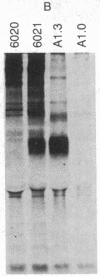
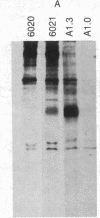
Abstract
Previously [Moore, K. W., Jardieu, P., Mietz, J. A., Trounstine, M. L., Kuff, E. L., Ishizaka, K. & Martens, C. L. (1986) J. Immunol. 136, 4283-4290], we examined a T-hybridoma-derived cDNA clone, 8.3, that encodes a biologically active murine IgE-binding factor (IgE-BF), and we showed that it was a variant member of the endogenous retroviral gene family related to mouse intracisternal A particles (IAPs). We have now characterized four more IgE-BF cDNA clones by heteroduplex and restriction enzyme analysis and found that they all represent different structural variants of the full-size IAP genomic element. In clones 8.3 and 10.2, which have been fully sequenced, the open reading frames span deletions 3.4 and 1.9 kilobases (kb) long, respectively, and specify different gag-pol fusion polypeptides. Clone 9.5 contains a 2.1-kb deletion entirely within the pol region. Two other clones (4.2 and 11.7) contain no internal deletion and may represent truncated cDNA copies of full-size (7.2 kb) IAP gene transcripts. Structural variants very similar to clone 10.2 are common in the mouse genome, and clone 9.5 is also probably not a unique gene form. The sequences of clones 8.3 and 10.2 are different in detail, but each is closely homologous to a randomly cloned mouse genomic IAP element throughout the gag-related portions of their open reading frames. Antibodies against two oligopeptides specified by the sequence of clone 8.3 immunoprecipitated IAP-related proteins from mouse neuroblastoma and myeloma cells, confirming that the IgE-BF produced by this clone shares sequence with expressed IAP elements in different cell types. Thus, information related to the IgE-BF is an integral part of the murine IAP retrotransposon gag gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Chiu I. M., Wong J. F., Tronick S. R., Roe B. A., Aaronson S. A., Schlom J. A new class of endogenous human retroviral genomes. Science. 1985 Jun 7;228(4704):1208–1211. doi: 10.1126/science.2408338. [DOI] [PubMed] [Google Scholar]
- Chiu I. M., Callahan R., Tronick S. R., Schlom J., Aaronson S. A. Major pol gene progenitors in the evolution of oncoviruses. Science. 1984 Jan 27;223(4634):364–370. doi: 10.1126/science.6197754. [DOI] [PubMed] [Google Scholar]
- Chiu I. M., Huang R. C., Aaronson S. A. Genetic relatedness between intracisternal A particles and other major oncovirus genera. Virus Res. 1985 Jul;3(1):1–11. doi: 10.1016/0168-1702(85)90036-x. [DOI] [PubMed] [Google Scholar]
- Christy R. J., Brown A. R., Gourlie B. B., Huang R. C. Nucleotide sequences of murine intracisternal A-particle gene LTRs have extensive variability within the R region. Nucleic Acids Res. 1985 Jan 11;13(1):289–302. doi: 10.1093/nar/13.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad D. H., Peterson L. H. The murine lymphocyte receptor for IgE. I. Isolation and characterization of the murine B cell Fc epsilon receptor and comparison with Fc epsilon receptors from rat and human. J Immunol. 1984 Feb;132(2):796–803. [PubMed] [Google Scholar]
- Finbloom D. S., Metzger H. Isolation of cross-linked IgE-receptor complexes from rat macrophages. J Immunol. 1983 Apr;130(4):1489–1491. [PubMed] [Google Scholar]
- Hojman-Montes de Oca F., Dianoux L., Peries J., Emanoil-Ravicovitch R. Intracisternal A particles: RNA expression and DNA methylation in murine teratocarcinoma cell lines. J Virol. 1983 Apr;46(1):307–310. doi: 10.1128/jvi.46.1.307-310.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff T. F., Uede T., Ishizaka K. Formation of rat IgE-binding factors by rat-mouse T cell hybridomas. J Immunol. 1982 Aug;129(2):509–514. [PubMed] [Google Scholar]
- Huff T. F., Yodoi J., Uede T., Ishizaka K. Presence of an antigenic determinant common to rat IgE-potentiating factor, IgE-suppressive factor, and Fc epsilon receptors on T and B lymphocytes. J Immunol. 1984 Jan;132(1):406–412. [PubMed] [Google Scholar]
