Abstract
Indices of functional connectivity in the default mode network (DMN) are promising neural markers of treatment response in late-life depression. We examined the differences in DMN functional connectivity between treatment-responsive and treatment-resistant depressed older adults. Forty-seven depressed older adults underwent MRI scanning pre- and post- pharmacotherapy. Forty-six never depressed older adults underwent MR scanning as comparison subjects. Treatment response was defined as achieving a Hamilton Depression Rating Scale of 10 or less post-treatment. We analyzed resting state functional connectivity using the posterior cingulate cortex as the seed region-of-interest. The resulting correlation maps were employed to investigate between-group differences. Additionally we examined the association between white matter hyperintensity burden and functional connectivity results. Comparison of pre- and post-treatment scans of depressed participants revealed greater post-treatment functional connectivity in the frontal precentral gyrus. Relative to treatment-responsive participants, treatment-resistant participants had increased functional connectivity in the left striatum. When adjusting for white matter hyperintensity burden, the observed differences lost significance for the PCC-prefrontal functional connectivity, but not for the PCC-striatum functional connectivity. The post-treatment “frontalization” of the DMN connectivity suggests a normalizing effect of antidepressant treatment. Moreover, our study confirms the central role of white matter lesions in disrupting brain functional connectivity.
Keywords: Late-life depression, Default Mode Network, treatment response, MRI, white matter hyperintensity
1. Introduction
Depression results in more years lived with disability than any other disease and ranks fourth in terms of disability-adjusted life years (Moussavi et al., 2007). As the population ages, successive cohorts of older adults will experience depressive disorders(Chapman and Perry, 2008). Compared with midlife depression, late-life depression (LLD) carries additional risk for suicide, medical comorbidity, disability, and family caregiving burden (Bruce et al., 2004; Charney et al., 2003). Successful antidepressant treatment is one of the most effective ways to reduce disability, prevent morbidity and improve quality of life in older depressed patients (Karp et al., 2009). However, LLD is often resistant to treatment and may exhibit slower symptom resolution than midlife depression (Whyte et al., 2004). This long response time in geriatric depression is one of the most challenging clinical features of LLD. Thus, in older adults it is particularly important to shorten this window, and to identify early the medication regimen will be the most effective for an individual patient. The identification of clinically actionable predictors of early treatment response is the focus of both personalized clinical care and translational bench-to-bedside research (Andreescu and Reynolds, 2011).
The Default-Mode Network (DMN) is an organized functional network comprised of several brain regions (posterior cingulate, medial prefrontal cortex, medial temporal cortex, and inferior parietal lobule) active during the resting state and inhibited during the performance of active tasks (Raichle et al., 2001). Analysis of resting state networks activity in clinical disorders such as major depression may enhance the understanding of the pathophysiological underpinning of mental illnesses (Greicius et al., 2003). Functional connectivity is operationally defined as temporally correlated neurophysiological events (Friston, 1994). Functional connectivity in resting state is based on the observation that brain regions show slow, correlated fluctuations during rest (Biswal et al., 1995; Fox and Raichle, 2007).
A number of studies have addressed the behavior of the DMN in major depressive disorder(Greicius et al., 2007; Hamilton et al., 2011; Sheline et al., 2009; Zhu et al., 2012). Given recent reports regarding the changes observed in the DMN with age (Koch et al., 2010; Tomasi and Volkow, 2012), it is more challenging to translate DMN changes observed in midlife depression into late-life depression. Thus, studies that focused on the default mode network in LLD (Wu et al., 2011) (Alexopoulos et al., 2012; Sexton et al., 2012), have described altered connectivity patterns in the subgenual anterior cingulate cortex (ACC), dorsomedial prefrontal cortex (dmPFC), orbitofrontal cortex (OFC) (Wu et al., 2011), precuneus and lateral parietal cortex (Alexopoulos et al., 2012). Previously, our group has reported (Wu et al., 2011) that this pattern of altered connectivity was correlated with the white-matter hyperintensity burden, supporting the role of vascular changes in the etiopathogenesis of at least some individuals with LLD (Alexopoulos, 2006; Sheline et al., 2010; Wu et al., 2011). In the same analysis, elderly depressed participants who remitted following antidepressant treatment exhibited improved functional connectivity compared to pretreatment, although alterations persisted in the anterior cingulate and the prefrontal cortex when remitted elderly depressed participants were compared with never-depressed older adults. However, the modest sample size did not allow us at that time to assess the relationship between treatment response and DMN functional connectivity patterns.
The use of resting state fMRI as a treatment predictor has several advantages including brevity and ease of scanning: resting state data are acquired in five-to-ten minutes and participants are not burdened by tasks. This advantage is more salient for older adults, who may have physical impairments that preclude longer acquisitions. Additionally, the acquisition is standardized across centers, eliminating the variability associated with differences in task designs and application. Thus, the identification of resting state predictors of treatment response may eventually be applicable clinically and contribute to future personalized treatment of LLD.
In this study we used a new and larger sample [forty seven depressed older adults, compared with twelve depressed older adults in the previous study (Wu et al., 2011)]. We sought to explore further the behavior of the DMN in LLD, focusing on the differences between responding and non-responding LLD participants. We hypothesized that treatment responsive participants would exhibit normalization of the DMN connectivity, while non-responders would maintain the pre-treatment pattern of altered functional connectivity.
2. Methods
2.1 Study participants
The data were collected from participants in the “Altered Functioning of Cognitive and Affective Circuits in LLD” study conducted at the University of Pittsburgh. The neuroimaging study was embedded in several open-label treatment intervention trials for LLD conducted at the University of Pittsburgh Advanced Center for Intervention and Services Research for Late-Life Mood Disorders (ACISR) from 2004 and 2011 (Karp et al., 2010; Reynolds et al., 2011; Reynolds et al., 2010). Additional description of these trials is provided in the online supplemental data. In brief, depressed older adult participants were diagnosed using the Structured Clinical Interview for DSM-IV (SCID) (First M, 1995) with a current non-psychotic, non-bipolar major depressive episode. Depressed participants had a baseline Hamilton Depression Rating Scale (HDRS) of 17 or higher. One of the parent studies used a threshold of 15 or higher on the Montgomery-Asberg Depression Rating Scale (Montgomery and Asberg, 1979), but also collected the HDRS, which was used as the outcome measure for this analysis. Other than MDD and anxiety disorders, all Axis I disorders served as exclusion criteria. Other exclusion criteria were: history of stroke or significant head injury, Alzheimer's, Parkinson's, or Huntington's disease. We excluded from the baseline scanning individuals who had taken psychotropic medications during the 2 weeks prior to imaging, except for as-needed lorazepam. Seven subjects had as-needed lorazeparm prescribed, but none took it within 24 hours before the scan. Medications other than psychotropic medications were acceptable, as medication use is common among older adults.
