Abstract
We developed a strategy that can prolong in vitro growth of T cell type of large granular lymphocyte (T-LGL) leukemia cells. Primary CD8+ lymphocytes from T-LGL leukemia patients were stably transduced with the retroviral tax gene derived from human T cell leukemia virus type 2. Expression of Tax overrode replicative senescence and promoted clonal expansion of the leukemic CD8+ T cells. These cells exhibit features characteristic of leukemic LGL, including resistance to FasL-mediated apoptosis, sensitivity to the inhibitors of sphingosine-1-phosphate receptor and IκB kinases as well as expression of cytotoxic gene products such as granzyme B, perforin and IFNγ. Collectively, these results indicate that this leukemia cell model can duplicate the main phenotype and pathophysiological characteristics of the clinical isolates of T-LGL leukemia. This model should be useful for investigating molecular pathogenesis of the disease and for developing new therapeutics targeting T-LGL leukemia.
Keywords: T-LGLL, Stat3, NF-κB, retroviral Tax oncoprotein
1. Introduction
Large granular lymphocyte (LGL) leukemia is a hematological malignancy of either T cells or natural killer (NK) cells [1]. T cell type of large granular lymphocyte (T-LGL) leukemia is the malignancy of CD8+ cytotoxic T cells, which usually occurs in elderly patients. The disease course is frequently associated with autoimmune diseases [2–5]. Many patients with T-LGL leukemia do not respond to the currently available immune-suppressive therapies [6]. Primary T-LGL leukemia cells display the CD3+/CD8+/CD57+ surface markers, representing activated cytotoxic T lymphocytes [7–9]. TCR rearrangement pattern indicates their clonal expansion [10, 11]. The primary leukemic cells exhibit constitutive activities of STAT3 and NF-κB, and both factors are critical mediators of oncogenic signaling and survival pathways [12, 13]. Activated Stat3 contributes to chemotherapy resistance as repression of STAT3 in LGL leukemic cells down-regulates a survival protein Mcl-1, resulting in an increased sensitivity to apoptosis [12]. Thus, STAT3 activation plays an essential role in promoting aberrant proliferation of the leukemic cells. In addition, NF-κB is constitutively activated in LGL leukemia, also playing a crucial role in promoting survival and proliferation of the leukemia cells [13]. It is of paramount significance to further verify therapeutic agents that target STAT3 and NF-κB for treatment of T-LGL leukemia in a well-defined leukemia model. It is also crucial to identify new survival signaling pathways that could serve as therapeutic target in treating T-LGL leukemia.
Current studies of T-LGL leukemia exclusively rely on fresh specimens collected from patients with T-LGL leukemia. There are no cell lines from these patients, although two CD4+ T-LGL cell lines from a single patient were previously reported [13a, 13b]. Previous efforts to establish CD8+ T-LGLL cell lines have not been successful, primarily because T-LGL leukemia cells are terminally differentiated cytotoxic T lymphocytes and undergo rapid replicative senescence. In the present study, we succeeded in generating T-LGL leukemia cell lines by stable expression of the retroviral protein Tax derived from human T cell leukemia virus type 2 (HTLV-2). Our study demonstrates that these established cell lines duplicate the phenotype of primary T-LGL leukemia cells from patients. This model should be valuable for studying the pathogenesis of T-LGL leukemia and developing novel therapeutics.
2. Materials and methods
2.1. Lentivirus vector, viral production and transduction of primary CD8 T cells
The tax gene from HTLV-2 was fused with enhanced GFP, and the tax2-gfp fusion fragment was cloned into the lentivirus vector pLCEF8 [14], in which the human elongation factor 1 alpha promoter drives expression of Tax2-GFP. The procedure for lentiviral production and concentration was described previously [15]. Human peripheral blood lymphocytes were isolated from healthy blood donors or from clinically confirmed T-LGL leukemia patients, and stimulated with PHA (1μg/ml) for 24 hours, followed by adding recombinant IL-2 (100u/ml) (AIDS Reagent Program). The activated lymphocytes were cultured for 5–7 days, and the CD8+ cells were enriched with anti-CD8 magnetic beads (Invitrogen). These purified CD8 T cells were then transduced with the lentivirus carrying the tax2-gfp expression cassette. The transduced cells were cultured continuously in complete media containing 20% fetal bovine serum and 100u/ml of recombinant IL-2.
2.2. Cell lines, antibodies and chemicals
MT-2 and SP cell lines were obtained from AIDS Reagent Program, and Jurkat T cell line was from ATCC. Antibodies for pERK1/2, ERK1, pMEK1, MEK1 and pAkt1 were purchased from Santa Cruz Biotechnology, and anti-Mcl-1 and pSTAT3, were from Cell Signaling. U0126, wortmanin, LY294002, BAY11-7082, 3-methyladenin and chloroquine were purchased from Sigma.
