Abstract
The cerebellum consists of parasagittal zones that define fundamental modules of neural processing. Each zone receives input from a distinct subdivision of the inferior olive (IO)—activity in one olivary subdivision will affect activity in one cerebellar module. To define functions of the cerebellar modules, we inactivated specific olivary subdivisions in six male cats with a glutamate receptor blocker. Olivary inactivation eliminates Purkinje cell complex spikes, which results in a high rate of Purkinje cell simple spike discharge. The increased simple spike discharge inhibits output from connected regions of the cerebellar nuclei. After inactivation, behavior was evaluated during a reach-to-grasp task and during locomotion. Inactivation of each subdivision produced unique behavioral deficits. Performance of the reach-to-grasp task was affected by inactivation of the rostral dorsal accessory olive (rDAO) and the rostral medial accessory olive (rMAO) and, possibly, the principal olive. rDAO inactivation produced paw drag during locomotion and a deficit in grasping the handle during the reach-to-grasp task. rMAO inactivation caused the cats to reach under the handle and produced severe limb drag during locomotion. Inactivation of the dorsal medial cell column, cell group β, or caudal medial accessory olive produced little deficit in the reach-to-grasp task, but each produced a different deficit during locomotion. In all cases, the cats appeared to have intact sensation, good spatial awareness, and no change of affect. Normal cerebellar function requires low rates of IO discharge, and each cerebellar module has a specific and unique function in sensory–motor integration.
Introduction
Climbing fiber projections from subdivisions of the inferior olive (IO) define parasagittal zones in cerebellar cortex (Groenewegen and Voogd, 1977; Groenewegen et al., 1979). Purkinje cells within a given zone converge onto the same region of the cerebellar output nuclei, and the nuclear projections from different zones do not overlap. Therefore, each olivary subdivision belongs to a unique module of cerebellar processing (Voogd and Bigare, 1980). In this study, we explored the contributions that different cerebellar modules make to movement control by inactivation of physiologically identified subdivisions of the IO with injections of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), a glutamate receptor antagonist.
During normal behavior, olivary neurons discharge at low irregular rates of a few spikes per second (Gellman et al., 1985). Despite the low rate, climbing fiber discharge strongly influences Purkinje cell simple spike discharge, and elimination of olivary input causes simple spike discharge to rapidly increase to a high regular rate (Colin et al., 1980; Rawson and Tilokskulchai, 1981; Montarolo et al., 1982; Strata and Montarolo, 1982). Simple spike discharge inhibits cerebellar nuclear cells (Ito et al., 1964), so a high rate of simple spike discharge effectively turns off cells in the cerebellar nuclei (Benedetti et al., 1983), which reduces discharge in cerebellar targets such as the magnocellular red nucleus (RNm) (Bardin et al., 1983; Billard and Daniel, 1985). Although the previous studies relied on anesthetized preparations, it is likely that the circuit operates in a similar fashion in the awake animal, since blocking glutamate receptors in the IO reduces discharge in the cerebellar nuclei of conscious rabbits (Zbarska et al., 2008). Therefore, elimination of complex spike discharge in one parasagittal zone will reduce or eliminate cerebellar output from the corresponding cerebellar module. Behavioral changes associated with eliminating output of a module provide insight into the contribution that the module makes to motor control.
Studies that have attempted to analyze cerebellar function with inactivation of the cerebellar nuclei (see Discussion) have produced variable results. Much of the variability probably arises from the involvement of multiple cerebellar modules. The topography of the IO in the cat allows selective inactivation of specific subdivisions. The cat IO consists of thin sheets of cells that are elongated over a large rostral–caudal extent, and the subdivisions are separated by layers of myelinated fibers.
In the current study, we used a reach-to-grasp task and a locomotion task to evaluate motor performance after inactivation of specific olivary subdivisions—inactivation of each subdivision produced a unique motor deficit. Despite disturbances of movement, the cats maintained strong appetites and showed no evidence of alterations in affect. They also maintained good spatial orientation in relation to desired targets, even when the loss of motor control made acquisition of the targets difficult. Our results provide a new level of detail about functional specialization within the cerebellum and emphasize the importance of low rates of olivary discharge for normal movement control.
Materials and Methods
Animals.
Experiments were performed using nine adult male cats. Three cats were used in an acute experiment to estimate the effect of CNQX injections on IO activity, and six cats were trained and tested on the behavioral tasks to evaluate behavioral effects of IO inactivation. All experiments were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with both the National Institutes of Health's Principles of Laboratory Animal Care (86-23, revised 1985) and the American Physiological Society's Guiding Principles in the Care and Use of Animals.
Acute experiments.
The three cats used in the acute experiment were anesthetized with an intramuscular injection of ketamine (15 mg/kg) followed by intravenous injections of sodium pentobarbital (5 mg, as needed) throughout the experiment. The skull overlying the cerebellum was removed and the dura mater was incised and reflected. Tungsten microelectrodes were used to map the IO. The rostral dorsal accessory olive (rDAO) was chosen to calibrate the effective area of the glutamate receptor antagonist CNQX diffusion because of its high sensitivity to somatosensory stimulation in the anesthetized preparation and well defined spatial map (Gellman et al., 1983).
Once the rDAO was mapped, a micropipette filled with CNQX was inserted into the chosen location within the rDAO. The area of effective spread of the CNQX was measured by recording spontaneous and evoked rDAO activity at different distances from the injection pipette before and after the CNQX injection. Cutaneous electrical stimulation (monophasic, square-wave, bipolar, 0.10 ms duration) was used to elicit olivary activity. Evoked olivary activity has variability in timing, polarity, and number of potentials, so the recordings were rectified, integrated (1 ms time constant), digitized (25 kHz sample rate), and averaged across 20 stimulus presentations to provide a reliable estimate of activity (Horn et al., 1996).
Polyamide-coated quartz micropipettes (tip opening ∼10 μm in diameter) were used to inject CNQX into the identified rDAO site. The same methodology, injection volume, and concentration were used for the later behavioral experiments. Injections were made with brief air pressure pulses while observing the meniscus of the fluid in the pipette with a calibrated microscope (for the behavioral experiments, injection volume was measured by meniscus movement along a length of calibrated small bore flexible tubing). Recordings of neural activity were made through the injection pipette to ensure accurate placement.
Shortly after the injection, the pipette was withdrawn to prevent the possible leakage of CNQX during the postinjection period. Recordings of spontaneous and evoked activity were made with a tungsten electrode that was positioned with a micrometer slide to enable accurate recording distances relative to the injection site. The same tungsten electrode was used for all postinjection recordings, and the depth of encountering rDAO neurons varied by <100 μm between penetrations. The injection pipette and recording electrode were cross-referenced to an optical reference point in the x, y, and z planes, and electrolytic lesions (−10 μA, 10 s) were placed at selected locations at the termination of the experiment for histological verification of recording locations.
A total of three (two cats, in one case both sides of the IO were used) CNQX injections (250 nl, 3.6 mm in saline) were placed in the rostral region (shoulder–trunk) of rDAO, and recordings of evoked and spontaneous activity in rDAO were made at various distances caudal to the injection site.
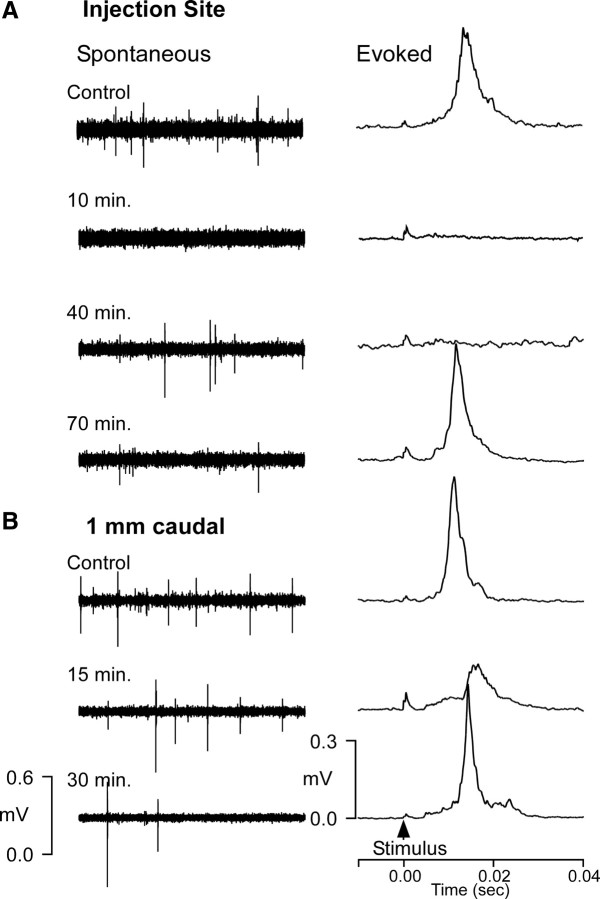
Figure 1A illustrates a parasagittal section through the brainstem of one of the acute experiments—several electrode tracks are visible. Figures 2 and 3 illustrate recordings made at specific times and distances after the CNQX injection. Immediately following injection, spontaneous and evoked activity at the injection site were absent in all cases. At sites 1 mm or more caudal to the injection, both spontaneous and evoked IO responses were unaffected over a 30 min postinjection period (Fig. 2B), although in one case there may have been some reduction in the 1 mm distant evoked response at 15 min (Fig. 3B, second trace). Recordings 0.4 mm caudal to the injection site indicated a complete loss of spontaneous and evoked activity for at least 45 min after injection (Fig. 2A, second and third traces) (recordings after 45 min were not made at this site). Moving the recording electrode deeper into the underlying rostral medial accessory olive (rMAO) revealed spontaneous activity that could be modulated with hard squeezes to the limb (Fig. 2C) (often, electrical stimulation is not an effective stimulus for rMAO neurons).
Figure 1.
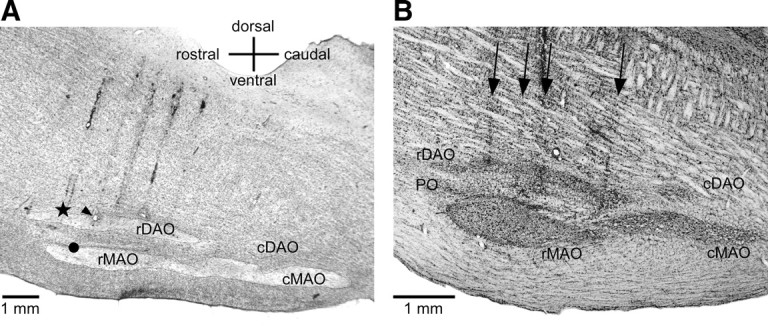
A, Sagittal section through the brainstem about 1.5 mm lateral to the midline from acute cat 1. The star shows the location of a 250 nl (3.6 mm) CNQX injection. The arrowhead indicates a recording location 1 mm caudal to the injection in rDAO (at the site of an electrolytic lesion) and the circle shows a recording site 1 mm ventral in rMAO. B, Sagittal section through the brainstem of one of the behavioral inactivation cases (cat 4) 1.2 mm lateral to the midline. The arrows show the locations of four electrode penetrations through the brainstem and IO. Gliosis is visible along the penetrations above and within the IO; however, CNQX injections did not appear to produce additional loss of IO neurons.