Participants were excluded if they received a diagnosis of dementia (this was corroborated with family members and medical records if dementia was considered). In this study all participants had MMSE above 24 (see Table 1).
Table 1. Demographic and clinical characteristic of the sample.
| Comparison group (N=46) | Depressed Elderly (baseline) N=47 | Group Comparison (depressed vs. nondepressed elderly) p-value | Depressed (post-treatment) N= 21 | Group comparison (treatment responsive vs. treatment resistant) | ||
|---|---|---|---|---|---|---|
| Treatment responsive depressed (N=10) | Treatment resistant depressed (N=ll) | |||||
| Age | 72.89±7.90 | 68.72±6.99 | p-value=0.008 | 67.9±4.86 | 68.45±7.85 | NS |
| Age at first episode | N/A | 48.63±21.97 (N=43) | N/A | 55.20±13.18 | 44.72±24.8 | NS |
| Recurrent vs Single episode (N) | N/A | 4 Recurrent/6 Single | 5 Recurrent/6 Single | NS | ||
| Gender [male/female] | 13/33 | 13/34 | 4/6 | 2/9 | ||
| Education (years, mean/SD) | 14.75±2.40(N =36)* | 14.48±2.65 (N=47) | NS | 14.75±2.75 | 16.81±2.18 | p-value=.003 |
| HDRS at baseline | 2.00±1.61 (N=ll)* | 19.06±3.90 (N=44) | p-value<0.001 | 19.90±3.51 | 20.54±2.80 | NS |
| HDRS post-treatment | N/A | N/A | 5.00±2.94 | 16.36±3.77 | p-value<.0001 | |
| WMH burden | 0.004±. 006 | 0.002±. 003 | p-value=0.165 | 0.003±. 005 | 0.001±. 002 | p-value=. 0.265 |
| MMSE | 28.97±1.26(N =44) | 27.91±2.25 (N=46) | p-value=0.007 | 28.40±0.96 | 28.54±1.21 | NS |
| CIRSG heart | 0.4±0.95 (N=25)* | 0.55±0.88 (N=43) | NS | 1.30±1.25 | 0.09±0.30 | p-value=.011 |
| CIRSG vascular | 1.48±0.87 (N=25)* | 1.76±0.71 (N=43) | NS | 1.90±0.73 | 1.72±0.64 | NS |
Legend: HDRS = Hamilton Depression Rating Scale; MMSE = Mini Mental State Examination; CIRS-G=Cumulative Illness Rating Scale for Geriatrics.
Education data, HDRS scores and CIRS-G scores were available only for a subgroup of comparison non-depressed participants. WMH=white matter hyperintensity
Depressed participants consented to a second MRI scan 12 weeks after the baseline scan. Treatment response was defined as attaining a score of 10 or lower on the HDRS at the time of the second scan. All of the depressed participants received monotherapy with SSRIs or SNRIs, with the exception of one participant who received augmenting treatment with bupropion. The antidepressants varied among studies: venlafaxine XR (mean dose =210 mg/day, N=15), escitalopram (mean dose = 15 mg/day, N=22), and duloxetine (mean dose = 100 mg/d, N =10). Never-depressed elderly participants were recruited from the community and from the healthy control registry of the University of Pittsburgh Alzheimer's Disease Research Center. The exclusion criterion for the comparison older adults was lifetime history of psychiatric disorder, with the exception of adjustment disorders.
All three intervention studies as well as the neuroimaging study were approved by the University of Pittsburgh IRB, and all participants provided written informed consent.
2.2. MRI acquisition
The baseline MR images were obtained at the time of subject enrollment, before initiation of pharmacotherapy for the depressed participants. Post-treatment scans for the depressed participants were obtained after 12 weeks of pharmacotherapy, while on a maintenance dose of antidepressant medication.
Resting state fMRI data were acquired during a five-minute interval while participants focused on fixation point on the screen. Participants were instructed to think of nothing in particular during this interval. Imaging data were collected with a 3Tesla Siemens Trio TIM scanner located in the MR Research Center at the University of Pittsburgh. For functional image alignment we used a T2-weighted magnetization-prepared rapid gradient echo (MPRAGE) sequence (TR/TE = 3000/101, in plane resolution 1 mm × 1 mm, 3mm slice thickness, 48 slices). T2*-weighted BOLD acquisitions were done using a gradient-echo echoplanar imaging (EPI) sequence: TR/TE = 2000/32, Matrix= 128×128×28, Voxel size = 2×2×3 mm3, axial acquisition (parallel to AC-PC). To assess for small vessel ischemic changes we used a T2-weighted FLAIR sequence (TR/TE = 9002/56ms Ef; TI = 2200 ms, NEX = 1) using an interleaved acquisition; 48 slices (3mm slice thickness, no gap).
For maximum brain coverage, the most superior slice was placed at the top of the cortex and the most inferior slice was located below the most inferior aspect of the temporal lobes.
2.3. Resting-state fMRI analyses
2.3.1. Preprocessing
Functional imaging data were analyzed with Statistical Parametric Mapping 5 (SPM5; http://www.fil.ion.ucl.ac.uk/spm/software/spm5) implemented in Matlab (Mathworks, Natick, MA). Motion correction was performed on each participant's time-series functional images by realigning the data to the first image in the series. Then, the T2-weighted structural image was segmented into three tissue types (grey, white, CSF) and the grey segmentation was registered to the functional images using a rigid-body model. The registered grey segmentation of the T2-weighted image and the functional images were then warped to match the SPM template image space. The resulting functional images were smoothed using a 10×10×10 mm Gaussian smoothing kernel. We chose the 10 mm kernel over the default 8 mm SPM5 smoothing kernel to account for greater morphologic variability in older adults (Reuter-Lorenz and Lustig, 2005).