2.3. Immunophenotype analysis, cell proliferation assay and TCR genotyping
The Immunophenotype of Tax2-immortalized CD8+ T cell line was determined with FACS. Cells were stained with allophycocyanin (APC) conjugated antibodies including anti-CD3, -CD4, -CD25, -TCRαβ, -CD45RO and -CD69 (eBioscience) according to the manufacturer’s instruction. The stained cells were subjected to FACS analysis. Cell proliferation assay was performed using tetrazolium compound based CellTiter 96® AQueous One Solution Cell Proliferation (MTS) assay (Promega). Quantitative PCR was used to examine TCR rearrangement using the method reported previously [16].
2.4. Electrophoretic mobility gel shift assay (EMSA) and real-time PCR
Nuclear extracts were prepared from various T cell lines using NE-PER nuclear and cytoplasmic extraction reagents (Pierce). The oligonucleotide was 5′-end labeled with biotin (Integrated DNA Technologies) and annealed to its complementary strand. The binding activities were examined by EMSA using Light Shift Chemiluminescent EMSA Kit (Pierce) following the protocol reported previously [15]. The real-time PCR analysis was performed according to the method as previously described [15].
3. Results
3.1. Establishment of T-LGLL-like model cell line
To establish long-term culture of T-LGL leukemia cells, we utilized the retroviral tax gene (tax2) derived from HTLV-2, a virus that preferentially infects CD8+ T cells. The tax2-gfp fusion gene was generated and constructed in a lentivirus vector in which the human elongation factor promoter drives constitutive expression of Tax2-GFP. CD8+ T cells from healthy donors or from clinically confirmed T-LGL leukemia patients were enriched through sorting with anti-CD8 magnetic beads, followed by lentiviral transduction. Roughly 30%–50% of cells were transduced by lentivirus expressing Tax2-GFP as evidenced by visualization with fluorescence imaging. About one month following transduction, nearly 100% of cells emitted green fluorescence, indicating that non-transduced cells lost growth potential and gradually disappeared during extended culture. The Tax2-GFP-expressing cells grew in clusters (data not shown). Untransduced CD8 T cells from healthy donors or T-LGL leukemia patients typically grow in culture for less than three weeks at normal conditions. The Tax2-GFP-transduced normal CD8 T cells only grew for about two months before dying. In contrast, the Tax2-GFP-transduced CD8+ T cells from T-LGL leukemia patients grew in culture for at least four months. One of the established T-LGL leukemia cell lines, named TL-1, was able to grow for over two years without losing growth potential. These findings demonstrate that Tax2 alone is not sufficient to immortalize normal CD8+ T cells, yet it can promote long-term expansion of the leukemic LGL cells.
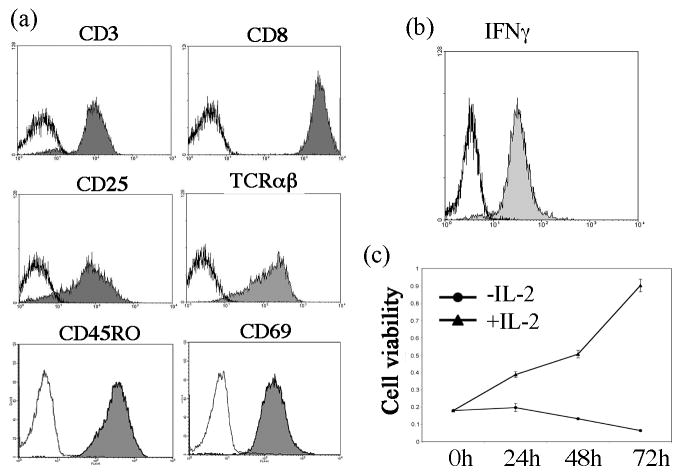
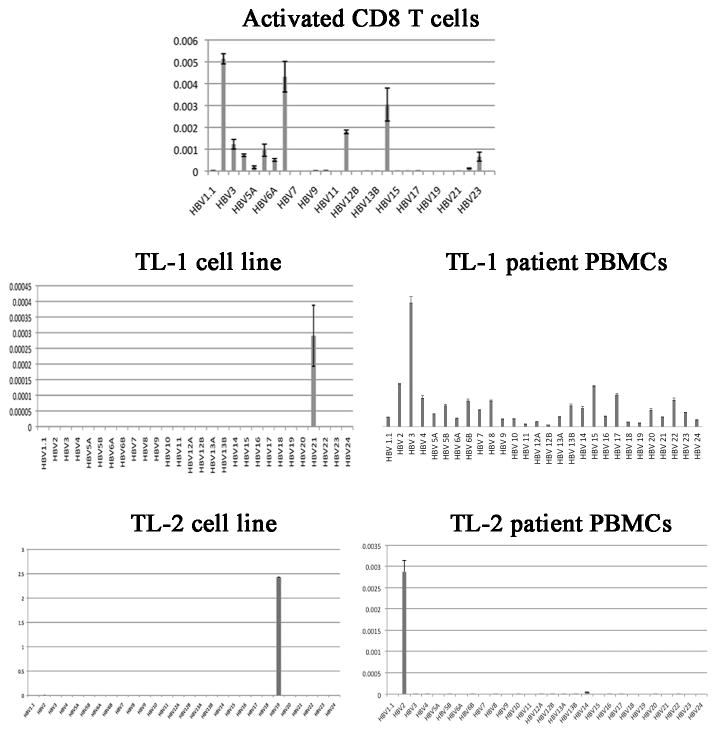
