Abstract
Human exposure to potentially neurotoxic methylmercury species is a public-health concern for many populations worldwide. Both fish and whale are known to contain varying amounts of methylmercury species. However studies of populations that consume large quantities of fish or whale have provided no clear consensus as to the extent of the risk. The toxicological profile of an element depends strongly on its chemical form. We have used X-ray absorption spectroscopy to investigate the comparative chemical forms of mercury and selenium in fish and whale skeletal muscle. The predominant chemical form of mercury in whale is found to closely resemble that found in fish. In the samples of skeletal muscle studied, no involvement of selenium in coordination of mercury is indicated in either whale or fish, with no significant inorganic HgSe or HgS type phases being detected. The selenium speciation in fish and whale shows that similar chemical types are present in each, but in significantly different proportions. Our results suggest that for equal amounts of Hg in skeletal muscle, the direct detrimental effects arising from the mercury content from consuming skeletal muscle from whale and fish should be similar if the effects of interactions with other components in the meat are not considered.
Introduction
Fish is consumed daily by billions of people around the world and is the major dietary source of potentially neurotoxic methylmercury species in many human populations.1 Predatory marine fish such as swordfish and shark contain sufficiently high levels of methylmercury species that in some regions consumers are currently advised to eat these fish less frequently than once a month, and not at all if pregnant.2 The nature of the methylmercury coordination in marine fish has been shown by X-ray absorption spectroscopy (XAS) to be an aliphatic thiolate, similar to the methylmercury-cysteine complex.3 More recently, it has been shown that this chemical form of mercury is not modified by digestion with simulated gastric fluid and that no molecular-level interactions with the selenium in the digest can be detected.4
The current limits for human consumption of fish borne mercury have in part been set with reference to data from studies on human populations with high dietary mercury from consumption of seafood. Three large studies of this nature have been reported. One study is located in the Faeroe Islands in the North Atlantic,5 a second in the Seychelles Islands in the Indian Ocean6 and the third in New Zealand.7 The Faeroes study is of a population with high dietary mercury primarily from pilot whale consumption. Faeroese traditional food also includes rendered pilot whale blubber, which contains high levels of polychlorinated biphenyls and dioxins,8-11 and well as other potentially toxic metals such as cadmium12 that may also affect health adversely.12 The primary source of exposure in the New Zealand study was battered and fried shark skeletal muscle typically prepared as “fish and chips”. The Seychelles study is of a population that consumes large quantities of marine fish that have mercury levels similar to those in oceanic fish consumed in North America and Europe. In all three studies, a cohort of pregnant women was evaluated for prenatal methylmercury exposure, and their children's development was examined at varying ages throughout childhood for neuro-developmental deficits. The three studies reach differing conclusions regarding the hazards of mercury exposure from seafood consumption. The Faeroes study reported statistically significant adverse effects and has been the basis for US fish consumption advisories.
The chemical form of mercury is key to its toxicological60 properties.13,14 It has been suggested that differences between the diets may be a factor in the apparent discrepancies.15 Recent work has used XAS to examine tissues from beluga whale16 and striped dolphin.17 These studies detected essentially only inorganic forms, in particular mercuric selenide, and the chemical nature of the non-inorganic mercury in whale meat remains uncertain. There are also well-established interactions between the toxicology of mercury and selenium in experimental animals.18 Ganther et al. showed that in quail, selenium naturally present in tuna could in part counteract the toxic effects of methylmercury hydroxide exposure.19 Moreover, when rats were given both methylmercury hydroxide and sodium selenite some of the toxic effects of the mercury were ameliorated.20,21 More recent work has emphasized the importance of the potential protective effects of selenium18,22-25 suggesting that an important factor in assessing the adverse effects of dietary methylmercury species is the mercury to selenium ratio.23,24 In particular it has been suggested that the high selenium levels naturally present in fish might actually serve to reduce the adverse effects of methylmercury.19,23,24 Again, the chemical form of selenium will be important to this.14 Here, we use XAS to compare the chemical nature of the mercury and selenium in skeletal muscle from whale and fish.
