Abstract
Sox2 is required for proper neuronal formation in the CNS, but the molecular mechanisms involved are not well characterized. Here, we addressed the role of Sox2 in neurogenesis of the developing chicken inner ear. Overexpressing Sox2 from a constitutive (β-actin) promoter induces the expression of the proneural gene, Neurogenin1 (Ngn1); however, the expression of a downstream target of Ngn1, Neurod1, is unchanged. As a result, there is a reduction of neural precursors to delaminate and populate the developing cochleo-vestibular ganglion. In contrast, overexpression of either Ngn1 or Neurod1 is sufficient to promote the neural fate in this system. These results suggest that high levels of Sox2 inhibit progression of neurogenesis in the developing inner ear. Furthermore, we provide evidence that Ngn1 and Neurod1 inhibit Sox2 transcription through a phylogenetically conserved Sox2 enhancer to mediate neurogenesis. We propose that Sox2 confers neural competency by promoting Ngn1 expression, and that negative feedback inhibition of Sox2 by Ngn1 is an essential step in the progression from neural precursor to nascent neuron.
Introduction
Sry-related HMG-box 2 (Sox2) is a transcription factor that contains a high-mobility-group (HMG) DNA-binding domain (Lefebvre et al., 2007). Its presumed role during vertebrate neural development is to maintain a proliferative neural progenitor pool and confer neural competency (Taranova et al., 2006; Pevny and Nicolis, 2010). The lack of Sox2 in neural tissues affects progenitor proliferation, expression of proneural genes, and neuronal differentiation (Ferri et al., 2004; Taranova et al., 2006). In addition, Sox2 is required for neural stem cell maintenance in vivo and in vitro, and neurospheres generated from neural stem cells that express a low level of Sox2 have reduced capacity to develop into mature neurons (Cavallaro et al., 2008; Favaro et al., 2009). As neural progenitors enter neurogenesis, downregulation of Sox2 is required, which is in part mediated by serine protease(s) (Bani-Yaghoub et al., 2006). Nonetheless, the underlying mechanisms that enable Sox2 to render neural competency, and facilitate initiation of neurogenesis remain largely uncharacterized (Van Raay et al., 2005; Taranova et al., 2006).
Neurons of the cochleovestibular ganglion (CVG, VIIIth cranial ganglion) innervate all sensory organs within the inner ear. Proper CVG formation requires the evolutionarily conserved basic helix-loop-helix genes Neurogenin1 (Ngn1) and Neurod1 (Liu et al., 2000; Ma et al., 2000; Matei et al., 2005), which in other neural tissues are required for neuronal fate specification, and differentiation, respectively (Ma et al., 1996; Ma et al., 1998, Bertrand et al., 2002; Chae et al., 2004). Beginning at the otic cup stage and continuing until the early stages of otocyst development, a subpopulation of cells in the neural sensory competent domain (NSD) of the otic epithelium sequentially expresses first Ngn1, and then Neurod1. These Ngn1- and Neurod1-positive neuroblasts soon exit from the NSD, and coalesce to form neurons of the CVG. After neuroblast delamination is complete, the remaining NSD is thought to gradually split to form the sensory hair cells, and supporting cells that comprise the sensory patches within the inner ear. The expression of Sox2 changes during this developmental period with high levels in the NSD, and its derived sensory patches but low levels in the delaminated neuroblasts, and sensory hair cells (Neves et al., 2007) (see Results). Mutant mice that have compromised Sox2 expression in the inner ear show deficits in both neural/neuronal, and sensory components (Kiernan et al., 2005; Puligilla et al., 2010). Similarly, mutations of SOX2 in humans can lead to sensorineural hearing loss in addition to brain defects, and anophthalmia (Hagstrom et al., 2005; Kelberman et al., 2006). Therefore, Sox2 has an important role in both brain, and sensory organ development but the molecular mechanisms involved are largely uncharacterized. Here we have examined the roles of Sox2 in neurogenesis of the developing inner ear using a transient gain-of-function approach. We find that overexpression of Sox2 readily induces Ngn1 expression in the otic epithelium, but proper neuroblast delamination requires Ngn1, and Neurod1 to transcriptionally downregulate Sox2 via interacting with a cis-regulatory repressive element within the nasal-otic placode-specific enhancer 1 (Nop-1) of Sox2.
Materials and Methods
Eggs, in ovo electroporation, and expression constructs.
Fertilized chicken eggs (B&E) were incubated at 38°C and staged according to Hamburger and Hamilton (1992). Full-length cDNA of chicken Sox2 was subcloned into the pMES-IRES-GFP expression vector, in which cDNA is driven by a chicken β-actin promoter. Mouse Ngn1 cDNA was subcloned into pMES-IRES-GFP and pCMV-DsRed-Express (Clontech) vector. Chicken Neurod1 cDNA was subcloned into the pCI-IRES-H2B-RFP vector. The Nop-1 and Nop1-Ebox Mutant (E-box sequence CAGGTG mutated to AGCTAA) regulatory elements of Sox2 were subcloned into an enhanced green fluorescent protein vector, ptkEGFPv2. Various plasmids were delivered to the right otic cup between 10 and 17 somite stages (E1.5) by electroporation. This was conducted by filling the right otic cup with plasmids at a concentration of 3–4 μg/μl tinted with fast green. Then, a negative platinum electrode was placed above the right otic cup and the positive electrode inserted underneath the embryo at the location of the left otic cup. Two to four pulses at 7 V with 100 ms duration and spacing were applied using a CUY21 electroporator (Bex). Then, the eggs were sealed and returned to the incubator for 6–48 h. For coelectroporation, the respective plasmid constructs were used at ∼3 μg/μl concentrations each.
In situ hybridization and immunohistochemistry.
In situ hybridization on cryosections was performed as previously described (Wu and Oh, 1996; Raft et al., 2007). Chicken digoxigenin-labeled α-sense RNA probes were generated for Sox2, GFP, Ngn1, Neurod1, and Lunatic fringe (Lfng). Similar cryosections for in situ hybridization were used for immunostaining. The primary antibodies used were rabbit polyclonal α-Sox2 (1:4000 Millipore Bioscience Research Reagents), goat polyclonal α-GFP-FITC-conjugated (1:400, GeneTex), rabbit polyclonal α-DsRed-Express (1:100, Clontech), and mouse monoclonal α-neuron-specific β-III Tubulin-Northern Lights 557 conjugated (1:25; TuJ-1-NL557, R&D Systems). The secondary antibodies used were goat α-rabbit Alexa Fluor 568 and 488 (1:250, Invitrogen). Antibody labeling was performed according to standard protocol (Raft et al., 2007), except the sections were subjected to antigen retrieval by citrate boiling for 5 min before immunostaining for α-Sox2.
Double-labeling of tissue sections with in situ and immunohistochemistry.
To analyze gene expression at a cellular level, ear sections were first probed for RNA transcripts (e.g., Ngn1), followed by labeling with rabbit polyclonal α-GFP antibody (1:500 Invitrogen) overnight at 4°C. During the in situ procedure, proteinase K exposure was reduced to 1 min and subsequent colorimetric development was carefully monitored and terminated when ectopic Ngn1 hybridization signals were apparent. The secondary antibody used was goat α-rabbit Alexa Fluor 488 (1:250 Invitrogen).
Ganglion size measurements, cell counts, and statistical analyses.
For analyzing the size of ganglia after electroporation, ear sections were subjected to Neurod1 in situ hybridization and photographed using a Zeiss microscope. The Neurod1-positive, ganglionic regions were traced with the NIH ImageJ analyzer and computed. The size of otocysts was estimated in a similar manner by tracing the outline of the otocyst in sections and summing their areas. Two-tailed Student's t tests were performed between control and treated samples with α levels of 0.05, 0.01, and 0.001.