We next examined the immunophenotype of the Tax2-GFP-established leukemia cells. We showed that TL-1 cells exhibited a CD3+/TCRαβ+/CD8+/CD25+/CD45RO+/CD69+ immunophenotype (Fig. 1a). CD45RO is the surface marker for memory T cells, while CD69 is a marker for activated T cells. Like activated CD8 T cells, interferon gamma was abundantly expressed in TL-1 cells (Fig. 1b). These results indicated that these cells were terminally differentiated, activated memory CD8 T cells, similar to the CD8+ leukemia cells seen from T-LGL leukemia patients. Furthermore, CD25, the IL-2 receptor alpha chain, was also detected in TL-1 cells (Fig. 1a), which rendered the cells responding to exogenous IL-2. Deprivation of IL-2 led to growth arrest and death of TL-1 cells (Fig. 1c). Another Tax2-GFP-established leukemia cell line, TL-2, also exhibited IL-2-dependent growth pattern (data not shown). However, the growth of TL-2 in culture was very slow, suggesting that TL-2 is not suitable for cancer cell model. Analysis of the TCR rearrangement showed that both TL-1 and TL-2 cell lines displayed a distinct peak of genotyping, whereas the normal activated CD8 T cells exhibited multiple peaks (Fig. 2). However, the genotypes of TL-1 and TL-2 cell lines were apparently distinct from their corresponding patients’ PBMCs (Fig. 2). Nevertheless, these data indicated that TL-1 and TL-2 were a clonal population of CD8+ leukemia cells.
Fig. 1.
Immunophenotype of Tax2-established T-LGL leukemia cells: (a) FACS analysis of surface markers on TL-1 cells; (b) Intracellular staining of IFNγ expression in TL-1 cells; (c) Cell viability assay to examine IL-2-dependent growth of TL-1 cells.
Fig. 2.
TCR Vβ genotyping of normal activated CD8+ cells, Tax2-established TL-1 and TL-2 cell lines and their corresponding patient PBMCs. Dilutions, RNA extraction, cDNA synthesis and qRT PCR are performed in triplicates.
3.2. Expression of cytotoxic gene products from the established T-LGLL cell line
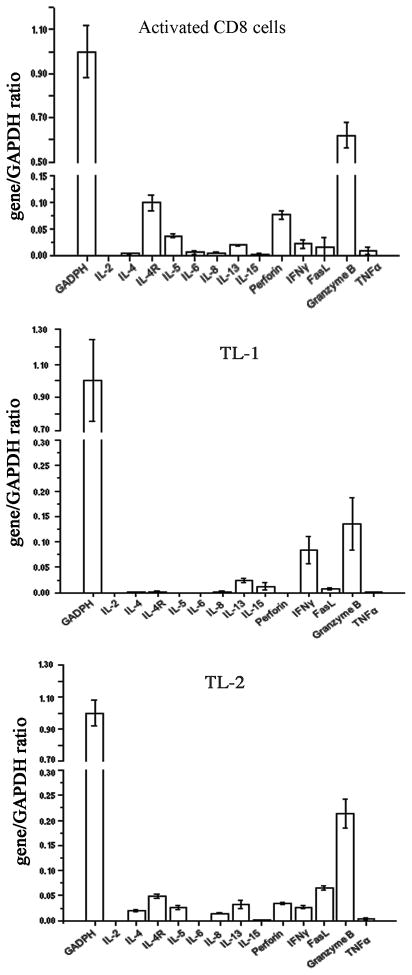
Primary T-LGL leukemia cells are derived from cytotoxic T cells. To determine whether the Tax2-established T-LGL cells present similar features, we analyzed cytokine and cytotoxic gene expression profiling on these cells by real-time PCR analysis. Similar to normal activated CD8+ T cells, the Tax-2-established leukemia cell lines TL-1 and TL-2 expressed cytotoxic gene products such as granzyme B, IFNγ and FasL (Fig. 3). Perforin was also expressed in TL-2 cells but not in TL-1 cells (Fig. 3), suggesting that some of T-LGL leukemia cells execute cytotoxic activity via indirect mechanism, probably by using IFNγ-mediated cytotoxic killing. Additionally, IL-13, an immune modulatory cytokine, was detected in both normal activated CD8 T cells and Tax2-established leukemia cells (Fig. 3). Although the presumptive antigen that these CD8+ CTLs recognize is not known, the expression of certain cytotoxic gene products and typical immunophenotype strongly support the notion that the Tax2-established CD8 T cells from T-LGL leukemia patients are leukemic CTLs.
Fig. 3.
Cytokine and cytotoxic gene expression profiles on Tax2-established T-LGL leukemia cell lines in comparison with normal activated CD8 T cells by real-time PCR.