2.3.2. Connectivity Analysis
We compared the DMN activity among older adult never-depressed participants and elderly depressed participants before and after 12 weeks of antidepressant treatment. We used the posterior cingulate cortex as the seed region to identify the DMN (Raichle et al., 2001). The left and right posterior cingulate from the Automated Anatomical Labeling atlas (Tzourio-Mazoyer et al., 2002) (1×1×1 mm) in Colin27 space was down sampled to a voxel resolution of 3.75 mm × 3.75 mm × 3.75 mm (left and right PCC combined, 200 voxels). A smaller region-of-interest (ROI) of 39 voxels, centered on the posterior cingulate, was created on template Colin27 by performing erosions (2 iterations, 6 connected, 2.5-dimensional) with a 3×3×3 voxel structuring element. The seed region was then resampled into SPM template space (2mm × 2mm × 2mm).
For each subject, a reference resting-state time-series was extracted by averaging the time-series for all voxels within the posterior cingulate ROI using the Marsbar plug-in in SPM5 (Brett M, 2002). The resultant time-series was used as the regressor in the first-level analysis in SPM5 to generate a correlation map for each participant.
We used a two-sample t-test of the correlation coefficient maps to test group differences between depressed elderly and comparison participants, and between treatment responsive and treatment resistant elderly depressed. We corrected for multiple comparison error using the small volume multiple correction embedded in SPM5. Corrected p values ≤ 0.05 were deemed significant.
2.4. White Matter Hyperintensity Assessment
An automated WMH localization and segmentation method was used to compute the normalized WMH volumes (7). For each participant, the calculated WMH volume was normalized using the overall brain volume. Further details regarding the automated WMH segmentation method are provided in the on-line supplemental data file.
Association between WMH and functional connectivity
We extracted the BOLD signal for the regions deemed significant in the group analyses (=ROIs) using REX (http://www.neuroimaging.org.au/nig/rex). For the group comparisons where the ROI data had a normal distribution, we fit a model using Multivariate analysis of variance (MANOVA) with the ROIs as outcomes and WMH and group (depressed vs. comparison/treatment responsive vs. treatment resistant) as predictors. For the group comparisons with outliers in the ROI data, we used a robust linear regression model with ROIs as outcome variable and group and WMH burden as predictors.
To test the demographic and clinical differences between depressed and never-depressed groups we used a two-sample t-test. Due to reduced sample size of responders and non-responders who underwent scanning post-treatment, we used Wilcoxon's rank sum test to determine whether there were any differences in clinical and demographic variables.
3. Results
Forty-six never-depressed older adult participants and 47 depressed older adult participants were included in this analysis. Twenty-seven of the 47 elderly depressed participants had a second imaging study at week 12 (nine participants withdrew consent from the parent study or the MRI study, five were lost to follow-up and six were unable to return for the second scan). Of the 27 elderly depressed participants who had a second imaging study at week 12, six had corrupted scans. Thus, twenty-one elderly depressed participants were included in the post-treatment analysis. Clinical and demographic characteristics of the sample are presented in Table 1. Table 2 presents a summary of the analyses and the MNI coordinates for the regions of interest. A flowchart depicting participant experience is presented in Fig 1.
Table 2. Summary of analyses and coordinates of regions of interest.
| Analysis | Direction | Region of Interest | T score, P value, cluster size(kE) | MNI coordinates (x/y/z) |
|---|---|---|---|---|
| Non-depressed vs. depressed elderly at baseline | Depressed>Non-depressed | Precuneus | T=3.2, p=0.001 uncor, kE =185 | 6.10/49.2/11.5 |
| Non-depressed>Depressed | Middle frontal gyrus (right &left) | T=3.17, p=0.001 uncor; kE= 33 (right)/20 (left) | 30/48/14 (right) −20/62/10 (left) | |
| Depressed elderly pre-treatment vs. post-treatment | Pre-treatment>post-treatment | Precuneus (right/left) | T=5.08, p<0.05 FDR-ROI, kE>5 | 16.6/−43,8/8 −2.3/−78.5/40 |
| Left Insula | T=3.55, p<0.05 FDR-ROI, kE>5 | −41.8/10.5/−6.8 | ||
| Left Hippocampus | T=4.8, p<0.05 FDR-ROI, kE>5 | −29.1/−38.4/2.01 | ||
| Post-treatment>Pre-treatment | Medial frontal gyrus (right/left) | T=6.55, p<0.05 FDR-ROI, kE>5 | 40/−16/64 −36/−16/66 | |
| Dorsal ACC | T=5.17, p<0.05 FDR-ROI, kE>5 | 5.4/4.4/46.9 | ||
| Treatment-responsive vs. treatment nonresponsive depressed elderly* | Treatment non-responsive> Treatment responsive | Cuneus | T=2.39, p=0.004, kE=1069 | 8/−88/14 |
| Dorsal ACC | T=2.16, p=0.02, kE=142 | −4/4/32 | ||
| Treatment responsive> Treatment nonresponsive | Medial prefrontal cortex | T=2.52, p=0.01, kE=75 | 2/66/0 | |
| Precuneus | T=2.97, p=0.004, kE= 722 | −2/−66/34 | ||
| Treatment-responsive vs. treatment nonresponsive depressed elderly** | Treatment nonresponsive > Treatment responsive | Left striatum | T=4.14, p<0.05 FDR-ROI, kE>5 | −11.9/5.1/−6.8 |
| Treatment responsive>Treatmentnonresponsive | dlPFC (right) | T =2.09, p=0.02, kE=17 | 50/10/48 | |
| Treatment-responsive vs. treatment nonresponsive depressed elderly, with RBANS as covariate | Treatment nonresponsive> Treatment responsive | Thalamus (right/left) | T =4.74, p= 0.001, kE=58(right)/31 (left) | 7.8/−26.8/−2 =−11.23/-25.4/−2 |
| Left Striatum | T =5.4, p< 0.001, kE=21 | −13.2/−0.3/−6.1 | ||
| Midbrain | T =4.56, p= 0.001, kE=20 | 6.4/−27.5/−1.3 | ||
| Treatment responsive> Treatment nonresponsive | Superior frontal gyrus | T= 4.53, p=0.001, kE =7 | 18.7/43.9/50 |
using baseline scan (T1) as outcome variable;
using the difference between the 12-week scan and the baseline scan (T2-T1) as outcome variable; Legend: MNI=Montreal Neurologic Institute; FDR=false discovery rate; ROI=region of interest; kE=cluster size; ACC = anterior cingulate cortex; dlPFC= dorsolateral prefrontal cortex.
