Pandolfi et al. provide an in-depth discussion on the synergism between all-trans-retinoic acid and arsenic trioxide treatment and their mechanisms of action on acute promyelocytic leukemia.
Abstract
Acute promyelocytic leukemia (APL) is a hematological malignancy driven by a chimeric oncoprotein containing the C terminus of the retinoic acid receptor-a (RARa) fused to an N-terminal partner, most commonly promyelocytic leukemia protein (PML). Mechanistically, PML-RARa acts as a transcriptional repressor of RARa and non-RARa target genes and antagonizes the formation and function of PML nuclear bodies that regulate numerous signaling pathways. The empirical discoveries that PML-RARa–associated APL is sensitive to both all-trans-retinoic acid (ATRA) and arsenic trioxide (ATO), and the subsequent understanding of the mechanisms of action of these drugs, have led to efforts to understand the contribution of molecular events to APL cell differentiation, leukemia-initiating cell (LIC) clearance, and disease eradication in vitro and in vivo. Critically, the mechanistic insights gleaned from these studies have resulted not only in a better understanding of APL itself, but also carry valuable lessons for other malignancies.
Acute promyelocytic leukemia (APL) was first described as an independent condition in 1957 (Hillestad, 1957) and presents clinically as a replacement of normal hematopoiesis in the bone marrow by an accumulation of promyelocytes, which are precursors of granulocytes. It is an aggressive disease associated with a high frequency of bleeding and thrombosis, and is rapidly fatal if not properly treated and managed. (Choudhry and DeLoughery, 2012; Kwaan and Huyck, 2010). The vast majority of APL cases are characterized by the fusion of the N-terminus of the promyelocytic leukemia protein (PML) to the C terminus of the retinoic acid receptor-a (RARa) transcription factor (Borrow et al., 1990; Chomienne et al., 1990; de Thé et al., 1990, 1991; Longo et al., 1990; Alcalay et al., 1991; Kakizuka et al., 1991; Pandolfi et al., 1991) as a result of a balanced chromosomal translocation, t(15;17)(q22;q12; Rowley et al., 1977). In variant forms of APL, RARa is fused to one of its alternative N-terminal partners including promyelocytic leukemia zinc finger (PLZF), nucleophosmin 1 (NPM1), nuclear mitotic apparatus (NUMA), signal transducer and activator of transcription 5B (STAT5b), protein kinase, cAMP-dependent, regulatory, type I, α (PRKAR1A), FIP1-like 1 (FIP1L1), BCL6 co-repressor (BCOR) and oligonucleotide/oligosaccharide-binding fold containing 2A (OBFC2A; Zelent et al., 2001; Redner, 2002; Catalano et al., 2007; Kondo et al., 2008; Yamamoto et al., 2010; Won et al., 2013). Although rare, these variant forms of APL have been very informative in elucidating the molecular mechanisms underlying the biological functions of the resulting fusion oncoproteins, especially PLZF-RARa, the most common and studied variant. This review is focused primarily on PML-RARa APL, and unless clearly stated, does not apply to the other translocations.
The molecular mechanism of transformation in APL has been extensively reviewed in numerous recent publications (Wang and Chen, 2008; de Thé and Chen, 2010; de Thé et al., 2012). In brief, RARa is a nuclear receptor that in the absence of its ligand retinoic acid (RA) represses the transcription of target genes by recruiting co-repressors and histone deacetylases. Physiological levels of RA convert RARa from a transcriptional repressor into a potent activator, driving expression of genes involved in myeloid differentiation. In contrast, PML-RARa remains an ineffectual transcriptional activator even in the presence of physiological RA levels. Additionally, by forming heterodimers with PML, PML-RARa antagonizes the formation of nuclear bodies (NBs), which are macromolecular structures that regulate the P53 pathway among many other activities. Thus, after a classical model of leukemogenesis, PML-RARa is thought to simultaneously contribute two oncogenic hits in one: the block of differentiation and the aberrant self-renewal of APL cells.
A variety of pharmacological interventions have proven effective against the disease. In particular, APL cells have been shown to be exquisitely sensitive to all-trans-RA (ATRA) and arsenic trioxide (ATO), which together with advances in chemotherapy (CT) and improvements in transfusion support therapy, have transformed the therapeutic landscape and converted a highly fatal disease into a highly curable one (Wang and Chen, 2008). Although widely regarded as “targeted” therapies because of their selective effects on the PML-RARa fusion protein, both ATRA and ATO are naturally derived compounds. Their clinical application preceded the detailed scientific understanding of their mechanisms of action and drove subsequent efforts to determine the molecular and biological processes underlying their tremendous efficacy. In this review, we will critically summarize current knowledge in the field of APL therapeutics, with particular emphasis on two interconnected questions that remain vigorously debated: (1) what is the contribution of reversing the block of differentiation of APL blasts vis-a-vis the elimination of the leukemia-initiating cell (LIC) pool on the effective treatment of the disease, and (2) is the reversal of transcriptional repression or the proteolytic degradation of PML-RARa more important?
THE ATRA REVOLUTION
ATRA induces remission without cure
ATRA was first shown to be capable of inducing differentiation in the cell line HL-60 and in primary APL specimens in vitro in 1981 (Breitman et al., 1981). The first description of ATRA as an APL therapy was subsequently published by a group from the Shanghai Institute of Hematology in 1988 (Huang et al., 1988). The study documented the use of ATRA as a single agent in induction therapy for 24 patients, 16 of whom were receiving their first treatment, along with 8 others who had previously undergone CT. Remarkably, all 24 patients achieved complete hematological remission (CHR) without developing bone marrow hypoplasia, with gains in coagulation parameters and a reduction in early mortality, a startling improvement for the first phase of therapy. Subsequently, other groups confirmed these results (Castaigne et al., 1990; Warrell et al., 1991), and demonstrated that granulocytic differentiation was the major driver of CHR in vivo (Warrell et al., 1991). In fact, the effect of ATRA on the morphology of APL cells was so striking that it subsequently became known as “differentiation therapy.”
Despite early promise however, it quickly became apparent that ATRA alone was not going to provide a cure for APL. Patients that achieved CHR after ATRA monotherapy almost inevitably relapsed, some of them within only a few months of remission (the only exception to date consists of a small number of patients cured by liposomal ATRA monotherapy; Tsimberidou et al., 2006). This outcome offered a challenge to the understanding of leukemia treatment at the time, because it totally uncoupled initial response from long-term outcome. The clue to understanding the inability of ATRA to eliminate APL came from the observation that patients in CHR after ATRA monotherapy retained a small number of APL cells, as indicated by the continued presence of PML-RARa mRNA (Warrell et al., 1991). This in turn led to the development of the concepts of minimal residual disease (MRD) and complete molecular remission (CMR) in APL and other malignancies. Indeed, currently, the elimination of MRD and the achievement of CMR (e.g., inability to detect the disease by a highly sensitive method such as PCR) is the goal of not only APL therapy (Grimwade et al., 2010), but treatment for acute lymphoblastic leukemia (Campana, 2009) and chronic myeloid leukemia (CML; O’Hare et al., 2012) as well.
Elimination of MRD and differentiation in APL therapy
That MRD reflects the persistence of LICs and predicts disease relapse is a concept that is now widely accepted. Though the cell surface markers, frequency, and pluripotent potential of LICs from different leukemias vary, they share several characteristics including high self-renewal capability, replicative quiescence and intrinsic resistance to differentiation and cell death (Magee et al., 2012). In patients, ATRA therapy induces differentiation of APL blasts into functional granulocytes, but as stated above, in the vast majority of cases does not produce a long-term cure. However, subsequent studies have reported great synergy between ATRA, anthracyclines and/or ATO (discussed in detail below), with various combinations capable of achieving CMR and consequently long-term remissions in >90% of patients (Mandelli et al., 1997; Sanz et al., 1999, 2004; Wang and Chen, 2008). Although ATO induces partial differentiation in vivo (Chen et al., 1997; Camacho et al., 2000), in comparison with ATRA, ATO and anthracyclines are poor cellular differentiation agents. Collectively, these data suggest that APL blast differentiation on its own is insufficient to cure APL.
