his story.
His daughter had severe regressive autism; it was heartbreaking. He himself is a successful businessman in the health and fitness industry. I listened to the story of this very successful man who loved his daughter, telling me with tears in his eyes that her behavior was normal until she was 18 months old, when she had a regression associated with severe GI troubles. Her behavior became absolutely horrible: complete loss of verbal ability, extreme self-injury, and aggression. She then needed restraints.
I discussed my thoughts that GI tract bacteria products could modulate brain metabolism and affect behavior. I spoke about my fledgling team, suggested what I thought was needed, and received the most unexpected reply of my medical career. He looked at me for a long moment and then said, “Seed money won't be enough for this project. What do you really want to do?” Patch has learned to go beyond his personal hardships and knows you gain more by giving. That initial meeting led Patch and me to found the Kilee Patchell-Evans Autism Research Group (http://psychology.uwo.ca/autism.htm). This group brought together a team of trusted experts from fields encompassing the disparate symptomatology of ASD. The research began with basic science and extended into emerging experimental approaches. With the assistance of his philanthropic team, especially Megan Cameron, they raised more than $4.5 million Canadian for our research program. We used novel research approaches to search for solutions for ASD with a trust in the scientific process and the free dissemination and exchange of information. Patchell-Evans received the Canadian Medical Association's Medal of Honor and Doctor of Laws Honoris Causa (Western University) for his personal contribution to the advancement of autism research in Canada. Without his help and support, none of this research would have been possible.
A GROUP OF DOCTORS AND THEIR RESEARCH
Patchell-Evans took me to a meeting with doctors looking at ASD from a different perspective. Jon Pangborn, PhD, a chemical engineer and a parent of a child with ASD, spoke on biochemical pathways, oxidative stress, methylation, and glutathione. Dr James was working with glutathione and oxidative stress, Dr Deth was working on methylation and dopamine biochemistry, Dr Finegold spoke about GI tract bacteria, and Dr Herbert talked about systems biology in ASD. I met Sidney Baker, MD, a pediatrician who somehow seemed to be getting many of these children better. The evidence was based largely on clinical observations without major clinical trials. As I began considering these topics, I began to see things I knew something about: stroke and oxidative stress, calcium, pH, immune function, oxidative stress and mitochondria, and GI-tract bacteria and their metabolites.
We are liars in medicine if we think we're experts in biochemistry. We didn't develop it, but we study it, memorize it, spit it out for the exam, and then forget it. I went back to my biochemistry books and saw how tinkering with oxidative stress and metabolism affected brain and the GI tract. I studied fat metabolism, energy utilization, and GI physiology, microbiology, and neurochemistry. Again, the idea at a very simplistic level: feed a GI tract bacteria, and that bacteria produces a neuroactive compound that affects brain function and behavior. I didn't know how some of these children were getting better; however, it seemed potentially rational and, more importantly, testable. It was also medicine as art. Physicians are delusional if we don't admit we are doing educated guesswork much of the time. When I did my epilepsy work, I thought I understood the biochemistry of these drugs. Then I realized it's an improvisation a bit like jazz, trying to figure out how to tailor drugs for an individual patient. We have a few blood markers, but even today, we rarely know how these drugs work. I had my expertise in medicine, neurometabolomics, neuropathology, and pharmacology. Other scientists and students came aboard, many of whom had family members with ASD.
BEHAVIOR
We began research with an animal model using rats. We gave them very small amounts of propionic acid, the metabolite of the GI bacteria that we suspected was the major problem. We were giving tiny amounts at the start, approximately the levels found in patients with propionic acidemia. We began with very simplistic first-level physiology—injecting tiny “puffs” of propionic acid into the cerebrospinal fluid of live adult rats through cannulae inserted into the cerebral ventricles. We could film their behavior and record electrographic activity. Low and behold: Tiny puffs, comparable to levels in patients with propionic acidemia, immediately made the rats hyperactive with repetitive behaviors (Figure 1).
Figure 1.
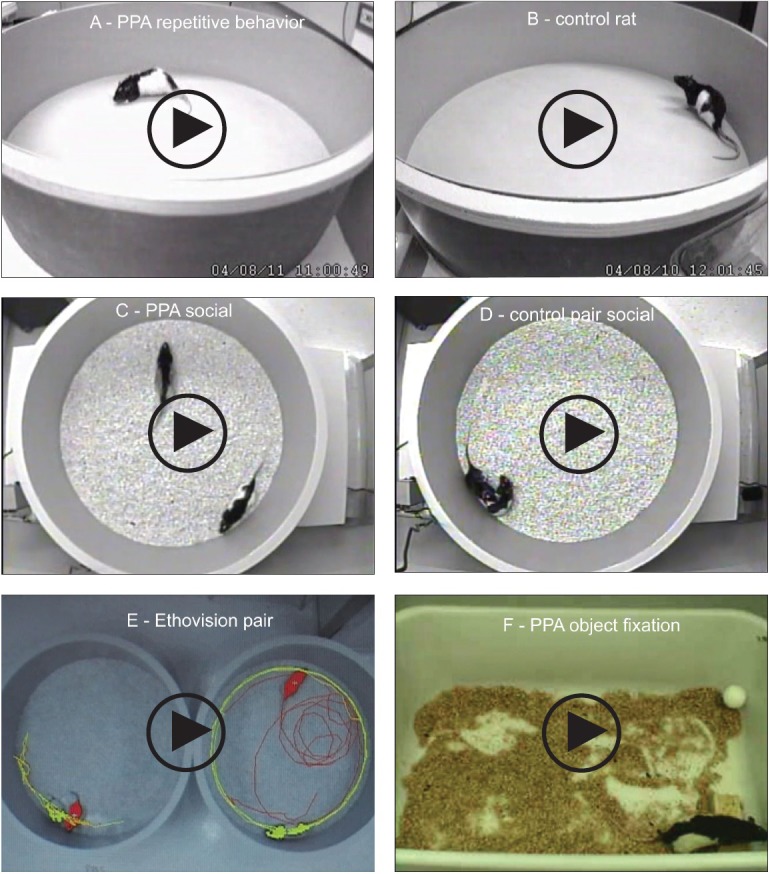
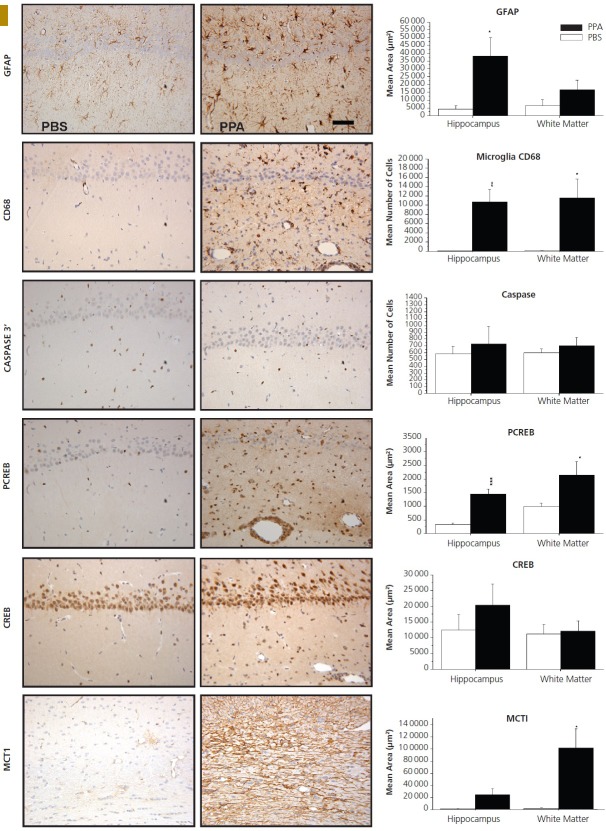
Behavioral videos of propionic acid infusions in rats. Single intracerebroventricular (ICV) infusions (4 μL of 0.26 M solution over 4 min) of propionic acid (PPA), a metabolic end product of autism-associated enteric bacteria, produce bouts of reversible hyperactive and repetitive behavior (A) in adult rats, compared with phosphate-buffered saline (PBS) vehicle–infused control rat (B). Rat pairs infused with PPA show markedly reduced social interaction and play behavior (C) compared with pairs of rats infused with PBS vehicle (D), which show typical social behavior. Ethovision behavioral tracking of control (left) and PPA-treated (right) rat pairs (E), showing further evidence of PPA-induced hyperactive, repetitive, and antisocial behavior. PPA-treated rat displays fixation on objects (F) and a specific object preference (ie, block vs sphere). PPA-infused rats also show turning, tics, dystonia, and retropulsion and electrographic evidence of complex partial seizures and basal ganglial spiking consistent with findings in patients with autism spectrum disorders. Modified with permission from MacFabe 2012, Microbial Ecology in Health and Disease.