- Ishizaka K. Regulation of IgE synthesis. Annu Rev Immunol. 1984;2:159–182. doi: 10.1146/annurev.iy.02.040184.001111. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Feenstra A., Lueders K., Smith L., Hawley R., Hozumi N., Shulman M. Intracisternal A-particle genes as movable elements in the mouse genome. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1992–1996. doi: 10.1073/pnas.80.7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Fewell J. W. Intracisternal A-particle gene expression in normal mouse thymus tissue: gene products and strain-related variability. Mol Cell Biol. 1985 Mar;5(3):474–483. doi: 10.1128/mcb.5.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Smith L. A., Lueders K. K. Intracisternal A-particle genes in Mus musculus: a conserved family of retrovirus-like elements. Mol Cell Biol. 1981 Mar;1(3):216–227. doi: 10.1128/mcb.1.3.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Wivel N. A., Lueders K. K. The extraction of intracisternal A-particles from a mouse plasma-cell tumor. Cancer Res. 1968 Oct;28(10):2137–2148. [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Comparison of the sequence organization of related retrovirus-like multigene families in three evolutionarily distant rodent genomes. Nucleic Acids Res. 1983 Jul 11;11(13):4391–4408. doi: 10.1093/nar/11.13.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Intracisternal A-particle genes: identification in the genome of Mus musculus and comparison of multiple isolates from a mouse gene library. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3571–3575. doi: 10.1073/pnas.77.6.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Sequences associated with intracisternal A particles are reiterated in the mouse genome. Cell. 1977 Dec;12(4):963–972. doi: 10.1016/0092-8674(77)90161-1. [DOI] [PubMed] [Google Scholar]
- Martens C. L., Huff T. F., Jardieu P., Trounstine M. L., Coffman R. L., Ishizaka K., Moore K. W. cDNA clones encoding IgE-binding factors from a rat-mouse T-cell hybridoma. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2460–2464. doi: 10.1073/pnas.82.8.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moore K. W., Jardieu P., Mietz J. A., Trounstine M. L., Kuff E. L., Ishizaka K., Martens C. L. Rodent IgE-binding factor genes are members of an endogenous, retrovirus-like gene family. J Immunol. 1986 Jun 1;136(11):4283–4290. [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Toh H., Miyata T., Awaya T. Nucleotide sequence of the Syrian hamster intracisternal A-particle gene: close evolutionary relationship of type A particle gene to types B and D oncovirus genes. J Virol. 1985 Aug;55(2):387–394. doi: 10.1128/jvi.55.2.387-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patarca R., Haseltine W. A. A major retroviral core protein related to EPA and TIMP. 1985 Nov 28-Dec 4Nature. 318(6044):390–390. doi: 10.1038/318390a0. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Segal S., Lueders K. K., Kuff E. L. RNA associated with murine intracisternal type A particles codes for the main particle protein. J Virol. 1978 Jul;27(1):118–126. doi: 10.1128/jvi.27.1.118-126.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power M. D., Marx P. A., Bryant M. L., Gardner M. B., Barr P. J., Luciw P. A. Nucleotide sequence of SRV-1, a type D simian acquired immune deficiency syndrome retrovirus. Science. 1986 Mar 28;231(4745):1567–1572. doi: 10.1126/science.3006247. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Kitasato H., Kawakami M., Ono M. Molecular cloning of retrovirus-like genes present in multiple copies in the Syrian hamster genome. Nucleic Acids Res. 1982 Oct 11;10(19):5733–5746. doi: 10.1093/nar/10.19.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh H., Ono M., Miyata T. Retroviral gag and DNA endonuclease coding sequences in IgE-binding factor gene. 1985 Nov 28-Dec 4Nature. 318(6044):388–389. doi: 10.1038/318388a0. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wujcik K. M., Morgan R. A., Huang R. C. Transcription of intracisternal A-particle genes in mouse myeloma and Ltk- cells. J Virol. 1984 Oct;52(1):29–36. doi: 10.1128/jvi.52.1.29-36.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yodoi J., Hirashima M., Ishizaka K. Regulatory role of IgE-binding factors from rat T lymphocytes. II. Glycoprotein nature and source of IgE-potentiating factor. J Immunol. 1980 Oct;125(4):1436–1441. [PubMed] [Google Scholar]