Analyses of Nop-1 activity were conducted by coelectroporating Nop1-GFP or Nop1-EboxMut-GFP with various plasmids Ngn1-DsRed, DsRed, Neurod1-RFP, or RFP as indicated. Ear sections were subjected to double-antibody labeling with α-GFP-FITC and α-DsRed-Express, counterstained with DAPI, and photographed using a Zeiss microscope. The images were merged in Adobe Photoshop and the total number of cells that coexpressed both GFP and DsRed per ear were counted. In some specimens, adjacent immunolabeled sections were probed for Ngn1 transcripts using in situ hybridization for identifying the NSD. Two-tailed Student's t tests were performed on the total number of coexpressing cells with α levels of 0.05 and 0.001, or a two-tailed χ2 test in a 2 × 2 contingency table was used for quantification as indicated.
Results
Sox2 expression is strong in the NSD but weak in the CVG
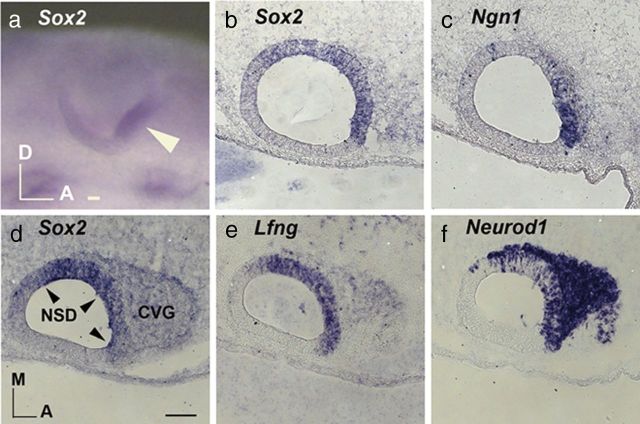
The NSD of the developing inner ear is defined by the overlapping expression domains of a number of genes such as Lfng, fibroblast growth factor 10 (Fgf10), and Sox2 (Cole et al., 2000; Alsina et al., 2004; Neves et al., 2007). Figure 1 illustrates the expression of Sox2 in the Lfng-positive NSD located primarily in the anteroventral region of the otic cup and otocyst (Fig. 1a,b,d,e). A subpopulation of cells in the NSD expresses Ngn1 and Neurod1, and these neuroblasts delaminate from the otic epithelium and coalesce to form the CVG. In the delaminated neuroblasts, Neurod1 but not Ngn1 transcripts are strongly expressed (Fig. 1c,f). Both Sox2 and Lfng transcripts are also much reduced in the CVG compared to the NSD (Fig. 1b,d,e). This reduction of Sox2 expression in the CVG suggests that Sox2 downregulation is required before neuronal differentiation, similar to its postulated role in other neural tissues.
Figure 1.
Endogenous expression of Sox2 in the developing chicken inner ear. a, Whole mount expression of Sox2 in the NSD of the otic cup (arrowhead). b and c and d–f, are 12 μm adjacent sections of otocysts at embryonic day 3 showing overlapping expression of Sox2 (b, d), Lfng (e), Ngn1 (c), and Neurod1 (f) in the NSD. Only Neuord1 (f) is strongly expressed in the neuroblasts of the CVG. A, Anterior; D, dorsal; M, medial. Scale bars, 100 μm.
Ectopic Sox2 causes a decrease in the size of CVG but an upregulation of Ngn1
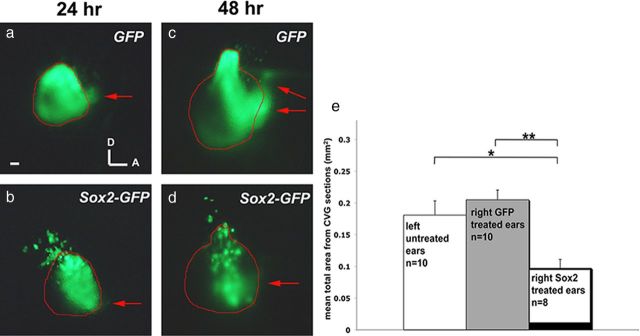
Based on the gene expression patterns and the postulated role of Sox2 during neurogenesis, we hypothesized that overexpression of Sox2 would inhibit neurogenesis in the inner ear. We tested this hypothesis by electroporating Sox2-IRES-GFP (referred as Sox2-GFP) or control IRES-GFP (referred as GFP) plasmid into the otic cup and analyzing the ears 24 and 48 h later. Sox2-GFP-treated ears appear to have a smaller CVG (Fig. 2b,d). Some of the 48 h specimens were sectioned, processed for in situ hybridization of Neurod1 transcripts, and used to quantify the size of the otocyst and ganglion (see Materials and Methods). Although there is no difference in the size of either the otocyst or the CVG between GFP electroporated and nonelectroporated ears, electroporation with Sox2-GFP resulted in ∼50% reduction in the size of CVG, compared to controls (Fig. 2e). These results support the hypothesis that overexpression of Sox2 inhibits neuronal formation.
Figure 2.
Overexpression of Sox2 leads to a decrease in the size of CVG. a–d, Otocysts at 24 h (a, b) and 48 h (c, d) after electroporation with GFP (a, c) or Sox2-GFP (b, d) between 10 and 17 somites stage. Brightfield images were used to outline the otocysts, shown in red, and red arrows point to the delaminated neuroblasts. There are more delaminated neuroblasts in GFP controls than Sox2-GFP ears at both time points. e, Quantitative analysis of the size of CVG (see Materials and Methods) indicates that the average size of CVG in GFP-treated ears is not significantly different from nonelectroporated control ears, whereas CVGs of Sox2-treated ears are ∼50% smaller than nonelectroporated and GFP-treated ears. Student's t test, *p = 0.0068; **p = 0.000084; error bars represent SEM. Scale bar, 100 μm.
In both chicken neural tube and Xenopus retina, overexpression of Sox2 inhibits neurogenesis, whereas proneural gene expression either remains unchanged or is downregulated in these tissues (Bylund et al., 2003; Van Raay et al., 2005). In contrast, Sox2-GFP-treated ears show ectopic Ngn1 expression 15–20 h after electroporation (Fig. 3a–d). Ectopic Ngn1 expression is observed outside of the NSD as well (Fig. 3d, white bracket). Double-labeling of Sox2-GFP and control ear sections with anti-GFP immunostaining and Ngn1 in situ hybridization indicates that there is a good correlation between GFP expression and Ngn1 upregulation in Sox2-GFP samples (Fig. 3k–l′, arrowheads), whereas no upregulation of Ngn1 is observed in GFP controls (Fig. 3i–j′, arrowheads). These results suggest a possible cell-autonomous induction of Ngn1 by Sox2. Furthermore, Ngn1 induction is rapid and is detectable within 6–7 h after Sox2 electroporation (Fig. 3m–p), consistent with the notion that Sox2 induces Ngn1 in a cell-autonomous fashion. Notably, despite the upregulation of Ngn1, there is no concomitant upregulation of Neurod1 in the Sox2-treated ears at 15–20 h or later time-points after electroporation (Fig. 3e–h; data not shown), suggesting that neurogenesis does not progress in these cells. This result is consistent with the observation of reduced CVG size (Fig. 2).
Figure 3.