Experimental
X-ray Absorption Spectroscopy
X-ray absorption spectroscopic (XAS) measurements were conducted at the Stanford Synchrotron Radiation Lightsource (SSRL) with the SPEAR storage ring containing 200 mA at 3.0 GeV. Mercury LIII-edge and selenium K-edge data were collected on the structural molecular biology XAS beamline 9-3 operating with a wiggler field of 2 T and employing a Si(220) double-crystal monochromator. Beamline 9-3 is equipped with a vertically collimating mirror upstream of the monochromator, and a downstream bent-cylindrical focusing mirror, both rhodium-coated. Harmonic rejection was accomplished by setting the cutoff angle of the mirrors to reject energies above 15 keV. To minimize radiation damage samples were maintained at a temperature of approximately 10K in a liquid helium flow cryostat (Oxford Instruments, Abingdon, UK). X-ray absorption spectra were measured as the Se Kα1,2 or Hg Lα1,2 fluorescence excitation spectra using a 30-element germanium array detector26 with analog electronics (Canberra Corporation, Meriden CT, USA) employing an amplifier shaping time of 0.125 μsec. To avoid problems with non-linearity of the detector due to high count-rates, X-ray filters (made of elemental As for Se, and Ga2O3 for Hg) were used to preferentially absorb scattered radiation, with silver Soller-slits (EXAFS Co., Pinoche NV, USA) optimally positioned between the sample and the detector. Incident and transmitted X-ray intensities were measured using nitrogen-filled ionization chambers. The mercury spectra were energy-calibrated with reference to LIII-edge spectrum of Hg-Sn amalgam foil measured simultaneously with the data, the lowest energy inflection of which was assumed to be 12285.0 eV. The selenium spectra were similarly energy calibrated with reference to the lowest energy inflection of a hexagonal elemental selenium foil which was assumed to be 12658.0 eV. XAS data were processed using standard techniques and employing the50 EXAFSPAK program suite.27 Normalization was done relative to the Hg LIII or Se K edge-jumps which are an indication of total Hg or Se, respectively and insensitive to chemical form. Edge-jumps were determined by using background removal and theoretical X-ray absorption cross sections using the EXAFSPAK program BACKSUB. Near-edge spectra were fitted to linear combinations of standard spectra using the EXAFSPAK program DATFIT and using the criteria previously described,4 which are that to be included in the fit the fitted fraction must be greater than 3 × the estimated standard deviation (i.e. the 99% confidence limit) obtained from the diagonal elements of the covariance matrix. Total selenium and mercury levels were estimated by measuring the absolute edge jump and comparing to standard solutions of known concentrations, as previously described.4
Sample preparation
Samples of skeletal muscle from an adult female pygmy sperm whale (Kogia breviceps) were obtained from a recently deceased specimen found beached. The samples did not show any signs of significant bacterial decay or degradation. Fish samples were gifts from a local fish market (Cook's Seafood, Menlo Park CA) and were taken from larger pieces of fish that were otherwise intended for human consumption. Tissue samples were cut into 3×2×20 mm3 pieces, loaded into acrylic XAS sample cuvettes, frozen in liquid nitrogen immediately and inserted in the liquid helium cryostat for XAS measurements. Standard solutions were prepared in aqueous buffer (50mM HEPES, pH 7.5) with 30% v/v glycerol to prevent ice diffraction artifacts places into acryallic 3×2×20 mm3 cuvettes and frozen in liquid nitrogen. Model compounds where commercially available were of the best quality available (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) or prepared as previously described.4,28
Results and Discussion
X-ray absorption spectra arise from photo-excitation of a core electron. The near-edge portion of the spectra can be defined as that within about 50 eV of the absorption edge. Near-edge spectra show structure arising from transitions from the core level into unoccupied molecular orbitals of the system. Intense transitions are dipole-allowed, Δl=±1; for K edges which result from 1s excitations, this yields final states with considerable p-orbital character. With LIII and LII edges transitions originate at 2p3/2 and 2p1/2, respectively, and yield final states of predominantly d or s orbital character. The 2p3/2→ns cross sections are only about 5% of the 2p3/2→nd, and the former are therefore often considered to be relatively unimportant. Near-edge spectra are therefore very sensitive to electronic structure, and can give a fingerprint of the chemical species of the metal or metalloid concerne.14,29 Using comparisons with standard spectra of known compounds, near-edge spectra can effectively be used to identify an overall chemical type, though often not the specific species involved. Thus, Hg2+ coordinated to four cysteine thiolate donors could be identified as Hg2+ bound to four aliphatic thiolates but no further identification of the aliphatic moiety would be expected. The nomenclature used for the near-edge spectrum is rather confused,29 and this region is alternatively referred to as the edge spectrum, the X-ray absorption near-edge fine structure or XANES, the near-edge X-ray absorption fine structure or NEXAFS and a variety of other acronyms.29 The region of the X-ray absorption spectrum above the absorption edge contains oscillatory modulations in the absorption coefficient known as the extended X-ray absorption fine structure or EXAFS. Analysis of the EXAFS requires higher concentrations than analysis of the near-edge spectra, and the tissue samples studied here are too low in concentration for EXAFS analysis.