It was not a subtle response. The rats also had brief complex partial seizures that sensitized with repeated exposures, had electrographic changes in their basal ganglia associated with tics, and were socially impaired. They also exhibited increased anxiety and remarkably, would fixate on objects vs other animals, even had “favorite” objects, and would exhibit further “rigid” perseverative behaviors, such as learn a maze without difficulty but not be able to “unlearn” it with maze reversal. All were consistent with the rigid, stereotypic, and impaired social behavior in ASD patients and their peculiar habit of “preferring” objects to people. But what was most remarkable was that we would give these compounds to an apparently neurologically intact animal; within 2 minutes, it would exhibit these behaviors, and within 30 minutes, it would revert back to apparently normal behavior. We were shocked. Control compounds such as propanol, the nonacidic alcohol analogue of propionic acid, were tested without effect.
Recovery could be explained by the fact that propionic acid as a fatty acid would get oxidized in the mitochondria, so the animals would first become hyperactive and ignore each other, then half an hour later their behavior would be apparently normal. On one level, we were showing that an intact nervous system could be hijacked by a metabolite—with implications not only for ASD but probably many other neuropsychiatric disorders. We also were potentially disproving the argument that these children with ASD only acted the way they did because their tummies were upset. That was undoubtedly part of it, but not all children with upset tummies show autistic behavior. The important thing, which isn't trivial, is that we were putting such a tiny amount into the CNS (not the GI tract) and eliciting ASD-like behaviors: this was a central effect. Furthermore, this would in a normal adult animal offer a potential explanation of the fluctuations of behaviors in ASD patients, where behavioral exacerbations occurred during carbohydrate diets (which theoretically would increase propionic acid production from ASD-associated GI bacteria), and remissions occurred following dietary carbohydrate restrictions (which would reduce propionic acid production by GI bacteria). Furthermore, it could offer an explanation of why eradication of GI bacteria by broad-spectrum antibiotics such as vancomycin or metronidazole could transiently improve symptoms in some patients.
I am indebted to the collaborative expertise of Drs Ossenkopp, Kavaliers, and Cain in teasing out and statistically quantifying these behaviors, which were dramatic from a clinician's viewpoint. We published a series of papers over the next few years with graduate students Jennifer Hoffman, Kelly Foley, and Sandy Shultz, and postdoctoral fellows Karina Rodriguez-Capote and Raymond Thomas.1,21,59–65 We had invalu able expertise from technologists Francis Boon, Andrew Franklin, and Lisa Tichenoff. Lastly, I coaxed Roy Taylor, a former hospital neuropathology technologist during my residency, to come out of retirement to join us.
NEUROINFLAMMATION AND CYCLIC ADENOSINE MONOPHOSPHATE RESPONSE ELEMENT-BINDING PROTEIN
Roy Taylor and I looked at the pathology of brains from the propionic acid–treated rats. A neuropathologist, Carlos Pardo, MD, at UCLA, showed that the brains of autopsied ASD patients, both young and old, who died accidentally were loaded with immunological cells—reactive astrocytes and microglia, as well as cytokines. These had been present throughout their lives,66,67 and the ongoing inflammatory process has some similarity to previous findings in epilepsy patients.68 This is a markedly different finding from what was assumed in the literature: prenatal irreversible brain synaptic alterations. Correspondingly, we looked at the brains of animals exposed to propionic acid and found the same inflammation and the same pathology21,59–62 (Figure 2). Intriguingly, neuroinflammation isn't completely a bad thing; activated microglia and astrocytes have been found to be instrumental in “synaptic shearing,” reorganizing neuronal pathways. It is also implicated in both seizure and in learning and memory.69
Figure 2.
Neuropathology (avidin–biotin complex immunohistochemistry) and semiquantitative image densitometry of coronal brain sections of dorsal hippocampus (CA2) and external capsule of adult rats with 14-day twice daily intracerebroventricular infusions of propionic acid (PPA) or phosphate-buffered saline (PBS). Propionic acid induced significant reactive astrogliosis (anti-GFAP) and microglial activation (anti-CD68) without apoptotic neuronal cell loss (anti-cleaved caspase 3) in rat hippocampus, similar to findings in autopsied brain from patients with autism. Nuclear translocation of anti-CREB and an increase of anti-phospho-CREB immunoreactivity are observed in neural, glial, and endovascular epithelia by propionic acid treatment, suggestive of gene induction. Propionic acid increases monocarboxylate transporter 1 (MCT1) immunoreactivity, primarily in white matter external capsule, suggestive of alterations in brain short–chain fatty acid transport/metabolism. Black bars indicate propionic acid–treated animals; white bars indicate PBS (vehicle)–treated animals. Horizontal measurement bar = 100 μ. Reproduced with permission from MacFabe (2012), Microbial Ecology in Health and Disease.
To further this concept, we found that brains from propionic acid–treated rodents have an increase in activated cyclic AMP response element-binding protein or “CREB,” a protein implicated in the molecular orchestration of the laying down of memory.21 It also has been implicated in seizure, mood and movement disorder, reward, and substance abuse.70 A GI bacterium appeared to be releasing a metabolite that is able to get the brain to “remember” where the bacteria was fed. This might be adaptive, but could overactivation of this pathway lead to “impaired forgetting,” obsessional behaviors, food interests, tics, and the enhanced restrictive memories found in ASD?