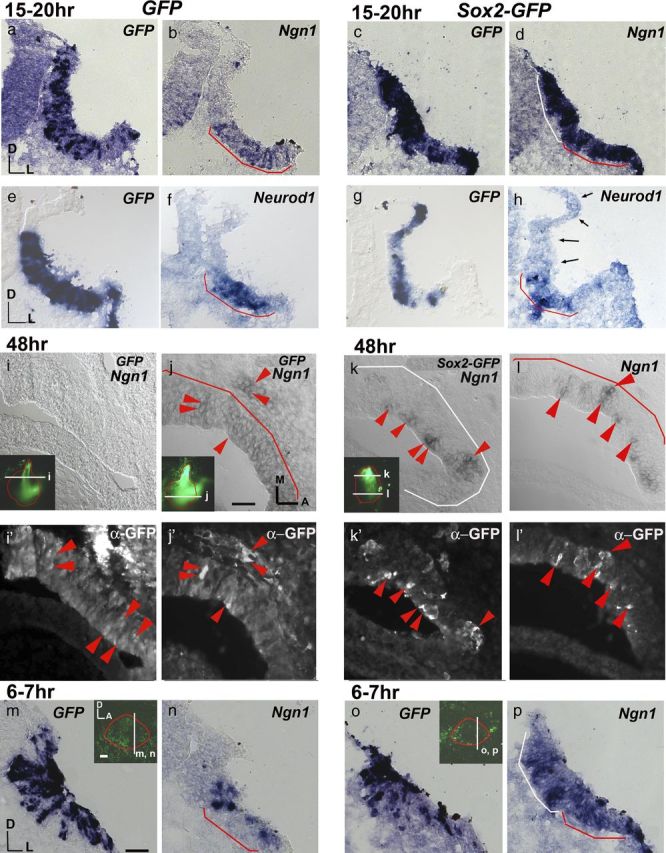
Sox2 upregulates Ngn1 expression but not Neurod1. a–h, GFP control (a, b, e, f) and Sox2-GFP-treated (c, d, g, h) otocysts 15–20 h after electroporation probed for GFP (a, c, e, g), Ngn1 (b, d) and Neurod1 (f, h) transcripts. In the GFP control ears, Ngn1 (b) and Neurod1 (f) are expressed only in the NSD (red bracket) but not in the region dorsal to the NSD (n = 7/7). c, d, g, h, In Sox2-GFP-treated ears, Ngn1 is strongly expressed in both the NSD (d, red bracket) and in a region of ectopic expression dorsal to the NSD (white bracket; n = 6/6), but no such upregulation is observed for Neurod1 (h, arrows; n = 7/7). i–l′, Sections of GFP controls (i–j′) and Sox2-GFP (k–l′)-treated ears double-labeled for ectopic Ngn1 transcripts and anti-GFP antibody 48 h after electroporation. GFP control ears show GFP immunostaining in the NSD with no ectopic Ngn1 expression (j,j′, red arrowheads). No Ngn1 transcript labeling is evident in dorsal sections of GFP controls (i), despite the presence of GFP immunostaining (i′). In Sox2-GFP ears, double-labeled cells (red arrowheads) are present in the dorsal non-NSD (k, k′) and ventral NSD region (l, l′) (n = 4/4). m–p, Otic cups electroporated with GFP control (m, n) or Sox2-GFP (o, p) and harvested after 6 to7 h. Adjacent sections were probed for GFP (m, o) and Ngn1 (n, p). Ngn1 expression is upregulated in the NSD (p, red bracket) as well as in regions outside the NSD (p, white bracket) of Sox2-treated ears (n = 7/7), compared with GFP control (n, NSD red bracket) (n = 7/7). Ears are outlined in red, and the level of section is indicated with white lines (insets). Scale bars, 100 μm.
Ngn1 is sufficient to promote neurogenesis and upregulate Neurod1
Despite the observed upregulation of Ngn1 in Sox2-treated ears, neurogenesis fails to proceed. These results seem contradictory, because Ngn is a potent inducer of neurogenesis in other neural systems (Ma et al., 1998; Bylund et al., 2003). Therefore, we investigated whether Ngn1 is equally potent in inducing neurogenesis in ear tissues. Electroporation experiments conducted with Ngn1-IRES-GFP (referred as Ngn1-GFP) show broad ectopic cell delamination from the entire otocyst within 24 h (Fig. 4c), compared to controls (Fig. 4a,b). Delamination is more evident at 48 h, to the extent that the otocysts are malformed and smaller in size (Fig. 4d, white arrows, g–h″, white arrowheads). Similar to control neuroblasts (Fig. 4e–f′, red arrowhead), these delaminated cells express Neurod1 (Fig. 4g–h′, white arrowheads). The ectopic expression of Neurod1 and the subsequent delamination of these cells could be a result of an expanded NSD. However, there is no obvious expansion of the Lfng expression domain (Fig. 4g″,h″) (n = 15) compared with controls (Fig. 4e″,f″) as would be expected if this were the case. Because a majority of the GFP-positive cells in the Ngn1-GFP specimens appear to have delaminated from the otic epithelium already by 48 h (Fig. 4g,h), we harvested some specimens 15 h after electroporation and confirmed the presence of ectopic Neurod1-positive cells within the otic epithelium at this earlier time point (Fig. 4i–j′). This ectopic Neurod1 expression pattern was not observed in Sox2-GFP specimens (Fig. 3e–h). Together, these results indicate that unlike Sox2-GFP, overexpressing Ngn1 is sufficient to upregulate Neurod1 and induce ectopic neurogenesis in the inner ear.
Figure 4.
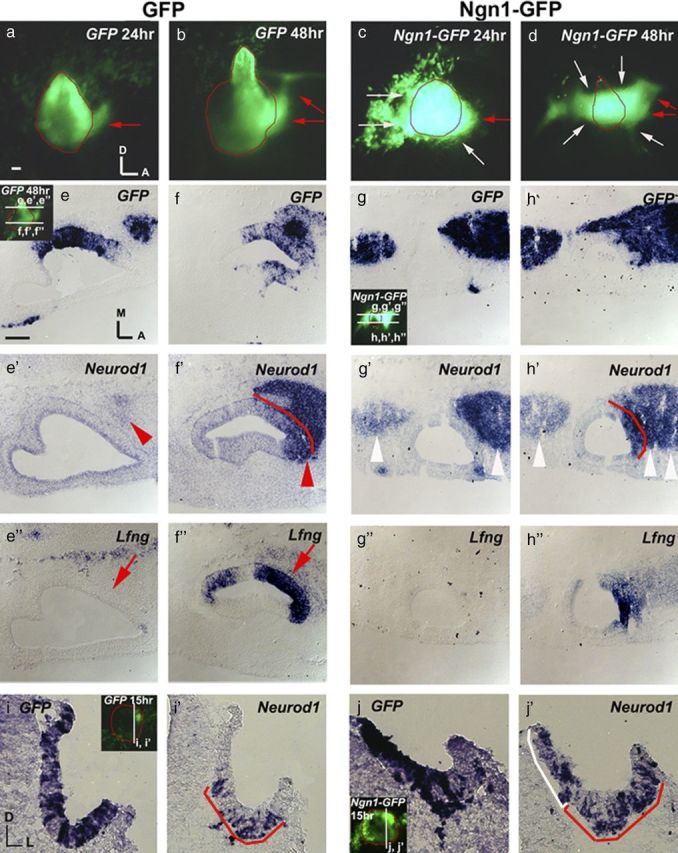
Ngn1 is sufficient to promote neurogenesis and upregulate Neurod1. a–d, Otic cup electroporated with GFP (a, b) or Ngn1-GFP (c, d) and harvested after 24 h (a, c) and 48 h (b, d). The outline of the otocyst is indicated in red. Red arrows point to the presumably delaminated neuroblasts. Neuroblasts delaminate from the NSD (c, d, red arrows) as well as ectopically from other regions of the otocyst in Ngn1-GFP ears (c, d, white arrows) compared with GFP ears (a, b). e–h″, Adjacent tissue sections probed for GFP, Neurod1, and Lfng transcripts in GFP control (e–f″) and Ngn1-GFP (g–h″)-treated ears 48 h after electroporation. In the GFP control ears, Neurod1 is expressed only in the CVG located anteromedial to the otocyst (e′, f′, red arrowheads) and the Lfng-positive NSD in the ventral otocyst (f′, f″, arrow), but not dorsal to the NSD (e′, e″, arrow), which is Lfng negative (n = 10/10). In Ngn1-GFP ears (g, h″), Neurod1-positive neuroblasts are detected both anterior and posterior to the otocyst in both dorsal (g′) and ventral (h′) sections (white arrowheads). However, Neurod1 expression in the otic epithelium is within the Lfng-positive NSD (h′, h″) (n = 4/4), similar to controls (f, f″). i–j′, Adjacent tissue sections probed for GFP and Neurod1 transcripts in GFP- (i, i′) and Ngn1-GFP- (j, j′) treated ears 15 h after electroporation. Neurod1 expression is upregulated in the ear epithelium in Ngn1-GFP-treated ears (j′, white bracket) (n = 6/8) but not in the GFP control (i′) (n = 6/6). Red brackets outline the NSD. Scale bars, 100 μm.