Fig 1.
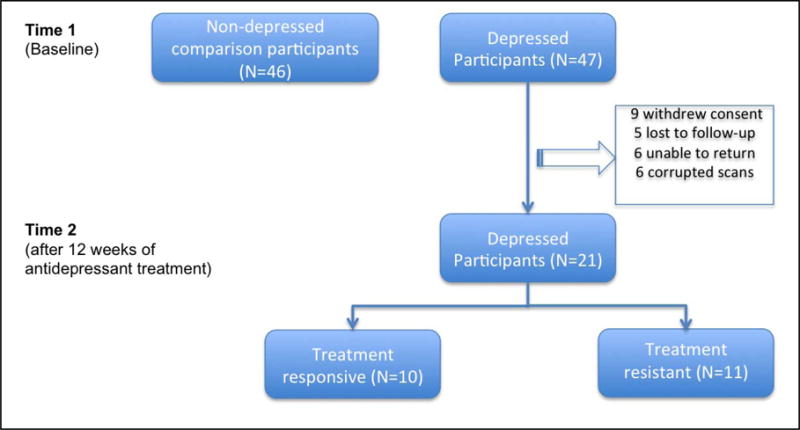
Flowchart of the participants in the study.
3.1. Group analysis at baseline
3.1.1. Compared with never-depressed older adults (N=46), elderly depressed participants (N=47) had greater functional connectivity in the precuneus (t=3.2, p=0.001 uncorrected, cluster size 185) (see figure 2).
Fig 2.
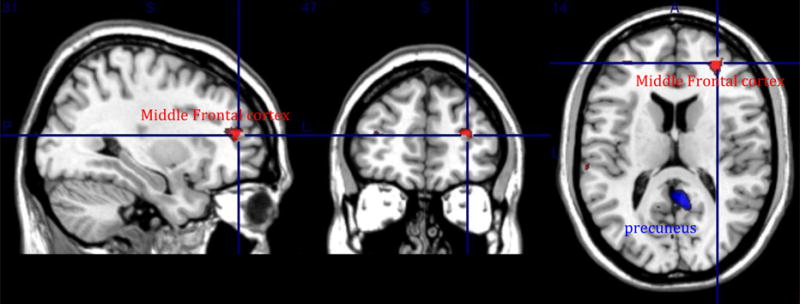
Group analysis at baseline between non-depressed elderly [N=46] and depressed elderly participants [N=47] Red: Non-depressed elderly participants > Depressed elderly participants (middle frontal cortex). Blue: Depressed elderly participants > Non-depressed elderly participants (precuneus).
3.1.2. Compared with elderly depressed, the never-depressed participants had greater functional connectivity in the middle frontal gyrus (right and left) [t=3.2, p=0.001 uncorrected, cluster size =33(right)/20(left)] (see figure 2).
Given the significant differences in age and cognitive status between comparison and depressed participants (see Table 1), we repeated the analysis controlling for age and MMSE score. The results were unchanged (maps available upon request).
When adjusting for the WMH in the MANOVA model (for the anterior cingulate, right and left middle frontal and posterior cingulate) or in the robust regression model (for the precuneus) no statistical difference was detected between the elderly depressed and the never-depressed older adult participants.
3.2. Treatment-effect analysis
3. 2.1. Relative to post-treatment, pre-treatment elderly depressed participants (N=47) had significantly higher functional connectivity in the right and left precuneus, left insula, and left hippocampus [t = from 3.55 to 5.08, p<0.05, FDR-ROI (see figure 3)].
Fig 3.
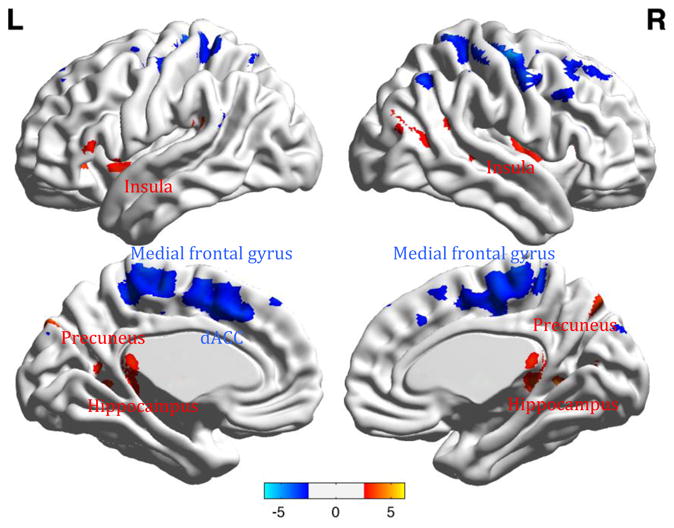
Group analysis of resting state functional connectivity between elderly depressed participants before [N=47] and after treatment [N=21].
Legend. Red: Pre-treatment>Post treatment contrast (precuneus, insula, hippocampus). Blue: Post-treatment>Pre-treatment contrast (medial frontal gyrus, dorsal Anterior Cingulate Cortex = ACC). Color bars list the range of t-values.
3.2.2. Relative to pre-treatment, post -treatment elderly depressed participants (N=21) had significantly higher functional connectivity in the right and left medial frontal gyrus, and dorsal anterior cingulate cortex (dACC) [t = 6.55 and 5.17 respectively, p<0.05, FDR-ROI (see figure 3)].
When adjusting for the WMH in the MANOVA model (for the right and left precuneus, left insula, left hippocampus) or in the robust regression model (for the right and left precentral gyrus) no statistical difference was detected between the elderly depressed before and after treatment.
3.3. Treatment response analysis
Treatment response was defined as a decrease in HDRS to 10 or less at twelve weeks (Andreescu et al., 2008b). After treatment, 11 depressed participants were defined as treatment resistant and 10 as treatment-responsive. We performed this analysis in two ways. First, we used the baseline functional connectivity (Tl) as outcome variable, in an effort to explore the predictive value of baseline functional connectivity for treatment response. Second, we used the difference in functional connectivity between the two time points (T2-T1) as the outcome variable; this difference reflects the increase in functional connectivity from baseline to week 12.