3.3. Expression of T cell receptor signaling molecules and oncogenic activation of the established T-LGLL cell line
We further examined expression of TCR signaling molecules using TL-1 as a model cell line. Jurkat cells are CD4+ T cells with intact TCR signaling response and as expected, the signaling molecules in this pathway, such as Lck, ZAP70, CD3ζ chain, LAT, MEK1, IKKβ and p85 subunit of PI3KC1, were abundantly expressed (Fig. 4a). MT-2 cells are HTLV-1-transformed CD4+ T cells and displayed down-regulated Lck, ZAP70 and CD3ζ (Fig. 4a), a pattern commonly found in ATL cells that usually do not respond to TCR engagement. SP cells are HTLV-1-immortalized CD8+ T cells, and their growth is dependent on a low level of exogenous IL-2. SP cells showed similar expression patterns of the TCR signaling molecules as Jurkat cells (Fig. 4a). An intact expression pattern of TCR signaling molecules was present in TL-1 cells (Fig. 4a), suggesting that these cells are likely reactive to antigen in vivo.
Fig. 4.
TL-1 cells exhibit constitutive activation of various oncogenic signaling molecules: (a) Expression profiles of TCR signaling molecules were shown in various T cell lines including Jurkat (lymphoblastic leukemia cells), MT-2 (HTLV-1-infected CD4 T cells), SP (HTLV-1-infected CD8+ T cells) and TL-1 (Tax-2-established T-LGL leukemia cells); (b) Expression of S1Ps, granzyme B and Mcl-1 were shown in TL-1 cells, RNK16 (rat NK leukemia cells) and NKL (human NK-LGL leukemia cells); (c) Immunoblot analysis for phosphorylated Akt, Stat3, ERK1, MEK1 in CD8-1 and CD8-2 (normal CD8+ T cells from two healthy donors), TL-1 and TL-2 (Tax2-established T-LGL leukemia cells) and SP (HTLV-1-immortalized CD8+ T cells); (d) EMSA for detecting NF-κB activity in Jurkat, MT-2, SP and TL-1 cells; (e) EMSA for Stat3 activity in TL-1 cells and NT8 cells (normal CD8+ cells transiently expressing Tax2-GFP); (F) EMSA for AP-1 and NF-ATc activities in MT-2, Jurkat, SP and TL-1 cells; (g) Cell viability of TL-1 cells treated with U012 (ERK inhibitor), Ly494002 (PI3K inhibitor), Wortmanin (PI3K inhibitor) or BAY11-7082 (IKK inhibitor) at indicated dose and time points.
LGL leukemia cells are known to express sphingosine-1-phosphare receptors (SIPRs), which render these leukemia cells sensitive to the inhibition of these receptors [13, 17]. We examined several isoforms of S1P receptors including S1PR1, S1PR2, S1PR3, S1PR4 and S1PR5 for their expression in TL-1 cells. Similar to the NK type of LGL leukemia cell lines, RNK16 and NKL, TL-1 cells expressed all five forms of S1PRs (Fig. 4b). In addition, Mcl-1, a pro-survival Bcl-2 family member, along with the cytotoxic gene produce granzyme B, was abundantly expressed in TL-2 cells (Fig. 4b). In Tax-2-established T-LGL leukemia cell lines TL-1 and TL-2, phosphorylated forms of Akt, Stat3, ERK1/2 and MEK1 were detected, indicating that these kinases were expressed in activated forms (Fig. 4c). The DNA-binding activities of NF-κB, STAT3 and AP-1 were also detected in TL-1 cells (Fig. 4d, 4e and 4f). Because these oncogenic activations are typically seen in primary T-LGL leukemia cells, our data demonstrated that TL-1 cells shared similar oncogenic activation patterns to the clinical isolates. Indeed, chemical inhibitors for ERK1/2, PI3K/Akt and IKK/NF-κB effectively reduced viability of TL-1 cells (Fig. 4g), indicating that these signaling pathways were crucial for promoting survival and proliferation of the established T-LGL leukemia cells in vitro.
3.4. Sensitivity of the established T-LGLL cell line to FasL and sphingosine-1-phosphate receptor inhibitor
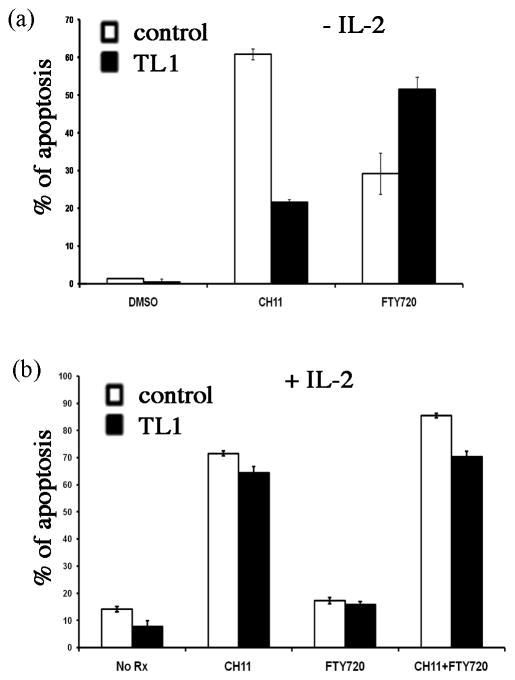
Primary T-LGL leukemia cells are resistant to FasL-mediated apoptosis and sensitive to sphingosine-1-phosphate receptor inhibition [18]. To test if TL-1 cells present features similar to clinical isolates, we treated TL-1 cells with CH11, a Fas stimulatory antibody that imitates FasL’s action, and FTY720, an inhibitor of S1PRs, either alone or in combination. The control cells were normal activated CD8+ T cells transiently expressing Tax2-GFP. In the absence of exogenous IL-2, TL-1 cells were resistant to CH11-induced apoptosis and sensitive to FTY720, as compared to the control cells that were treated at the same condition (Fig. 5a). Addition of exogenous IL-2 in culture restored the sensitivity of TL-1 cells to CH11 (Fig. 5b). Both TL-1 cells and the control cells were equally resistant to FTY720 in the presence of IL-2, and the combination of CH11 and FTY720 restored the sensitivity of these cells to FTY720 (Fig. 5b).