Figure 2.
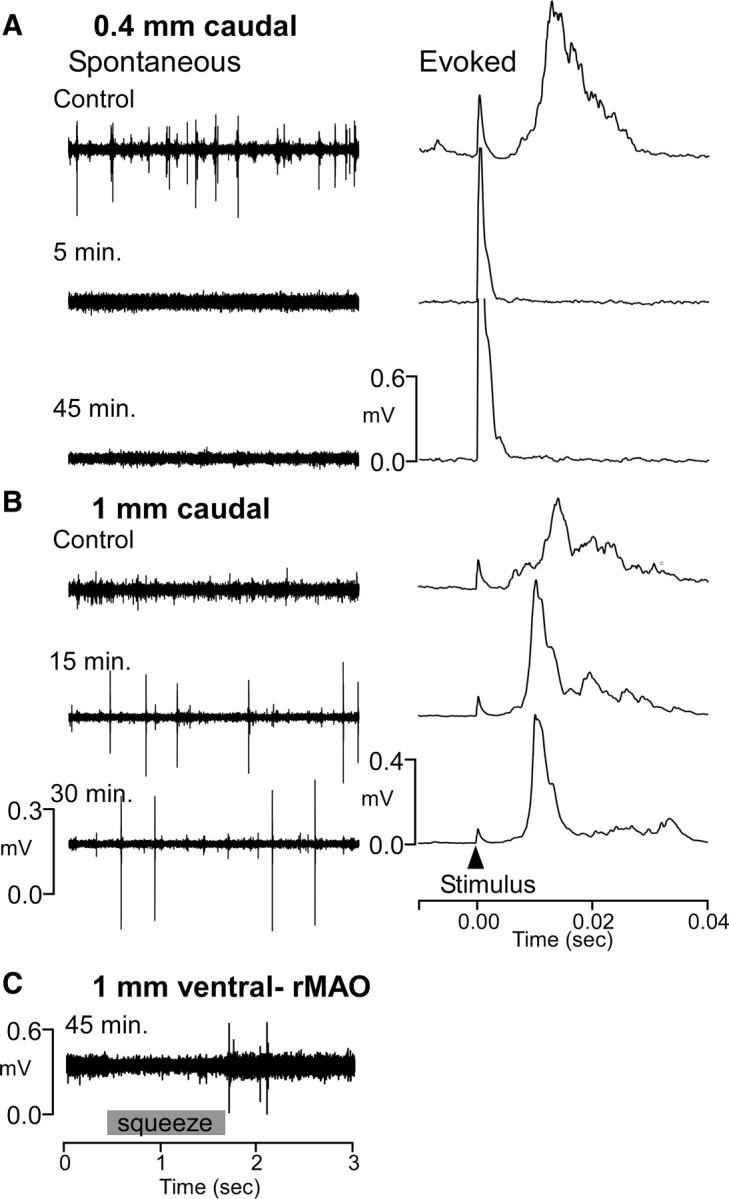
Acute cat 1: Inactivation lasts at least 45 min and spreads <1 mm from injection site. A, Recordings 0.4 mm caudal to CNQX injection. Upper trace illustrates spontaneous (left) and average evoked (right) activity before injection. Lower traces show no spontaneous or evoked activity 5 and 45 min after injection despite higher stimulus strength (larger artifact). B, Recordings 1 mm caudal to injection show no loss of spontaneous or evoked activity. C, Recordings ventral in rMAO at 45 min after injection show IO activity that responded to squeezes of the forelimb.
Figure 3.
Acute cat 2: Partial loss at 1 mm. A, Recordings at the injection site showed complete loss of activity 10 min after injection with recovery of spontaneous but not evoked activity 40 min after injection. At 70 min after injection there was complete recovery of all activity. B, Recordings 1 mm distant showed a partial suppression of evoked but not spontaneous activity 15 min after injection. No loss was seen after 30 min.
In a second case, spontaneous and evoked activity were absent 10 min following the injection (Fig. 3A, trace 2). At 40 min after injection, spontaneous, but not evoked, activity had recovered (Fig. 3A, trace 3), and both spontaneous and evoked activity recovered by 70 min (Fig. 3A, trace 4). No loss of activity was seen 1 mm caudal to the injection (Fig. 3B). In the third case, spontaneous and evoked activity were absent immediately following the injection, but both had recovered by 60 min after injection. No loss of spontaneous or evoked activity was seen 1 mm caudal to the injection site. Together, the results demonstrate that a 250 nl injection produced an inactivated area within rDAO with a radius of somewhere between 0.4 and 1.0 mm for ∼45 min.
To separate the effects of CNQX injection from effects of fluid injection, 250 nl injections of the carrier fluid (normal saline) were made in a third cat. For these injections, the injection pipettes were used for recording at and caudal to the injection sites. As with CNQX, there was an immediate loss of evoked and spontaneous activity at the injection site. However, spontaneous and evoked activity returned within 12 min after injection, and full evoked potentials were recorded at 20 min after injection. Neither spontaneous nor evoked activity was lost 0.5 mm caudal to a saline injection. In summary, 250 nl injections of saline briefly suppress activity at the injection site but have little or no effect 0.5 mm distant to the injection.
There is also the possibility that the pressure injections damaged areas of the IO near the injection sites. Histological examination of the olive after the acute injections showed no loss of neurons (Fig. 1A), but the survival period may have been too short to demonstrate damage. Histological examination at the termination of behavioral studies showed gliosis along the electrode tracks both within and external to the IO, but there appeared to be no additional damage to the IO that could be attributed to the injections. Figure 1B illustrates a histological section from cat 4. There were four injections made in rDAO within 0.3 mm of this section and three injections into rMAO. Although gliosis can be seen along some of the tracks, there appears to be no cell loss within the IO caused by the injections.
Our acute findings appear to contrast with those reported by Lang (2001), who concluded that CNQX injections blocked evoked but not spontaneous activity in the IO. However, the findings of the two studies are not that disparate: In one case, we did see recovery of spontaneous discharge before recovery of the evoked response, and Lang's report (2001) included Purkinje cells that showed a complete loss of complex spikes, suggesting that at least some areas of the IO lost spontaneous as well as evoked activity. Our acute experiments were meant to demonstrate that it is possible to inactivate a relatively confined area of the IO with CNQX using our injection protocol, regardless of the cause. The experiments do not address the issue of whether or not olivary cells can generate action potentials in the absence of synaptic activity.
The acute experiments indicated that the CNQX injections eliminated IO activity in a radius of 0.5 mm to 1.0 mm from the injection site for an extended period of time, which is in good agreement with the behavioral results (see later): Behaviorally, rDAO injections can produce deficits confined to the forelimb region of rDAO. The total width of rDAO is ∼2.0 mm, and it is about equally divided by forelimb and hindlimb regions (Gellman et al., 1983). Behavioral effects confined to one limb (see rDAO results, Fig. 9) suggest that the effective area of IO inactivation is not larger than 1 mm in diameter.
Figure 9.
rDAO inactivation produced paw drag of the forelimb contralateral to the injection. The floor was sprinkled with carbon black before walking. Neither the ipsilateral forelimb nor hindlimbs showed darkening greater than seen before injection (2-32).
Behavioral experiments.
Six cats were tested using two behavioral tasks. The first task was an operant task that required the cats to reach forward and then grasp and retrieve a handle upon presentation of a tone cue (Fig. 4A). The cats received a small quantity of pureed chicken and cod liver oil extruded from the end of the handle upon successful performance of the reach-to-grasp task. Accurate placement of injections was vital to the success of the experiment, so all injections were made during performance of the reach-to-grasp task, which involved some restraint of the head with a flexible rubber mount.
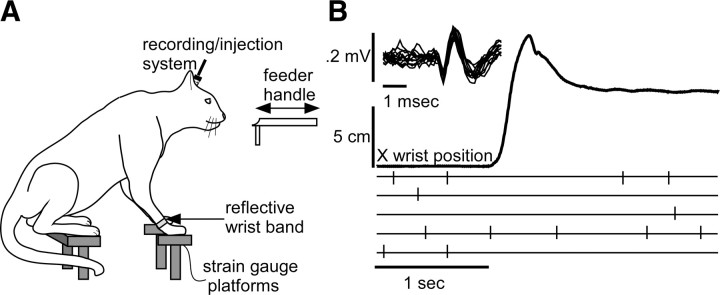
Figure 4.
A, The reach-to-grasp task. A reflective wrist band allows tracking of the x–y–z wrist position coordinates during the task. B, Injection pipette recordings of rDAO activity during the task before a CNQX injection. Upper inset illustrates overplotted IO cell waveforms. The second trace shows the average wrist position in the x-axis (extension) for five trials. Lower rasters show discriminated spike discharge for the five trials.
The second task was a simple open-field, locomotor task. Cats were trained to walk between two food bowls placed 3 m apart on a runway grid to obtain a few pellets of cat chow in the bowl and to walk across a sandbox to reach a food bowl. All cats received supplemental food in their home cages to maintain their body weights between 85 and 100% of their pretraining, free-feeding weight.
Implant surgery.
Surgery was performed in an American Association of Laboratory Animal Care approved surgical suite using aseptic techniques. Each cat was initially anesthetized with an intramuscular injection of ketamine (15 mg/kg) followed by supplemental injections of sodium pentobarbital (5 mg, intravenous as needed) for the duration of the implant surgery. The skull overlying the cerebellum was removed, bone channels were sealed with bone wax, and a Narishige-type recording chamber and a head restraint device were fastened to the skull with stainless steel screws and dental acrylic.
Recordings and inactivation.
Neural activity within the IO was recorded with varnish-insulated, tungsten microelectrodes (10–30 μm tip exposure length). Olivary spike frequency, waveform, and amplitude are very distinctive when compared to other brainstem nuclei. The dorsalmost division of the IO, the rDAO, responds to somatosensory stimulation and is somatotopically organized. Cells responding to face–forelimb–hindlimb are organized in a medial to lateral progression. Spontaneous IO activity in rDAO is low (usually <1 Hz) as shown in Figure 4B.
After the IO had been positively identified and mapped, recordings with tungsten electrodes were discontinued, and micropipettes made of polyamide-coated quartz tubing were used to record neural activity and inject CNQX into the various subdivisions of the IO during the reach-to-grasp testing of the experimental sessions. To ensure no residual effects, injections were made no more frequently than every other day. During the experiment, a CNQX-loaded micropipette was advanced to 1 mm dorsal to the IO and control data files of the reach-to-grasp were collected and stored for later analysis. On some trials, we placed injections at the 1 mm dorsal site, and no behavioral effects were observed from these injections (see Results). Upon completion of collecting control data, the pipette was advanced to the division of the olive to be injected, and a volume of CNQX (250 nl, 3.6 mm) was injected across 2 min. The pipette remained in place, and data files were collected of neural activity and behavior (in figures, data files are identified by animal number followed by track number).