Overexpressing Ngn1 leads to downregulation of endogenous Sox2
In the chicken neural tube, overexpression of Ngn2 leads to the downregulation of Sox2 (Bylund et al., 2003). To determine whether Ngn1 inhibits Sox2 expression in the inner ear, we analyzed immunostaining of Sox2 in Ngn1-GFP-treated ears 15 h after electroporation. Sox2 immunostaining is significantly reduced in Ngn1-GFP ears (Fig. 5c–d′, asterisk) compared to GFP control ears (Fig. 5a,b′). Thus, whereas ectopic Ngn1 drives neuroblast formation, Sox2 expression is concomitantly downregulated. These results are consistent with the observation that the endogenous Sox2 transcripts are reduced in the CVG (Fig. 1b,d) and suggest that Sox2 downregulation by Ngn1 is required for neurogenesis to proceed normally.
Figure 5.
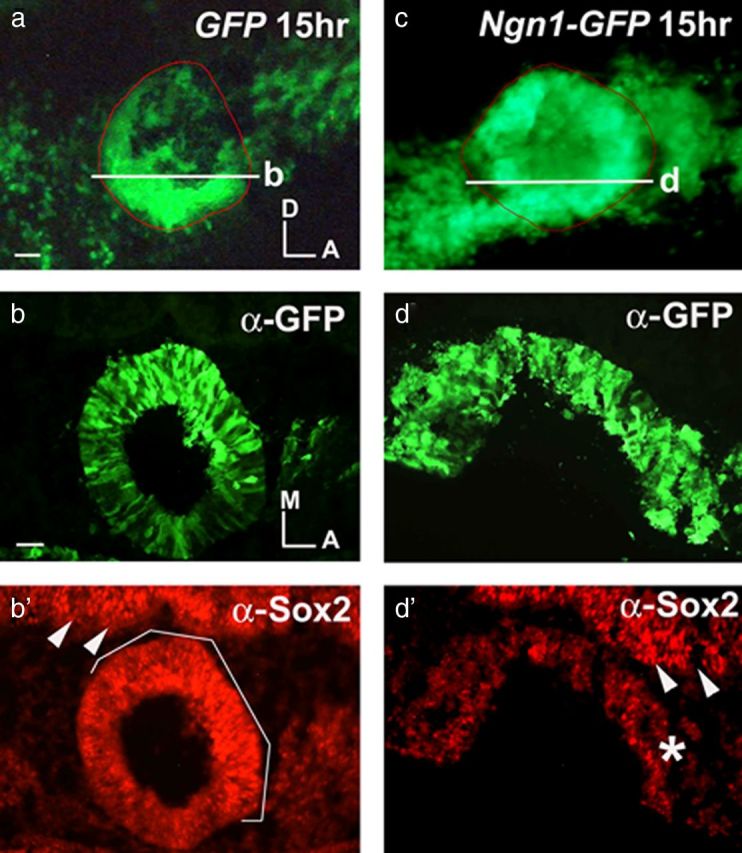
Overexpressing Ngn1 leads to downregulation of Sox2. a, c, Otic cups were electroporated with GFP (a) and Ngn1-GFP (c) and harvested at 15 h. Ears shown in a and c were sectioned (b and d, respectively) and double-labeled with anti-GFP (b, d) and anti-Sox2 (b, d′) antibodies. b, b′, Sox2 immunostaining in the NSD of GFP-treated ears (b′, white bracket) (n = 4/4) indicates that some of the cells are positive for both Sox2 and GFP. d′, The GFP-positive region in Ngn1-GFP ears shows reduced Sox2 immunostaining (asterisk) (n = 4/5). In contrast, staining in the neural tube is similar in-treated and control samples (b′, d′, white arrowheads). Ngn1-GFP electroporated ears often show a delay in otic cup closure (c–d′). The ears are outlined in red (a, c), and the white lines in a and c indicate the level of section in b and d, respectively. Scale bars, 100 μm.
Nop-1 regulatory element of Sox2 is inhibited by Ngn1
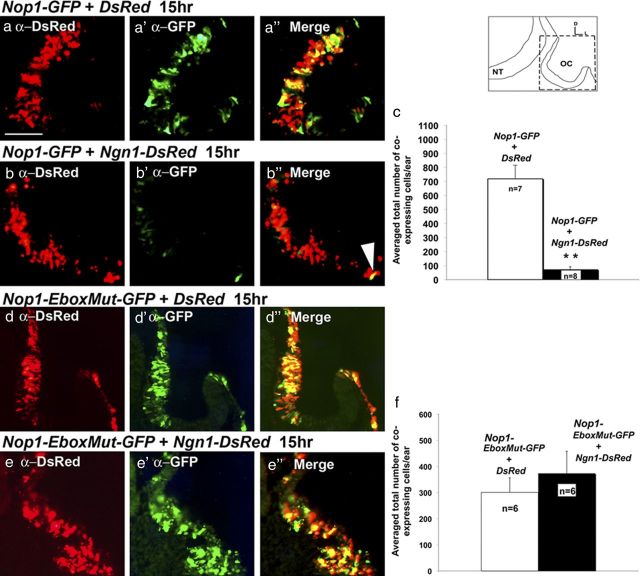
The tissue-specific expression of Sox2 is mediated by many cis-regulatory elements including Nop-1, which confers expression in the developing chicken inner ear (Uchikawa et al., 2003). This Nop-1 enhancer has a repressive domain (Sugahara et al., unpublished results). This repressive domain contains an E-box (CANNTG) consensus sequence motif (Blackwell et al., 1993; Bertrand et al., 2002; Seo et al., 2007), which can be bound by E-proteins in complex with proneural bHLH transcription factors, such as Ngn1 or Neurod1 (Markus et al., 2002). To test whether Ngn1 can directly interact with Nop-1 to inhibit Sox2 transcription, a Nop-1 enhancer driven EGFP (Nop1-GFP) was electroporated into the otic cup in the presence of Ngn1-DsRed or DsRed alone and analyzed 15 h later (Fig. 6). Based on the total number of GFP and DsRed coexpressing cells, the Nop1-GFP enhancer activity is significantly reduced in the presence of Ngn1-DsRed compared to DsRed controls (Fig. 6a–c). In contrast, the number of double-labeled cells is not significantly decreased when a plasmid with a mutated E-box in Nop-1 (Nop1-EboxMut-GFP) was used (Fig. 6d–f). These findings indicate that Ngn1 inhibits Nop-1 activity and it may inhibit Sox2 transcription in vivo by binding to the E-box of Nop-1.
Figure 6.