Fig. 5.
TL-1 cells are resistant to FasL-mediated apoptosis and sensitive to the inhibitor for sphingosine-1-phosphate receptor: (a) Apoptosis induction by CH11 (2μg/ml, FasL mimetics) or FTY720 (5μM, a.k.a. fingolimod, a structural analog of sphingosine) for 8h after IL-2 withdraw (16h), as measured by FACS analysis of annexin V-APC stained cells. NT8, normal CD8 T cells transiently expressing Tax-2-GFP, was used as control; (b) Apoptosis induction by CH11 (2μg/ml) or FTY720 (5μM) or the combination of CH11 and FTY720 in cells treated with IL-2 for 24h. The control cells are normal activated CD8 T cells transiently expressing Tax-2-GFP.
3.5. Constitutive activity of autophagy in the established T-LGLL cell line
Our previous study demonstrated that Tax-2 is able to activate autophagy pathway in supporting T cell survival [15]. We examined the autophagic activity in Tax-2-established T-LGL leukemia cells, and found a significant accumulation of LC3-II, a marker for autophagy, in TL-1 cells regardless of the presence of recombinant IL-2 (Fig. 6a). Suppression of autophagy pathway with the chemical autophagy inhibitor, 3-MA or chloroquine, effectively reduced viability of TL-1 cells (Fig. 6b), implying a critical role of Tax2-mediated autophagy for the survival and growth of Tax2-established T-LGL leukemia cells in culture.
Fig. 6.
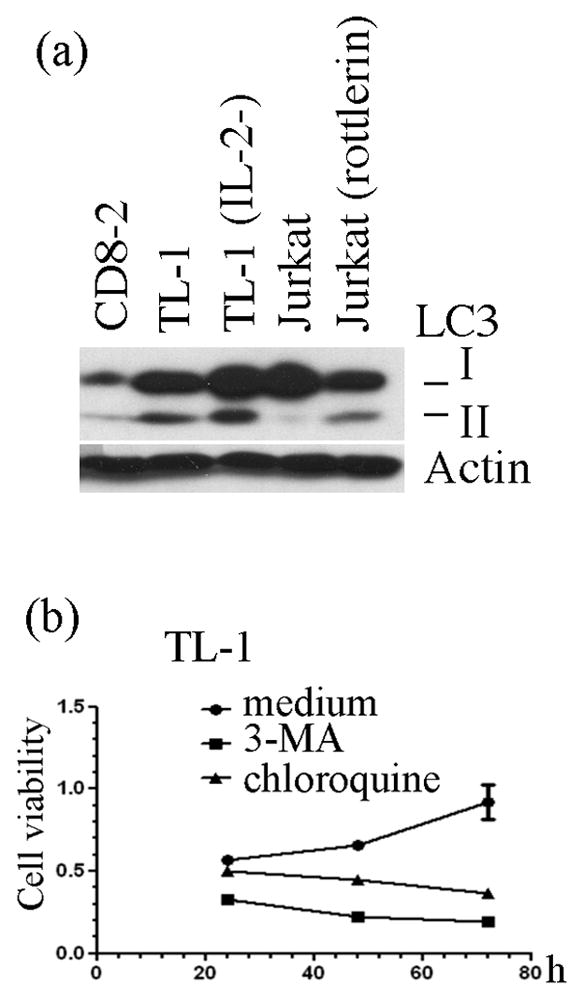
Constitutive activity of autophagy in TL-1 cells: (a) Conversion of LC3-I to LC3-II in CD8-2 cells (normal, PHA-activated CD8+ T cells from donor 2), TL-1 cells (cultured in the presence of IL-2), TL-1 (IL-2-) (TL-1 cells cultured without IL-2 for 24 hours), Jurkat cells without or with rottlerin (5μM for 6 hours) was examined with anti-LC3 immunoblot. β-actin is used for protein loading control; (b) Cell viability of TL-1 cells treated with autophagy inhibitors, 3-MA and chloroquine, at indicated time points.
4. Discussion
In the present study, we established T-LGL leukemia cell lines that exhibit characteristic features of primary T-LGL leukemia. The Tax2-established T-LGL cells are clonal population with mature CD8+ CTL phenotype, oncogenic activation and drug-sensitivity profiles that resemble clinical isolates. Thus far, only TL-1 cell line has exhibited optimal growth rate suitable for both in vitro and in vivo experiments to investigate the pathogenesis of T-LGL leukemia. Considering all the previous difficulties in establishing a T-LGL leukemia model, our study succeeded by introducing Tax2 into leukemic LGL cells.