OXIDATIVE STRESS, GLUTATIONE, CARNITINE, AND OMEGA-3 FATTY ACIDS
Fred Possmayer, PhD, is a lipid biochemist who developed artificial surfactant to help premature babies breath easier.71 We began to try to further validate the biochemistry of the propionic acid animal model by looking at oxidative stress and lipid profiles found by Dr James and the Drs Chauhan.30,32,72 We showed that propionic acid increases oxidative stress markers and reduces brain glutathione levels similar to findings in ASD patients. The reduced glutathione could theoretically make the brain more “sensitive” to a variety of other environmental toxins (metals, xenobiotics) proposed to further contribute to the disorder.59–63,65 As in neuroinflammation, even brief exposures to oxidative stress have been implicated in neuroplasticity and memory.73 Furthermore, propionic acid conceptually puts a “wrench” in mitochondria by inhibiting the first half of citric acid cycle and increasing the other half (Figure 3). Propionic acid also impairs the metabolism of carnitine, the compound that brings fatty acids into the mitochondria for beta oxidation.1,63,74,75, 76
Figure 3.
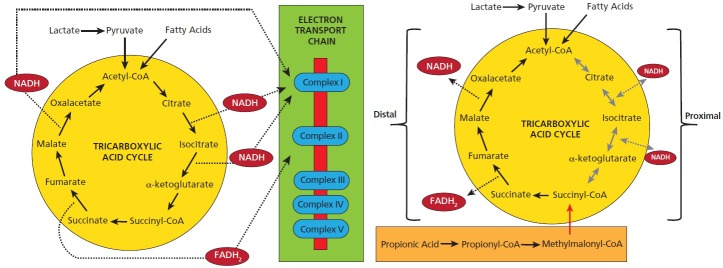
Metabolism of the tricarboxylic-acid cycle during (a) typical metabolism and (b) with high levels of propionic acid (PPA). PPA is metabolized to propionyl-CoA, which inhibits the proximal portion of the tricarboxylic-acid cycle and enhances the distal portion of the tricarboxylic-acid cycle (see discussion for details). Modified from Frye, Melnyk, and MacFabe 2013, Translational Psychiatry.
Abbreviations: FADH2, flavin adenine dinucleotide; NADH, nicotinamide adenine dinucleotide.
Carnitine has an underappreciated role in brain development and function.77 Many patients with ASD have a relative carnitine deficiency,78 deriving from inherited79 and acquired factors. Long-term antibiotic use may not only reduce carnitine by increased production of GI bacterial propionic acid but also directly inhibit carnitine reuptake from the GI tract and kidney by blocking carnitine-specific organic cation transporters novel transporters.80 We propose that the onset of regressive autism may be the result of a “carnitine crash” from a variety of these causes during brain development.1,75
Propionic acid and to a lesser extent butyric acid lower the levels of omega-3 fatty acids and increase the levels of omega-6s, also consistent with findings in ASD patients, leading to the altered membrane fluidity, antioxidant capacity, and lipid mediated neuronal signaling in the condition.64,65 It also offers credibility to explain why omega-3s, carnitine, and N-acetyl cysteine (which increases glutathione levels) may hold promise in treating ASD.1,76,81 Propionic acid also increased brain expression of monocarboxylate transporters, important in fatty acid and ketone transport into the brain.41,42
Findings in the rodent model, so far, have been identical to those in autistic patients, but there is much more work to be done. Rodent models of disease are absolutely essential and offer an ethical way to test mechanism and therapeutics that are impossible to test in humans.1 It is our group's feeling that this approach, along with others like it, is necessary to bridge the gap to understanding the mechanisms for patient findings, notably how patients regress, why they improve, who is “at risk,” and why many proposed treatments do or do not work. We are now finding that early brief systemic exposures of propionic acid, pre- or postnatally and in adulthood, can have long-term effects on brain development and behavior.64 Other groups around the world are working on this. Afaf Al-Ansary, PhD, a biochemist from Saudi Arabia, and graduate student Yasmin Al-Jaddani invited me to King Saud University as a visiting professor because of the alarming increase of ASD in the region. They have further contributed to our understanding of propionic acid's effects on brain and its relation to ASD.82–84
AUTISM: THE “DIABETES” OF THE GASTROINTESTINAL TRACT
As I was finding more metabolic disturbances, I saw a cacophony of methylations, glutathione, methyl B12, methylmalonic acid, and markers of oxidative stress. I began to understand that if you put propionic acid—this GI tract bacteria metabolite—into the system, it could produce identical metabolic disturbances. Some empirical treatments for these patients also made sense. For example, if you cut dietary carbohydrates (this is plausible but not entirely proven), it might reduce symptoms by not feeding the bacteria that make propionic acid.85 Dr Finegold found that giving an antibiotic such as vancomycin or metronidazole that killed GI tract bacteria transiently caused some children to begin talking for a few weeks until the antibiotic was discontinued and the bacteria, as spore formers, reappeared. It is plausible that these antibiotics work by killing bacteria that made propionic acid, allowing the child to improve. If you give patients probiotics, you are possibly putting “good bacteria” in to keep bad bacteria out. Giving carnitine or methyl B12 to promote the breakdown of the propionic acid or giving omega-3 or n-acetyl cysteine to restore the respective lipid or glutathione imbalances may help as well.
The mitochondrial disorders observed in ASD—studied extensively by Dan Rossignol, MD, Rossignol Medical Center, Irvine, California, and Richard Frye, MD, PhD, University of Arkansas,86,87—appeared to occur largely through environmental and not inherited means. These disorders might be caused by or at least worsened by enteric short-chain fatty acids, including propionic acid, from GI tract bacteria. I am indebted to the brilliant collaborative work of Dr Frye, who reviewed his patient population and found a large subset with the lipid (acylcarnitine) and biochemical (citric acid, glutathione) findings predicted by the propionic rodent model.76 There was an absence of genetic abnormalities to explain these changes, suggesting that the biochemical findings stemmed from environmental factors and were not inherited.