3.3.1. T1 as outcome variable (see Fig 4)
Fig 4.
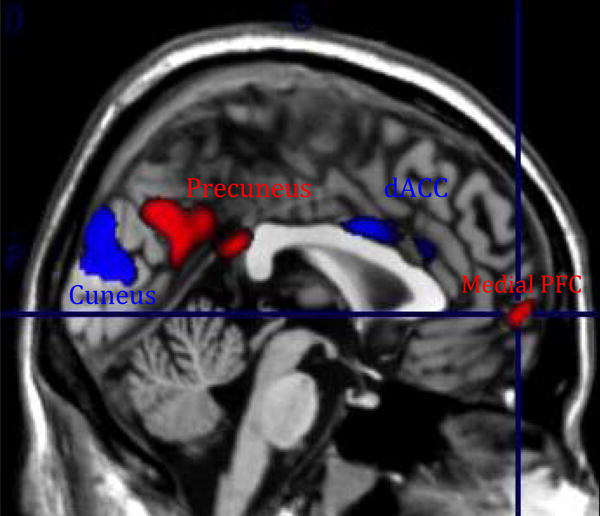
Treatment effect analysis using the baseline scan (T1) as outcome variable.
Legend. In red: Responders (N=10)>Non-responders (N=11)[medial prefrontal cortex, precuneus]. In blue: Non-responders (N=11) > Responders (N=10)[cuneus, dorsal ACC]
3.3.1.1. Relative to treatment-nonresponsive participants, treatment-responsive depressed participants had at baseline greater connectivity between the PCC seed and medial prefrontal cortex (t=2.52, p=0.01, cluster size =75) and the precuneus (t=2.97, p=0.004, clusteteize-722).
3.3.1.2. Relative to treatment-responsive participants, treatment-nonresponsive depressed participants had at baseline greater connectivity between the PCC seed and the cuneus (t=2.39, p=0.004, cluster size =1069) and dorsal ACC (t=2.16, p=0.02, cluster size = 142). The Ia and Ib results did not survive multiple comparison correction.
3.3.2. T2-T1 as outcome variable
3.3.2.1. Relative to treatment-responsive participants, treatment-nonresponsive depressed participants had steeper increase in resting state functional connectivity in the left striatum [t=4.14, p<0.05, FDR-ROI (see figure 5)].
Fig 5.
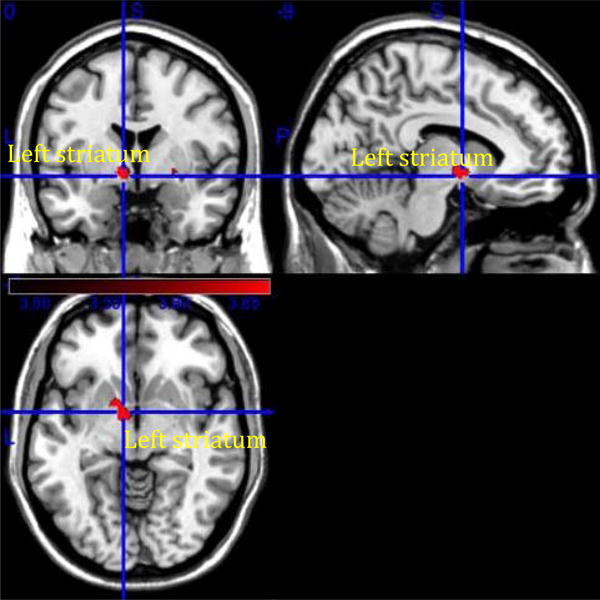
Treatment effect analysis using the difference between the 12-week scan and the baseline scan (T2-T1) as outcome variable.
Non-responders [N=11] > Responders [N=10] [left striatum].
When adjusting for the WMH in the robust regression model for the left striatum, the results remained statistically significant (two-tail t=3.148, p<0.001).
3.3.2.2. Relative to treatment-nonresponsive participants, treatment-responsive depressed participants had a steeper increase in functional connectivity in the left dorsolateral prefrontal cortex (t=2.09,p=0.02 uncorrected, cluster size 17). The results did not survive multiple comparison correction.
When adjusting for the WMH in the robust regression model for the left dorsolateral prefrontal cortex no statistical difference was detected between the treatment-nonresponsive and treatment-responsive elderly depressed participants.
4. Discussion
In this analysis of resting state functional connectivity in LLD, we report treatment-related differences in DMN connectivity. In our previous report, we described improved PCC-medial prefrontal cortex connectivity following 12 weeks of antidepressant treatment (Wu et al., 2011). In this new and larger sample we describe a more complex behavior of the DMN following treatment. Before treatment, depressed participants exhibit greater connectivity in various posterior regions (precuneus, hippocampus). Post-treatment, the same participants exhibit increased connectivity in the anterior, frontal nodes of the DMN. This post-treatment “frontalization” of the DMN connectivity – resembling the DMN functional connectivity in never-depressed participants- suggests a possible normalizing effect of antidepressant treatment. We also confirm the role of WMH burden in altering the functional connectivity in LLD. There are however areas of divergence with our previous report, mainly in the group contrasts between never-depressed comparison participants and depressed participants: in the Wu et al report depressed participants exhibited decreased connectivity in the subgenual ACC but increased connectivity in the dorsomedial prefrontal cortex and the orbitofrontal cortex. In our current study, depressed participants exhibited greater connectivity in the posterior nodes of the DMN, and lower connectivity in the anterior nodes of the DMN. These areas of divergence will require further research with larger samples.
While descriptive changes of the DMN are relevant for in-depth understanding of the neural basis of LLD, the identification of neural markers of treatment response may have a more immediate translational effect [e.g. reducing the lengthy treatment trials often required in clinical practice]. In this study, poor treatment response was associated with increased functional connectivity in the left striatum after 12 weeks of treatment, a result that remained significant after adjusting for WMH burden. Several investigators, including our group, have described structural and functional modifications in the striatum, associated with cognitive task-related activity in LLD (Aizenstein et al., 2005; Butters et al., 2009). Recently, increased functional connectivity in the striatum during resting state has been described in amnestic mild cognitive impairment (Bai et al., 2008). Several studies have described the role of cognitive impairment in treatment resistance in LLD (Alexopoulos et al., 2004; Lloyd et al., 2001; Reynolds et al., 2011). Other studies have observed that non-remitting depressed elderly have shown slower processing speed, worse episodic memory and executive function (Sheline et al., 2010). Taken together, these findings suggest that depressed participants with poor treatment response may have subtle functional changes reflecting a pre-clinical neurodegenerative process (Andreescu et al., 2008a; Steffens et al., 2007; Steffens et al., 2003). Dysfunction in connectivity due to neurodegeneration or white matter changes may mediate susceptibility to treatment response (Aizenstein et al., 2011; Sheline et al., 2010).