Long-term culture of antigen-specific CTL lines has been reported by introducing human telomerase reverse transcriptase (hTERT) gene into these cells [19]. hTERT is a catalytic subunit of the enzyme telomerase that adds TTAGGG nucleotide repeat sequences, also known as telomeres, to the ends of chromosomal DNA [20]. However, another group reported that hTERT failed to immortalize primary CD8+ T cells [21], and our group was also unable to utilize hTERT to establish T-LGL leukemia cell line (data not shown). Like any other cancer cells, T-LGL leukemia cells exhibit constitutive activity of hTERT and therefore, other unknown factors likely cause replicative senescence in primary T-LGL leukemia cells. Primary T-LGL leukemia cells are activated CD8+ cells primarily due to their reactive capacity to a presumptive antigen in patients. However, these activation features may not be maintained because of lack of such antigen in culture, which would contribute to their replicative senescence. Therefore, the primary T-LGL leukemia cells need to be engineered in order to imitate antigen-stimulated proliferation.
The Tax protein appears to be a favorable choice. HTLV-2 preferentially infects CD8+ T cells and is capable of immortalizing primary CD8+ T cells from healthy donors. Our results indicated that Tax2 alone is not able to immortalize normal CD8+ T cells but selectively transforms leukemic CD8+ cells. There are several factors that may contribute to successful development of T-LGL leukemia model. First, Tax-2 induces expression of CD25, the IL-2Rα, which renders the Tax-2-expressing cells responsive to exogenous IL-2, providing an autocrine loop in stimulating T cell growth. Second, Tax2 constitutively activates IκB kinases, causing a hyperactivity of autophagy that promotes T cell survival. Lastly, Tax2 maintains the activated phenotypes of primary T-LGL leukemia cells, leading to their sustainable growth. It is noted that autophagy is induced in response to antigen stimulation in activated T cells [22, 23]. Primary T-LGL leukemia cells may exhibit transient activity of autophagy (data not shown), because they are likely antigen-simulated. Following prolonged culture, the autophagy activity is expected to disappear in primary T-LGL leukemia cells because of lack of such antigen in culture. Thus, Tax2 is capable of supplementing autophagy activity feature in the primary T-LGL leukemia cells, leading to their continuous growth in vitro even in the absence of antigen. In addition, Tax2 activates a variety of oncogenic signaling molecules including Stat3, PI3K/Akt, IKK/NF-κB and MAPK/ERK that overlap with the oncogenic activation present in primary T-LGL leukemia cells. The activated features of T-LGL cells, therefore, can be maintained in culture for prolonged time.
It should be noted that there are several drawbacks of this leukemia model. First, the genotypes of the established T-LGL cell lines were apparently distinct from their corresponding patient samples. The mechanism of the genotype shift of these leukemia cell lines is currently not clear. The genotype shift could be caused by prolonged in vitro culture [24]. Another possibility is that there might exist minor populations of the leukemia cells that acquire growth advantage in this culture model. Second, the immunophenotype of CD45RA+/CD45RO− is typically present in primary specimens [7–9], however the established leukemia cell lines exhibit CD45RA−/CD45RO+ (data not shown). This phenotypic change might be caused by long-term culture or expression of Tax2. This possibility needs further exploration. Lastly, although the purified CD8 T cells from patients displayed a CD8+/CD4− phenotype, the established T-LGL leukemia cell lines TL-1 and TL-2 showed expression of CD4 in addition to the abundant levels of CD8. Our data indicate that unlike immature double-positive T cells, the established T-LGL leukemia cell lines are mature CD8+ T cells with unique immunophenotype and cytokine expression patterns. Indeed, expression of CD4 on mature CD8+ T cells has been previously reported, which could be seen following prolonged activation of CD8 T cells [25–31]. Likewise, our established leukemia cell lines represent terminally differentiated, constitutively activated memory CD8+ T cells.
It would be certainly interesting to see if such T-LGL leukemia-like model cell line could be used to establish an in vivo mouse model as well. An additional advantage of the Tax2-GFP-established T-LGL leukemia model is that these cells continuously emit green fluorescence, which would differentiate transplanted cells from endogenous cells in trying to establish an in vivo mouse model. This LGL leukemia model could be further modified by inducible expression of Tax2-GFP in primary T-LGL leukemia cells. Collectively, the establishment of T-LGL leukemia model is important for further investigation into disease pathogenesis and development of new therapeutics.
Acknowledgments
We thank Dr. Susan Nyland, Ranran Zhang and Aijun Liao at Penn State Hershey Cancer Institute for technical assistance. This work is supported by a fund from Penn State Hershey Cancer Institute and in part by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under award number RO1AI090113 to Hua Cheng, and the National Cancer Institute under award number RO1 CA94872 to Dr. Thomas Loughran. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Contributions
Tong Ren designed and performed experiments as well as wrote the manuscript, Jun Yang and Katie Broeg participated in the experiment of genotyping, Xin Liu and Thomas Loughran participated in data analysis, and Hua Cheng designed and analyzed data.