Ten minutes after the conclusion of the injection, the pipette was removed from the brain, the chamber cleaned and the cat was moved to the open-field room for locomotion testing. A record of the paw placements was made by having the cat walk across a smooth sand surface and photographs made of the footprints and foot drag locations. Cats were monitored in the open-field test for 15–20 min. In some instances, the cats were returned to the reach-to-grasp apparatus to observe any performance changes that might have occurred in the reach-to-grasp over time. When behavioral testing was completed, the cats were returned to their home cages.
Data analysis.
Reflective tape (1.5 cm width) attached to the wrist of the cats provided measurements of limb position in space by using a digital three-dimensional optical position tracking system (DynaSight, Origin Instruments). Digital x–y–z position signals were transformed to analog voltages at 64 Hz by a custom digital-to-analog voltage converter and sampled using a 1401+ (Cambridge Electronic Devices). Position signals were plotted relative to each other after the recording sessions using plotting software (SigmaPlot).
Digital video records of cats in the reach-to-grasp apparatus were acquired using a Canon GL2 video camera placed 1.5 m lateral to the apparatus. Video data files (720 × 480 pixels, 30 frames/s) were created and stored for later analyses. In a similar fashion, records of the cats performing the locomotor task were recorded from the cat's side (Canon XL1) and above (Hitachi RPM1U). The videos from the top view were first saved on tape (Sony Hi8, CVD-1000) and later exported to video files for further analysis (640 × 480 pixel, 30 frames/s). A Canon (EOS1) digital camera was used to capture higher resolution images during both preinjection and postinjection trials on both tasks.
Statistical analyses of the reach-to-grasp and locomotion in rDAO, rMAO, caudal medial accessory olive (cMAO), dorsomedial cell column (DMCC), and cell group β (β) were made by comparing averages of 10 trials (reach-to-grasp and locomotion) immediately before the injections with the averages of 10 trials after a minimum of 5 min after injection. For each IO inactivation, repeated-measures t tests (using Bonferroni's correction for multiple tests) were completed on the number of regrasps during the reach-to-grasp, the swing height during locomotion (measured from video fields, 30/s), and the number of steps taken on the 3 m runway (counted from video records). For more subjective measures, such as collapsing, slipping, etc., the video records were scored by two independent observers.
Verification of injection sites.
Marking lesions (−10 μA for 10 s) in the IO were placed at the end of the experimental series of injections. Before perfusion, cats received an intramuscular injection of ketamine (15 mg/kg) followed by a lethal dose of sodium pentobarbital (∼25 mg/kg) delivered in a single rapid intravenous bolus. The circulatory system was rinsed with 9.25% sucrose at 300 mmHg followed by 2 L of 10% Formalin at 120 mmHg. The brains were equilibrated in 30% sucrose, frozen, and sectioned at 40 μm. Every section through areas of interest was collected and stained with either cresyl violet or neutral red/Luxol blue. Locations of unmarked injection sites were reconstructed relative to the electrolytic lesions.
Results
The restricted spread of the CNQX injections allowed selective inactivation of the cMAO, DMCC, and β. Some regions of the rDAO are very close to the dorsal lamella of the principal olive (PO), and the most medial regions of rDAO fuse with the ventral lamella of the PO. By placing injections into the most dorsal part of forelimb rDAO, we attempted to obtain relatively pure inactivation of this subdivision. The ventral lamella of the PO lies just dorsal to the rMAO, and it is likely that some, perhaps all, injections into rMAO also involved the PO.
Ten control injections (six cats, 250 nl of 3.6 mm CNQX per injection) were placed into the reticular formation 1 mm dorsal to the rDAO. None of these injections produced any change in performance of the reach-to-grasp task or in locomotion. One reticular injection led to a temporary increase in vocalization, and one produced a brief period of nystagmus. In five instances, the pipettes were advanced into the IO, but blocked tips prevented injection; these incidents produced no behavioral effects.
General observations
Although each injection produced unique effects depending upon its location, a few general observations apply to all injections: Injections of CNQX into small regions of the IO produced rapid (within minutes) alterations in motor behavior. The injected cats, regardless of injection site, maintained good spatial awareness—they would accurately orient their heads toward food or people and steps were often initially oriented toward the desired objects, although a lack of balance would result in movement or falling in a different direction. Sensory responses appeared to be intact, and slight touches to foot hairs continued to elicit rapid limb withdrawals, even when the limb dangled to one side of the stance platform (as with rMAO injections, see later). Affective responses showed no alteration—the cats responded normally to petting and scratching and showed no signs of fear, aggression, or agitation. Appetites remained normal except for injections into β and, to a lesser extent, DMCC, which led to autonomic behaviors reminiscent of motion sickness. Recovery was totally uneventful and normal motor control returned within ∼2 h after injection.
rDAO injections
Due to its high cutaneous sensitivity and orderly somatotopy (Gellman et al., 1983, 1985), the rDAO is the easiest subdivision to identify. Accordingly, we first mapped the location of rDAO in all behavioral cases using metal electrodes. This map then provided a reference to identify the coordinates for other IO subdivisions. Although we always recorded through the injection pipette, the IO recordings were not always of high quality, which may have been due to slight leakage of CNQX or by accumulation of protein at the tip of the pipette. However, even with compromised recordings, it was possible to identify when the IO was entered. Additionally, all pipettes were cross referenced to an optical zero point to provide uniform depth coordinates. When the pipettes did provide good cell isolation, the properties agreed well with those predicted by metal and the other pipette recordings. Figure 4B illustrates rDAO unit recordings made with an injection pipette. Despite high cutaneous sensitivity on the paw of the forelimb, this rDAO neuron displays a low discharge rate (∼1 Hz) unrelated to the reach-to-grasp movement. Reconstructions located 27 injections (six cats) to rDAO, all but one of which produced a behavioral deficit.
Reach-to-grasp task
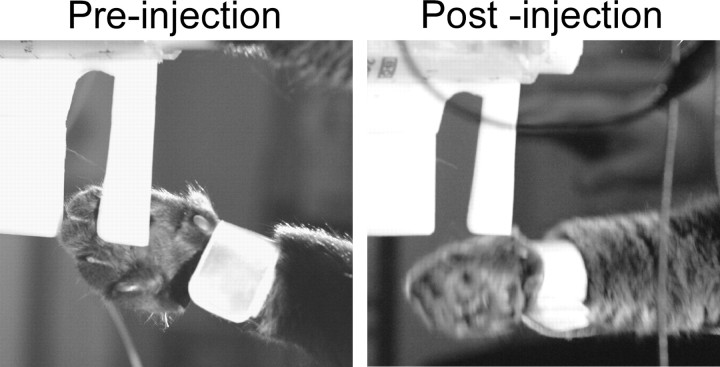
After collecting a series of normal reach-to-grasp trials, an injection of CNQX was placed into the forelimb region of rDAO. Within a few minutes, several obvious behavioral changes occurred in the performance of the task: The cats now had difficulty in grasping the handle and often had to make several grasp attempts before being able to retrieve the handle. The number of grasping attempts after CNQX injections was significantly greater when compared to control grasps before the injections (t = 3.90, p < 0.05, n = 27 injections). The wrist trajectories (especially the top view) plotted in Figure 5A demonstrated loops formed by multiple attempts to grasp the handle. The handle would also frequently slip out of the paw while the cats licked the food. Although the wrist starting location was more variable after injection, the trajectory of the wrist to the handle was little changed. The end location, however, was extended farther beyond the handle when compared to preinjection end points.
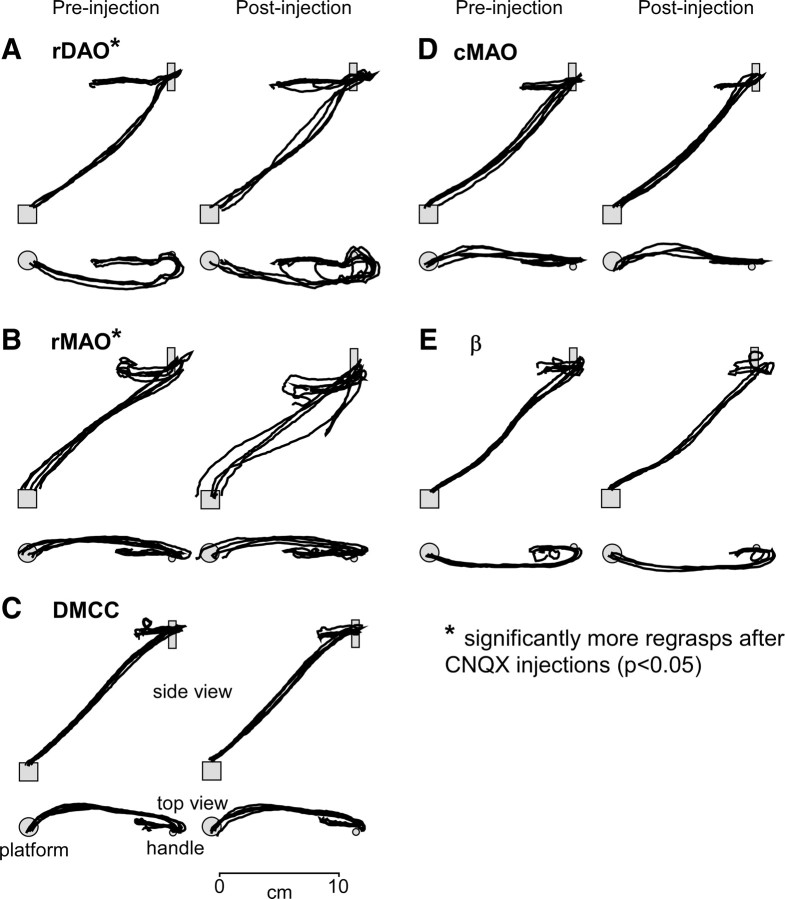
Figure 5.
Overplotted x–y–z wrist trajectories during the reach-to-grasp (5 trials). The first column in each panel shows reaches before CNQX injections and the second column shows after the CNQX injections. A, Forelimb rDAO inactivation produced repeated grasp attempts, slipping off the handle, and long reaching with relatively normal reach trajectory (2-43). B, rMAO inactivation produced low reaching and grasping difficulties (4-15). C–E, DMCC, cMAO, and β inactivation produced no reaching or grasping deficits (cases 5-34, 6-57, and 3-34, respectively).