Ngn1 inhibits Nop-1 activity. a–b″, Nop1-GFP was co-electroporated with DsRed (a–a″) or Ngn1-DsRed (b–b″). Fifteen hours after electroporation, ears were harvested, sectioned, and double-labeled with anti-GFP and anti-DsRed antibodies, and counterstained with DAPI (data not shown). Images were merged. Whereas many cells in the control samples coelectroporated with DsRed (a″) are double-labeled, few double-labeled cells are present in ears that were coelectroporated with Ngn1-DsRed (b″, arrowhead). c, Quantification of GFP and DsRed colabeled cells indicates that the number of colabeled cells is significantly reduced in the presence of Ngn1-DsRed compared to DsRed control (p = 0.00036). d–e″, Nop1-EboxMut-GFP was coelectroporated with DsRed (d–d″) or Ngn1-DsRed (e–e″). In contrast, the number of colabeled cells of Nop1-EboxMut-GFP is not significantly affected in the Ngn1-DsRed ears compared with control ears (f) (p = 0.49). Error bars represent SEM. Orientation of sections is indicated in the schematic. NT, Neural tube; OC, otic cup. Scale bar, 100 μm.
Neurod1 is sufficient to promote neurogenesis and inhibit Nop-1 activity
Neurod1, which is activated by Ngn1 (Ma et al., 1996), is required for the formation of the CVG (Liu et al., 2000). To test whether Neurod1 induces neurogenesis similar to Ngn1, Neurod1-IRES-RFP (Neurod1-RFP), or IRES-RFP (RFP) were electroporated at the otic cup stage and harvested 48 h later (Fig. 7a–f″). Neurod1-RFP ears show ectopic neuroblast delamination that is positive for a neuronal marker, TuJ1 (Fig. 7e,e′,f,f′) compared with RFP controls (Fig. 7b–c″). Therefore, expression of Neurod1 is also sufficient to mediate neurogenesis in the developing inner ear.
Figure 7.
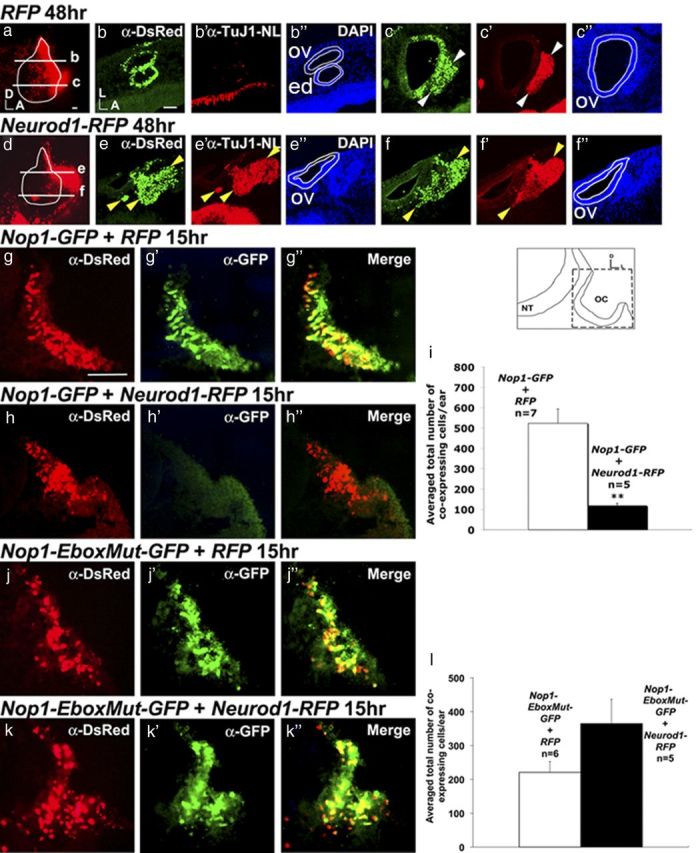
Neurod1 is sufficient to promote neurogenesis and inhibits Nop-1 activity. a, d, Otocysts electroporated with RFP (a) or Neurod1-RFP (d) at 48 h after electroporation. Ears are outlined and levels of sections are indicated in white. b–c″, Ear sections were double-labeled with anti-DsRed (b, c) and anti-TuJ1 (b′, c′, and counterstained with DAPI (b″, c″). Only ventral sections of RFP controls (n = 4/4) show delaminated DsRed-positive and Tuj1-positive neuroblasts (c–c″). e–f″, In Neurod1-RFP-treated ears, double-labeled anti-DsRed and anti-TuJ1-positive neuroblast delamination is observed in both dorsal (e, e′, yellow arrowheads) and ventral sections (f, f′, yellow arrowheads) (n = 3/3). g–h″, Nop1-GFP coelectroporated with RFP control (g–g″) or Neurod1-RFP (h–h″). Embryos were harvested 15 h after electroporation, sectioned, double-labeled with anti-GFP and anti-DsRed, and counterstained with DAPI (data not shown). (g″) and (h″) are merged images. i, Quantification of the number of GFP and DsRed colabeled cells. The number of double-labeled cells is significantly reduced in the presence of Neurod1-RFP compared to controls (**p = 0.0013). j–k″, Nop1-EboxMut-GFP is co-electroporated with RFP (j) or Neurod1-RFP (k). l, The number of colabeled cells is not significantly affected by Neurod1-RFP compared with controls (p = 0.12). Error bars represent SEM. Orientation of the sections is indicated in the schematic. NT, Neural tube; OC, otic cup; ov, otic vesicle; ed, endolymphatic duct. Scale bars, 100 μm.
In addition, we tested whether Neurod1 is also capable of inhibiting Nop-1 activity. Nop1-GFP and Nop1-EboxMut-GFP plasmids were coelectroporated with Neurod1-RFP (Fig. 7g–l). The Nop1-GFP enhancer activity is significantly reduced in the presence of Neurod1-RFP compared with controls (Fig. 7i) based on the reduced number of colabeled cells. However, the number of colabeled cells is not significantly different between Neurod1-RFP and RFP controls when Nop1-EboxMut-GFP was used (Fig. 7l). These findings suggest that Neurod1 is capable of inhibiting Nop-1 activity in a similar manner as Ngn1.
Nop-1 activity is inhibited within the NSD
Next, to determine whether inhibition of Sox2 transcription through Nop-1 is biologically relevant, we tested whether the presence of endogenous proneural bHLH factors within the NSD could alter Nop1-GFP expression. We electroporated specimens with Nop1-GFP and RFP and adjacent sections were processed for immunostaining and Ngn1 in situ hybridization. Using the Ngn1 expression domain to demarcate the NSD, colabeled cells inside and outside of the NSD were counted (Fig. 8). The number of colabeled cells in the NSD (Fig. 8b″, arrows, i) was significantly reduced compared with colabeled cells outside of the NSD (Fig. 8b′,d″, arrowheads), suggesting that the Nop1-GFP enhancer activity is inhibited in the NSD. This reduction in the number of colabeled cells was not observed when the Nop1-EboxMut-GFP plasmid was used (Fig. 8e–h″, arrowheads). Consistently, the proportion of double-labeled cells outside the NSD is not significantly different between Nop1-GFP and Nop1-EboxMut-GFP specimens (Fig. 8j). Together, these results suggest that Nop-1 activity can be inhibited by some endogenous factors within the NSD. Although these endogenous factors have not been identified, taking into consideration the known functions of Ngn1 and Neurod1 in the CNS, they are the most likely candidates expressed in the NSD that interact with the E-box binding site in Nop-1.
Figure 8.