The presence of LICs in APL and their resistance to ATRA monotherapy has also been confirmed in faithful mouse models using transplantation experiments that functionally dissect LICs as cells capable of initiating disease when transferred to a syngeneic recipient (Brown et al., 1997; Grisolano et al., 1997; He et al., 1997, 1998). In an ex vivo study, Zheng et al. demonstrated that unlike ATO, ATRA does not abolish the self-renewal or engraftment potential of APL LICs (Zheng et al., 2007). Similarly, in an elegant in vivo study, Nasr et al. (2008) showed that low doses of ATRA that efficiently induce terminal differentiation are unable to target LICs or clear the disease. Intriguingly, high doses of ATRA reduced LIC frequency, but did not eradicate LICs, whereas the combination of ATRA and cAMP enhanced the effect on LICs (Guillemin et al., 2002; Nasr et al., 2008). Collectively, these observations underscore the significance of LIC eradication in curing APL.
Despite the inability of ATRA to cure APL as a monotherapy, it is important to note that it remains a mainstay of APL treatment precisely because of its pro-differentiation effects (albeit in combination with CT or ATO with which it can synergize to eliminate LICs and MRD; Fig. 1). In fact, a recent recommendation from an expert European panel states that ATRA should be administered in cases where APL is suspected, even if definitive diagnosis based on karyotyping or other molecular methods is not immediately available (Sanz et al., 2009). The leading cause of early death in APL is bleeding caused by a coagulopathy driven by procoagulant and fibrinolytic factors (e.g., Annexin A2 and tissue factor) present in APL blasts and blast-derived microparticles (Koyama et al., 1994; Menell et al., 1999). Proapoptotic agents such as ATO and CT can promote the release of these factors, thereby accentuating the problem (Sanz and Montesinos, 2010). ATRA-driven differentiation, on the other hand, minimizes the release of these factors by converting APL blasts into mature granulocytes. Indeed, Wang and Chen (2008) compared ATRA induced differentiation with the ancient Chinese Philosophy on the control of society, exemplified by the famous Confucius’ saying: “If you use laws to direct the people, and punishments to control them, they will merely try to evade the laws, and will have no sense of shame. But if by virtue you guide them, and by the rites you control them, there will be a sense of shame and of right.”
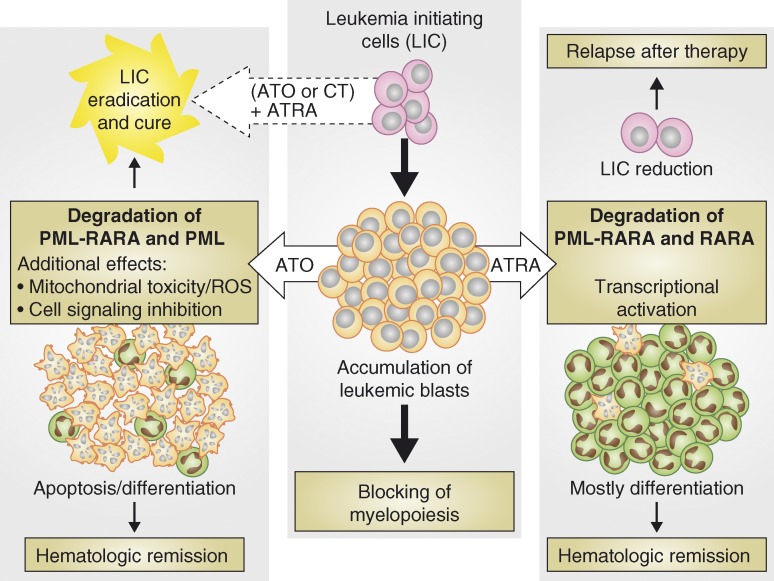
Figure 1.
Elimination of leukemic blasts and LICs is necessary for definitive cure of APL. LICs posses high self-renewal capability and give rise to leukemic blasts that form the bulk of the disease (middle). Both ATRA and ATO promote remission of disease by targeting leukemic blasts. Pharmacological doses of ATRA predominantly drive differentiation of leukemic blasts, but are unable to efficiently eliminate LICs that can drive disease relapse (right). In contrast, ATO induces both apoptosis and differentiation of leukemic blasts and can also target LICs and is therefore more effective that ATRA as a monotherapy (left). ATRA acts synergistically with ATO and/or CT, and these combination therapies definitively cure APL in the vast majority of cases (dashed arrow).
Uncoupling PML-RARa transactivation and degradation
The molecular mode of action of ATRA remains a subject of intense research. At pharmacological doses, ATRA converts PML-RARa from a transcriptional repressor to a transcriptional activator and induces its proteolysis (Wang and Chen, 2008). Under most conditions these two processes are tightly coupled, a mechanism that applies not only to PML-RARa and RA receptors, but also to other nuclear receptors including estrogen receptor, thyroid hormone receptor and peroxisome proliferator-activated receptors (Nawaz et al., 1999; Zhu et al., 1999; Hauser et al., 2000; Kopf et al., 2000; Tanaka et al., 2001). Indeed linkage of ligand-dependent activation and subsequent degradation is a general feature of nuclear receptor signaling, providing a negative feedback loop that ensures that signaling is shut off once the ligand is no longer present. In the case of PML-RARa, spontaneous mutations that render APL cells resistant to ATRA in patients or in vitro almost invariably occur in the ligand-binding domain (LBD; Raelson et al., 1996; Rosenauer et al., 1996; Marasca et al., 1999; Côté et al., 2000). Additionally, targeted mutational analysis has revealed that alterations to either the ligand-binding domain or the AF2 domain of PML-RARa that abolish the transactivation of PML-RARa by ATRA, also abolish receptor catabolism (Zhu et al., 1999). Interestingly, ATRA resistance mediated by LBD mutations can be at least partially reversed by genetic or pharmacological activation of the lysine demethylase PHF8, which serves as an essential co-activator of PML-RARa transcription after pharmacological administration of ATRA (Arteaga et al., 2013).
Several groups have devoted considerable effort to uncoupling transactivation and proteolysis of PML-RARa on the one hand, and APL blast differentiation and disease clearance in vivo on the other. Low doses of ATRA that efficiently induce terminal differentiation are unable to target LICs or clear the disease in mouse models of APL (Nasr et al., 2008). Additionally, PML-RARa Ser873, a residue that is phosphorylated by protein kinase A (PKA) and was shown to be important for ATRA-mediated PML-RARa degradation but not transactivation, is essential for ATRA-mediated clearance of APL (Nasr et al., 2008), (note however, that the equivalent residue in RARa, Ser369, has been implicated in transcriptional activation of wild-type RARa (Gaillard et al., 2006)). Finally, in a recent issue of The Journal of Experimental Medicine, Ablain et al. (2013) showed that the synthetic retinoids Etretinate and NRX195183, compounds that drive transcription of PML-RARa–dependent genes at levels comparable to ATRA, but do not cause the oncoprotein to be degraded, induce granulocytic differentiation at levels comparable to ATRA, but are far less effective at clearing the disease in vivo. Viewed along with clinical observations and experimental data discussed earlier, these results suggest that: (1) transcriptional transactivation of PML-RARa is the main driver of differentiation; (2) differentiation alone is not sufficient to induce disease clearance; and (3) the clinical efficacy of ATRA is a result of its ability to link transactivation of PML-RARa and its subsequent proteolysis.
CURATIVE MONOTHERAPY
The first definitive evidence of ATOs efficacy in APL again came from a well-controlled study conducted in Shanghai, in which ATO remarkably induced CR in 9 out of 10 relapsed patients, most of whom had already received ATRA therapy (Shen et al., 1997). The only patient that did not achieve CR presented with a loss of PML-RARa during the course of initial treatment. In contrast to ATRA, ATO monotherapy produced long-term cures, even when used in the setting of relapsed disease (Shen et al., 1997; Soignet et al., 1998). Patients treated with ATO as a single agent in frontline therapy presented a 3-yr disease-free survival (DFS) rate of 87% (Mathews et al., 2006) and a 5-yr DFS rate of 80% (Mathews et al., 2010). When this analysis was restricted to the major group of patients considered to have a lower risk of relapse, the DFS at 3 and 5 yr was 100% (Mathews et al., 2006, 2010). A recent phase II study similarly reported a CR rate of 85% and a 5-yr DFS of 66.7% for ATO as a single agent, though a relatively high number of deaths caused by differentiation syndrome (13.2%) were observed (Ghavamzadeh et al., 2011).