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sokol L, Loughran TP., Jr Large granular lymphocyte leukemia. Oncologist. 2006;11:263–73. doi: 10.1634/theoncologist.11-3-263. [DOI] [PubMed] [Google Scholar]
- 2.Rose MG, Berliner N. T-cell large granular lymphocyte leukemia and related disorders. Oncologist. 2004;9:247–58. doi: 10.1634/theoncologist.9-3-247. [DOI] [PubMed] [Google Scholar]
- 3.Shah A, Diehl LF, St Clair EW. T cell large granular lymphocyte leukemia associated with rheumatoid arthritis and neutropenia. Clin Immunol. 2009;132:145–52. doi: 10.1016/j.clim.2009.03.515. [DOI] [PubMed] [Google Scholar]
- 4.Bleesing JJ, Janik JE, Fleisher TA. Common expression of an unusual CD45 isoform on T cells from patients with large granular lymphocyte leukaemia and autoimmune lymphoproliferative syndrome. Br J Haematol. 2003;120:93–6. doi: 10.1046/j.1365-2141.2003.04034.x. [DOI] [PubMed] [Google Scholar]
- 5.Loughran TP, Jr, Kadin ME, Starkebaum G, Abkowitz JL, Clark EA, Disteche C, et al. Leukemia of large granular lymphocytes: association with clonal chromosomal abnormalities and autoimmune neutropenia, thrombocytopenia, and hemolytic anemia. Ann Intern Med. 1985;102:169–75. doi: 10.7326/0003-4819-102-2-169. [DOI] [PubMed] [Google Scholar]
- 6.Lamy T, Loughran TP., Jr How I treat LGL leukemia. Blood. 2011;117:2764–74. doi: 10.1182/blood-2010-07-296962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundell R, Hartung L, Hill S, Perkins SL, Bahler DW. T-cell large granular lymphocyte leukemias have multiple phenotypic abnormalities involving pan-T-cell antigens and receptors for MHC molecules. Am J Clin Pathol. 2005;124:937–46. [PubMed] [Google Scholar]
- 8.O’Malley DP. T-cell large granular leukemia and related proliferations. Am J Clin Pathol. 2007;127:850–9. doi: 10.1309/A8FHDA0VVRJ05GJP. [DOI] [PubMed] [Google Scholar]
- 9.Bigouret V, Hoffmann T, Arlettaz L, Villard J, Colonna M, Ticheli A, et al. Monoclonal T-cell expansions in asymptomatic individuals and in patients with large granular leukemia consist of cytotoxic effector T cells expressing the activating CD94:NKG2C/E and NKD2D killer cell receptors. Blood. 2003;101:3198–204. doi: 10.1182/blood-2002-08-2408. [DOI] [PubMed] [Google Scholar]
- 10.Langerak AW, van Den Beemd R, Wolvers-Tettero IL, Boor PP, van Lochem EG, Hooijkaas H, et al. Molecular and flow cytometric analysis of the Vbeta repertoire for clonality assessment in mature TCRalphabeta T-cell proliferations. Blood. 2001;98:165–73. doi: 10.1182/blood.v98.1.165. [DOI] [PubMed] [Google Scholar]
- 11.Lima M, Almeida J, Santos AH, dos Anjos Teixeira M, Alguero MC, Queiros ML, et al. Immunophenotypic analysis of the TCR-Vbeta repertoire in 98 persistent expansions of CD3(+)/TCR-alphabeta(+) large granular lymphocytes: utility in assessing clonality and insights into the pathogenesis of the disease. Am J Pathol. 2001;159:1861–8. doi: 10.1016/s0002-9440(10)63032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epling-Burnette PK, Liu JH, Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest. 2001;107:351–62. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang R, Shah MV, Yang J, Nyland SB, Liu X, Yun JK, et al. Network model of survival signaling in large granular lymphocyte leukemia. Proc Natl Acad Sci U S A. 2008;105:16308–13. doi: 10.1073/pnas.0806447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Mizutani N, Ito H, Hagiwara K, Kobayashi M, Hoshikawa A, Nishida Y, Takagi A, Kojima T, Suzuki M, Osawa Y, Ohnishi K, Daibata M, Murate T. Involvement of KRAS G12A mutation in the IL-2-independent growth of a human T-LGL leukemia cell line, PLT-2. Nagoya J Med Sci. 2012;74(3–4):261–71. [PMC free article] [PubMed] [Google Scholar]
- 13b.Daibata M, Matsuo Y, Machida H, Taguchi T, Ohtsuki Y, Taguchi H. Differential gene-expression profiling in the leukemia cell lines derived from indolent and aggressive phases of CD56+ T-cell large granular lymphocyte leukemia. Int J Cancer. 2004;108(6):845–51. doi: 10.1002/ijc.11647. [DOI] [PubMed] [Google Scholar]
- 14.Huang J, Ren T, Guan H, Jiang Y, Cheng H. HTLV-1 Tax is a critical lipid raft modulator that hijacks IkappaB kinases to the microdomains for persistent activation of NF-kappaB. J Biol Chem. 2009;284:6208–17. doi: 10.1074/jbc.M806390200. [DOI] [PubMed] [Google Scholar]