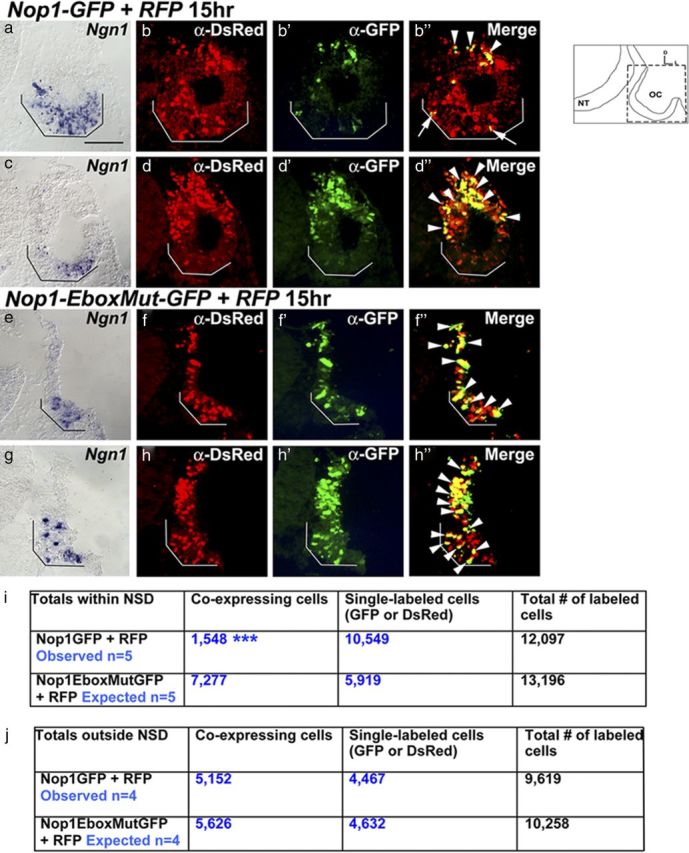
The activity of Nop-1 is inhibited within the endogenous Ngn1 and Neurod1-positive NSD. a–h″, Ears coelectroporated with Nop1-GFP and RFP (a–d″) or Nop1-EboxMut-GFP and RFP as controls (e–h″) 15 h after electroporation. Adjacent sections probed for either Ngn1 transcripts (a, c, e, g; serving as an indicator for the NSD, black brackets) or double-labeled with anti-DsRed and anti-GFP (b–b″, d–d″, f–f″, h–h″) and counterstained with DAPI (data not shown). b″, d″, f″, and h″ are merged images. b″, d″, Doubled-labeled cells are present outside of the NSD (arrowheads), but fewer are present within the NSD (b″ white bracket and arrows) of Nop-1-treated ears. In contrast, ears electroporated with the Nop1-EboxMut-GFP plasmid show double-labeled cells outside (f–f″, h–h″, arrowheads) as well as inside the NSD (f–f″, h–h″, arrowheads within white brackets). i, j, Quantification of GFP and DsRed double-labeled cells within (i) and outside (j) the NSD using χ2 tests. The data indicate that the number of colabeled cells in Nop1-GFP ears is significantly reduced within the NSD compared with Nop1-EboxMut-GFP ears (p < 0.0001; χ2 = 4982.53), whereas no significant difference was observed between the two treatments outside the NSD (p = 0.0693; χ2 = 3.299). The level of sections is indicated in the schematic. NT, Neural tube; OC, otic cup. Scale bar, 100 μm.
Discussion
The role of Sox2 in mediating neural competence
Increasing evidence suggests that neural development from progenitor state to the formation of neuronal subtypes is a tightly regulated cascade of molecular events. The factors that define neural competency and the roles of Sox2 in this process are not clear. Sox2 may mediate neural competency by facilitating the initiation of neurogenesis. Evidence for Sox2's role in inner ear neurogenesis first came from analyses of the mouse mutants, ysb and lcc, in which Sox2 expression in the inner ear is reduced or absent, respectively (Kiernan et al., 2005; Puligilla et al., 2010). Neuronal formation is affected in the lcc mutants even though the underlying molecular mechanisms have not been thoroughly investigated (Puligilla et al., 2010). Ectopic neuronal formation in cochlear explants by Sox2 is additional supporting evidence for a role of Sox2 in inner ear neurogenesis (Puligilla et al., 2010). Our results demonstrate a rapid induction of Ngn1 by Sox2, suggesting a direct, cell-autonomous action via transcriptional regulation. In support of this notion, Sox2 has been shown to upregulate Ngn1 expression in a cochlear cell line and recently in chicken otocysts (Jeon et al., 2011; Neves et al., 2012), and a Sox2 binding site has been identified in the 5′ promoter region of Ngn1 (Jeon et al., 2011). Similarly, a Sox2 binding site has also been identified in the cis-regulatory element of Ngn1 in zebrafish embryos using chromatin immunoprecipitation (Okuda et al., 2010). Therefore, the binding of Sox2 to a regulatory element of the Ngn1 promoter may be one of the requirements for acquiring neural competence.
Thus far, there is no direct evidence that Sox2 induces proneural genes in other neural systems. Nevertheless, data from several studies place Sox2 upstream of proneural genes. For example, Sox2 is thought to function downstream from Frizzled 5 but upstream of the proneural gene, Xash3, in mediating retina formation in Xenopus (Van Raay et al., 2005). In addition, Sox3, another SoxB1 group family member, is postulated to be downstream of Lef1 in inducing the proneural gene, Zash1a, in the developing zebrafish hypothalamus (Lee et al., 2006). In both cases, overexpression of Sox2 or Sox3 rescues the neural phenotype caused by knockdown of Frizzled 5 and Lef1, respectively (Van Raay et al., 2005; Lee et al., 2006). The lack of a direct causal relationship between overexpressing Sox and induction of proneural genes in these systems (in contrast to the inner ear results shown here) could be due to the cellular context of the tissue at the time of the experiment. It is clear that the onset of proneural gene expression is highly regulated by multiple activators and repressors. The presence of repressors may mask the positive effects of Sox2 on proneural gene expression. For example, the Notch signaling pathway, which is thought to be active at the same developmental time as Sox2, has been shown to inhibit Ngn2 expression in the chicken neural tube by upregulating Hes1 and Hes5 (Holmberg et al., 2008). Therefore, Notch and Sox proteins may function in an antagonistic manner to coordinate the timing of neural progression. Furthermore, if proneural genes are already activated in vivo at the time of the experiment, overexpression of Sox2 would inhibit rather than promotes expression of proneural/neuronal genes (Agathocleous et al., 2009). These results are also consistent with our finding that neural progression is inhibited by overexpression of Sox2.
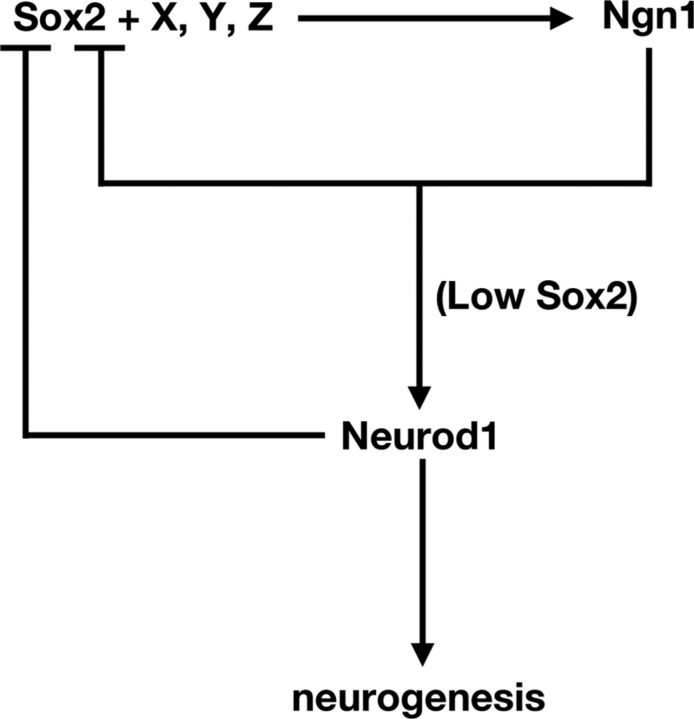
Although we demonstrate that the cellular context of the otic cup is conducive to Ngn1 induction by exogenous Sox2, it is unlikely to be the sole factor in inducing Ngn1 in vivo (Fig. 9). It has been shown recently that Ngn1 and Neurod1 transcripts are downregulated in Eya1- or Six1-null inner ears, and overexpression of both Eya1 and Six1, but not either factor alone, is able to induce neurogenesis in mouse embryos in vitro (Ahmed et al., 2012b). Sox2, which is known to partner with multiple proteins and transcription factors (Wilson and Koopman, 2002; Uchikawa et al., 2011), has also been shown to physically interact with Eya1 in several embryonic cell lines (Zou et al., 2008). Therefore, it is possible that Sox2 interacts with factors such as Eya1 and Six1 in regulating Ngn1 expression. In support of this notion, Sox2 has been recently shown to interact with Eya1 and Six1 and activate another proneural gene, Atoh1, in cochlear cultures (Ahmed et al., 2012a).