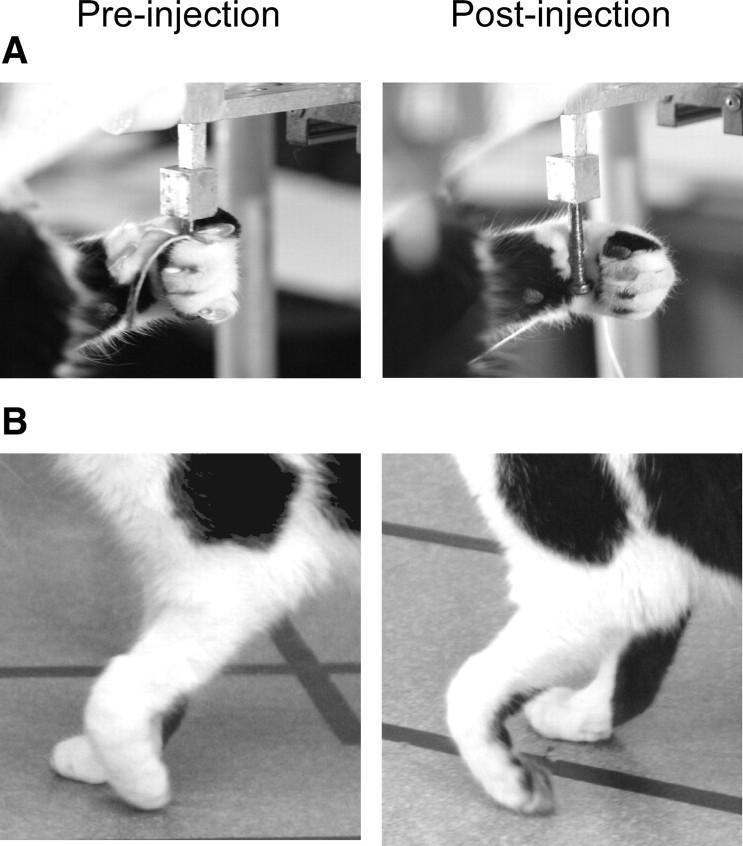
Figure 6A illustrates the change in grasp following rDAO injections. Normally, the cats used their toes to firmly grasp the handle for retrieval (Fig. 6A, left), but after rDAO injection, the handle was hooked with the wrist rather than the toes (Fig. 6A, right). The reaching past the handle may, at least in part, be due to this change in grasping, since using the wrist to hook the handle requires further extension of the limb.
Figure 6.
rDAO inactivation produced alterations in grasping and locomotion with contralateral forelimb (2-32). A, Inactivation led to hooking with wrist rather than grasping with toes. B, The forepaw dragged during early swing.
The variable starting position appeared to be due to an inability to secure a firm stance on the platform. After injection the paw would usually be placed with accuracy onto the platform, but it would then slide forward until the toes hung over the front edge (Fig. 7A). The sliding also led to frequent readjustments of limb position on the platform (Table 1 summarizes this and other deficits seen with injections into different IO subdivisions). Occasionally, the cats would lift their feet during platform placement, apparently in an attempt to gain a secure footing. The lifting might indicate a loss of sensation, but the cats withdrew their limb briskly in response to slight touches of the foot hair.
Figure 7.
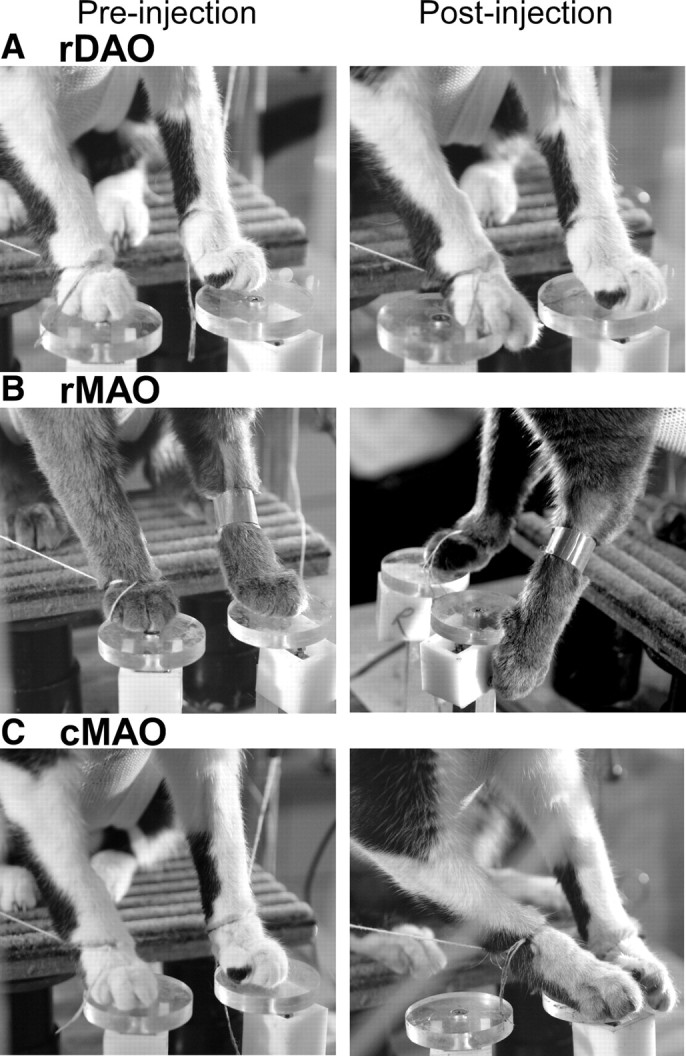
Alterations in stance. A–C, Locations of feet on platforms before (left column) and after (right column) a CNQX injection of left rDAO (2-15; A), right rMAO (7-23; B), and left cMAO (2-12; C).
Table 1.
Percentage of injections in IO that produced a given behavioral effect
| rDAO | rMAO | cMAO | DMCC | β | |
|---|---|---|---|---|---|
| Reach-to-grasp | |||||
| Long reach | 85 | 0 | 6 | 0 | 0 |
| Low reach | 7 | 27 | 0 | 0 | 0 |
| Grasp problems | 74 | 30 | 6 | 11 | 0 |
| Multiple adjustments | 67 | 20 | 0 | 11 | 0 |
| Miss platform—dangle contra fl | 11 | 67 | 0 | 0 | 0 |
| Slide on platform—contra fl | 41 | 6 | 0 | 11 | 0 |
| Bilateral fl displacement platforms | 7 | 3 | 69 | 0 | 27 |
| Locomotion | |||||
| Paw drag (exclusive) | 93 | 3 | 6 | 11 | 0 |
| Limp/leg | 0 | 58 | 19 | 0 | 0 |
| Waddle | 0 | 28 | 6 | 0 | 0 |
| Lateropulsion | 0 | 12 | 69 | 11 | 27 |
| Collapse | 0 | 0 | 0 | 78 | 0 |
| Unsteady-autonomic* | 0 | 0 | 0 | 11 | 64 |
| Number of cats | 6 | 6 | 4 | 4 | 4 |
| Number of injections | 27 | 33 | 16 | 9 | 11 |
| Number of ineffective injections | 1 | 2 | 0 | 1 | 1 |
Effects that occurred greater than 50% of the time are bold. Notice that the effects seen after injection of each subdivision are unique in that no two subdivisions share an effect that occurred in more than 50% of the cases, and all subdivisions had at least one effect that occurred in more than 50% of the cases. fl, Forelimb; contra, contralateral.
*Loss of appetite, emesis, and excessive licking.
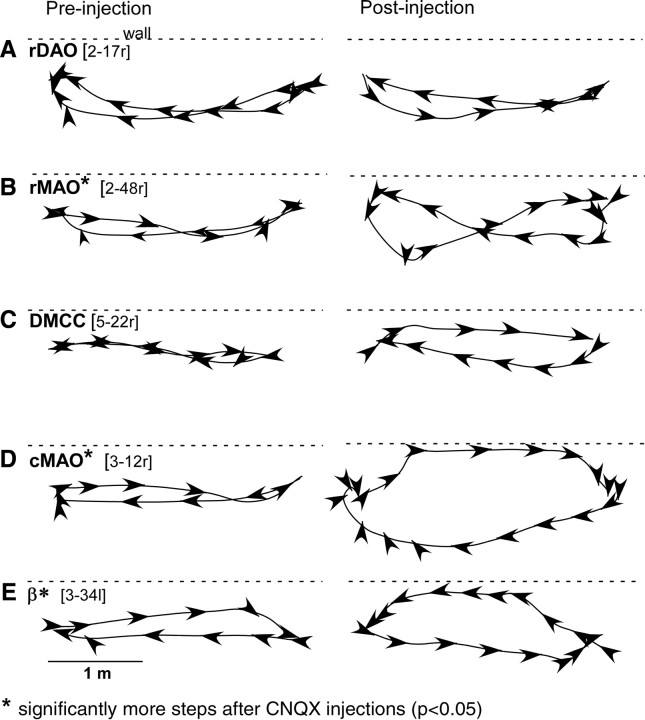
Locomotion
On casual inspection, inactivation of rDAO produced little deficit in locomotion; the cats walked quickly and directly between food bowls. Figure 8A illustrates walking paths for a cat before and after rDAO injection. The paths are very similar, and this cat even walked slightly faster after injection when compared to before injection. However, closer examination using video recording revealed that the dorsum of the paw contralateral to the injection turned under and dragged during locomotion. Figure 6B illustrates the position of the paw in midswing. The preinjection photo shows that the paw barely touches the floor, whereas the dorsum clearly drags after injection. Trails left in the sandbox as well as slow-motion video review during walking were used to score the presence or absence of foot drag before and after inactivation.
Figure 8.
Overhead locomotor paths during walking between food bowls. The first column shows a cycle of walking between the bowls before a CNQX injection and the second column shows after the CNQX injection. Each arrowhead represents contralateral forelimb paw placement, and the direction of the arrow indicates the orientation of the paw at placement. Dashed lines show the location of the wall of the room.
For illustrative purposes, carbon black (flour or chalk dust for cats with dark paws) was lightly sprinkled on the floor to demonstrate drag. The photographs in Figure 9 display the forelimb and hindlimb paws of a cat following rDAO inactivation. Notice that the toes of the left paw (contralateral to the injection site) have become blackened on the dorsal surface due to dragging. The toes of the right paw are only slightly darkened on the front, which was normal for this cat. Neither of the hindlimb paws showed darkening, which suggests that the effects of the inactivation were largely confined to forelimb rDAO. More lateral rDAO injections produced dragging of the dorsum of the contralateral hindlimb as well as the forelimb.
Figure 10A illustrates the average height of the paw from the floor for a normalized step cycle before and after rDAO injection. Comparison of the step cycles for the rDAO injections showed a significant difference between paw height before and after CNQX injections (t = 3.16, p < 0.05, n = 27 injections). Around midswing the paw is barely above the floor, and this is the point where the dorsum typically contacts the floor. However, an examination of the traces illustrates that the initial lift of the limb during the step cycle is severely reduced in amplitude, which may account for the dragging of the dorsum. The second peak of the step cycle is relatively normal in amplitude but starts from a lower initial position. The initial reduction in limb lift may result from an absence of a push off from the paw.
Figure 10.