In school, I was always reminded that in 1923, Frederick Banting, MD, and Charles Best, MD, from the University of Western Ontario and the University of Toronto were awarded the Nobel Prize for Medicine for discovering the role of insulin in diabetes. Diabetes became our model for looking at a parallel with ASD. Like ASD, there are multiple causes for diabetes (eg, autoimmune, other endocrine disorders, medications), but they follow a general conceptual theme. In diabetes, you can't metabolize sugar; could it be that in ASD, you can't metabolize some dietary sugars (eg, refined, wheat-based carbohydrates) because they feed the bacteria that make propionic acid? In both diabetes and ASD, diet is very important but only part of the picture, and it has varying effects depending on the type of diabetes (ie, it is more beneficial in type II diabetes). Both disorders crave carbohydrates. Diet modification doesn't cure diabetes, but what happens when a diabetic changes his or her diet tells you a lot about the disease. It is a disorder of glucose metabolism, not excess glucose. Something similar may be occurring in some types of ASD—a disorder of enteric fatty acid metabolism in which propionic acid, or something like it, is the substance in excess. In diabetes, we use insulin or glyberide to improve carbohydrate metabolism. By analogy, in ASD, we are using carnitine and methyl B12 to improve fatty acid metabolism or in the future, eradicating/reducing ASD-associated propionic acid–producing GI-tract bacteria. In diabetes, there are biomarkers (blood or urine glucose, glycosylated hemoglobin), and in ASD, there are potential biomarkers (oxidative, stress, glutathione, lipid/acylcarnitine profiles, citric acid–cycle abnormalities, potential bacterial populations). These need further verification. In diabetes, there are associated comorbidities—peripheral nerve damage, cardiac and vision complications, altered immunity and CNS dysfunction, and infections all make the condition worse. In ASD, in addition to the CNS changes, there are associated comorbidities—immunological changes, sensitivities, and infections, mostly arising from the GI tract. This conceptual framework for ASD is new and in its infancy, but it seems to fit.
THE MICROBIOME: WINDOW INTO THE FUTURE OF MEDICINE
If you were to conceive yourself as a spaceship going into the microscopic milieu inside of your digestive tract, it would be like looking at a rainforest. You would see certain kinds of bacteria huddled in this little corner interacting with others; you would see things equivalent to big rainforest trees and branches interacting. You would see other bacteria—like insects and birds—living inside of the tree. You would see a complex interaction between predator and prey. You would also see these interactions that we all see in biology, different organisms in a rainforest interacting to their mutual benefit.
When a rainforest is clear-cut, it causes massive damage to the ecosystem; the same can be true for chronic antibiotic use, both in the individual patient, in the hospital, and in society at large. Chronic antibiotic use can cause major alterations in the microbiome that affect immune function, normal brain development, and whole body development—particularly in the first few months of life.
It is an underappreciated fact that when we come into this world, we have no bacteria in our digestive tracts. The first bacteria, good or bad, come from mom. Other bacteria arrive that alter this flora for better or worse—breastfeeding, Cesarean section, the hospital, the food we eat, and acquired infections. Breast milk provides a special set of polysaccharides that have an exclusive relationship with lactobacilli that emerge to be nourished by milk and in turn nourish the infant. Many infant formulas alter this balance. Forces come into play when the microbiome is altered; some effects are temporary, and some are permanent. These may be a trigger for a number of diseases.
The bacteria that live in our GI tract may change who and what we are, all the way from disease susceptibility or resistance to our brain, behavior, and even personality. These bacteria need fuel, and like us, they produce waste products. Some of these waste products, at certain levels and at certain times of development, can be beneficial to us—that's what we and others have been studying with short-chain fatty acids. They also produce a myriad of other products that modulate immune function and metabolism that have positive and negative effects. These bacteria interact with each other. They may compete with one another for the same food substrate. One bacteria's waste product is another bacteria's food. It is very complex.
A number of recent clinical studies have shown increased propionic acid in the stool,88 clostridia-associated urinary metabolites,52 unique bacterial populations, and altered carbohydrate and short-chain fatty acid metabolism in GI-tract biopsies in ASD patients.89 Rodents raised in a germ-free environment with an absence of GI-tract microbiome behave differently than normal rodents, and early introduction of different microbiota can alter behavior.90,91 Early long-term antibiotic exposure in rodents alters GI-tract flora, increases GI-tract short-chain fatty acids, and modulates host fatty acid metabolism.92 Ongoing work by Dr Finegold has identified further novel bacterial populations (ie, Desulfovibrio), which in addition to making propionic acid also produce hydrogen sulphide to damage mitochondrial function, thus potentiating propionic acid's effects.16 Emma Allen-Vercoe, PhD, at the University of Guelph, is examining microbial ecosystems, as opposed to single organisms, in the study of inflammatory bowel diseases and the use of complex microbial populations to “restore” abnormal GI tract bacterial populations in C difficile colitis and hopefully ASD.93
I became aware of the overrepresentation of ASD in Somalian newcomers to Western society from the literature and the media. Every Somali population, whether they emigrated to the United States, Canada, the United Kingdom, or Scandinavia,56 has noticed an increased prevalence of ASD in their community. They call it the “Western disease” since it is not endemic in East Africa. Once in the West, Somalis eat a Western diet and may acquire a Western microbiome or metabolites. And in a similar fashion, when Westerners travel to other parts of the world, they acquire certain GI tract infections. This subject was made into a documentary—the “Autism Enigma” by the Canadian Broadcasting Corporation (http://cogentbenger.com/autism/). We are currently collaborating with Dr Allen-Vercoe on pilot studies examining the microbiota of Somali children with and without ASD and their families.
Tore Midtvedt, MD, PhD, one of the pioneers of the microbiome in health and disease, invited me to speak on ASD at a Nobel Symposium1 at Sweden's Karolinska Institute. Dr Midtvedt has examined the changing metabolites of the developing infant microbiome55 and the effects of antibiotics and other factors in GI development prospectively in a large cohort of patients.94 Along with Drs Finegold, Allen-Vercoe, and Frye, we have followed up with a Toronto Symposium on Gut-Brain Connections in Autism (http://www.autismcanada.org/scientificsymposium/).
SUMMARY: LIMITATIONS, CAUTION, AND HOPE FOR THE FUTURE
During the last decade of research, we have taken what is thought to be a hopeless disease and offered potential avenues for discovery. We are finding patients who act strange and want strange things to eat, and the things they want actually make them worse. The treatment may lie with the last thing one would ever think of: the metabolism of bacteria and their interaction with us, their host. One of the most recent happenings in this field is respectfully questioning the dogmatic view that ASD is genetic. Studying genetics is not trivial; it's absolutely essential, and I would defend to my death the absolute necessity of looking into this. But in all honesty, we are not finding a direct genetic cause in the vast majority of patients. Recently, from our work with Bistra Nankova, PhD, from New York Medical College, we are finding that short chain fatty acids, including propionic acid, are histone deacetylase inhibitors and thus are switches for genes, particularly those involved in the metabolism of catecholamines, important in anxiety, arousal, movement disorder, aggression, and craving.35 There is the potential that bacteria can control and tinker with our metabolism and even our genes.
The paradox in ASD patients is that they do some things better than the general population. As mentioned, there are diseases with which you can't remember and diseases with which you can't forget, which get us into potentially understanding the savantism or high memory retention common in autistic children. Another crucial fact about the microbiome is that our internal bacteria outnumber our cells 100:1 and have at least 10 times more genetic material. Some now argue that these bacteria, through natural selection, may be controlling or modulating our behavior. This may serve the host well until environmental factors, such as the Western diet or overuse of antibiotics, reset the microbiome to produce alterations of this behavior—the obsessions, perseverations, food fixations, and tics, but also at times enhanced memory associated with ASD.