While several studies have addressed the neural markers of treatment response in midlife MDD (Keedwell et al., 2009; Lisiecka et al., 2011; Mayberg et al., 1997; Pizzagalli, 2011), the increased connectivity in the striatum is one of the first reports of neural biomarkers of treatment response in LLD. Further, it extends the substantial literature suggesting impaired cognitive function is an index of treatment resistance.
The findings reported in this study as well as in the Wu et al (2011) and other recent studies (Aizenstein et al., 2011; Alexopoulos et al., 2012; Kenny et al., 2010; Sexton et al., 2012; Steffens et al., 2011), point toward specific functional connectivity alterations in LLD.
Given the changes in results when adjusting for WMH burden, our study confirms the central role of the white matter lesions in disrupting brain functional connectivity (Aizenstein et al., 2011; Steffens et al., 2011). However, the lack of difference between groups with regard to WMH burden points toward a complex model in which the association between functional connectivity and treatment response in late-life depression is the final result of the interplay of multiple factors including compromised white matter tracts, age-related neurodegeneration (e.g. cortical atrophy, amyloid deposition), and genetic factors (e.g. APOE or BDNF status)(Ballmaier et al., 2004; Taylor et al., 2008b). As we did not measure other factors, we can only speculate that the results that survived WMH burden correction may indicate a greater weight of grey matter lesions in predicting treatment response (e.g. striatum), while the results contingent on WMH (e.g. vmPFC) indicate a greater weight of the white matter lesions in predicting treatment response (Gunning-Dixon et al., 2010; Taylor et al., 2008a). This hypothesis is supported by the structural underpinning of the default mode network (Greicius et al., 2009) in which longer tracts connecting the posterior seed with the anterior nodes of the network (e.g. vmPFC) may be more vulnerable to small vessel disease, as these tracts pass through the deep and periventricular white matter regions (Krishnan et al., 2006).
Our study has several strengths: a large sample that enabled us to perform sub-group analysis, a longitudinal design that allowed us to compare participants pre- and post-treatment, and a comprehensive neuropsychological evaluation that allowed us to assess multiple cognitive domains.
Several limitations are worth noting. As participants were pooled from three different clinical trials, they received different antidepressant pharmacotherapy during the 12-week intervention. However, with the exception of one participant who received augmenting treatment with Bupropion, all of the antidepressant medications used in these trials were selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs). The efficacy difference between various SSRIs and SNRIs is still a matter of debate, with some (Taylor et al., 2006; Taylor and Doraiswamy, 2004) but not other (Papakostas et al., 2007; Thase et al., 2011) studies reporting no efficacy difference between these two categories of antidepressants. Several participants were lost to follow-up, which reduced the post-treatment sample. The baseline group analysis results (depressed versus never-depressed) did not survive multiple correction analysis, indicating an increased heterogeneity in the current sample of depressed older adult participants. Other possible limitations include the use of a five-minute acquisition period for the resting state fMRI and the use of seed-based connectivity analysis. Data-driven analysis, such as independent component analysis (ICA) is a potential venue for further exploring the functional connectivity in this database. Lastly, we used WMH burden as a global biomarker of overall white matter disease and we only computed the whole brain WMH burden. Thus, we cannot make more specific inferences regarding the region-specific correlation between WMH burden and ROI functional connectivity indices.
Recently, it has been shown that functional MRI changes may be sufficiently specific to be clinical useful. Thus, distinct network disruptions have been described for specific neurodegenerative disorders such as Alzheimer's disease, semantic dementia and frontotemporal dementia(Seeley et al., 2009). DMN changes are relatively easy to use as biomarkers of treatment response as the resting state acquisition is brief and does not require complex activation tasks. Collecting brain imaging data along with clinical treatment can provide several types of biomarkers: diagnostic markers, prognostic markers, markers of disease progression and markers of neural changes that result from treatment (Cullen, 2012). Our present study provides groundwork regarding the neural markers of treatment response in LLD, but further research is warranted, including a standardized treatment randomization design. Further research is also needed to disentangle the role of the numerous other neural contributors, such as different biomarker cascades (e.g., inflammation, neurotrophic support, endocrine-metabolic dysfunction) and neurotransmitter systems modified by SSRIs or SNRIs and the impact of these changes on resting state connectivity.
Acknowledgments
Supported by NIMH MH 086686, MH 071944, MH080240, R01 MH080240, P30 MH90333, R21 NS060184, R37 AG025516, AG033575, P01 AG025204, the Brain and Behavior Research Foundation (NARSAD) Young Investigator Award (Dr. Andreescu), the UPMC Endowment in Geriatric Psychiatry, and the John A. Hartford Foundation.
The authors would like to thank the Geriatric Psychiatry Neuroimaging Lab staff for their support.
Footnotes
Financial disclosures: Carmen Andreescu, Dana Tudorascu, Erica Tamburo, Julie Price and Meenal Patel do not have any potential conflict of interest to acknowledge. Meryl A. Butters received lecture fees from the University of Texas Southwestern Medical Center, the Southern Illinois University School of Medicine, and the Fundació ACE (Barcelona, Spain). Jordan F. Karp has received medication supplies for investigator initiated trials from Pfizer and Reckitt Benckiser, and owns stock in Corcept. Charles F. Reynolds III has received research support from Pfizer Inc., Eli Lilly and Co., Bristol Meyers Squibb, Forest Pharmaceuticals, and Wyeth Pharmaceuticals. Howard Aizenstein has received research support from Novartis Pharmaceuticals.