Figure 9.
A model showing Sox2 and other unknown factors (X–Z) initiate neurogenesis by upregulating Ngn1. Ngn1 inhibits Sox2 transcription to upregulate Neurod1 and promote progression of neurogenesis. Neurod1 further inhibits Sox2 transcription and mediates neuronal differentiation.
Neurogenesis proceeds only after Sox2 is downregulated
Overexpression of Ngn1 is sufficient to initiate neurogenesis. However, despite the fact that overexpressing Sox2 induces Ngn1 in the otocyst, Neuord1 expression and neuroblast delamination from the otic epithelium are inhibited. We attribute the lack of Neuord1 upregulation in Sox2-treated specimens to high levels of Sox2 driven by the β-actin promoter, which cannot be effectively inhibited by Ngn1. This result is also consistent with previous findings that Sox2 downregulation is required for neurogenesis to proceed (Bylund et al., 2003; Bani-Yaghoub et al., 2006). Furthermore, we provide the first evidence that both Ngn1 and Neurod1 can inhibit Sox2 at the transcriptional level. Ngn1 and Neurod1 function via interacting with the Nop-1 enhancer, which is conserved between chickens and mammals (Uchikawa et al., 2003). Moreover, our results indicate that a key step of neural progression is the induction of Neurod1, which requires Ngn1, as well as a reduced level of Sox2. This notion is consistent with the reduced Sox2 hybridization signals in the CVG compared with the NSD (Fig. 1).
Notably, in neural stem cells of the adult mouse hippocampus, Sox2 functions as a repressor of Neurod1, and the removal of Sox2 is required before Wnt-signaling can activate Neurod1 (Kuwabara et al., 2009). Based on these results, we postulate that a key component of neural progression is the transcriptional repression of Sox2, which in turn leads to the alleviation of repression on Neurod1. Although both Ngn1 and Neurod1 are capable of engaging in an inhibitory feedback loop of Sox2 transcription, the earlier onset of Ngn1 expression relative to that of Neurod1 suggests that Ngn1 is a more important factor in repressing Sox2 activity. This inhibitory action on Sox2 does not necessarily have to be mediated through binding to Nop-1 alone.
In addition to the provided evidence that high levels of exogenous Sox2 block progression of neurogenesis cell-autonomously, there is likely to be a non-cell-autonomous contribution to the reduction in the size of the CVG in Sox2 overexpressed specimens. Ngn1 is thought to positively regulate Delta1 expression, which activates the Notch signaling pathway in neighboring cells to inhibit the neural fate (Ma et al., 1996; Ma et al., 1998, Brooker et al., 2006). Our preliminary results indicate that Delta1 expression is also upregulated in the ectopic Sox2 specimens, suggesting that lateral inhibition of the neural fate via Delta-Notch signaling could contribute to the reduced CVG size as well.
Sox2, Ngn1, and Neurod1 form a regulatory network within the NSD
The ability of Ngn1, Neurod1, and Sox2 (to some extent) to drive neural fates outside the NSD indicates that these genes can override extrinsic signaling by factors, such as Wnts, Sonic hedgehog, and retinoic acid that normally interact to limit the NSD to the anteroventral region of the otic cup (Riccomagno et al., 2005; Bok et al., 2007, 2011). Given the well established relationship between Sox and the Wnt signaling pathway in other systems (Agathocleous et al., 2009; Kormish et al., 2010) and the importance of Wnt signaling in dorsal-ventral patterning of the inner ear (Riccomagno et al., 2005), it is likely that exogenous Sox2 is overriding the Wnt signaling that normally restricts the neural sensory competent region to the ventral otic cup and otocyst. This hypothesis warrants further investigation.
In conclusion, we have identified a molecular mechanism whereby Sox2 mediates neural competency and progression in the inner ear (Fig. 9). Given the similarity in the postulated role of Sox2 between the inner ear and other neural systems (Bylund et al., 2003; Van Raay et al., 2005; Bani-Yaghoub et al., 2006), this mechanism may be generally applicable to other neural tissues. The feedforward and feedback control between Sox2 and proneural genes elucidate a piece of the puzzle in this complex program of neural development.
Footnotes
This work was supported by the Intramural Program of NIDCD (to D.K.W.), Grants-in-Aid for Scientific Research 23770248 (to M.U.) and MEXT Japan 22247035 (to H.K.). We thank Michael Mulheisen, Nicole Huang, Nathan Hsieh, Eric Yi, and Andrew Yatteau for technical assistance. We thank Dr. Lisa Taneyhill at the University of Maryland at College Park for valuable advice throughout this project, Dr. Jonas Muhr for providing the Sox2 plasmid, Dr. Jean-Marc Matter for the Neurod1 plasmid, and Dr. Marianne Bronner-Fraser for the pCI-IRES-H2B-RFP plasmid. We also thank Drs. Lisa Cunningham, Tom Friedman, Matthew Kelley, James Keller, and Steven Raft for critical reading of this manuscript.
The authors declare no competing financial interests.