The people who have taught me the most are the parents who refuse to believe what they were told—that their child's regression and associated symptoms were all in their imagination. Parents know their children. This work could never have been done unless I worked with these special-needs people, saw the giftedness as well as the tragedy of these disorders, and had the training from so many gifted clinicians and scientists and the support of parents and many others from childhood until now. I must stress that we absolutely need the scientific rigor of asking the proper questions and designing the proper experiments and controls, but we also need the art of medicine and the ability to actually observe and listen to patients. The spark for understanding autism and the GI tract could come only from seeing the patient as an individual, not as a collection of symptoms, and seeing some of the assets as well as the disabilities in this condition. Deducing whether clinical expertise or science is more important in addressing enormous problems like ASD is like asking which half of a pair of scissors are more important. We need both.
It is important to note that propionic acid affects multiple systems at different developmental periods in a complex manner, and the evidence of increased propionic acid or other short-chain enteric fatty acids being involved in the pathophysiology of ASD, although compelling, is still circumstantial at this stage (Table).
Table.
Potential Causes and Consequences of Increased Enteric Short-chain Fatty Acid Production and/or Decreased Breakdown and Their Relation to Autism Spectrum Disordera
| Causes | Consequences of SCFAs |
|---|---|
| Long-term antibiotics for routine infection (maternal/infant) treatment of maternal ß hemolytic strep | Gut dysmotility/inflammation/carbohydrate malabsorption/altered gut permeability (tight junction impairment) |
| Hospitalization (colonization of nosocomial bacteria), ie, Cesarean section, neonatal distress | Active uptake of SCFA to CNS (monocarboxylate transporters) |
| Prenatal drugs (valproate, ethanol) | pH-dependent intracellular concentration of SCFA |
| Opportunistic infection (Clostridium spp, Desulfovibrio spp) | Neurotransmitter synthesis and release (catecholamines, enkephalins) CNS/sympathetic nervous system |
| Maternal/infant gut dysbiosis | Receptor activity (+NMDA, -GABA) SCFA G protein coupled receptors/Ca++ influx |
| Organic acidemias (propionic/methylmalonic, biotinidase/holocarboxylase deficiency) | Gap junction closure, altered neurodevelopment, neuroinflammation |
| B12/biotin deficiency | Impaired mitochondrial function/increased oxidative stress |
| Genetic/acquired impaired carnitine synthesis/absorption (TMLHE/OCTN2 genes, ß-lactam antibiotics) | Reduced glutathione/increased sensitivity to xenobiotics (ie, acetaminophen) |
| Mitochondrial disorder/dysfunction (inherited/acquired) | Decreased carnitine/altered lipid metabolism/membrane fluidity |
| Colitis (impaired barrier/SCFA metabolism), ie, celiac disease, Met-receptor tyrosine kinase mutation | Altered gene expression (CREB activation, histone deacetylase inhibition) |
| Increased refined carbohydrate consumption—substrate for bacterial fermentation | Antisocial/perseverative/anxiety-like behavior, seizure/movement disorder, restrictive food interests/carbohydrate craving |
Abbreviations: CNS, central nervous system; CREB, cAMP response element-binding protein; GABA, gamma-aminobutyric acid; NMDA, N-methyl-D-aspartate; OCTN2, organic cation transporter; SCFA, short-chain fatty acid; TMLHE, trimethyllysine hydroxylase, epsilon.
These findings, which are not mutually exclusive, may contribute to the pathophysiology, behavioral symptoms, and comorbidities of autism. Modified with permission from MacFabe (2012), Microbial Ecology in Health and Disease.
Future studies should identify additional parallels between the propionic acid rodent model of ASD and individuals with ASD who manifest similar biomarkers, including acylcarnitines, redox abnormalities, and altered stool short-chain fatty acids and microbial populations. Further study of this model and this subgroup of autistic patients should improve our understanding of the pathophysiology and potential risk factors that lead to the metabolic, brain, and behavior abnormalities associated with ASD.80
We need to tread carefully regarding many empiric treatments that seem to help some ASD patients but may have no effect on or worsen others. More importantly, we need to know the exact mechanism behind why these treatments may or may not work. Long-term broad-spectrum antibiotic treatment, although initially improving symptoms, may be ineffective or even worsen the condition by further altering the already damaged microbiome and may risk a superinfection by selecting for antibiotic-resistant strains. Nonetheless, the pioneering work of Dr Finegold and the phenomena of transient improvement following oral vancomycin16 must be reexamined with the new techniques and biomarkers described above. However, eradication of ASD bacteria may not be the answer—the condition may not be caused by unique bacteria; it may indeed be caused, as Dr Midtvedt suggests, by the absence of certain bacteria. Here the role of probiotics, which need much more scientific rigor to substantiate possible therapeutic claims, may be useful. The consideration of fecal transplant, or synthetic stool, gleaned from encouraging early studies in C difficile patients,93 holds promise. However, we do not know the suitability of an appropriate donor (ie, there is evidence family members may be a carrier state), and the effect of such an introduced microbiome in an immune-altered ASD patient is not yet known. Judicious practice of standard hygiene (ie, handwashing with soap and water to inhibit in particular lipid envelope infectious agents) throughout hospitals and clinics (particularly obstetrics, neonatology, pediatrics) but also by family members, daycares, and institutions to reduce fecal oral spread16 and more rational use of antibiotics andantimicrobials are warranted. This, coupled with early presymptomatic screening (ie, acyclarnitine profiles, redox, identification of “at risk” populations) and treatment, may hold the most promise, as was shown with phenylketonuria, another apparently hopeless neurodevelopmental disorder.
Finally, it is important to note that propionic and related short-chain fatty acids can elicit behaviors that are anxiety-like, perseverative, repetitive, ritualistic, and antisocial. These behaviors are common to many other neuropsychiatric conditions (obsessive compulsive, mood, anxiety, attention deficit/hyperactive and eating disorders, irritable bowel syndrome, and schizophrenia) where infectious agents have been proposed.95–97 Indeed, the growing incidence of ASD and ASD-related conditions, coupled with the observed alterations in the human microbiome secondary to dietary, medical, and agricultural factors, and their potential effect on human and animal behavior should be further examined. Professor Jared Diamond contended in his book Guns, Germs, and Steel that the impact of human migration and urbanization, domestication of plant and animals, and resultant human diseases shaping cultures is not trivial.98 At this stage, it is not so far-fetched to say that Western society has altered human microbial populations, which in turn may be altering human behavior and culture.
Regardless of people's view of ASD and their individual scientific approach, clinicians and researchers all care about trying to help these families. Miracles can happen when people start to understand metabolism and how things are connected to each other in this chain. Like the tiny denizens that dwell in our intestines, we have to share and work together. Considering the continual increase in ASD and the toll it places on individuals, families, and society, I think the future of humanity depends on it. Other countries around the world, adopting our Western food, agricultural, and medical practices are rapidly seeing the increase in what my African friends call “The Western Disease.” In my opinion, the future of autism and I think of many disorders will be the 3Ms: metabolism, mitochondria, and the microbiome. Microbes are not our enemies; a stable, healthy microbiome may be one the best friends we will ever have. I am delighted by the results to date and hopeful for the future.
