Abstract
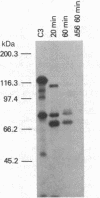
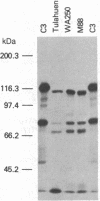
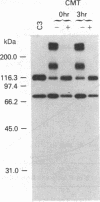
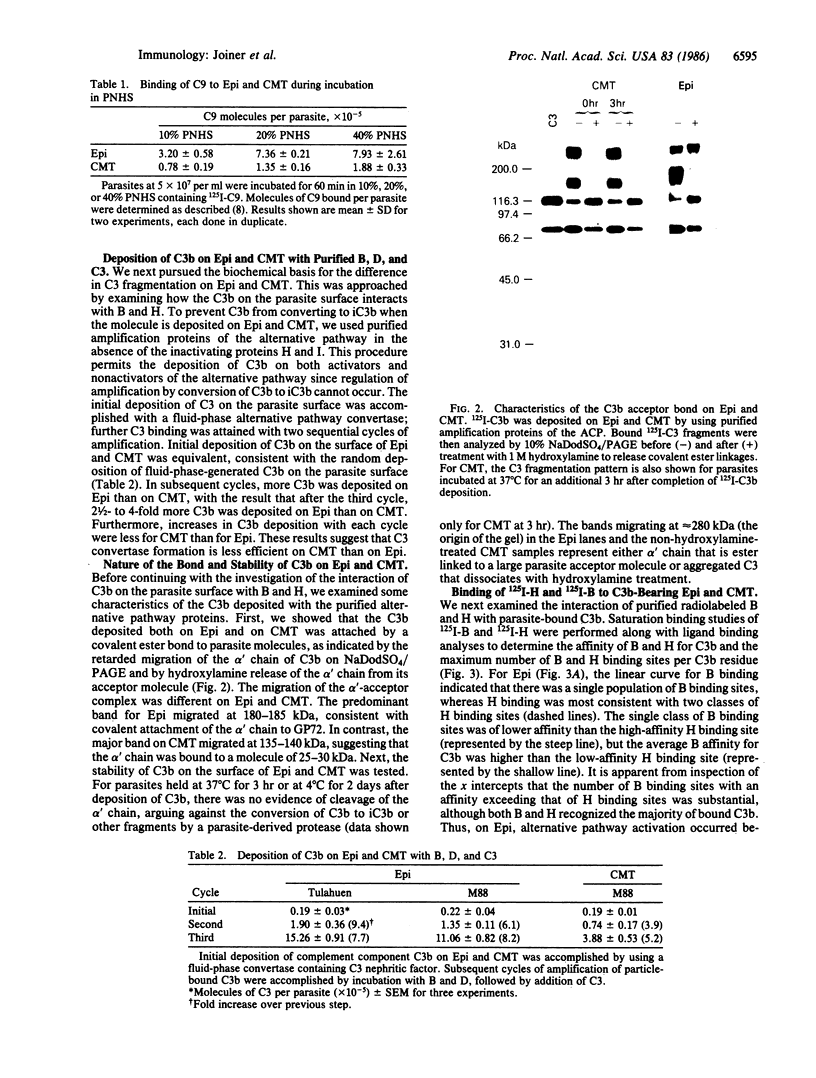
During its differentiation in the insect vector to a stage infective for the mammalian host, Trypanosoma cruzi becomes resistant to lysis by the alternative pathway of complement. To elucidate the mechanism of complement evasion, we studied control of complement activation on the surface of the noninfective epimastigote and the infective culture-derived metacyclic trypomastigote stages (CMT) of T. cruzi. It was found that the predominant form of complement component C3 on epimastigotes is C3b, whereas the majority of C3 on CMT is in the form of the hemolytically inactive fragment iC3b, which cannot participate in C5 convertase formation or lead to deposition of the lytic C5b-9 complex. Our results also showed that C3 binds by a covalent ester linkage to surface molecules of different molecular weight in the epimastigote stage and CMT. Binding studies with purified complement components indicated that CMT do not support efficient formation of an alternative pathway C3 convertase. C3b on the parasite surface fails to bind the amplification component, factor B, rather than showing enhanced binding of the control component, factor H. These results identify the biochemical basis for evasion of complement-mediated killing in T. cruzi and reveal a mechanism for developmental regulation of complement activation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown E. J., Joiner K. A., Gaither T. A., Hammer C. H., Frank M. M. The interaction of C3b bound to pneumococci with factor H (beta 1H globulin), factor I (C3b/C4b inactivator), and properdin factor B of the human complement system. J Immunol. 1983 Jul;131(1):409–415. [PubMed] [Google Scholar]
- Brown E. J., Joiner K. A., Gaither T. A., Hammer C. H., Frank M. M. The interaction of C3b bound to pneumococci with factor H (beta 1H globulin), factor I (C3b/C4b inactivator), and properdin factor B of the human complement system. J Immunol. 1983 Jul;131(1):409–415. [PubMed] [Google Scholar]
- Contreras V. T., Salles J. M., Thomas N., Morel C. M., Goldenberg S. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol Biochem Parasitol. 1985 Sep;16(3):315–327. doi: 10.1016/0166-6851(85)90073-8. [DOI] [PubMed] [Google Scholar]
- Fearon D. T. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5867–5871. doi: 10.1073/pnas.76.11.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman H. A. Mathematical theory of complex ligand-binding systems of equilibrium: some methods for parameter fitting. Anal Biochem. 1972 Aug;48(2):317–338. doi: 10.1016/0003-2697(72)90084-x. [DOI] [PubMed] [Google Scholar]
- Ferrante A., Allison A. C. Alternative pathway activation of complement by African trypanosomes lacking a glycoprotein coat. Parasite Immunol. 1983 Sep;5(5):491–498. doi: 10.1111/j.1365-3024.1983.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Franke E. D., McGreevy P. B., Katz S. P., Sacks D. L. Growth cycle-dependent generation of complement-resistant Leishmania promastigotes. J Immunol. 1985 Apr;134(4):2713–2718. [PubMed] [Google Scholar]
- Gaither T. A., Hammer C. H., Frank M. M. Studies of the molecular mechanisms of C3b inactivation and a simplified assay of beta 1H and the C3b inactivator (C3bINA). J Immunol. 1979 Sep;123(3):1195–1204. [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Harrison R. A., Lachmann P. J. The physiological breakdown of the third component of human complement. Mol Immunol. 1980 Jan;17(1):9–20. doi: 10.1016/0161-5890(80)90119-4. [DOI] [PubMed] [Google Scholar]