References
- Agathocleous M, Iordanova I, Willardsen MI, Xue XY, Vetter ML, Harris WA, Moore KB. A directional Wnt/beta-catenin-Sox2-proneural pathway regulates the transition from proliferation to differentiation in the Xenopus retina. Development. 2009;136:3289–3299. doi: 10.1242/dev.040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M, Wong EY, Sun J, Xu J, Wang F, Xu PX. Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Dev Cell. 2012a;22:377–390. doi: 10.1016/j.devcel.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M, Xu J, Xu PX. EYA1 and SIX1 drive the neuronal developmental program in cooperation with the SWI/SNF chromatin-remodeling complex and SOX2 in the mammalian inner ear. Development. 2012b;139:1965–1977. doi: 10.1242/dev.071670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsina B, Abelló G, Ulloa E, Henrique D, Pujades C, Giraldez F. FGF signaling is required for determination of otic neuroblasts in the chick embryo. Dev Biol. 2004;267:119–134. doi: 10.1016/j.ydbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Bani-Yaghoub M, Tremblay RG, Lei JX, Zhang D, Zurakowski B, Sandhu JK, Smith B, Ribecco-Lutkiewicz M, Kennedy J, Walker PR, Sikorska M. Role of Sox2 in the development of the mouse neocortex. Dev Biol. 2006;295:52–66. doi: 10.1016/j.ydbio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Blackwell TK, Huang J, Ma A, Kretzner L, Alt FW, Eisenman RN, Weintraub H. Binding of myc proteins to canonical and noncanonical DNA sequences. Mol Cell Biol. 1993;13:5216–5224. doi: 10.1128/mcb.13.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok J, Chang W, Wu DK. Patterning and morphogenesis of the vertebrate inner ear. Int J Dev Biol. 2007;51:521–533. doi: 10.1387/ijdb.072381jb. [DOI] [PubMed] [Google Scholar]
- Bok J, Raft S, Kong KA, Koo SK, Dräger UC, Wu DK. Transient retinoic acid signaling confers anterior-posterior polarity to the inner ear. Proc Natl Acad Sci U S A. 2011;108:161–166. doi: 10.1073/pnas.1010547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development. 2006;133:1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1–3 activity. Nat Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- Cavallaro M, Mariani J, Lancini C, Latorre E, Caccia R, Gullo F, Valotta M, DeBiasi S, Spinardi L, Ronchi A, Wanke E, Brunelli S, Favaro R, Ottolenghi S, Nicolis SK. Impaired generation of mature neurons by neural stem cells from hypomorphic Sox2 mutants. Development. 2008;135:541–557. doi: 10.1242/dev.010801. [DOI] [PubMed] [Google Scholar]
- Chae JH, Stein GH, Lee JE. NeuroD: the predicted and the surprising. Mol Cells. 2004;18:271–288. [PubMed] [Google Scholar]
- Cole LK, Le Roux I, Nunes F, Laufer E, Lewis J, Wu DK. Sensory organ generation in the chicken inner ear: contributions of bone morphogenetic protein 4, serrate1, and lunatic fringe. J Comp Neurol. 2000;424:509–520. doi: 10.1002/1096-9861(20000828)424:3<509::aid-cne8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Favaro R, Valotta M, Ferri AL, Latorre E, Mariani J, Giachino C, Lancini C, Tosetti V, Ottolenghi S, Taylor V, Nicolis SK. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci. 2009;12:1248–1256. doi: 10.1038/nn.2397. [DOI] [PubMed] [Google Scholar]
- Ferri AL, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala M, DeBiasi S, Nicolis SK. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- Hagstrom SA, Pauer GJ, Reid J, Simpson E, Crowe S, Maumenee IH, Traboulsi EI. SOX2 mutation causes anophthalmia, hearing loss, and brain anomalies. Am J Med Genet A. 2005;138A:95–98. doi: 10.1002/ajmg.a.30803. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Holmberg J, Hansson E, Malewicz M, Sandberg M, Perlmann T, Lendahl U, Muhr J. SoxB1 transcription factors and Notch signaling use distinct mechanisms to regulate proneural gene function and neural progenitor differentiation. Development. 2008;135:1843–1851. doi: 10.1242/dev.020180. [DOI] [PubMed] [Google Scholar]
- Jeon SJ, Fujioka M, Kim SC, Edge AS. Notch signaling alters sensory or neuronal cell fate specification of inner ear stem cells. J Neurosci. 2011;31:8351–8358. doi: 10.1523/JNEUROSCI.6366-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelberman D, Rizzoti K, Avilion A, Bitner-Glindzicz M, Cianfarani S, Collins J, Chong WK, Kirk JM, Achermann JC, Ross R, Carmignac D, Lovell-Badge R, Robinson IC, Dattani MT. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Invest. 2006;116:2442–2455. doi: 10.1172/JCI28658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kormish JD, Sinner D, Zorn AM. Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev Dyn. 2010;239:56–68. doi: 10.1002/dvdy.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12:1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Wu SF, Goering LM, Dorsky RI. Canonical Wnt signaling through Lef1 is required for hypothalamic neurogenesis. Development. 2006;133:4451–4461. doi: 10.1242/dev.02613. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Dumitriu B, Penzo-Méndez A, Han Y, Pallavi B. Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. Int J Biochem Cell Biol. 2007;39:2195–2214. doi: 10.1016/j.biocel.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai MJ. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev. 2000;14:2839–2854. doi: 10.1101/gad.840500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus M, Du Z, Benezra R. Enhancer-specific modulation of E protein activity. J Biol Chem. 2002;277:6469–6477. doi: 10.1074/jbc.M110659200. [DOI] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller Inner Ear Sensory Epithelia in Neurog1 Null Mice Are Related to Earlier Hair Cell Cycle Exit. Developmental Dynamics. 2005;234:633–650. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves J, Kamaid A, Alsina B, Giraldez F. Differential expression of Sox2 and Sox3 in neuronal and sensory progenitors of the developing inner ear of the chick. J Comp Neurol. 2007;503:487–500. doi: 10.1002/cne.21299. [DOI] [PubMed] [Google Scholar]
- Neves J, Uchikawa M, Bigas A, Giraldez F. The prosensory function of Sox2 in the chicken inner ear relies on the direct regulation of Atoh1. PLoS One. 2012;7:e30871. doi: 10.1371/journal.pone.0030871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda Y, Ogura E, Kondoh H, Kamachi Y. B1 SOX coordinate cell specification with patterning and morphogenesis in the early zebrafish embryo. PLoS Genet. 2010;6:e1000936. doi: 10.1371/journal.pgen.1000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny LH, Nicolis SK. Sox2 roles in neural stem cells. Int J Biochem Cell Biol. 2010;42:421–424. doi: 10.1016/j.biocel.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Puligilla C, Dabdoub A, Brenowitz SD, Kelley MW. Sox2 induces neuronal formation in the developing mammalian cochlea. J Neurosci. 2010;30:714–722. doi: 10.1523/JNEUROSCI.3852-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft S, Koundakjian EJ, Quinones H, Jayasena CS, Goodrich LV, Johnson JE, Segil N, Groves AK. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134:4405–4415. doi: 10.1242/dev.009118. [DOI] [PubMed] [Google Scholar]
- Riccomagno MM, Takada S, Epstein DJ. Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 2005;19:1612–1623. doi: 10.1101/gad.1303905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Lim JW, Yellajoshyula D, Chang LW, Kroll KL. Neurogenin and NeuroD direct transcriptional targets and their regulatory enhancers. EMBO J. 2007;26:5093–5108. doi: 10.1038/sj.emboj.7601923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taranova OV, Magness ST, Fagan BM, Wu Y, Surzenko N, Hutton SR, Pevny LH. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006;20:1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchikawa M, Ishida Y, Takemoto T, Kamachi Y, Kondoh H. Functional analysis of chicken Sox2 enhancers highlights an array of diverse regulatory elements that are conserved in mammals. Dev Cell. 2003;4:509–519. doi: 10.1016/s1534-5807(03)00088-1. [DOI] [PubMed] [Google Scholar]
- Uchikawa M, Yoshida M, Iwafuchi-Doi M, Matsuda K, Ishida Y, Takemoto T, Kondoh H. B1 and B2 Sox gene expression during neural plate development in chicken and mouse embryos: universal versus species-dependent features. Dev Growth Differ. 2011;53:761–771. doi: 10.1111/j.1440-169X.2011.01286.x. [DOI] [PubMed] [Google Scholar]
- Van Raay TJ, Moore KB, Iordanova I, Steele M, Jamrich M, Harris WA, Vetter ML. Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron. 2005;46:23–36. doi: 10.1016/j.neuron.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Wilson M, Koopman P. Matching SOX: partner proteins and co-factors of the SOX family of transcriptional regulators. Curr Opin Genet Dev. 2002;12:441–446. doi: 10.1016/s0959-437x(02)00323-4. [DOI] [PubMed] [Google Scholar]
- Wu DK, Oh SH. Sensory organ generation in the chick inner ear. J Neurosci. 1996;16:6454–6462. doi: 10.1523/JNEUROSCI.16-20-06454.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Erickson C, Kim EH, Jin D, Fritzsch B, Xu PX. Eya1 gene dosage critically affects the development of sensory epithelia in the mammalian inner ear. Hum Mol Genet. 2008;17:3340–3356. doi: 10.1093/hmg/ddn229. [DOI] [PMC free article] [PubMed] [Google Scholar]