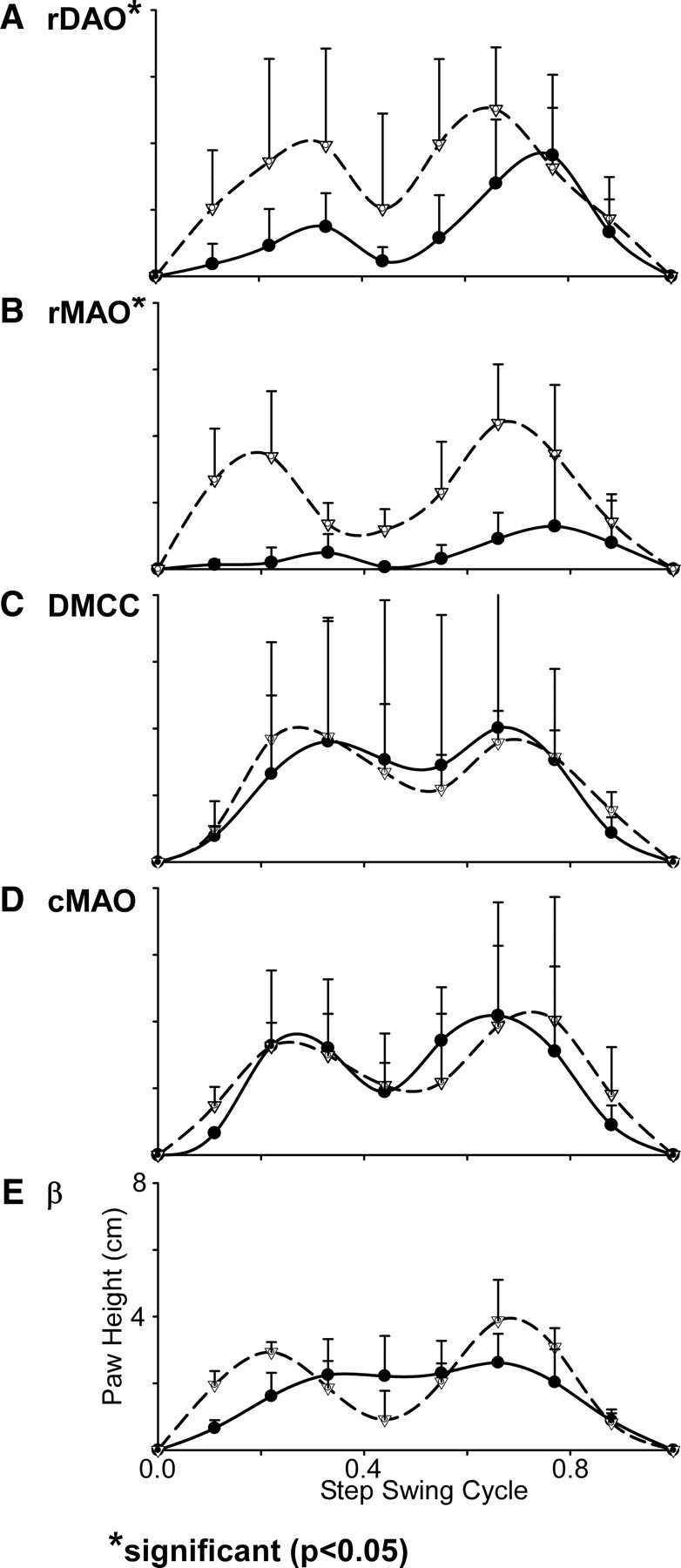
Altered stepping: Averages of 10 steps (contralateral forelimb paw height) before (dashed lines and open triangles) and after CNQX (solid lines and solid circles) injections. SDs for each tenth of the swing phase are shown with vertical bars. A, rDAO inactivation produced lowered paw height in early swing with a midswing drag (2-32). B, rMAO inactivation produced severe height reduction and drag throughout the swing (7-23, limp case). C, D, With wall support, neither DMCC (5-22) nor cMAO (4-16) inactivation produced changes in the step cycle. E, β inactivation resulted in shorter steps with less midswing dip (2-16).
Inactivation of hindlimb rDAO also produced dragging of the limb contralateral to the injection, which appeared as trails in the sandbox and was evident in the video records. The deficit appeared to be similar to that seen for forelimb rDAO inactivation.
In summary, rDAO inactivation produced a deficit in toe use contralateral to the injection site during grasping, in lifting of the paw during locomotion and in maintaining limb position during stance. Except for reaching past the handle and the multiple grasping attempts, there was little effect on reach trajectory. Although the paw dorsum contacted the floor during locomotion, overall, locomotion was efficient. Dragging of the paw during locomotion and reaching beyond the handle may have been due to decreased flexion of several limb joints, but it was clear that toe use was severely compromised during handle grip and stance.
rMAO injections
Although the rMAO is a large target, it is not a homogeneous region like rDAO. The rostral tip of rMAO projects to lateral posterior interpositus (NIP), which projects to the superior colliculus (May et al., 1990) rather than RNm. It is likely that the rostral tip of rMAO is involved in eye movement control. Three injections were placed into the rostral tip of rMAO—none produced deficits in reaching or locomotion.
An additional complication of rMAO injections is that they probably include the ventral lamella of the PO, which at some locations lies adjacent to the dorsal surface of rMAO. Therefore, it is possible that the deficits seen with rMAO injections are at least partly due to PO inactivation. Thirty-three injections (six cats) were localized to limb related regions of rMAO; 31 injections produced a behavioral deficit.
Reach-to-grasp task
Injections into rMAO disturbed several aspects of the reach-to-grasp. Stance was severely disturbed and the cats frequently dangled the limb contralateral to the injection off to one side of the platform (Fig. 7B, Table 1). The deficit in stance differed from that seen with rDAO; with rDAO injections the paw placement would hit the platform and then slide after placement, and the cats would often make corrective placements. rMAO injections led to inaccurate placement of the paw to one side of the platform, which the cats, typically, did not correct. In the most severely affected cats, both front paws would deviate to the injected side during stance.
The trajectories of the reaches with the limb contralateral to the injection were variable (Fig. 5B), and the cats would frequently end with the paw under the handle (Fig. 11) and would need to correct their movement to grasp and retrieve the handle. The number of attempts to grasp the handle significantly increased after CNQX injections (t = 6.70, p < 0.05, n = 33 injections) in comparison to trials before the injections. Despite stance being affected on both sides, only the reach with the limb contralateral to the injection was disturbed. The rMAO injections did not produce the over reach that was seen with rDAO injections. In some instances the cats would hook the handle with their wrists, but grasping problems were not as commonly seen as with the rDAO injections (Table 1).
Figure 11.
Inactivation of rMAO (7-23, limp case) produced low reaching by the contralateral forelimb.
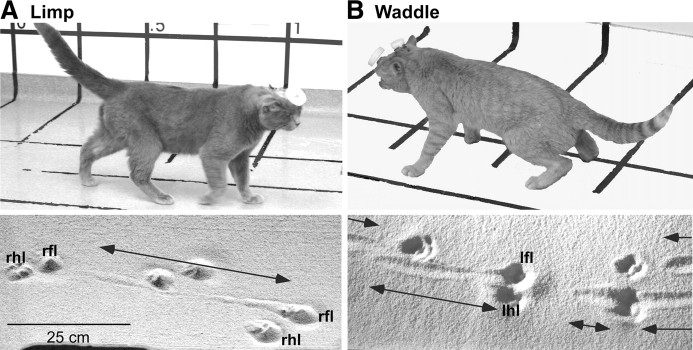
Locomotion
Locomotion was severely disrupted by rMAO injections. In several cases (58%, Table 1), rMAO injections produced a discreet limp of one or both of the contralateral legs. In other cases (28%, Table 1), the cats would walk with a widespread waddling motion with dragging of several limbs (waddle and paw drag are treated as exclusive categories in Table 1). Figure 12 provides a comparison of the postures following an injection into rMAO that produced a limp (Fig. 12A) versus one that produced a waddle (Fig. 12B). Figure 12 also illustrates traces left in a sandbox, and it is clear that the limp led to dragging of one limb, whereas the waddle resulted in dragging of multiple limbs.
Figure 12.
rMAO inactivation produced either of two deficits in locomotion. A, Fifty-eight percent of the injections produced a pronounced limp of the contralateral limb that led to extensive limb dragging during swing (7-23). A record of the contralateral forelimb drag is shown in the sandbox photograph. B, Twenty-eight percent of the rMAO injections produced waddling during locomotion with dragging of all limbs and swaying of the body axis from side to side (case 3-38). The upper photograph was processed to show greater contrast between the cat and floor.
Axial support of the body appeared to be compromised—during eating, the cats would sit in a slumped posture with midline sag. During walking the cats that were waddling had difficulty maintaining a straight path, and their limbs would often cross. The resulting walking paths between the food bowls described a wide figure eight (Fig. 8B) that was not seen following the injections that led to limping behavior. Significantly more steps were taken after the CNQX injections when compared to performance before the rMAO injections (t = 3.81, p < 0.05, n = 33 injections). Measurements of paw height during walking revealed significantly lower height along the step cycle (t = 4.02, p < 0.05, n = 33 injections) with frequent contact to the floor (Fig. 10B) during either limping or waddling injections.
In six cases with severe waddling, the cats were returned to apparatus for additional reach-to-grasp testing, since it seemed likely that reaching would be disturbed more than that seen with the testing shortly after injection. The forelimb contralateral to the injection showed no greater deficits in grasping the handle than during earlier testing, and the ipsilateral forelimb showed no reaching deficits. Mapping the locations of rMAO injections that produced waddling and those that produced limping showed no obvious spatial segregation within rMAO, although this may reflect the low spatial resolution afforded by the injections or, possibly, variable involvement of PO.
DMCC injections
The dorsomedial cell column is a subnucleus that is a dorsomedial extension of rostral rMAO. Although DMCC is physically close to the rMAO, PO, and rDAO, the behavioral effects of inactivation are unique and differ greatly from inactivation of the other subdivisions. A total of nine injections (four cats) were localized to DMCC (Table 1); eight of the injections produced a behavioral deficit.
Reach-to-grasp task
Inactivation of DMCC produced no obvious abnormality in the reach-to-grasp. Trajectory of the reach was undisturbed and the cats grasped the handle efficiently and securely using their toes (Fig. 5C). Placement of the foot onto the pedestal was accurate and no slippage occurred during stance.
Locomotion
Locomotion following DMCC inactivation was relatively normal. The step cycle was somewhat distorted in that the mid dip during swing was not as deep as normal (Fig. 10C), and the cats often appeared to walk somewhat more slowly with their heads kept relatively immobile. Interstep interval was similar to before injection, and the walking paths were similar to before injection. Walking paths directly approached the food bowls but the postinjection paths diverged somewhat more than normal (Fig. 8C), and, in some instances, the cats used the wall for additional bracing of the body. However, the number of steps were not significantly different compared to the preinjection condition (p > 0.05, n = 9 injections).