Clinical registration trials: NCT00696292 (Maintenance therapies in late-lifedepression-III), NCT00177294 (Geriatric Depression: Gettingbetter, gettingwell),NCT00177671 (ADAPT: Addressing Depression and Pain together).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizenstein HJ, Andreescu C, Edelman KL, Cochran JL, Price J, Butters MA, Karp J, Patel M, Reynolds CF., 3rd fMRI Correlates of White Matter Hyperintensities in Late-Life Depression. Am J Psychiatry. 2011 doi: 10.1176/appi.ajp.2011.10060853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein HJ, Butters MA, Figurski JL, Stenger VA, Reynolds CF, 3rd, Carter CS. Prefrontal and striatal activation during sequence learning in geriatric depression. Biol Psychiatry. 2005;58:290–296. doi: 10.1016/j.biopsych.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006;60:1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Murphy C, Heo M. Executive dysfunction, heart disease burden, and remission of geriatric depression. Neuropsychopharmacology. 2004;29:2278–2284. doi: 10.1038/sj.npp.1300557. [DOI] [PubMed] [Google Scholar]
- Andreescu C, Butters MA, Begley A, Rajji T, Wu M, Meltzer CC, Reynolds CF, 3rd, Aizenstein H. Gray matter changes in late life depression--a structural MRI analysis. Neuropsychopharmacology. 2008a;33:2566–2572. doi: 10.1038/sj.npp.1301655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C, Mulsant BH, Houck PR, Whyte EM, Mazumdar S, Dombrovski AY, Pollock BG, Reynolds CF., 3rd Empirically derived decision trees for the treatment of late-life depression. Am J Psychiatry. 2008b;165:855–862. doi: 10.1176/appi.ajp.2008.07081340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C, Reynolds CF., 3rd Late-life depression: evidence-based treatment and promising new directions for research and clinical practice. Psychiatr Clin North Am. 2011;34:335–355. doi: 10.1016/j.psc.2011.02.005. vii-iii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Zhang Z, Yu H, Shi Y, Yuan Y, Zhu W, Zhang X, Qian Y. Default-mode network activity distinguishes amnestic type mild cognitive impairment from healthy aging: a combined structural and resting-state functional MRI study. Neurosci Lett. 2008;438:111–115. doi: 10.1016/j.neulet.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Kumar A, Thompson PM, Narr KL, Lavretsky H, Estanol L, Deluca H, Toga AW. Localizing gray matter deficits in late-onset depression using computational cortical pattern matching methods. Am J Psychiatry. 2004;161:2091–2099. doi: 10.1176/appi.ajp.161.11.2091. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Brett M, A JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox, 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- Bruce ML, Ten Have TR, Reynolds CF, 3rd, Katz II, Schulberg HC, Mulsant BH, Brown GK, McAvay GJ, Pearson JL, Alexopoulos GS. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291:1081–1091. doi: 10.1001/jama.291.9.1081. [DOI] [PubMed] [Google Scholar]
- Butters MA, Aizenstein HJ, Hayashi KM, Meltzer CC, Seaman J, Reynolds CF, 3rd, Toga AW, Thompson PM, Becker JT. Three-dimensional surface mapping of the caudate nucleus in late-life depression. Am J Geriatr Psychiatry. 2009;17:4–12. doi: 10.1097/JGP.0b013e31816ff72b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DP, Perry GS. Depression as a major component of public health for older adults. Prev Chronic Dis. 2008;5:A22. [PMC free article] [PubMed] [Google Scholar]
- Charney DS, Reynolds CF, 3rd, Lewis L, Lebowitz BD, Sunderland T, Alexopoulos GS, Blazer DG, Katz IR, Meyers BS, Arean PA, Borson S, Brown C, Bruce ML, Callahan CM, Charlson ME, Conwell Y, Cuthbert BN, Devanand DP, Gibson MJ, Gottlieb GL, Krishnan KR, Laden SK, Lyketsos CG, Mulsant BH, Niederehe G, Olin JT, Oslin DW, Pearson J, Persky T, Pollock BG, Raetzman S, Reynolds M, Salzman C, Schulz R, Schwenk TL, Scolnick E, Unutzer J, Weissman MM, Young RC. Depression and Bipolar Support Alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in late life. Arch Gen Psychiatry. 2003;60:664–672. doi: 10.1001/archpsyc.60.7.664. [DOI] [PubMed] [Google Scholar]
- Cullen K. Imaging Adolescent Depression Treatment. Am J Psychiatry. 2012;169:348–350. doi: 10.1176/appi.ajp.2012.12010057. [DOI] [PubMed] [Google Scholar]
- First M, S R, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P) 2.0 1995. [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Friston K. Functional and Effective Connectivity in Neuroimaging: A synthesis. Hum Brain Mapp. 1994;2:56–78. [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulated cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Walton M, Cheng J, Acuna J, Klimstra S, Zimmerman ME, Brickman AM, Hoptman MJ, Young RC, Alexopoulos GS. MRI signal hyperintensities and treatment remission of geriatric depression. J Affect Disord. 2010 doi: 10.1016/j.jad.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp JF, Skidmore E, Lotz M, Lenze E, Dew MA, Reynolds CF., 3rd Use of the late-life function and disability instrument to assess disability in major depression. J Am Geriatr Soc. 2009;57:1612–1619. doi: 10.1111/j.1532-5415.2009.02398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp JF, Weiner DK, Dew MA, Begley A, Miller MD, Reynolds CF., 3rd Duloxetine and care management treatment of older adults with comorbid major depressive disorder and chronic low back pain: results of an open-label pilot study. Int J Geriatr Psychiatry. 2010;25:633–642. doi: 10.1002/gps.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell P, Drapier D, Surguladze S, Giampietro V, Brammer M, Phillips M. Neural markers of symptomatic improvement during antidepressant therapy in severe depression: subgenual cingulate and visual cortical responses to sad, but not happy, facial stimuli are correlated with changes in symptom score. J Psychopharmacol. 2009;23:775–788. doi: 10.1177/0269881108093589. [DOI] [PubMed] [Google Scholar]
- Kenny ER, O'Brien JT, Cousins DA, Richardson J, Thomas AJ, Firbank MJ, Blamire AM. Functional connectivity in late-life depression using resting-state functional magnetic resonance imaging. Am J Geriatr Psychiatry. 2010;18:643–651. doi: 10.1097/JGP.0b013e3181cabd0e. [DOI] [PubMed] [Google Scholar]