ACKNOWLEDGMENTS
This manuscript was prepared as a summary of a discussion with Dr Sidney Baker following the Beth Israel Medical Center “Current Trends in Autism Research” Conference, New York City, 2013. The author wishes to thank all the members and collaborators of the Kilee Patchell-Evans Autism Research Group and the countless families and caregivers who have shared their stories. I would also extend our utmost thanks to David Patchell-Evans for his tireless devotion to people with autism, and his daughter, Kilee. This research was supported by contributions from GoodLife Children's Charities, Autism Canada, and Autism Research Institute to the author.
Disclosure Dr MacFabe completed the ICMJE Disclosure Form for Potential Conflicts of Interest and had no conflicts related to this work to disclose.
REFERENCES
- 1.MacFabe DF. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb Ecol Health Dis. 2012;23:19260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart DJ, MacFabe DF, Vanderwolf CH. Cholinergic activation of the electro-corticogram: role of the substantia innominata and effects of atropine and quinuclidinyl benzilate. Brain Res. 1984;322(2):219–32 [DOI] [PubMed] [Google Scholar]
- 3.Stewart DJ, MacFabe DF, Leung LW. Topographical projection of cholinergic neurons in the basal forebrain to the cingulate cortex in the rat. Brain Res. 1985;358(1-2):404–7 [DOI] [PubMed] [Google Scholar]
- 4.Borst JG, Leung LW, MacFabe DF. Electrical activity of the cingulate cortex. II. Cholinergic modulation. Brain Res. 1987;407(1):81–93 [DOI] [PubMed] [Google Scholar]
- 5.Engeland CG, Nielsen DV, Kavaliers M, Ossenkopp KP. Locomotor activity changes following lipopolysaccharide treatment in mice: a multivariate assessment of behavioral tolerance. Physiol Behav. 2001;72(4):481–91 [DOI] [PubMed] [Google Scholar]
- 6.Coltart I, Tranah TH, Shawcross DL. Inflammation and hepatic encephalopathy. Arch Biochem Biophys. 2013;536(2):189–96 [DOI] [PubMed] [Google Scholar]
- 7.Marth T. New insights into Whipple's disease—a rare intestinal inflammatory disorder. Dig Dis. 2009;27(4):494–501 [DOI] [PubMed] [Google Scholar]
- 8.Moores A, Fox S, Lang A, Hirschfield GM. Wilson disease: Canadian perspectives on presentation and outcomes from an adult ambulatory setting. Can J Gastroenterol. 2012;26(6):333–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frantseva MV, Kokarovtseva L, Naus CG, Carlen PL, MacFabe D, Perez Velazquez JL. Specific gap junctions enhance the neuronal vulnerability to brain traumatic injury. J Neurosci. 2002;22(3):644–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rorig B, Sutor B. Serotonin regulates gap junction coupling in the developing rat somatosensory cortex. Eur J Neurosci. 1996;8(8):1685–95 [DOI] [PubMed] [Google Scholar]
- 11.Perez-Velazquez JL, Frantseva MV, Naus CC. Gap junctions and neuronal injury: protectants or executioners? Neuroscientist. 2003;9(1):5–9 [DOI] [PubMed] [Google Scholar]
- 12.Karuri AR, Dobrowsky E, Tannock IF. Selective cellular acidification and toxicity of weak organic acids in an acidic microenvironment. Br J Cancer. 1993;68(6):1080–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brock M, Buckel W. On the mechanism of action of the antifungal agent propionate. Eur J Biochem. 2004;271(15):3227–41 [DOI] [PubMed] [Google Scholar]
- 14.Finegold SM, Molitoris D, Song Y, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002September1;35(Suppl 1):S6–S16 [DOI] [PubMed] [Google Scholar]
- 15.Sandler RH, Finegold SM, Bolte ER, et al. Short-term benefit from oral vancomycin treatment of regressive-onset autism. J Child Neurol. 2000;15(7):429–35 [DOI] [PubMed] [Google Scholar]
- 16.Finegold SM. State of the art; microbiology in health and disease. Intestinal bacterial flora in autism. Anaerobe. 2011;17(6):367–8 [DOI] [PubMed] [Google Scholar]
- 17.Stackebrandt E, Rainey FA. Phylogenetic relationships Rood JI, McClane BA, Songer JG, Titball RW, The clostridia, molecular biology and pathogenesis. Waltham, MA: Academic Press; 1997:3–19 [Google Scholar]
- 18.Ameis SH, Szatmari P. Imaging-genetics in autism spectrum disorder: advances, translational impact, and future directions. Front Psychiatry. 2012May15;3:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu VW, Nguyen A, Kim KS, et al. Gene expression profiling of lymphoblasts from autistic and nonaffected sib pairs: altered pathways in neuronal development and steroid biosynthesis. PLoS One. 2009;4(6):e5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbert MR. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr Opin Neurol. 2010;23(2):103–10 [DOI] [PubMed] [Google Scholar]
- 21.MacFabe DF, Cain DP, Rodriguez-Capote K, et al. Neurobiological effects of intraventricular propionic acid in rats: possible role of short-chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res. 2007;176(1):149–69 [DOI] [PubMed] [Google Scholar]
- 22.Anders JJ. Lactic acid inhibition of gap junctional intercellular communication in in vitro astrocytes as measured by fluorescence recovery after laser photo-bleaching. Glia. 1988;1(6):371–9 [DOI] [PubMed] [Google Scholar]
- 23.Moore H, Grace AA. A role for electrotonic coupling in the striatum in the expression of dopamine receptor-mediated stereotypies. Neuropsychopharmacology. 2002;27(6):980–92 [DOI] [PubMed] [Google Scholar]
- 24.Juszczak GR, Swiergiel AH. Properties of gap junction blockers and their behavioural, cognitive and electrophysiological effects: animal and human studies. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):181–98 [DOI] [PubMed] [Google Scholar]
- 25.Wiencken-Barger AE, Djukic B, Casper KB, McCarthy KD. A role for Connexin43 during neurodevelopment. Glia. 2007;55(7):675–86 [DOI] [PubMed] [Google Scholar]
- 26.Nagasawa K, Chiba H, Fujita H, et al. Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J Cell Physiol. 2006;208(1):123–32 [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Li N, Neu J. Tight junctions, leaky intestines, and pediatric diseases. Acta Paediatr. 2005;94(4):386–93 [DOI] [PubMed] [Google Scholar]
- 28.Zarate G, Gonzalez S, Chaia AP. Assessing survival of dairy propionibacteria in gastrointestinal conditions and adherence to intestinal epithelia. Methods Mol Biol. 2004;268:423–32 [DOI] [PubMed] [Google Scholar]
- 29.James SJ, Cutler P, Melnyk S, et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004;80(6):1611–7 [DOI] [PubMed] [Google Scholar]