- Iida K., Nussenzweig V. Complement receptor is an inhibitor of the complement cascade. J Exp Med. 1981 May 1;153(5):1138–1150. doi: 10.1084/jem.153.5.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. II. C8 and C9 release C5b67 from the surface of Salmonella minnesota S218 because the terminal complex does not insert into the bacterial outer membrane. J Exp Med. 1982 Mar 1;155(3):809–819. doi: 10.1084/jem.155.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K., Hieny S., Kirchhoff L. V., Sher A. gp72, the 72 kilodalton glycoprotein, is the membrane acceptor site for C3 on Trypanosoma cruzi epimastigotes. J Exp Med. 1985 May 1;161(5):1196–1212. doi: 10.1084/jem.161.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum F., Lima M. F. Susceptibility of insect-borne, metacyclic forms of Trypanosoma cruzi to antibody-mediated mechanisms of destruction. Am J Trop Med Hyg. 1983 Nov;32(6):1236–1241. doi: 10.4269/ajtmh.1983.32.1236. [DOI] [PubMed] [Google Scholar]
- Kipnis T. L., David J. R., Alper C. A., Sher A., da Silva W. D. Enzymatic treatment transforms trypomastigotes of Trypanosoma cruzi into activators of alternative complement pathway and potentiates their uptake by macrophages. Proc Natl Acad Sci U S A. 1981 Jan;78(1):602–605. doi: 10.1073/pnas.78.1.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Law S. K., Levine R. P. Interaction between the third complement protein and cell surface macromolecules. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2701–2705. doi: 10.1073/pnas.74.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Nogueira N., Bianco C., Cohn Z. Studies on the selective lysis and purification of Trypanosoma cruzi. J Exp Med. 1975 Jul 1;142(1):224–229. doi: 10.1084/jem.142.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Morrison D. C., Schreiber R. D., Müller-Eberhard H. J. Activation of the alternative complement pathway: recognition of surface structures on activators by bound C3b. J Immunol. 1980 Feb;124(2):977–982. [PubMed] [Google Scholar]
- Pangburn M. K., Müller-Eberhard H. J. Relation of putative thioester bond in C3 to activation of the alternative pathway and the binding of C3b to biological targets of complement. J Exp Med. 1980 Oct 1;152(4):1102–1114. doi: 10.1084/jem.152.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J Exp Med. 1981 Sep 1;154(3):856–867. doi: 10.1084/jem.154.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Trombold J. S., Müller-Eberhard H. J. Paroxysmal nocturnal hemoglobinuria: deficiency in factor H-like functions of the abnormal erythrocytes. J Exp Med. 1983 Jun 1;157(6):1971–1980. doi: 10.1084/jem.157.6.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm L. E., Whitlow M. B., Koski C. L., Shin M. L., Mayer M. M. Elimination of complement channels from the plasma membranes of U937, a nucleated mammalian cell line: temperature dependence of the elimination rate. J Immunol. 1983 Sep;131(3):1411–1415. [PubMed] [Google Scholar]
- Sturtevant J. E., Balber A. E. Externally disposed membrane polypeptides of intact and protease-treated Trypanosoma lewisi correlated with sensitivity to alternate complement pathway-mediated lysis. Infect Immun. 1983 Dec;42(3):869–875. doi: 10.1128/iai.42.3.869-875.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. J. Metacyclogenesis of Trypanosoma cruzi in vitro: a simplified procedure. Trans R Soc Trop Med Hyg. 1982;76(3):300–303. doi: 10.1016/0035-9203(82)90173-0. [DOI] [PubMed] [Google Scholar]
- Volanakis J. E., Bhown A., Bennett J. C., Mole J. E. Partial amino acid sequence of human factor D:homology with serine proteases. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1116–1119. doi: 10.1073/pnas.77.2.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler J. M., Daha M. R., Austen K. F., Fearon D. T. Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3268–3272. doi: 10.1073/pnas.73.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley K., Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976 Nov 2;144(5):1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmer T., Sims P. J. Effect of complement proteins C5b-9 on blood platelets. Evidence for reversible depolarization of membrane potential. J Biol Chem. 1985 Jul 5;260(13):8014–8019. [PubMed] [Google Scholar]