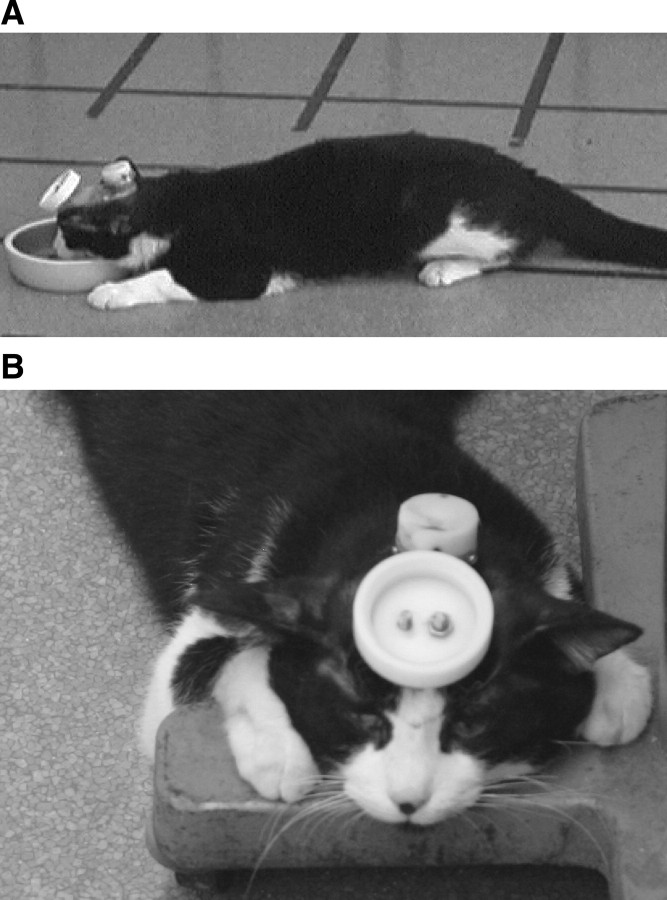
Feeding from the food bowls revealed a striking behavioral deficit. As the cats attempted to eat food near the edges of the bowl, they would rotate their heads on the roll axis to reach food along the inside wall of the bowl; the rotation produced what appeared to be a positive feedback situation. The rotations of the head to one side and then the other rapidly built in amplitude and the cats would collapse and remain motionless with their forelimbs splayed horizontally onto the floor (Fig. 13A). The same collapse would occur when the head was rotated out of the horizontal such as during rubbing of the ear on a vertical pole (Fig. 13B) or when the head was passively rotated by the experimenter. Sudden collapsing was never observed with inactivation of other olivary regions (Table 1). Horizontal eye and head movements appeared to have some target overshoot, but these were not systematically studied. After inactivation, the cats quickly learned to hold their heads in a fixed horizontal position when walking about the room. Although head rotation in the roll plane produced collapsing, changes along the pitch axis, as when the cats bent their necks to eat from the bowl, produced no disturbance.
Figure 13.
DMCC inactivation produced collapsing when the head rotated in the roll axis. A, Position assumed by the cat shortly after rotating the head to eat food in the corner of the bowl (5-34). B, Posture assumed after rotation of the head to rub on a vertical pole (5-22). Head movements in the horizontal or pitch axes produced no disturbance.
In summary, DMCC inactivation produced no clear deficit until the cats rotated their heads out of the horizontal. The rotation caused immediate counter-rotation and collapsing to the floor, where the cats appeared to attempt to cling to the floor and maintain their heads in a horizontal position.
cMAO injections
The caudal cMAO constitutes a large injection target (Fig. 2) with little overlying IO, so injections into this subdivision are unlikely to affect other subdivisions. Sixteen injections (four cats) were localized to cMAO; all produced a behavioral deficit.
Reach-to-grasp task
cMAO inactivation had no effect on the reach-to-grasp task. In fact, during the first inactivation case, the undisturbed reach-to-grasp led us to suspect that the injection had failed and we made additional injections (Fig. 14B). Although limb trajectory and grasping were not affected (Fig. 5D) there was a shift in forelimb position during stance with one or both limbs displaced to the direction of the injected side (Fig. 7C). These findings were characteristic of the cMAO injection group (Table 1).
Figure 14.
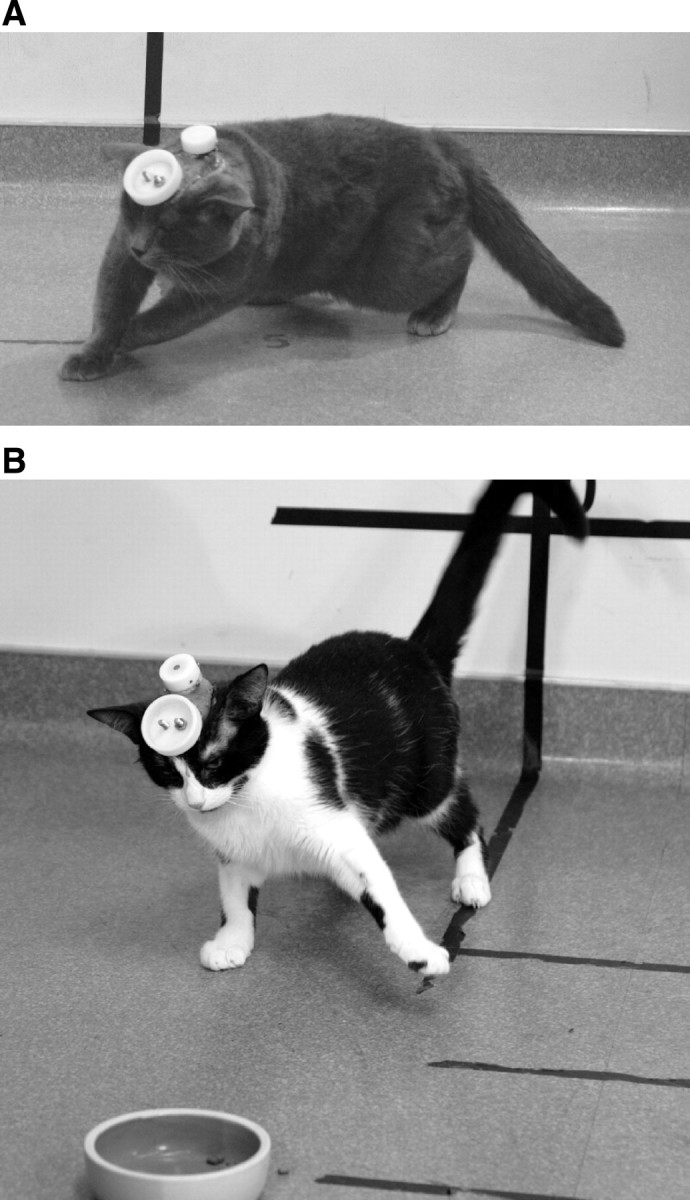
cMAO inactivation produced imbalance between body sides. A, Cat falling to the side following a 250 nl injection into the right cMAO (7-30). B, Locomotion after a 750 nl injection into the left cMAO (case 2-12, data not used in data analysis or Table 1). Despite the imbalance, the cat maintained good orientation to the food bowl, and the reach-to-grasp was undisturbed.
Locomotion
When the cats were removed from the testing apparatus and placed on the floor, it was immediately clear that there was an imbalance between the sides of the body. Severely affected cats fell to the side contralateral to the injection and were unable to walk more than a step or two (Fig. 14A). If the food bowl was placed within reach, the cats ate readily from their prone position. Less strongly affected cats showed lateropulsion away from the side of the injection. In some instances, the cats attempted to hop to reach the food bowls, but they were unbalanced in both the push off and landing, and the attempts were futile.
Figure 8D illustrates walking paths for one cMAO injection. Notice that as the cat walked between the two food bowls, the walking path curved strongly outward (away from the injected side) and then spiraled in to the left bowl. The change in locomotor path was significantly different after the CNQX injections in comparison to control paths (t = 6.17, p < 0.05, n = 16 injections). The direction of the stepping (foot orientation indicated by arrowheads, Fig. 8D) remained oriented to the bowl, although the cat moved laterally to keep from falling to that side. During the return to the right food bowl, this cat used the room wall for body support and rapidly walked along the wall. During this period of locomotion, the alternating limb pattern and step cycle appeared normal, and plots of paw height did not significantly differ from preinjection step height (p > 0.05, n = 16 injections) (Fig. 10D).
In summary, cMAO injections produce lateropulsion during walking, which appeared to be due to an imbalance between the sides of the body with the side contralateral to the injection being weaker. Independent use of the limbs during the reach-to-grasp was unaffected, and the normal alternating step cycle during locomotion was intact.
β injections
β constitutes a relatively large region medial to the rostral pole of cMAO. A total of 11 injections (four cats) were localized to β; 10 injections produced a behavioral deficit.
Reach-to-grasp task
The reach-to-grasp was unaffected by β injections. The limb trajectories were unchanged, grasping was efficient (Fig. 5E), and limb placement on the pedestals was normal. One noticeable change was an excessive amount of licking around the mouth and lips, which we believe was due to some regurgitation of eaten food, but this was not documented in detail.
Locomotion
Locomotion was severely disturbed by β inactivation. The cats adopted a widespread stance and were unsteady and lurched from side to side. The less affected cats were still interested in food and the walking paths from a cat with a β injection are shown in Figure 8E. Notice that this cat took small uneven steps, even when using the room wall to brace the body. As with cMAO injections, several cats displayed lateropulsion to the side contralateral to the injection when walking (Table 1). Significantly more steps were taken after the CNQX injections to reach the food bowls in comparison to the preinjection condition (t = 5.46, p < 0.05, n = 11 injections). The additional steps may be due to a combination of longer walking paths as well as shorter swing cycles during locomotion.
The more severely disturbed cats were not interested in food and spent much of the time sitting on their hindlimbs licking about their mouths and showed a reluctance to walk. When they did attempt to walk, they were unsteady and stumbled. Even slight vestibular stimulation would elicit regurgitation of food. Despite their motion sickness, the cats accurately oriented to objects within the room.
Discussion
Our observations that IO inactivation affected neither sensation nor spatial localization echo early cerebellar findings: Flourens (1842) removed the cerebellum from a rooster and presented the rooster with a chicken. The rooster recognized the chicken and attempted to mount but lacked sufficient muscular coordination. The cerebellar patients of Holmes (1917) retained accurate localization of their limbs in space and suffered no sensory deficits. Neither investigator saw evidence for regional specialization within the cerebellum, which is probably due to involvement of multiple cerebellar modules by the lesions.
rDAO inactivation
Studies of structures related to rDAO indicate that olivary inactivation is equivalent to removing cerebellar output. rDAO projects to the C1 and C3 zones of cerebellar cortex (Groenewegen et al., 1979), which project to anterior interpositus (NIA). NIA projects to the RNm (Courville, 1966), which projects to the spinal cord (Pompeiano and Brodal, 1957).
RNm inactivation produces effects similar to rDAO inactivation: the cats hook the lever with their wrists, reach beyond the lever, slip on the stance platforms, and drag their paws during walking. Reach trajectory is relatively undisturbed and locomotion is efficient (Gibson et al., 1994). Lesions of the RNm produce deficits in toe use for grasping food (Sybirska and Górska, 1980), as do lesions of the rat rubrospinal tract (Schrimsher and Reier, 1993). Deficits in toe use for grasping, stance, and walking suggest that, at least for this module, processing is focused on musculature rather than specific actions.