- 30.James SJ, Melnyk S, Jernigan S, et al. Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. Am J Med Genet B Neuropsychiatr Genet. 2006August17;141B(8):947–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deth R, Muratore C, Benzecry J, Power-Charnitsky VA, Waly M. How environmental and genetic factors combine to cause autism: A redox/methylation hypothesis. Neurotoxicology. 2008;29(1):190–201 [DOI] [PubMed] [Google Scholar]
- 32.Chauhan A, Audhya T, Chauhan V. Brain region-specific glutathione redox imbalance in autism. Neurochem Res. 2012;37(8):1681–9 [DOI] [PubMed] [Google Scholar]
- 33.Rorig B, Klausa G, Sutor B. Intracellular acidification reduced gap junction coupling between immature rat neocortical pyramidal neurones. J Physiol. 1996;490 (pt-1):31–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakao S, Moriya Y, Furuyama S, Niederman R, Sugiya H. Propionic acid stimulates superoxide generation in human neutrophils. Cell Biol Int. 1998;22(5):331–7 [DOI] [PubMed] [Google Scholar]
- 35.DeCastro M, Nankova BB, Shah P, et al. Short-chain fatty acids regulate tyrosine hydroxylase gene expression through a cAMP-dependent signaling pathway. Brain Res Mol Brain Res. 2005;142(1):28–38 [DOI] [PubMed] [Google Scholar]
- 36.Wajner M, Latini A, Wyse AT, Dutra-Filho CS. The role of oxidative damage in the neuropathology of organic acidurias: insights from animal studies. J Inherit Metab Dis. 2004;27(4):427–48 [DOI] [PubMed] [Google Scholar]
- 37.Hara H, Haga S, Aoyama Y, Kiriyama S. Short-chain fatty acids suppress cholesterol synthesis in rat liver and intestine. J Nutr. 1999;129(5):942–8 [DOI] [PubMed] [Google Scholar]
- 38.Le Poul E, Loison C, Struyf S, et al. Functional characterization of human receptors for short-chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278(28):25481–9 [DOI] [PubMed] [Google Scholar]
- 39.Parab S, Nankova BB, La Gamma EF. Differential regulation of the tyrosine hydroxylase and enkephalin neuropeptide transmitter genes in rat PC12 cells by short-chain fatty acids: concentration-dependent effects on transcription and RNA stability. Brain Res. 2007;1132(1):42–50 [DOI] [PubMed] [Google Scholar]
- 40.Tamai I, Takanaga H, Maeda H, et al. Participation of a proton-cotransporter, MCT1, in the intestinal transport of monocarboxylic acids. Biochem Biophys Res Commun. 1995;214(2):482–9 [DOI] [PubMed] [Google Scholar]
- 41.Bergersen L, Rafiki A, Ottersen OP. Immunogold cytochemistry identifies specialized membrane domains for monocarboxylate transport in the central nervous system. Neurochem Res. 2002;27(1-2):89–96 [DOI] [PubMed] [Google Scholar]
- 42.Conn AR, Fell DI, Steele RD. Characterization of alpha-keto acid transport across blood-brain barrier in rats. Am J Physiol 1983;245(3):E253–E260 [DOI] [PubMed] [Google Scholar]
- 43.Mitsui R, Ono S, Karaki S, Kuwahara A. Neural and non-neural mediation of propionate-induced contractile responses in the rat distal colon. Neurogastroenterol Motil. 2005;17(4):585–94 [DOI] [PubMed] [Google Scholar]
- 44.Perez B, Desviat LR, Rodriguez-Pombo P, et al. Propionic acidemia: identification of twenty-four novel mutations in Europe and North America. Mol Genet Metab. 2003;78(1):59–67 [DOI] [PubMed] [Google Scholar]
- 45.Yorifuji T, Kawai M, Muroi J, et al. Unexpectedly high prevalence of the mild form of propionic acidemia in Japan: presence of a common mutation and possible clinical implications. Hum Genet. 2002;111(2):161–5 [DOI] [PubMed] [Google Scholar]
- 46.Kim KC, Kim P, Go HS, et al. The critical period of valproate exposure to induce autistic symptoms in Sprague-Dawley rats. Toxicol Lett. 20115;201(2):137–42 [DOI] [PubMed] [Google Scholar]
- 47.Coulter DL. Carnitine, valproate, and toxicity. J Child Neurol. 1991;6(1):7–14 [DOI] [PubMed] [Google Scholar]
- 48.Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–7 [DOI] [PubMed] [Google Scholar]
- 49.Atladóttir HO, Thorsen P, Østergaard L, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010December;40(12):1423–30 [DOI] [PubMed] [Google Scholar]
- 50.Atladottir HO, Thorsen P, Schendel DE, Østergaard L, Lemcke S, Parner ET. Association of hospitalization for infection in childhood with diagnosis of autism spectrum disorders: a Danish cohort study. Arch Pediatr Adolesc Med. 2010;164(5):470–7 [DOI] [PubMed] [Google Scholar]
- 51.Patterson PH. Maternal infection and autism. Brain Behav Immun. 2012;26(3):393. [DOI] [PubMed] [Google Scholar]
- 52.Yap IK, Angley M, Veselkov KA, Holmes E, Lindon JC, Nicholson JK. Urinary metabolic phenotyping differentiates children with autism from their unaffected siblings and age-matched controls. J Proteome Res. 2010June4;9(6):2996–3004 [DOI] [PubMed] [Google Scholar]
- 53.Forsythe P, Sudo N, Dinan T, Taylor VH, Bienenstock J. Mood and gut feelings. Brain Behav Immun. 2010;24(1):9–16 [DOI] [PubMed] [Google Scholar]
- 54.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Midtvedt AC, Midtvedt T. Production of short-chain fatty acids by the intestinal microflora during the first 2 years of human life. J Pediatr Gastroenterol Nutr. 1992;15(4):395–403 [DOI] [PubMed] [Google Scholar]
- 56.Barnevik-Olsson M, Gillberg C, Fernell E. Prevalence of autism in children of Somali origin living in Stockholm: brief report of an at-risk population. Dev Med Child Neurol. 2010;52(12):1167–8 [DOI] [PubMed] [Google Scholar]
- 57.Kavaliers M, Choleris E, Agmo A, Pfaff DW. Olfactory-mediated parasite recognition and avoidance: linking genes to behavior. Horm Behav. 2004;46(3):272–83 [DOI] [PubMed] [Google Scholar]
- 58.Kaushik M, Lamberton PH, Webster JP. The role of parasites and pathogens in influencing generalised anxiety and predation-related fear in the mammalian central nervous system. Horm Behav. 2012;62(3):191–201 [DOI] [PubMed] [Google Scholar]
- 59.MacFabe DF, Rodriguez-Capote K, Hoffman JE, et al. A novel rodent model of autism: intraventricular infusions of propionic acid increase locomotor activity and induce neuroinflammation and oxidative stress in discrete regions of adult rat brain. Am J Biochem Biotech. 2008;4(2):146–66 [Google Scholar]
- 60.MacFabe DF, Cain NE, Boon F, Ossenkopp KP, Cain DP. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: relevance to autism spectrum disorder. Behav Brain Res. 2011;217(1):47–54 [DOI] [PubMed] [Google Scholar]
- 61.Shultz SR, MacFabe DF, Ossenkopp KP, et al. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism. Neuropharmacology. 2008;54(6):901–11 [DOI] [PubMed] [Google Scholar]