- Koch W, Teipel S, Mueller S, Buerger K, Bokde AL, Hampel H, Coates U, Reiser M, Meindl T. Effects of aging on default mode network activity in resting state fMRI: does the method of analysis matter? Neuroimage. 2010;51:280–287. doi: 10.1016/j.neuroimage.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Krishnan MS, O'Brien JT, Firbank MJ, Pantoni L, Carlucci G, Erkinjuntti T, Wallin A, Wahlund LO, Scheltens P, van Straaten EC, Inzitari D. Relationship between periventricular and deep white matter lesions and depressive symptoms in older people. The LADIS Study. Int J Geriatr Psychiatry. 2006;21:983–989. doi: 10.1002/gps.1596. [DOI] [PubMed] [Google Scholar]
- Lisiecka D, Meisenzahl E, Scheuerecker J, Schoepf V, Whitty P, Chaney A, Moeller HJ, Wiesmann M, Frodl T. Neural correlates of treatment outcome in major depression. Int J Neuropsychopharmacol. 2011;14:521–534. doi: 10.1017/S1461145710001513. [DOI] [PubMed] [Google Scholar]
- Lloyd AJ, Grace JB, Jaros E, Perry RH, Fairbairn AF, Swann AG, O'Brien JT, McKeith IG. Depression in late life, cognitive decline and white matter pathology in two clinico-pathologically investigated cases. Int J Geriatr Psychiatry. 2001;16:281–287. doi: 10.1002/gps.328. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Thase ME, Fava M, Nelson JC, Shelton RC. Are antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depressive disorder? A meta-analysis of studies of newer agents. Biol Psychiatry. 2007;62:1217–1227. doi: 10.1016/j.biopsych.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, 3rd, Butters MA, Lopez O, Pollock BG, Dew MA, Mulsant BH, Lenze EJ, Holm M, Rogers JC, Mazumdar S, Houck PR, Begley A, Anderson S, Karp JF, Miller MD, Whyte EM, Stack J, Gildengers A, Szanto K, Bensasi S, Kaufer DI, Kamboh MI, DeKosky ST. Maintenance treatment of depression in old age: a randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Arch Gen Psychiatry. 2011;68:51–60. doi: 10.1001/archgenpsychiatry.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CF, 3rd, Dew MA, Martire LM, Miller MD, Cyranowski JM, Lenze E, Whyte EM, Mulsant BH, Pollock BG, Karp JF, Gildengers A, Szanto K, Dombrovski AY, Andreescu C, Butters MA, Morse JQ, Houck PR, Bensasi S, Mazumdar S, Stack JA, Frank E. Treating depression to remission in older adults: a controlled evaluation of combined escitalopram with interpersonal psychotherapy versus escitalopram with depression care management. Int J Geriatr Psychiatry. 2010;25:1134–1141. doi: 10.1002/gps.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Allan CL, Le Masurier M, McDermott LM, Kalu UG, Herrmann LL, Maurer M, Bradley KM, Mackay CE, Ebmeier KP. Magnetic Resonance Imaging in Late-Life Depression: Multimodal Examination of Network DisruptionMRI in Late-Life Depression. Arch Gen Psychiatry. 2012;69:680–689. doi: 10.1001/archgenpsychiatry.2011.1862. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Pieper CF, Barch DM, Welsh-Boehmer K, McKinstry RC, MacFall JR, D'Angelo G, Garcia KS, Gersing K, Wilkins C, Taylor W, Steffens DC, Krishnan RR, Doraiswamy PM. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67:277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, Potter GG, McQuoid DR, MacFall JR, Payne ME, Burke JR, Plassman BL, Welsh-Bohmer KA. Longitudinal magnetic resonance imaging vascular changes, apolipoprotein E genotype, and development of dementia in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry. 2007;15:839–849. doi: 10.1097/JGP.0b013e318048a1a0. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Taylor WD, Denny KL, Bergman SR, Wang L. Structural integrity of the uncinate fasciculus and resting state functional connectivity of the ventral prefrontal cortex in late life depression. PLoS One. 2011;6:e22697. doi: 10.1371/journal.pone.0022697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, Taylor WD, Krishnan KR. Progression of subcortical ischemic disease from vascular depression to vascular dementia. Am J Psychiatry. 2003;160:1751–1756. doi: 10.1176/appi.ajp.160.10.1751. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Freemantle N, Geddes JR, Bhagwagar Z. Early onset of selective serotonin reuptake inhibitor antidepressant action: systematic review and meta-analysis. Arch Gen Psychiatry. 2006;63:1217–1223. doi: 10.1001/archpsyc.63.11.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Doraiswamy PM. A systematic review of antidepressant placebo-controlled trials for geriatric depression: limitations of current data and directions for the future. Neuropsychopharmacology. 2004;29:2285–2299. doi: 10.1038/sj.npp.1300550. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Kuchibhatla M, Payne ME, Macfall JR, Sheline YI, Krishnan KR, Doraiswamy PM. Frontal white matter anisotropy and antidepressant remission in late-life depression. PLoS One. 2008a;3:e3267. doi: 10.1371/journal.pone.0003267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Zuchner S, McQuoid DR, Payne ME, MacFall JR, Steffens DC, Speer MC, Krishnan KR. The brain-derived neurotrophic factor VAL66MET polymorphism and cerebral white matter hyperintensities in late-life depression. Am J Geriatr Psychiatry. 2008b;16:263–271. doi: 10.1097/JGP.0b013e3181591c30. [DOI] [PubMed] [Google Scholar]
- Thase ME, Ninan PT, Musgnung JJ, Trivedi MH. Remission with venlafaxine extended release or selective serotonin reuptake inhibitors in depressed patients: a randomized, open-label study. The primary care companion to CNS disorders. 2011;13 doi: 10.4088/PCC.10m00979blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Aging and functional brain networks. Mol Psychiatry. 2012;17:549–558. doi: 10.1038/mp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Whyte EM, Dew MA, Gildengers A, Lenze EJ, Bharucha A, Mulsant BH, Reynolds CF. Time course of response to antidepressants in late-life major depression: therapeutic implications. Drugs Aging. 2004;21:531–554. doi: 10.2165/00002512-200421080-00004. [DOI] [PubMed] [Google Scholar]
- Wu M, Andreescu C, Butters MA, Tamburo R, Reynolds CF, 3rd, Aizenstein H. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res. 2011 doi: 10.1016/j.pscychresns.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, Yao S. Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71:611–617. doi: 10.1016/j.biopsych.2011.10.035. [DOI] [PubMed] [Google Scholar]