A deficit in toe use following rDAO inactivation is consistent with a selective RNm projection to C8 motor neuronal pools that innervate digit muscles (McCurdy et al., 1987; Robinson et al., 1987; Holstege et al., 1988; Fujito et al., 1991; Fujito and Aoki, 1995; Pong et al., 2002), and recording studies of the RNm emphasize the importance of hand movements for cell discharge (Gibson et al., 1985a,b; van Kan and McCurdy, 2001).
Inactivation of NIA produces deficits similar to rDAO and RNm inactivation, but studies using interpositus inactivation are not entirely consistent. Some reports indicate that interpositus inactivation produces paw drag, hypermetria, underreaching, slipping, and deficits in grasping (Kolb et al., 1997; Milak et al., 1997; Bracha et al., 1999), which is similar to rDAO inactivation. In contrast, Cooper et al. (2000) reported alterations in reach trajectory but no grasping deficits.
Muscimol injections into monkey interpositus have also produced variable findings. Mason et al. (1998) report that NIA inactivation produces a deficit in preshaping of the fingers for grasping, which is consistent with our rDAO injections and recording studies of NIA (van Kan et al., 1994). Thach et al. (1992) reported no deficit in finger movements but did see walking on the paw dorsum. Goodkin and Thach (2003) reported deficits in all types of limb movements including finger movements, while Monzée et al. (2004) reported deficits in upper limb but not finger movements. Muscimol can spread large distances (Martin, 1991), and the different reports may reflect inactivation of different regions of the nuclei.
It is possible that our injections into the IO only produced a partial set of deficits, but the similarity of deficits produced by rDAO inactivation and RNm inactivation suggest that the IO injections provided a relatively comprehensive view of motor involvement of a given subregion. Overall, the behavioral effects of inactivation of circuits related to rDAO (and findings from acute studies, see Introduction) indicate that IO inactivation suppresses cerebellar output; they also support a focus of rDAO and its related structures on toe and finger use for a variety of behaviors.
rMAO inactivation
The caudal 2/3 of rMAO contains cells that respond to squeezes of the limbs and receives input from motor cortex via nucleus Darkschewitsch (Porter et al., 1993; Onodera and Hicks, 1995). Output is to the C2 zone of cerebellar cortex (Groenewegen et al., 1979) and medial NIP, which projects to surround cells in RNm (Robinson et al., 1987).
rMAO injections produced either limping or waddling. It is likely that these effects arise from different regions of rMAO or from involvement of PO. The waddling appeared to be similar to that described for quadrupedal locomotion of the monkey after plugging of the semicircular canals (Cohen et al., 2009). Neither limping nor waddling is seen after RNm inactivation (Gibson et al., 1994), which suggests that PO, NIP, or projections to areas other than RNm may be responsible for these effects.
rMAO injections produced low and variable reach trajectories for the limb contralateral to the injections. Handle hooking was observed with some rMAO injections, which could be due to spread upward into rDAO (and the intervening PO).
Martin et al. (2000) reported that NIA muscimol injections produced underreaching, but NIP injections produced high reaching and faster reaches, which we did not see. It is possible that their NIP injections included some cerebellar cortex, which could produce higher activity in the nuclei. In agreement with our findings, Mason et al. (1998) reported that NIP inactivation in the monkey produced inaccurate reaching.
DMCC inactivation
DMCC inactivation produced no deficits in the reach-to-grasp or locomotion, when the head was held in a horizontal position. However, head rotations quickly led to collapsing, which surprised us (and seemed to surprise the cats).
The importance of DMCC for maintaining posture with the head rotated is consistent with its input. The DMCC receives input from the otoliths (Kaufman et al., 1991), which signal head orientation in relation to gravity. The DMCC projects to a lateral strip of the nodulus and uvula of the vermis (Voogd and Bigare, 1980; Akaogi et al., 1994), which also receives canal input from the vestibular system (Barmack, 2003).
Collapsing only occurred with movements of the head in the roll axis. For adaptation of the vestibulo-ocular reflex of the monkey, roll gain has a gravity-dependent component not seen for yaw and pitch (Yakushin et al., 2009), which is consistent with roll being dependent upon otolith input and neural processing separate from that of tilt and pitch.
After DMCC inactivation, head rotation produced an overcorrection that led to oscillation, which suggests a mismatch between head movement and sensory feedback. The overcorrection may result from inappropriate movement gain, since a misrepresentation of head position would be likely to produce a head tilt, which we did not observe. It has been shown that Purkinje cells in the nodulus and uvula combine otolith and canal signals to selectively encode head translation (Yakusheva et al., 2007). DMCC inactivation may disable this mechanism and produce inappropriate vestibular reflexes in response to head tilt.
Injections into caudal subdivisions
Injections into the caudal IO produced severe disturbances in balance with no effect on reaching and grasping, which agrees with effects reported for fastigial inactivation (Martin et al., 2000).
Injections into β and cMAO both produced balance disturbances, but the cMAO injections primarily affected balance between the sides of the body. cMAO injections did not produce strong autonomic effects, and, even when the cats could not stand, they ate readily when the food bowls were placed within reach. Cats with β inactivation lost interest in food and spent a large amount of time sitting still and licking. Although β injections sometimes produced curved walking paths, the cats were often unsteady and lurched to either side when they attempted to walk.
cMAO injections had little or no effect on the step cycle, and, when leaning against the wall, they walked quickly to the food bowl with a normal gait. Guillaume et al. (2000) reported curved walking paths after inactivation of the fastigial nucleus, which was interpreted as a deficit in spatial representation. cMAO injections did not appear to produce a general spatial deficit. The cats oriented accurately to the food bowls during walking, and, during reaching to grasp, there was no misdirection of the limb, which might be expected from a general spatial deficit.
Function of the inferior olive
Our data emphasize the importance of low rates of IO discharge for ongoing motor control: Without that discharge, the simple spike rate of the Purkinje cell rapidly increases to a high rate (Colin et al., 1980), which eliminates output from the cerebellum. Conversely, slightly higher rates of IO discharge eliminate simple spikes (Colin et al., 1980; Rawson and Tilokskulchai, 1981; Montarolo et al., 1982; Strata and Montarolo, 1982); a remarkably small change in complex spike rate controls the total range of simple spike rate. The IO is inhibited by cells in the cerebellar nuclei (Andersson et al., 1988; Lang et al., 1996; Gibson et al., 2002, 2004; Bengtsson et al., 2004), which are inhibited by simple spike discharge. Therefore, the olivo-cerebellar-olivary circuit comprises a negative feedback loop regulating simple spike discharge—this regulation may maintain the operational state of the Purkinje cell and, perhaps, set the gain of movements produced by the cerebellum.
In summary, our results indicate a unique function for each cerebellar module. Although behavioral deficits do not directly translate to function, they do provide a comprehensive guide to cerebellar regional specialization.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke Grant 5R01NS44592 to A. R. Gibson, and by the Barrow Neurological Foundation. We thank James F. Baker, Mitchell Glickstein, and Enrico Mugnaini for their comments and advice on this manuscript.
References
- Akaogi K, Sato Y, Ikarashi K, Kawasaki T. Zonal organization of climbing fiber projections to the nodulus in the cat. Brain Res. 1994;638:1–11. doi: 10.1016/0006-8993(94)90626-2. [DOI] [PubMed] [Google Scholar]
- Andersson G, Garwicz M, Hesslow G. Evidence for a GABA-mediated cerebellar inhibition of the inferior olive in the cat. Exp Brain Res. 1988;72:450–456. doi: 10.1007/BF00250590. [DOI] [PubMed] [Google Scholar]
- Bardin JM, Batini C, Billard JM, Buisseret-Delmas C, Conrath-Verrier M, Corvaja N. Cerebellar output regulation by the climbing and mossy fibers with and without the inferior olive. J Comp Neurol. 1983;213:464–477. doi: 10.1002/cne.902130409. [DOI] [PubMed] [Google Scholar]
- Barmack NH. Central vestibular system: vestibular nuclei and posterior cerebellum. Brain Res Bull. 2003;60:511–541. doi: 10.1016/s0361-9230(03)00055-8. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Montarolo PG, Strata P, Tempia F. Inferior olive inactivation decreases the excitability of the intracerebellar and lateral vestibular nuclei in the rat. J Physiol. 1983;340:195–208. doi: 10.1113/jphysiol.1983.sp014758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson F, Svensson P, Hesslow G. Feedback control of Purkinje cell activity by the cerebello-olivary pathway. Eur J Neurosci. 2004;20:2999–3005. doi: 10.1111/j.1460-9568.2004.03789.x. [DOI] [PubMed] [Google Scholar]
- Billard JM, Daniel H. Inferior olive destruction induces dysfacilitation of the red nucleus activity. Brain Res. 1985;336:372–375. doi: 10.1016/0006-8993(85)90671-7. [DOI] [PubMed] [Google Scholar]
- Bracha V, Kolb FP, Irwin KB, Bloedel JR. Inactivation of interposed nuclei in the cat: classically conditioned withdrawal reflexes, voluntary limb movements and the action primitive hypothesis. Exp Brain Res. 1999;126:77–92. doi: 10.1007/s002210050718. [DOI] [PubMed] [Google Scholar]
- Cohen B, Xiang Y, Yakushin SB, Kunin M, Raphan T, Minor L, Della Santina CC. Effect of canal plugging on quadrupedal locomotion in monkey. Ann N Y Acad Sci. 2009;1164:89–96. doi: 10.1111/j.1749-6632.2009.03845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin F, Manil J, Desclin JC. The olivocerebellar system. I. Delayed and slow inhibitory effects: an overlooked salient feature of cerebellar climbing fibers. Brain Res. 1980;187:3–27. doi: 10.1016/0006-8993(80)90491-6. [DOI] [PubMed] [Google Scholar]