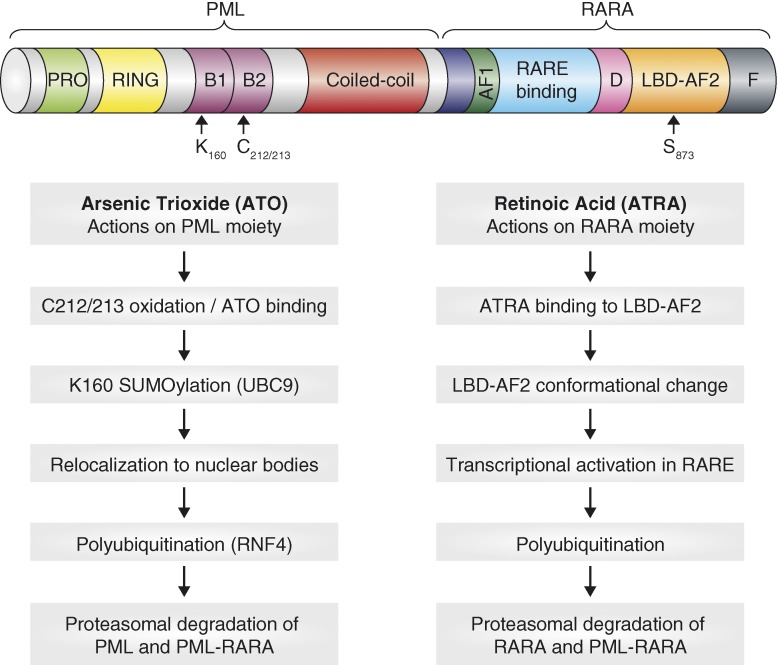
On the basis of these striking data, the mechanism of action of ATO was put under intense scrutiny. ATO was shown to degrade PML-RARa via its PML moiety further reinforcing the idea that APL is addicted to the PML-RARa oncoprotein (Lallemand-Breitenbach et al., 2012). We now know that ATO binds directly to PML and PML-RARa via two cysteines (C212/213) in the B2 domain and induces oxidation of these residues and the formation of intermolecular disulfide bonds (Jeanne et al., 2010; Zhang et al., 2010). This is followed by sumoylation of a lysine residue (K160) and the subsequent reorganization of PML/PML-RARa from a diffuse/microspeckled nuclear pool into matrix-associated macromolecular structures termed PML-nuclear bodies (NB; Jeanne et al., 2010). It is within these structures that PML/PML-RARa is polyubiquitinated by the Sumo-dependent E3 ubiquitin ligase RNF4 and subsequently degraded by the proteasome (Lallemand-Breitenbach et al., 2008; Tatham et al., 2008; Fig. 2). The few characterized cases of ATO resistance displayed missense mutations in the B2 domain of PML-RARa, rendering the oncoprotein resistant to sumoylation and degradation (Goto et al., 2011) and confirming the importance of this domain for the efficacy of ATO in APL.
Figure 2.
Mechanisms of proteolysis of PML-RARa by ATRA and ATO. ATRA induces degradation of RARa and PML-RARa via the RARa moiety. ATO induces degradation of PML and PML-RARa via the PML moiety.
It should be noted that ATO can also induce degradation of PML/PML-RARa not only through direct binding, but also indirectly through its effects on the mitochondria and generation of ROS. ROS promotes oxidation of PML and its assembly into NBs, and numerous compounds that induce ROS production have shown activity against APL. Alpha tocopheryl succinate (α-TOS) induces apoptosis of APL cells and clearance of disease in a murine APL model by directly targeting the mitochondrial respiratory chain, leading to accumulation of ROS and at least partial degradation of PML-RARa (dos Santos et al., 2012). Likewise, paraquat, a dangerous poison that produces massive amounts of ROS (Morán et al., 2010), is highly effective at degrading PML-RARa and clearing APL in the mouse (Jeanne et al., 2010). Notably, anthracyclines generate ROS, which may partially explain their increased efficacy in APL in comparison with other types of AML.
ATO VERSUS ATRA
The evidence from mice and men firmly demonstrates that whereas both ATO and ATRA target the PML-RARa oncoprotein for proteolysis, only ATO is superior at achieving a complete cure, as a monotherapy, by eliminating residual LICs. One possible explanation of these findings is that ATO is simply more effective at degrading PML-RARa than ATRA. It should be noted that ATRA, which is typically administered orally and is rapidly metabolized, may not reach optimal concentration in patient serum to ensure killing of all APL cells. Indeed when administered intravenously via injection of a liposomal-encapsulated preparation, and thereby bypassing initial hepatic metabolism, ATRA may be more effective and sufficient to cure some APL patients. However, another intriguing possibility is that, in addition to degrading PML-RARa, ATO also targets other pathways that are essential for LIC maintenance and self-renewal. In APL cells, ATRA degrades PML-RARa, restoring the function of the remaining wild-type PML allele, whereas ATO targets both PML-RARa and PML for degradation. Recent evidence has shown that PML regulates hematopoietic stem cell maintenance via mTOR and peroxisome proliferator-activated receptor-d (PPAR-d)–fatty-acid oxidation (FAO) pathways (Ito et al., 2008, 2012). Likewise, PML regulates maintenance of LICs transformed by the presence of the CML oncogene BCR-ABL, which rapidly exhaust upon loss of PML (Ito et al., 2008). Ablation of PML in APL LICs treated with ATO may have similar effects, driving them from their quiescent state and promoting loss of self-renewal capabilities and clearance.
Other molecular pathways triggered by ATO likely synergize with its effects on PML-RARa and PML. At clinically relevant concentrations, ATO can promote apoptosis in APL cells by several mechanisms. Oxidative stress triggered by ATO can reverse the inhibitory effect of glutathione transferase P1-1 (GSTP1-1) on the c-Jun N-terminal kinase (JNK) proapoptotic signaling cascade (Bernardini et al., 2006). ATO treatment can also induce expression of caspase-10 through its effects on chromatin at the Caspase-10 locus (Li et al., 2002). Likewise, treatment of the APL cell line NB4 with ATO has been reported to repress expression of hTERT, MYC, and C17 through oxidation of the transcription factor Sp1 (Chou et al., 2005). However, to date, these studies have been performed in vitro, and it remains to be determined which of these mechanisms contribute to LIC elimination and which function predominantly in removing the bulk APL blast population during ATO treatment.
ATO/ATRA COMBINATION THERAPY
While the discovery of the efficacy of ATRA and ATO in APL was originally based on clinical studies, preclinical studies in mouse models of APL proved that ATRA and ATO treatments are synergistic and highly curative. Notably, in vivo mouse modeling experiments proved decisive in reversing the erroneous notion that ATRA and ATO would oppose each other, an idea based on misleading in vitro experiments using primary APL cells and cell lines (Shao et al., 1998; Lallemand-Breitenbach et al., 1999; Jing et al., 2001; Rego et al., 2000). The superior effectiveness of combining ATRA and ATO was initially demonstrated by two studies using transgenic mouse models of PML-RARa APL (Lallemand-Breitenbach et al., 1999; Rego et al., 2000). PLZF-RARa associated APL in the mouse did not respond to this combination (see below; Rego et al., 2000). Critically, these studies emphasized the relevance of ATO and ATRA converging on the degradation of PML-RARa via targeting the PML and RARa moieties, respectively. Later clinical reports began to point to the synergistic interaction of this combination (Kennedy et al., 2000; Au et al., 2002; Visani et al., 2003) and its benefit was subsequently demonstrated in a randomized study in both short- (Shen et al., 2004) and long-term analyses (Hu et al., 2009). Most recently, ATRA plus ATO was shown to be at least as efficacious as the standard protocol of ATRA plus idarubicin in non–high risk patients (2 yr DFS of 97% versus 86.7% for ATRA plus ATO and ATRA plus idarubicin, respectively; Lo-Coco et al., 2013). That ATO and ATRA synergize against APL and are among the most successful leukemia treatments are now widely accepted as facts (Sanz et al., 2009; Tallman and Altman, 2009; Sanz and Lo-Coco, 2011; Mi et al., 2012).