- 62.Shultz SR, MacFabe DF, Martin S, et al. Intracerebroventricular injections of the enteric bacterial metabolic product propionic acid impair cognition and sensorimotor ability in the Long-Evans rat: further development of a rodent model of autism. Behav Brain Res. 2009;200(1):33–41 [DOI] [PubMed] [Google Scholar]
- 63.Thomas RH, Foley KA, Mepham JR, Tichenoff LJ, Possmayer F, MacFabe DF. Altered brain phospholipid and acylcarnitine profiles in propionic acid infused rodents: further development of a potential model of autism spectrum disorders. J Neurochem. 2010;113(2):515–29 [DOI] [PubMed] [Google Scholar]
- 64.Ossenkopp KP, Foley KA, Gibson J, et al. Systemic treatment with the enteric bacterial fermentation product, propionic acid, produces both conditioned taste avoidance and conditioned place avoidance in rats. Behav Brain Res. 2012;227(1):134–41 [DOI] [PubMed] [Google Scholar]
- 65.Thomas RH, Meeking MM, Mepham JR, et al. The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: further development of a rodent model of autism spectrum disorders. J Neuroinflammation. 2012;9(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67–81 [DOI] [PubMed] [Google Scholar]
- 67.Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathol. 2007;17(4):434–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Araujo FM, Rossetti F, Chanda S, Yourick D. Exposure to nerve agents: from status epilepticus to neuroinflammation, brain damage, neurogenesis and epilepsy. Neurotox. 2012;33(6):1476–90 [DOI] [PubMed] [Google Scholar]
- 69.Frick LR, Williams K, Pittenger C. Microglial dysregulation in psychiatric disease. Clin Dev Immunol. 2013;2013:608654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28(8):436–45 [DOI] [PubMed] [Google Scholar]
- 71.Zuo YY, Veldhuizen RA, Neumann AW, Petersen NO, Possmayer F. Current perspectives in pulmonary surfactant—inhibition, enhancement and evaluation. Biochim Biophys Acta 2008;1778(10):1947–77 [DOI] [PubMed] [Google Scholar]
- 72.Chauhan A, Chauhan V. Oxidative stress in autism. Pathophysiology. 2006;13(3):171–81 [DOI] [PubMed] [Google Scholar]
- 73.Kohman RA, Rhodes JS. Neurogenesis, inflammation and behavior. Brain Behav Immun. 2013;27(1):22–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brass EP, Beyerinck RA. Effects of propionate and carnitine on the hepatic oxidation of short- and medium-chain-length fatty acids. J Biochem. 1988;250(3):819–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frye RE. Biomarkers of abnormal energy metabolism in children with autism spectrum disorder. N A J Med Sci. 2012;5(3):141–7 [Google Scholar]
- 76.Frye RE, Melnyk S, MacFabe DS. Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Transl Psychiatry. 2013;3:e220 doi:10.1038/tp.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jones LL, McDonald DA, Borum PR. Acylcarnitines: role in brain. Prog Lipid Res. 2010;49(1):61–75 [DOI] [PubMed] [Google Scholar]
- 78.Filipek PA, Juranek J, Nguyen MT, Cummings C, Gargus JJ. Relative carnitine deficiency in autism. J Autism Dev Disord. 2004;34(6):615–23 [DOI] [PubMed] [Google Scholar]
- 79.Celestino-Soper PB, Violante S, Crawford EL, et al. A common X-linked inborn error of carnitine biosynthesis may be a risk factor for nondysmorphic autism. Proc Nat Acad Sci U S A. 2012;109(21):7974–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pochini L, Galluccio M, Scumaci D, et al. Interaction of beta-lactam antibiotics with the mitochondrial carnitine/acylcarnitine transporter. Chem Biol Interact. 2008;173(3):187–94 [DOI] [PubMed] [Google Scholar]
- 81.Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. 2013;34(3):167–77 [DOI] [PubMed] [Google Scholar]
- 82.El-Ansary AK, Ben BA, Kotb M. Etiology of autistic features: the persisting neurotoxic effects of propionic acid. J Neuroinflammation. 2012;9(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.El-Ansary A, Al-Daihan S, Al-Dbass A, Al-Ayadhi L. Measurement of selected ions related to oxidative stress and energy metabolism in Saudi autistic children. Clin Biochem. 2010;43(1-2):63–70 [DOI] [PubMed] [Google Scholar]
- 84.Al-Gadani Y, El-Ansary A, Attas O, Al-Ayadhi L. Metabolic biomarkers related to oxidative stress and antioxidant status in Saudi autistic children. Clin Biochem. 2009;42(10-11):1032–40 [DOI] [PubMed] [Google Scholar]
- 85.Gottschall E. Breaking the vicious cycle: intestinal health through diet Baltimore, Ontario, Canada: The Kirkton Press; 1994 [Google Scholar]
- 86.Frye RE, Rossignol DA. Mitochondrial dysfunction can connect the diverse medical symptoms associated with autism spectrum disorders. Pediatr Res. 2011;69:41R–47R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rossignol DA, Frye RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry. 2011January25;17:290–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci. 2012August;57(8):2096–102 [DOI] [PubMed] [Google Scholar]
- 89.Williams BL, Hornig M, Buie T, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One. 2011;6(9):e24585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saulnier DM, Ringel Y, Heyman MB, et al. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes. 2013;4(1):17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–12 [DOI] [PubMed] [Google Scholar]
- 92.Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Petrof EO, Claud EC, Gloor GB, Allen-Vercoe E. Microbial ecosystems therapeutics: a new paradigm in medicine? Benef Microbes. 2013;4(1):53–65 [DOI] [PubMed] [Google Scholar]
- 94.White RA, Bjornholt JV, Baird DD, et al. Novel developmental analyses identify longitudinal patterns of early gut microbiota that affect infant growth. PLoS Comput Biol. 2013;9(5):e1003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Suren P, Bakken IJ, Aase H, et al. Autism spectrum disorder, ADHD, epilepsy, and cerebral palsy in norwegian children. Pediatrics. 2012;130(1):e152–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sokol MS. Infection-triggered anorexia nervosa in children: clinical description of four cases. J Child Adolesc Psychopharmacol. 2000;10(2):133–45 [DOI] [PubMed] [Google Scholar]
- 97.Kerbeshian J, Burd L. Is anorexia nervosa a neuropsychiatric developmental disorder? An illustrative case report. World J Biol Psychiatry. 2009;10(4 Pt 2):648–57 [DOI] [PubMed] [Google Scholar]
- 98.Diamond J. Guns, germs, and steel New York, NY: W.W. Norton & Company, Inc; 1997 [Google Scholar]