- Cooper SE, Martin JH, Ghez C. Effects of inactivation of the anterior interpositus nucleus on the kinematic and dynamic control of multijoint movement. J Neurophysiol. 2000;84:1988–2000. doi: 10.1152/jn.2000.84.4.1988. [DOI] [PubMed] [Google Scholar]
- Courville J. Somatotopical organization of the projection from the nucleus interpositus anterior of the cerebellum to the red nucleus. An experimental study in the cat with silver impregnation methods. Exp Brain Res. 1966;2:191–215. doi: 10.1007/BF00236713. [DOI] [PubMed] [Google Scholar]
- Flourens P. Ed 2. Paris: Bailliere; 1842. Recherches experimentales sur les proprietes et les fonctions du systeme nerveux dans les animaux vertebres. [Google Scholar]
- Fujito Y, Aoki M. Monosynaptic rubrospinal projections to distal forelimb motoneurons in the cat. Exp Brain Res. 1995;105:181–190. doi: 10.1007/BF00240954. [DOI] [PubMed] [Google Scholar]
- Fujito Y, Imai T, Aoki M. Monosynaptic excitation of motoneurons innervating forelimb muscles following stimulation of the red nucleus in cats. Neurosci Lett. 1991;127:137–140. doi: 10.1016/0304-3940(91)90913-e. [DOI] [PubMed] [Google Scholar]
- Gellman R, Houk JC, Gibson AR. Somatosensory properties of the inferior olive of the cat. J Comp Neurol. 1983;215:228–243. doi: 10.1002/cne.902150210. [DOI] [PubMed] [Google Scholar]
- Gellman R, Gibson AR, Houk JC. Inferior olivary neurons in the awake cat: detection of contact and passive body displacement. J Neurophysiol. 1985;54:40–60. doi: 10.1152/jn.1985.54.1.40. [DOI] [PubMed] [Google Scholar]
- Gibson AR, Houk JC, Kohlerman NJ. Relation between red nucleus discharge and movement parameters in trained macaque monkeys. J Physiol. 1985a;358:551–570. doi: 10.1113/jphysiol.1985.sp015566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson AR, Houk JC, Kohlerman NJ. Magnocellular red nucleus activity during different types of limb movement in the macaque monkey. J Physiol. 1985b;358:527–549. doi: 10.1113/jphysiol.1985.sp015565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson AR, Horn KM, Van Kan PL. Grasping cerebellar function. In: Bennett KMB, Castiello U, editors. Insights into the reach to grasp. Amsterdam: Elsevier; 1994. pp. 85–108. [Google Scholar]
- Gibson AR, Horn KM, Pong M. Inhibitory control of olivary discharge. Ann N Y Acad Sci. 2002;978:219–231. doi: 10.1111/j.1749-6632.2002.tb07569.x. [DOI] [PubMed] [Google Scholar]
- Gibson AR, Horn KM, Pong M. Activation of climbing fibers. Cerebellum. 2004;3:212–221. doi: 10.1080/14734220410018995. [DOI] [PubMed] [Google Scholar]
- Goodkin HP, Thach WT. Cerebellar control of constrained and unconstrained movements. I. Nuclear inactivation. J Neurophysiol. 2003;89:884–895. doi: 10.1152/jn.00114.2002. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Voogd J. The parasagittal zonation within the olivocerebellar projection. I. Climbing fiber distribution in the vermis of cat cerebellum. J Comp Neurol. 1977;174:417–488. doi: 10.1002/cne.901740304. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Voogd J, Freedman SL. The parasagittal zonation within the olivocerebellar projection. II. Climbing fiber distribution in the intermediate and hemispheric parts of cat cerebellum. J Comp Neurol. 1979;183:551–601. doi: 10.1002/cne.901830307. [DOI] [PubMed] [Google Scholar]
- Guillaume A, Goffart L, Courjon JH, Pelisson D. Altered visuo-motor behavior during inactivation of the caudal fastigial nucleus in the cat. Exp Brain Res. 2000;132:457–463. doi: 10.1007/s002210000359. [DOI] [PubMed] [Google Scholar]
- Holmes G. The symptoms of acute cerebellar injuries due to gunshot wounds. Brain. 1917;40:461–535. [Google Scholar]
- Holstege G, Blok BF, Ralston DD. Anatomical evidence for red nucleus projections to motoneuronal cell groups in the spinal cord of the monkey. Neurosci Lett. 1988;95:97–101. doi: 10.1016/0304-3940(88)90639-8. [DOI] [PubMed] [Google Scholar]
- Horn KM, Van Kan PL, Gibson AR. Reduction of rostral dorsal accessory olive responses during reaching. J Neurophysiol. 1996;76:4140–4151. doi: 10.1152/jn.1996.76.6.4140. [DOI] [PubMed] [Google Scholar]
- Ito M, Yoshida M, Obata K. Monosynaptic inhibition of the intracerebellar nuclei induced from the cerebellar cortex. Experientia. 1964;20:575–576. doi: 10.1007/BF02150304. [DOI] [PubMed] [Google Scholar]
- Kaufman GD, Anderson JH, Beitz A. Activation of a specific vestibulo-olivary pathway by centripetal acceleration in rat. Brain Res. 1991;562:311–317. doi: 10.1016/0006-8993(91)90637-b. [DOI] [PubMed] [Google Scholar]
- Kolb FP, Irwin KB, Bloedel JR, Bracha V. Conditioned and unconditioned forelimb reflex systems in the cat: involvement of the intermediate cerebellum. Exp Brain Res. 1997;114:255–270. doi: 10.1007/pl00005634. [DOI] [PubMed] [Google Scholar]
- Lang EJ. Organization of olivocerebellar activity in the absence of excitatory glutamatergic input. J Neurosci. 2001;21:1663–1675. doi: 10.1523/JNEUROSCI.21-05-01663.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang EJ, Sugihara I, Llinás R. GABAergic modulation of complex spike activity by the cerebellar nucleoolivary pathway in rat. J Neurophysiol. 1996;76:255–275. doi: 10.1152/jn.1996.76.1.255. [DOI] [PubMed] [Google Scholar]
- Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett. 1991;127:160–164. doi: 10.1016/0304-3940(91)90784-q. [DOI] [PubMed] [Google Scholar]
- Martin JH, Cooper SE, Hacking A, Ghez C. Differential effects of deep cerebellar nuclei inactivation on reaching and adaptive control. J Neurophysiol. 2000;83:1886–1899. doi: 10.1152/jn.2000.83.4.1886. [DOI] [PubMed] [Google Scholar]
- Mason CR, Miller LE, Baker JF, Houk JC. Organization of reaching and grasping movements in the primate cerebellar nuclei as revealed by focal muscimol inactivations. J Neurophysiol. 1998;79:537–554. doi: 10.1152/jn.1998.79.2.537. [DOI] [PubMed] [Google Scholar]
- May PJ, Hartwich-Young R, Nelson J, Sparks DL, Porter JD. Cerebellotectal pathways in the macaque: implications for collicular generation of saccades. Neuroscience. 1990;36:305–324. doi: 10.1016/0306-4522(90)90428-7. [DOI] [PubMed] [Google Scholar]
- McCurdy ML, Hansma DI, Houk JC, Gibson AR. Selective projections from the cat red nucleus to digit motor neurons. J Comp Neurol. 1987;265:367–379. doi: 10.1002/cne.902650306. [DOI] [PubMed] [Google Scholar]
- Milak MS, Shimansky Y, Bracha V, Bloedel JR. Effects of inactivating individual cerebellar nuclei on the performance and retention of an operantly conditioned forelimb movement. J Neurophysiol. 1997;78:939–959. doi: 10.1152/jn.1997.78.2.939. [DOI] [PubMed] [Google Scholar]
- Montarolo PG, Palestini M, Strata P. The inhibitory effect of the olivocerebellar input on the cerebellar Purkinje cells in the rat. J Physiol. 1982;332:187–202. doi: 10.1113/jphysiol.1982.sp014409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzée J, Drew T, Smith AM. Effects of muscimol inactivation of the cerebellar nuclei on precision grip. J Neurophysiol. 2004;91:1240–1249. doi: 10.1152/jn.01124.2002. [DOI] [PubMed] [Google Scholar]
- Onodera S, Hicks TP. Patterns of transmitter labelling and connectivity of the cat's nucleus of Darkschewitsch: a wheat germ agglutinin-horseradish peroxidase and immunocytochemical study at light and electron microscopical levels. J Comp Neurol. 1995;361:553–573. doi: 10.1002/cne.903610402. [DOI] [PubMed] [Google Scholar]
- Pompeiano O, Brodal A. Experimental demonstration of a somatotopical origin of rubrospinal fibers in the cat. J Comp Neurol. 1957;108:225–251. doi: 10.1002/cne.901080204. [DOI] [PubMed] [Google Scholar]
- Pong M, Horn KM, Gibson AR. Spinal projections of the cat parvicellular red nucleus. J Neurophysiol. 2002;87:453–468. doi: 10.1152/jn.00950.2000. [DOI] [PubMed] [Google Scholar]
- Porter CM, van Kan PLE, Horn KM, Bloedel JR, Gibson AR. Functional divisions of cat rMAO. Soc Neurosci Abstr. 1993;19:499–10. [Google Scholar]
- Rawson JA, Tilokskulchai K. Suppression of simple spike discharges of cerebellar Purkinje cells by impulses in climbing fibre afferents. Neurosci Lett. 1981;25:125–130. doi: 10.1016/0304-3940(81)90319-0. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Houk JC, Gibson AR. Limb specific connections of the cat magnocellular red nucleus. J Comp Neurol. 1987;257:553–577. doi: 10.1002/cne.902570406. [DOI] [PubMed] [Google Scholar]
- Schrimsher GW, Reier PJ. Forelimb motor performance following dorsal column, dorsolateral funiculi, or ventrolateral funiculi lesions of the cervical spinal cord in the rat. Exp Neurol. 1993;120:264–276. doi: 10.1006/exnr.1993.1060. [DOI] [PubMed] [Google Scholar]
- Strata P, Montarolo PG. Functional aspects of the inferior olive. Arch Ital Biol. 1982;120:321–329. [PubMed] [Google Scholar]
- Sybirska E, Górska T. Effects of red nucleus lesions on forelimb movements in the cat. Acta Neurobiol Exp (Wars) 1980;40:821–841. [PubMed] [Google Scholar]
- Thach WT, Kane SA, Mink JW, Goodkin HP. Cerebellar output: multiple maps and modes of control in movement coordination. In: Llinas R, Sotelo C, editors. The cerebellum revisited. New York: Springer; 1992. pp. 283–300. [Google Scholar]
- van Kan PL, McCurdy ML. Role of primate magnocellular red nucleus neurons in controlling hand preshaping during reaching to grasp. J Neurophysiol. 2001;85:1461–1478. doi: 10.1152/jn.2001.85.4.1461. [DOI] [PubMed] [Google Scholar]
- van Kan PL, Horn KM, Gibson AR. The importance of hand use to discharge of interpositus neurones of the monkey. J Physiol. 1994;480:171–190. doi: 10.1113/jphysiol.1994.sp020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voogd J, Bigare F. Topographical distribution of olivary and cortico nuclear fibers in the cerebellum: a review. In: Courville J, Montigny DEC, Lamarre Y, editors. Inferior olivary nucleus: anatomy and physiology. New York: Raven; 1980. pp. 207–235. [Google Scholar]
- Yakusheva TA, Shaikh AG, Green AM, Blazquez PM, Dickman JD, Angelaki DE. Purkinje cells in posterior cerebellar vermis encode motion in an inertial reference frame. Neuron. 2007;54:973–985. doi: 10.1016/j.neuron.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Yakushin SB, Xiang Y, Cohen B, Raphan T. Dependence of the roll angular vestibuloocular reflex (aVOR) on gravity. J Neurophysiol. 2009;102:2616–2626. doi: 10.1152/jn.00245.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbarska S, Bloedel JR, Bracha V. Cerebellar dysfunction explains the extinction-like abolition of conditioned eyeblinks after NBQX injections in the inferior olive. J Neurosci. 2008;28:10–20. doi: 10.1523/JNEUROSCI.3403-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]