ONCOPROTEIN DEGRADATION MAY NOT SUFFICE
Although APL associated with t(11;17)(q23;q21) chromosomal translocation is a rare subtype, the study of its associated fusion protein PLZF-RARa and its comparison with PML-RARa, have offered important insights into the mechanisms underlying the pathogenesis of APL (He et al., 1998). APL patients harboring the t(11;17)(q23;q21) chromosomal translocation have a significantly worse prognosis than patients with t(15:17) APL and show little or no response to either ATO or ATRA (Licht et al., 1995; Koken et al., 1999). Not surprisingly ATO is unable to induce the proteolysis of PLZF-RARa in vitro or in vivo (Koken et al., 1999; Rego et al., 2000). In contrast, pharmacological doses of ATRA do produce efficient degradation of PLZF-RARa and are sufficient to induce phenotypic differentiation of APL blasts, albeit less effectively than in PML-RARa leukemic cells (Koken et al., 1999; Rego et al., 2000; Nasr et al., 2008). However, unlike in t(15;17) APL, ATRA treatment does not restore normal hematopoiesis or induce temporary remission in either humans or mice with PLZF-RARa APL (Licht et al., 1995; Rego et al., 2000; Nasr et al., 2008). Indeed, PLZF-RARa–transformed cells retain the ability to grow, even in the absence of detectable levels of PLZF-RARa protein (Rego et al., 2000), demonstrating that oncogene degradation may not be an effective strategy in all malignancies (Ablain et al., 2011). Notably, ATRA in combination with histone deacetylase inhibitors has shown efficacy in cell lines, transgenic mouse models, and some patients (Warrell et al., 1998; He et al., 2001), suggesting that PLZF-RARa establishes an aberrant chromatin state that is maintained independently of the continued presence of the fusion protein. This, in turn, implies that (in the case of PLZF-RARa) the sole degradation of the oncoprotein does not suffice, perhaps due to its long-lasting effects on chromatin remodeling toward transcriptional repression, and that these effects have to be reversed for complete remission and disease eradication to occur. Nevertheless, experiments using transgenic models where the PLZF-RARa oncoprotein can be genetically ablated are needed to fully understand the mechanisms underlying the apparent lack of oncogene-addiction in PLZF-RARa APL. Furthermore, in human t(11;17)(q23;q21) APL, the reciprocal product RARa-PLZF is also expressed and can contribute to the block in hematopoietic differentiation and the unresponsiveness to therapy, as shown in dual transgenic model that coexpress the PLZF-RARa and RARa-PLZF fusion genes (He et al., 2000).
BEYOND APL
Two important themes have emerged from our therapeutic conquest of APL and the subsequent analysis of the molecular basis underpinning the remarkable clinical efficacy of ATRA and ATO: first, cure requires the elimination of the bulk of the tumor, as well as a small residual population of cells capable of self-renewal and disease reinitiation; Second, activation of pathways that results in degradation of key driving oncoproteins might represent a highly effective method for targeting cancer cells when/if the cancer cell is addicted to its continued expression.
That ATO is curative in >70% of APL patients is likely a combination of its effects on both the bulk blast population, via degradation of PML-RARa; and LICs, at least in part via PML-dependent/PML-RARa–independent mechanism. Though ATO has shown poor efficacy as a single agent in many other malignancies, its effects on self-renewal of quiescent cells may potentiate the effects of other therapeutic modalities that are able to effectively eliminate rapidly dividing cells. Indeed, in this role, ATO has shown promise in at least two other hematopoietic malignancies, namely adult T cell leukemia/lymphoma driven by HTLV-1 infection (in combination with interferon) and CML (in combination with tyrosine kinase inhibitor, Fig. 3). In both cases, ATO is able to induce at least partial proteolysis of the driving oncoprotein (the viral transactivator Tax in HTLV-1 and the Bcr-Abl fusion protein in CML) and, importantly, transplantation experiments in mouse models have revealed that ATO treatment can result in profound inhibition of LIC function (Ito et al., 2008; El Hajj et al., 2010). In the case of CML, these effects are also due to ATO’s ability to target PML, as shown by genetic inactivation of the Pml gene itself (Ito et al., 2008).
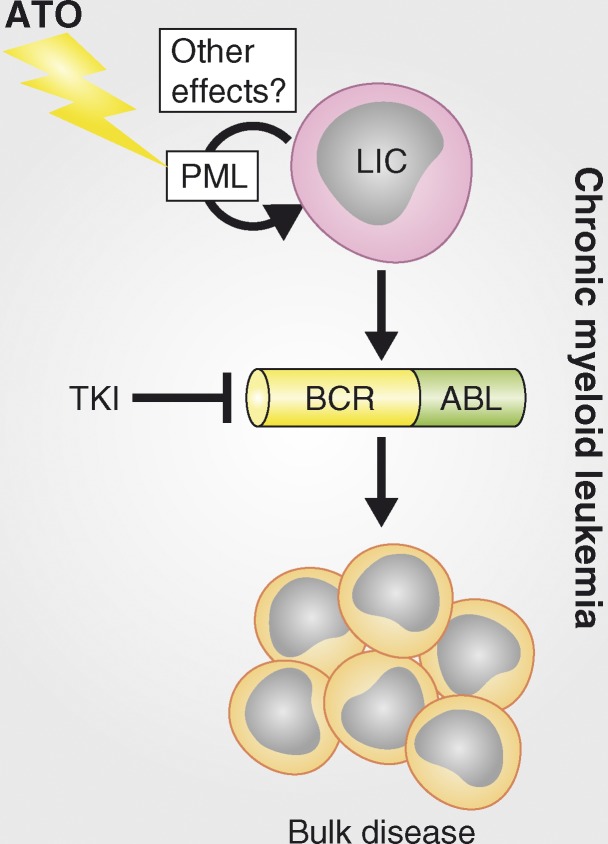
Figure 3.
Rationale for using arsenic trioxide ATO to eliminate LICs in CML. CML is driven by the Bcr-Abl oncoprotein that displays aberrant tyrosine kinase signaling. Tyrosine kinase inhibitors (TKI) such as imatinib can block aberrant signaling and prevent the formation of leukemic cells, but they do not eliminate LICs, thus requiring continuous treatment, inevitably resulting in resistance and relapse. ATO may synergize with TKI to eliminate LICs, thereby curing the disease.
Moving beyond PML, numerous other cellular pathways have been shown to be key for LIC maintenance. The polycomb group protein Bmi-1 is essential for the proliferation and self-renewal of both hematopoietic stem cells (HSCs) and LICs. Genetic deletion of Bmi-1 in a HoxA9/Meis1a transduction model of AML has no effect on the primary disease, but abrogates its ability to be transplanted to secondary recipients (Lessard and Sauvageau, 2003). Conversely, overexpression of Bmi-1 can cooperate with Bcr-Abl in promoting the transformation of CML B-lymphoid progenitors into self-renewing LICs in B cell acute lymphoblastic leukemia (Sengupta et al., 2012).
LICs have recently been shown to depend on high levels of Bcl2 and oxidative phosphorylation for their energy needs. Although these cells possess low levels of ROS, at least in part mediating their resistance to ROS-inducing chemotherapy, they can be efficiently targeted by pharmacological inhibition of Bcl2 (Lagadinou et al., 2013). LICs also appear to be dependent on elevated Wnt–B-catenin and mTOR signaling, potentially providing other therapeutic targets for their elimination (Wang et al., 2010). The Wnt–B-catenin pathway is dispensable for maintenance of normal HSCs, but is essential for both the formation and maintenance of LICs (Wang et al., 2010). Hyperactivation of mTOR signaling leads to exhaustion of HSCs on the one hand, and the formation of LICs on the other (Yilmaz et al., 2006; Zhang et al., 2006). Notably, indomethacin and rapamycin, which inhibit Wnt–B-catenin and mTOR, respectively, have been shown to promote survival in animal models (Heidel et al., 2012; Zhang et al., 2006).
CONCLUDING REMARKS
To date, APL remains the only leukemia that is routinely cured by an oncoprotein-targeted therapy. Some researchers have argued that the biology of APL is unique and that our success in overcoming this once deadly foe is the result of its distictive genetics. While this may be true, in part, research over the past 15 yr has revealed that APL shares many features with other types of leukemia (and indeed solid cancers), namely aberrant self-renewal of a rare stem/progenitor-like cell, a block of differentiation, and the addiction of the malignant cells to key oncogenic drivers. The discovery of two agents that synergize to target both of these processes in a hematopoietic cell transformed by the presence of the PML-RARa fusion protein was crucial from a clinical standpoint. Subsequent efforts to understand the basis for the efficacy of these drugs are providing valuable lessons for our fight against other malignancies.
Acknowledgments
We thank Pandolfi laboratory members for critical discussions, Thomas Garvey for editing of the manuscript, and Priscila Scheucher for drawing the illustrations.