- 15.Ren T, Dong W, Takahashi Y, Xiang D, Yuan Y, Liu X, et al. HTLV-2 Tax immortalizes human CD4+ memory T lymphocytes by oncogenic activation and dysregulation of autophagy. J Biol Chem. 2012;287:34683–93. doi: 10.1074/jbc.M112.377143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochsenreither S, Fusi A, Busse A, Nagorsen D, Schrama D, Becker J, et al. Relative quantification of TCR Vbeta-chain families by real time PCR for identification of clonal T-cell populations. J Transl Med. 2008;6:34. doi: 10.1186/1479-5876-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah MV, Zhang R, Irby R, Kothapalli R, Liu X, Arrington T, et al. Molecular profiling of LGL leukemia reveals role of sphingolipid signaling in survival of cytotoxic lymphocytes. Blood. 2008;112:770–81. doi: 10.1182/blood-2007-11-121871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu JH, Wei S, Lamy T, Li Y, Epling-Burnette PK, Djeu JY, et al. Blockade of Fas-dependent apoptosis by soluble Fas in LGL leukemia. Blood. 2002;100:1449–53. [PubMed] [Google Scholar]
- 19.Hooijberg E, Ruizendaal JJ, Snijders PJ, Kueter EW, Walboomers JM, Spits H. Immortalization of human CD8+ T cell clones by ectopic expression of telomerase reverse transcriptase. J Immunol. 2000;165:4239–45. doi: 10.4049/jimmunol.165.8.4239. [DOI] [PubMed] [Google Scholar]
- 20.Weinrich SL, Pruzan R, Ma L, Ouellette M, Tesmer VM, Holt SE, et al. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat Genet. 1997;17:498–502. doi: 10.1038/ng1297-498. [DOI] [PubMed] [Google Scholar]
- 21.Migliaccio M, Amacker M, Just T, Reichenbach P, Valmori D, Cerottini JC, et al. Ectopic human telomerase catalytic subunit expression maintains telomere length but is not sufficient for CD8+ T lymphocyte immortalization. J Immunol. 2000;165:4978–84. doi: 10.4049/jimmunol.165.9.4978. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Wang LX, Yang G, Hao F, Urba WJ, Hu HM. Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res. 2008;68:6889–95. doi: 10.1158/0008-5472.CAN-08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clemente MJ, Wlodarski MW, Makishima H, Viny AD, Bretschneider I, Shaik M, et al. Clonal drift demonstrates unexpected dynamics of the T-cell repertoire in T-large granular lymphocyte leukemia. Blood. 2011;118:4384–93. doi: 10.1182/blood-2011-02-338517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan YB, Landay AL, Zack JA, Kitchen SG, Al-Harthi L. Upregulation of CD4 on CD8+ T cells: CD4dimCD8bright T cells constitute an activated phenotype of CD8+ T cells. Immunology. 2001;103:270–80. doi: 10.1046/j.1365-2567.2001.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zloza A, Sullivan YB, Connick E, Landay AL, Al-Harthi L. CD8+ T cells that express CD4 on their surface (CD4dimCD8bright T cells) recognize an antigen-specific target, are detected in vivo, and can be productively infected by T-tropic HIV. Blood. 2003;102:2156–64. doi: 10.1182/blood-2002-07-1972. [DOI] [PubMed] [Google Scholar]
- 27.Zloza A, Al-Harthi L. Multiple populations of T lymphocytes are distinguished by the level of CD4 and CD8 coexpression and require individual consideration. J Leukoc Biol. 2006;79:4–6. doi: 10.1189/jlb.0805455. [DOI] [PubMed] [Google Scholar]
- 28.Kitchen SG, Korin YD, Roth MD, Landay A, Zack JA. Costimulation of naive CD8(+) lymphocytes induces CD4 expression and allows human immunodeficiency virus type 1 infection. J Virol. 1998;72:9054–60. doi: 10.1128/jvi.72.11.9054-9060.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitchen SG, LaForge S, Patel VP, Kitchen CM, Miceli MC, Zack JA. Activation of CD8 T cells induces expression of CD4, which functions as a chemotactic receptor. Blood. 2002;99:207–12. doi: 10.1182/blood.v99.1.207. [DOI] [PubMed] [Google Scholar]
- 30.Kitchen SG, Jones NR, LaForge S, Whitmire JK, Vu BA, Galic Z, et al. CD4 on CD8(+) T cells directly enhances effector function and is a target for HIV infection. Proc Natl Acad Sci U S A. 2004;101:8727–32. doi: 10.1073/pnas.0401500101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitchen SG, Whitmire JK, Jones NR, Galic Z, Kitchen CM, Ahmed R, et al. The CD4 molecule on CD8+ T lymphocytes directly enhances the immune response to viral and cellular antigens. Proc Natl Acad Sci U S A. 2005;102:3794–9. doi: 10.1073/pnas.0406603102. [DOI] [PMC free article] [PubMed] [Google Scholar]