Guilherme Augusto dos Santos was supported by a beginner investigator fellowship from ACS/UICC (ACSBI). L. Kats was supported by an Overseas Postdoctoral Fellowship from the National Health and Medical Research Council of Australia. Research in the Pandolfi laboratory related to this article is supported by National Institutes of Health grants to P.P.P. (R01CA102142, R01CA142874 and R01CA142780).
The authors declare no competing financial interests.
References
- Ablain J., Nasr R., Bazarbachi A., de Thé H. 2011. The drug-induced degradation of oncoproteins: an unexpected Achilles’ heel of cancer cells? Cancer Discov. 1:117–127 10.1158/2159-8290.CD-11-0087 [DOI] [PubMed] [Google Scholar]
- Ablain J., Leiva M., Peres L., Fonsart J., Anthony E., de Thé H. 2013. Uncoupling RARA transcriptional activation and degradation clarifies the bases for APL response to therapies. J. Exp. Med. 210:647–653 10.1084/jem.20122337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcalay M., Zangrilli D., Pandolfi P.P., Longo L., Mencarelli A., Giacomucci A., Rocchi M., Biondi A., Rambaldi A., Lo-Coco F., et al. 1991. Translocation breakpoint of acute promyelocytic leukemia lies within the retinoic acid receptor alpha locus. Proc. Natl. Acad. Sci. USA. 88:1977–1981 10.1073/pnas.88.5.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga M.F., Mikesch J.H., Qiu J., Christensen J., Helin K., Kogan S.C., Dong S., So C.W. 2013. The histone demethylase PHF8 governs retinoic acid response in acute promyelocytic leukemia. Cancer Cell. 23:376–389 10.1016/j.ccr.2013.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au W.Y., Chim C.S., Lie A.K., Liang R., Kwong Y.L. 2002. Combined arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia recurring from previous relapses successfully treated using arsenic trioxide. Br. J. Haematol. 117:130–132 10.1046/j.1365-2141.2002.03409.x [DOI] [PubMed] [Google Scholar]
- Bernardini S., Nuccetelli M., Noguera N.I., Bellincampi L., Lunghi P., Bonati A., Mann K., Miller W.H., Jr, Federici G., Lo-Coco F. 2006. Role of GSTP1-1 in mediating the effect of As2O3 in the Acute Promyelocytic Leukemia cell line NB4. Ann. Hematol. 85:681–687 10.1007/s00277-006-0139-8 [DOI] [PubMed] [Google Scholar]
- Borrow J., Goddard A.D., Sheer D., Solomon E. 1990. Molecular analysis of acute promyelocytic leukemia breakpoint cluster region on chromosome 17. Science. 249:1577–1580 10.1126/science.2218500 [DOI] [PubMed] [Google Scholar]
- Breitman T.R., Collins S.J., Keene B.R. 1981. Terminal differentiation of human promyelocytic leukemic cells in primary culture in response to retinoic acid. Blood. 57:1000–1004 [PubMed] [Google Scholar]
- Brown D., Kogan S., Lagasse E., Weissman I., Alcalay M., Pelicci P.G., Atwater S., Bishop J.M. 1997. A PMLRARalpha transgene initiates murine acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA. 94:2551–2556 10.1073/pnas.94.6.2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho L.H., Soignet S.L., Chanel S., Ho R., Heller G., Scheinberg D.A., Ellison R., Warrell R.P., Jr 2000. Leukocytosis and the retinoic acid syndrome in patients with acute promyelocytic leukemia treated with arsenic trioxide. J. Clin. Oncol. 18:2620–2625 [DOI] [PubMed] [Google Scholar]
- Campana D. 2009. Role of minimal residual disease monitoring in adult and pediatric acute lymphoblastic leukemia. Hematol. Oncol. Clin. North Am. 23:1083–1098: vii (vii.) 10.1016/j.hoc.2009.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaigne S., Chomienne C., Daniel M.T., Ballerini P., Berger R., Fenaux P., Degos L. 1990. All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I. Clinical results. Blood. 76:1704–1709 [PubMed] [Google Scholar]
- Catalano A., Dawson M.A., Somana K., Opat S., Schwarer A., Campbell L.J., Iland H. 2007. The PRKAR1A gene is fused to RARA in a new variant acute promyelocytic leukemia. Blood. 110:4073–4076 10.1182/blood-2007-06-095554 [DOI] [PubMed] [Google Scholar]
- Chen G.Q., Shi X.G., Tang W., Xiong S.M., Zhu J., Cai X., Han Z.G., Ni J.H., Shi G.Y., Jia P.M., et al. 1997. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 89:3345–3353 [PubMed] [Google Scholar]
- Chomienne C., Ballerini P., Balitrand N., Huang M.E., Krawice I., Castaigne S., Fenaux P., Tiollais P., Dejean A., Degos L., et al. 1990. The retinoic acid receptor alpha gene is rearranged in retinoic acid-sensitive promyelocytic leukemias. Leukemia. 4:802–807 [PubMed] [Google Scholar]
- Chou W.C., Chen H.Y., Yu S.L., Cheng L., Yang P.C., Dang C.V. 2005. Arsenic suppresses gene expression in promyelocytic leukemia cells partly through Sp1 oxidation. Blood. 106:304–310 10.1182/blood-2005-01-0241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry A., DeLoughery T.G. 2012. Bleeding and thrombosis in acute promyelocytic leukemia. Am. J. Hematol. 87:596–603 10.1002/ajh.23158 [DOI] [PubMed] [Google Scholar]
- Côté S., Zhou D., Bianchini A., Nervi C., Gallagher R.E., Miller W.H., Jr 2000. Altered ligand binding and transcriptional regulation by mutations in the PML/RARalpha ligand-binding domain arising in retinoic acid-resistant patients with acute promyelocytic leukemia. Blood. 96:3200–3208 [PubMed] [Google Scholar]
- de Thé H., Chen Z. 2010. Acute promyelocytic leukaemia: novel insights into the mechanisms of cure. Nat. Rev. Cancer. 10:775–783 10.1038/nrc2943 [DOI] [PubMed] [Google Scholar]
- de Thé H., Chomienne C., Lanotte M., Degos L., Dejean A. 1990. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature. 347:558–561 10.1038/347558a0 [DOI] [PubMed] [Google Scholar]
- de Thé H., Lavau C., Marchio A., Chomienne C., Degos L., Dejean A. 1991. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 66:675–684 10.1016/0092-8674(91)90113-D [DOI] [PubMed] [Google Scholar]
- de Thé H., Le Bras M., Lallemand-Breitenbach V. 2012. The cell biology of disease: Acute promyelocytic leukemia, arsenic, and PML bodies. J. Cell Biol. 198:11–21 10.1083/jcb.201112044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos G.A., Abreu e Lima R.S., Pestana C.R., Lima A.S., Scheucher P.S., Thomé C.H., Gimenes-Teixeira H.L., Santana-Lemos B.A., Lucena-Araujo A.R., Rodrigues F.P., et al. 2012. (+)α-Tocopheryl succinate inhibits the mitochondrial respiratory chain complex I and is as effective as arsenic trioxide or ATRA against acute promyelocytic leukemia in vivo. Leukemia. 26:451–460 10.1038/leu.2011.216 [DOI] [PubMed] [Google Scholar]
- El Hajj H., El-Sabban M., Hasegawa H., Zaatari G., Ablain J., Saab S.T., Janin A., Mahfouz R., Nasr R., Kfoury Y., et al. 2010. Therapy-induced selective loss of leukemia-initiating activity in murine adult T cell leukemia. J. Exp. Med. 207:2785–2792 10.1084/jem.20101095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard E., Bruck N., Brelivet Y., Bour G., Lalevée S., Bauer A., Poch O., Moras D., Rochette-Egly C. 2006. Phosphorylation by PKA potentiates retinoic acid receptor alpha activity by means of increasing interaction with and phosphorylation by cyclin H/cdk7. Proc. Natl. Acad. Sci. USA. 103:9548–9553 10.1073/pnas.0509717103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavamzadeh A., Alimoghaddam K., Rostami S., Ghaffari S.H., Jahani M., Iravani M., Mousavi S.A., Bahar B., Jalili M. 2011. Phase II study of single-agent arsenic trioxide for the front-line therapy of acute promyelocytic leukemia. J. Clin. Oncol. 29:2753–2757 10.1200/JCO.2010.32.2107 [DOI] [PubMed] [Google Scholar]
- Goto E., Tomita A., Hayakawa F., Atsumi A., Kiyoi H., Naoe T. 2011. Missense mutations in PML-RARA are critical for the lack of responsiveness to arsenic trioxide treatment. Blood. 118:1600–1609 10.1182/blood-2011-01-329433 [DOI] [PubMed] [Google Scholar]
- Grimwade D., Vyas P., Freeman S. 2010. Assessment of minimal residual disease in acute myeloid leukemia. Curr. Opin. Oncol. 22:656–663 10.1097/CCO.0b013e32833ed831 [DOI] [PubMed] [Google Scholar]
- Grisolano J.L., Wesselschmidt R.L., Pelicci P.G., Ley T.J. 1997. Altered myeloid development and acute leukemia in transgenic mice expressing PML-RAR alpha under control of cathepsin G regulatory sequences. Blood. 89:376–387 [PubMed] [Google Scholar]
- Guillemin M.C., Raffoux E., Vitoux D., Kogan S., Soilihi H., Lallemand-Breitenbach V., Zhu J., Janin A., Daniel M.T., Gourmel B., et al. 2002. In vivo activation of cAMP signaling induces growth arrest and differentiation in acute promyelocytic leukemia. J. Exp. Med. 196:1373–1380 10.1084/jem.20021129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S., Adelmant G., Sarraf P., Wright H.M., Mueller E., Spiegelman B.M. 2000. Degradation of the peroxisome proliferator-activated receptor gamma is linked to ligand-dependent activation. J. Biol. Chem. 275:18527–18533 10.1074/jbc.M001297200 [DOI] [PubMed] [Google Scholar]
- He L.Z., Tribioli C., Rivi R., Peruzzi D., Pelicci P.G., Soares V., Cattoretti G., Pandolfi P.P. 1997. Acute leukemia with promyelocytic features in PML/RARalpha transgenic mice. Proc. Natl. Acad. Sci. USA. 94:5302–5307 10.1073/pnas.94.10.5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L.Z., Guidez F., Tribioli C., Peruzzi D., Ruthardt M., Zelent A., Pandolfi P.P. 1998. Distinct interactions of PML-RARalpha and PLZF-RARalpha with co-repressors determine differential responses to RA in APL. Nat. Genet. 18:126–135 10.1038/ng0298-126 [DOI] [PubMed] [Google Scholar]
- He L.Z., Bhaumik M., Tribioli C., Rego E.M., Ivins S., Zelent A., Pandolfi P.P. 2000. Two critical hits for promyelocytic leukemia. Mol. Cell. 6:1131–1141 10.1016/S1097-2765(00)00111-8 [DOI] [PubMed] [Google Scholar]
- He L.Z., Tolentino T., Grayson P., Zhong S., Warrell R.P., Jr, Rifkind R.A., Marks P.A., Richon V.M., Pandolfi P.P. 2001. Histone deacetylase inhibitors induce remission in transgenic models of therapy-resistant acute promyelocytic leukemia. J. Clin. Invest. 108:1321–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidel F.H., Bullinger L., Feng Z., Wang Z., Neff T.A., Stein L., Kalaitzidis D., Lane S.W., Armstrong S.A. 2012. Genetic and pharmacologic inhibition of β-catenin targets imatinib-resistant leukemia stem cells in CML. Cell Stem Cell. 10:412–424 10.1016/j.stem.2012.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillestad L.K. 1957. Acute promyelocytic leukemia. Acta Med. Scand. 159:189–194 10.1111/j.0954-6820.1957.tb00124.x [DOI] [PubMed] [Google Scholar]
- Hu J., Liu Y.F., Wu C.F., Xu F., Shen Z.X., Zhu Y.M., Li J.M., Tang W., Zhao W.L., Wu W., et al. 2009. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA. 106:3342–3347 10.1073/pnas.0813280106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.E., Ye Y.C., Chen S.R., Chai J.R., Lu J.X., Zhoa L., Gu L.J., Wang Z.Y. 1988. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 72:567–572 [PubMed] [Google Scholar]
- Ito K., Bernardi R., Morotti A., Matsuoka S., Saglio G., Ikeda Y., Rosenblatt J., Avigan D.E., Teruya-Feldstein J., Pandolfi P.P. 2008. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 453:1072–1078 10.1038/nature07016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Carracedo A., Weiss D., Arai F., Ala U., Avigan D.E., Schafer Z.T., Evans R.M., Suda T., Lee C.H., Pandolfi P.P. 2012. A PML–PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat. Med. 18:1350–1358 10.1038/nm.2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanne M., Lallemand-Breitenbach V., Ferhi O., Koken M., Le Bras M., Duffort S., Peres L., Berthier C., Soilihi H., Raught B., de Thé H. 2010. PML/RARA oxidation and arsenic binding initiate the antileukemia response of As2O3. Cancer Cell. 18:88–98 10.1016/j.ccr.2010.06.003 [DOI] [PubMed] [Google Scholar]
- Jing Y., Wang L., Xia L., Chen G.Q., Chen Z., Miller W.H., Waxman S. 2001. Combined effect of all-trans retinoic acid and arsenic trioxide in acute promyelocytic leukemia cells in vitro and in vivo. Blood. 97:264–269 10.1182/blood.V97.1.264 [DOI] [PubMed] [Google Scholar]
- Kakizuka A., Miller W.H., Jr, Umesono K., Warrell R.P., Jr, Frankel S.R., Murty V.V., Dmitrovsky E., Evans R.M. 1991. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell. 66:663–674 10.1016/0092-8674(91)90112-C [DOI] [PubMed] [Google Scholar]
- Kennedy G.A., Marlton P., Cobcroft R., Gill D. 2000. Molecular remission without blood product support using all-trans retinoic acid (ATRA) induction and combined arsenic trioxide/ATRA consolidation in a Jehovah’s Witness with de novo acute promyelocytic leukaemia. Br. J. Haematol. 111:1103–1105 10.1046/j.1365-2141.2000.02480.x [DOI] [PubMed] [Google Scholar]
- Koken M.H., Daniel M.T., Gianni M., Zelent A., Licht J., Buzyn A., Minard P., Degos L., Varet B., de Thé H. 1999. Retinoic acid, but not arsenic trioxide, degrades the PLZF/RARalpha fusion protein, without inducing terminal differentiation or apoptosis, in a RA-therapy resistant t(11;17)(q23;q21) APL patient. Oncogene. 18:1113–1118 10.1038/sj.onc.1202414 [DOI] [PubMed] [Google Scholar]
- Kondo T., Mori A., Darmanin S., Hashino S., Tanaka J., Asaka M. 2008. The seventh pathogenic fusion gene FIP1L1-RARA was isolated from a t(4;17)-positive acute promyelocytic leukemia. Haematologica. 93:1414–1416 10.3324/haematol.12854 [DOI] [PubMed] [Google Scholar]
- Kopf E., Plassat J.L., Vivat V., de Thé H., Chambon P., Rochette-Egly C. 2000. Dimerization with retinoid X receptors and phosphorylation modulate the retinoic acid-induced degradation of retinoic acid receptors alpha and gamma through the ubiquitin-proteasome pathway. J. Biol. Chem. 275:33280–33288 10.1074/jbc.M002840200 [DOI] [PubMed] [Google Scholar]
- Koyama T., Hirosawa S., Kawamata N., Tohda S., Aoki N. 1994. All-trans retinoic acid upregulates thrombomodulin and downregulates tissue-factor expression in acute promyelocytic leukemia cells: distinct expression of thrombomodulin and tissue factor in human leukemic cells. Blood. 84:3001–3009 [PubMed] [Google Scholar]
- Kwaan H.C., Huyck T. 2010. Thromboembolic and bleeding complications in acute leukemia. Expert Rev Hematol. 3:719–730 10.1586/ehm.10.71 [DOI] [PubMed] [Google Scholar]
- Lagadinou E.D., Sach A., Callahan K., Rossi R.M., Neering S.J., Minhajuddin M., Ashton J.M., Pei S., Grose V., O’Dwyer K.M., et al. 2013. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 12:329–341 10.1016/j.stem.2012.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand-Breitenbach V., Guillemin M.C., Janin A., Daniel M.T., Degos L., Kogan S.C., Bishop J.M., de Thé H. 1999. Retinoic acid and arsenic synergize to eradicate leukemic cells in a mouse model of acute promyelocytic leukemia. J. Exp. Med. 189:1043–1052 10.1084/jem.189.7.1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand-Breitenbach V., Jeanne M., Benhenda S., Nasr R., Lei M., Peres L., Zhou J., Zhu J., Raught B., de Thé H. 2008. Arsenic degrades PML or PML-RARalpha through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat. Cell Biol. 10:547–555 10.1038/ncb1717 [DOI] [PubMed] [Google Scholar]
- Lallemand-Breitenbach V., Zhu J., Chen Z., de Thé H. 2012. Curing APL through PML/RARA degradation by As2O3. Trends Mol. Med. 18:36–42 10.1016/j.molmed.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Lessard J., Sauvageau G. 2003. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 423:255–260 10.1038/nature01572 [DOI] [PubMed] [Google Scholar]
- Li J., Chen P., Sinogeeva N., Gorospe M., Wersto R.P., Chrest F.J., Barnes J., Liu Y. 2002. Arsenic trioxide promotes histone H3 phosphoacetylation at the chromatin of CASPASE-10 in acute promyelocytic leukemia cells. J. Biol. Chem. 277:49504–49510 10.1074/jbc.M207836200 [DOI] [PubMed] [Google Scholar]
- Licht J.D., Chomienne C., Goy A., Chen A., Scott A.A., Head D.R., Michaux J.L., Wu Y., DeBlasio A., Miller W.H., Jr, et al. 1995. Clinical and molecular characterization of a rare syndrome of acute promyelocytic leukemia associated with translocation (11;17). Blood. 85:1083–1094 [PubMed] [Google Scholar]
- Lo-Coco F., Avvisati G., Vignetti M., Thiede C., Orlando S.M., Iacobelli S., Ferrara F., Fazi P., Cicconi L., Di Bona E., et al. ; Gruppo Italiano Malattie Ematologiche dell’Adulto; German-Austrian Acute Myeloid Leukemia Study Group; Study Alliance Leukemia 2013. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N. Engl. J. Med. 369:111–121 10.1056/NEJMoa1300874 [DOI] [PubMed] [Google Scholar]
- Longo L., Pandolfi P.P., Biondi A., Rambaldi A., Mencarelli A., Lo-Coco F., Diverio D., Pegoraro L., Avanzi G., Tabilio A., et al. 1990. Rearrangements and aberrant expression of the retinoic acid receptor alpha gene in acute promyelocytic leukemias. J. Exp. Med. 172:1571–1575 10.1084/jem.172.6.1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee J.A., Piskounova E., Morrison S.J. 2012. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 21:283–296 10.1016/j.ccr.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelli F., Diverio D., Avvisati G., Luciano A., Barbui T., Bernasconi C., Broccia G., Cerri R., Falda M., Fioritoni G., et al. 1997. Molecular remission in PML/RAR alpha-positive acute promyelocytic leukemia by combined all-trans retinoic acid and idarubicin (AIDA) therapy. Gruppo Italiano-Malattie Ematologiche Maligne dell’Adulto and Associazione Italiana di Ematologia ed Oncologia Pediatrica Cooperative Groups. Blood. 90:1014–1021 [PubMed] [Google Scholar]
- Marasca R., Zucchini P., Galimberti S., Leonardi G., Vaccari P., Donelli A., Luppi M., Petrini M., Torelli G. 1999. Missense mutations in the PML/RARalpha ligand binding domain in ATRA-resistant As(2)O(3) sensitive relapsed acute promyelocytic leukemia. Haematologica. 84:963–968 [PubMed] [Google Scholar]
- Mathews V., George B., Lakshmi K.M., Viswabandya A., Bajel A., Balasubramanian P., Shaji R.V., Srivastava V.M., Srivastava A., Chandy M. 2006. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood. 107:2627–2632 10.1182/blood-2005-08-3532 [DOI] [PubMed] [Google Scholar]
- Mathews V., George B., Chendamarai E., Lakshmi K.M., Desire S., Balasubramanian P., Viswabandya A., Thirugnanam R., Abraham A., Shaji R.V., et al. 2010. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: long-term follow-up data. J. Clin. Oncol. 28:3866–3871 10.1200/JCO.2010.28.5031 [DOI] [PubMed] [Google Scholar]
- Menell J.S., Cesarman G.M., Jacovina A.T., McLaughlin M.A., Lev E.A., Hajjar K.A. 1999. Annexin II and bleeding in acute promyelocytic leukemia. N. Engl. J. Med. 340:994–1004 10.1056/NEJM199904013401303 [DOI] [PubMed] [Google Scholar]
- Mi J.Q., Li J.M., Shen Z.X., Chen S.J., Chen Z. 2012. How to manage acute promyelocytic leukemia. Leukemia. 26:1743–1751 10.1038/leu.2012.57 [DOI] [PubMed] [Google Scholar]
- Morán J.M., Ortiz-Ortiz M.A., Ruiz-Mesa L.M., Fuentes J.M. 2010. Nitric oxide in paraquat-mediated toxicity: A review. J. Biochem. Mol. Toxicol. 24:402–409 10.1002/jbt.20348 [DOI] [PubMed] [Google Scholar]
- Nasr R., Guillemin M.C., Ferhi O., Soilihi H., Peres L., Berthier C., Rousselot P., Robledo-Sarmiento M., Lallemand-Breitenbach V., Gourmel B., et al. 2008. Eradication of acute promyelocytic leukemia-initiating cells through PML-RARA degradation. Nat. Med. 14:1333–1342 10.1038/nm.1891 [DOI] [PubMed] [Google Scholar]
- Nawaz Z., Lonard D.M., Dennis A.P., Smith C.L., O’Malley B.W. 1999. Proteasome-dependent degradation of the human estrogen receptor. Proc. Natl. Acad. Sci. USA. 96:1858–1862 10.1073/pnas.96.5.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare T., Zabriskie M.S., Eiring A.M., Deininger M.W. 2012. Pushing the limits of targeted therapy in chronic myeloid leukaemia. Nat. Rev. Cancer. 12:513–526 10.1038/nrc3317 [DOI] [PubMed] [Google Scholar]
- Pandolfi P.P., Grignani F., Alcalay M., Mencarelli A., Biondi A., Lo-Coco F., Grignani F., Pelicci P.G. 1991. Structure and origin of the acute promyelocytic leukemia myl/RAR alpha cDNA and characterization of its retinoid-binding and transactivation properties. Oncogene. 6:1285–1292 [PubMed] [Google Scholar]
- Raelson J.V., Nervi C., Rosenauer A., Benedetti L., Monczak Y., Pearson M., Pelicci P.G., Miller W.H., Jr 1996. The PML/RAR alpha oncoprotein is a direct molecular target of retinoic acid in acute promyelocytic leukemia cells. Blood. 88:2826–2832 [PubMed] [Google Scholar]
- Redner R.L. 2002. Variations on a theme: the alternate translocations in APL. Leukemia. 16:1927–1932 10.1038/sj.leu.2402720 [DOI] [PubMed] [Google Scholar]
- Rego E.M., He L.Z., Warrell R.P., Jr, Wang Z.G., Pandolfi P.P. 2000. Retinoic acid (RA) and As2O3 treatment in transgenic models of acute promyelocytic leukemia (APL) unravel the distinct nature of the leukemogenic process induced by the PML-RARalpha and PLZF-RARalpha oncoproteins. Proc. Natl. Acad. Sci. USA. 97:10173–10178 10.1073/pnas.180290497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenauer A., Raelson J.V., Nervi C., Eydoux P., DeBlasio A., Miller W.H., Jr 1996. Alterations in expression, binding to ligand and DNA, and transcriptional activity of rearranged and wild-type retinoid receptors in retinoid-resistant acute promyelocytic leukemia cell lines. Blood. 88:2671–2682 [PubMed] [Google Scholar]
- Rowley J.D., Golomb H.M., Dougherty C. 1977. 15/17 translocation, a consistent chromosomal change in acute promyelocytic leukaemia. Lancet. 1:549–550 10.1016/S0140-6736(77)91415-5 [DOI] [PubMed] [Google Scholar]
- Sanz M.A., Lo-Coco F. 2011. Modern approaches to treating acute promyelocytic leukemia. J. Clin. Oncol. 29:495–503 10.1200/JCO.2010.32.1067 [DOI] [PubMed] [Google Scholar]
- Sanz M.A., Montesinos P. 2010. Open issues on bleeding and thrombosis in acute promyelocytic leukemia. Thromb. Res. 125(Suppl 2):S51–S54 10.1016/S0049-3848(10)70013-X [DOI] [PubMed] [Google Scholar]
- Sanz M.A., Martín G., Rayón C., Esteve J., González M., Díaz-Mediavilla J., Bolufer P., Barragán E., Terol M.J., González J.D., et al. 1999. A modified AIDA protocol with anthracycline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RARalpha-positive acute promyelocytic leukemia. PETHEMA group. Blood. 94:3015–3021 [PubMed] [Google Scholar]
- Sanz M.A., Martín G., González M., León A., Rayón C., Rivas C., Colomer D., Amutio E., Capote F.J., Milone G.A., et al. ; Programa de Estudio y Traitmiento de las Hemopatías Malignas 2004. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood. 103:1237–1243 10.1182/blood-2003-07-2462 [DOI] [PubMed] [Google Scholar]
- Sanz M.A., Grimwade D., Tallman M.S., Lowenberg B., Fenaux P., Estey E.H., Naoe T., Lengfelder E., Büchner T., Döhner H., et al. 2009. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 113:1875–1891 10.1182/blood-2008-04-150250 [DOI] [PubMed] [Google Scholar]
- Sengupta A., Ficker A.M., Dunn S.K., Madhu M., Cancelas J.A. 2012. Bmi1 reprograms CML B-lymphoid progenitors to become B-ALL-initiating cells. Blood. 119:494–502 10.1182/blood-2011-06-359232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao W., Fanelli M., Ferrara F.F., Riccioni R., Rosenauer A., Davison K., Lamph W.W., Waxman S., Pelicci P.G., Lo-Coco F., et al. 1998. Arsenic trioxide as an inducer of apoptosis and loss of PML/RAR alpha protein in acute promyelocytic leukemia cells. J. Natl. Cancer Inst. 90:124–133 10.1093/jnci/90.2.124 [DOI] [PubMed] [Google Scholar]
- Shen Z.X., Chen G.Q., Ni J.H., Li X.S., Xiong S.M., Qiu Q.Y., Zhu J., Tang W., Sun G.L., Yang K.Q., et al. 1997. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 89:3354–3360 [PubMed] [Google Scholar]
- Shen Z.X., Shi Z.Z., Fang J., Gu B.W., Li J.M., Zhu Y.M., Shi J.Y., Zheng P.Z., Yan H., Liu Y.F., et al. 2004. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc. Natl. Acad. Sci. USA. 101:5328–5335 10.1073/pnas.0400053101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soignet S.L., Maslak P., Wang Z.G., Jhanwar S., Calleja E., Dardashti L.J., Corso D., DeBlasio A., Gabrilove J., Scheinberg D.A., et al. 1998. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N. Engl. J. Med. 339:1341–1348 10.1056/NEJM199811053391901 [DOI] [PubMed] [Google Scholar]
- Tallman M.S., Altman J.K. 2009. How I treat acute promyelocytic leukemia. Blood. 114:5126–5135 10.1182/blood-2009-07-216457 [DOI] [PubMed] [Google Scholar]
- Tanaka T., Rodríguez de la Concepción M.L., De Luca L.M. 2001. Involvement of all-trans-retinoic acid in the breakdown of retinoic acid receptors alpha and gamma through proteasomes in MCF-7 human breast cancer cells. Biochem. Pharmacol. 61:1347–1355 10.1016/S0006-2952(01)00600-1 [DOI] [PubMed] [Google Scholar]
- Tatham M.H., Geoffroy M.C., Shen L., Plechanovova A., Hattersley N., Jaffray E.G., Palvimo J.J., Hay R.T. 2008. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat. Cell Biol. 10:538–546 10.1038/ncb1716 [DOI] [PubMed] [Google Scholar]
- Tsimberidou A.M., Tirado-Gomez M., Andreeff M., O’Brien S., Kantarjian H., Keating M., Lopez-Berestein G., Estey E. 2006. Single-agent liposomal all-trans retinoic acid can cure some patients with untreated acute promyelocytic leukemia: an update of The University of Texas M. D. Anderson Cancer Center Series. Leuk. Lymphoma. 47:1062–1068 10.1080/10428190500463932 [DOI] [PubMed] [Google Scholar]
- Visani G., Piccaluga P.P., Martinelli G., Rossi M., Malagola M., Baccarani M. 2003. Sustained molecular remission in advanced acute promyelocytic leukemia with combined pulsed retinoic acid and arsenic trioxide. Clinical evidence of synergistic effect and real-time quantification of minimal residual disease. Haematologica. 88:ELT15. [PubMed] [Google Scholar]
- Wang Z.Y., Chen Z. 2008. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 111:2505–2515 10.1182/blood-2007-07-102798 [DOI] [PubMed] [Google Scholar]
- Wang Y., Krivtsov A.V., Sinha A.U., North T.E., Goessling W., Feng Z., Zon L.I., Armstrong S.A. 2010. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 327:1650–1653 10.1126/science.1186624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrell R.P., Jr, Frankel S.R., Miller W.H., Jr, Scheinberg D.A., Itri L.M., Hittelman W.N., Vyas R., Andreeff M., Tafuri A., Jakubowski A., et al. 1991. Differentiation therapy of acute promyelocytic leukemia with tretinoin (all-trans-retinoic acid). N. Engl. J. Med. 324:1385–1393 10.1056/NEJM199105163242002 [DOI] [PubMed] [Google Scholar]
- Warrell R.P., Jr, He L.Z., Richon V., Calleja E., Pandolfi P.P. 1998. Therapeutic targeting of transcription in acute promyelocytic leukemia by use of an inhibitor of histone deacetylase. J. Natl. Cancer Inst. 90:1621–1625 10.1093/jnci/90.21.1621 [DOI] [PubMed] [Google Scholar]
- Won D., Shin S.Y., Park C.J., Jang S., Chi H.S., Lee K.H., Lee J.O., Seo E.J. 2013. OBFC2A/RARA: a novel fusion gene in variant acute promyelocytic leukemia. Blood. 121:1432–1435 10.1182/blood-2012-04-423129 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Tsuzuki S., Tsuzuki M., Handa K., Inaguma Y., Emi N. 2010. BCOR as a novel fusion partner of retinoic acid receptor alpha in a t(X;17)(p11;q12) variant of acute promyelocytic leukemia. Blood. 116:4274–4283 10.1182/blood-2010-01-264432 [DOI] [PubMed] [Google Scholar]
- Yilmaz O.H., Valdez R., Theisen B.K., Guo W., Ferguson D.O., Wu H., Morrison S.J. 2006. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 441:475–482 10.1038/nature04703 [DOI] [PubMed] [Google Scholar]
- Zelent A., Guidez F., Melnick A., Waxman S., Licht J.D. 2001. Translocations of the RARalpha gene in acute promyelocytic leukemia. Oncogene. 20:7186–7203 10.1038/sj.onc.1204766 [DOI] [PubMed] [Google Scholar]
- Zhang J., Grindley J.C., Yin T., Jayasinghe S., He X.C., Ross J.T., Haug J.S., Rupp D., Porter-Westpfahl K.S., Wiedemann L.M., et al. 2006. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 441:518–522 10.1038/nature04747 [DOI] [PubMed] [Google Scholar]
- Zhang X.W., Yan X.J., Zhou Z.R., Yang F.F., Wu Z.Y., Sun H.B., Liang W.X., Song A.X., Lallemand-Breitenbach V., Jeanne M., et al. 2010. Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML. Science. 328:240–243 10.1126/science.1183424 [DOI] [PubMed] [Google Scholar]
- Zheng X., Seshire A., Rüster B., Bug G., Beissert T., Puccetti E., Hoelzer D., Henschler R., Ruthardt M. 2007. Arsenic but not all-trans retinoic acid overcomes the aberrant stem cell capacity of PML/RARalpha-positive leukemic stem cells. Haematologica. 92:323–331 10.3324/haematol.10541 [DOI] [PubMed] [Google Scholar]
- Zhu J., Gianni M., Kopf E., Honoré N., Chelbi-Alix M., Koken M., Quignon F., Rochette-Egly C., de Thé H. 1999. Retinoic acid induces proteasome-dependent degradation of retinoic acid receptor alpha (RARalpha) and oncogenic RARalpha fusion proteins. Proc. Natl. Acad. Sci. USA. 96:14807–14812 10.1073/pnas.96.26.14807 [DOI] [PMC free article] [PubMed] [Google Scholar]