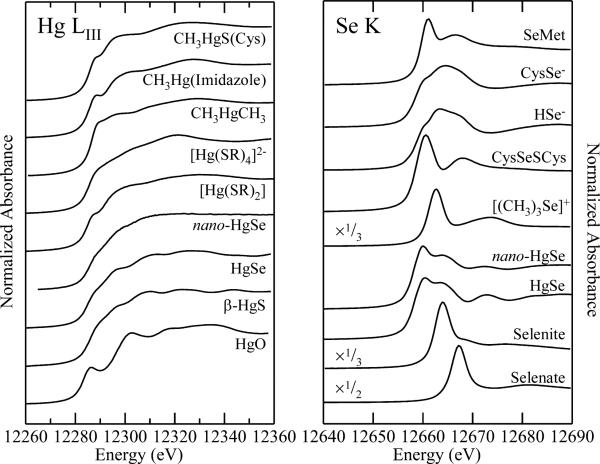
Fig. 1 shows the Hg LIII and Se K near-edge spectra of selected standard compounds. As we have previously commented3,4,30 the differences between individual Hg LIII spectra are more subtle than for Se K spectra. In the case of Se K-edges the valence orbitals have mainly p-character, giving rise to intense dipole-allowed structure in the spectra and rich chemical variability. In contrast, for Hg LIII edges, there is only minor involvement of d-orbitals in the valence orbitals, and thus only subtle variability in the spectra. The consequence of this is that significantly better signal to noise ratios are required for Hg. Despite this the spectra can still give the desired information concerning speciation of an unknown. We note that certain selenium compounds such as selenate are subject to photo-reduction by the X-ray beam. In such cases progressive changes in the spectra are observed in sequential scans, and care must be taken to obtain chemically relevant spectra. Several experimental strategies can help protect against this, including the use of liquid helium temperatures and translating the sample during data acquisition so that the beam interrogates only unexposed regions. Photo-reduced spectra are clearly evident on inspection of the data reported by other workers,31 although often this is not commented upon.
Fig. 1.
Comparison of Hg LIII and Se K near-edge spectra of selected standard species. Most species were measured as dilute (ca. 1 mM) aqueous solutions buffered at physiological pH (7-7.5) in the presence of 30% v/v glycerol using X-ray fluorescence detection. Exceptions were nano-HgSe which was run as a colloidal suspension in buffered water with 30% v/v glycerol, HgSe, HgO and β-HgS which were run as solids by monitoring transmittance, and CH3HgCH3 which was run in isopropanol solution due to its low water solubility.
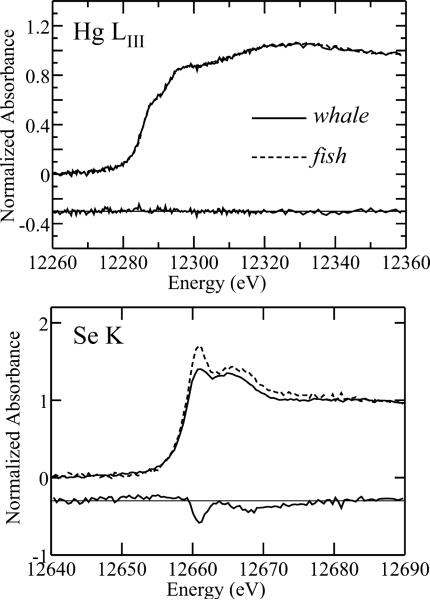
Fig. 2 shows a comparison of the Hg LIII and Se K near-edge spectra of whale muscle with those of a sample of swordfish (Xiphias gladius) skeletal muscle,3,4 plus difference spectra to illustrate the difference in speciation. Elemental concentrations can be estimated directly from X-ray absorption spectra by comparing the edge-jumps from un-normalized background-subtracted spectra with edge-jumps measured from spectra of solutions containing the element of interest (either Se or Hg) at known concentrations under the same conditions.4 When this was done for samples of whale, swordfish and Pacific bluefin tuna (Thunnus orientalis) we obtained approximate mercury concentrations of 2.3, 5.0 and 2.5 μM for whale, swordfish and tuna skeletal muscle, respectively. Similar calculations for selenium gave approximate selenium concentrations of 4.7, 5.1 and 5.8 μM for whale, swordfish and tuna skeletal muscle, respectively. These levels are similar to those previously reported.23 Comparison of the Hg LIII spectra of whale with that of swordfish and tuna shows that they are identical within the noise of the data, the difference spectrum showing only noise with no recognizable spectroscopic differences (Fig. 2). In contrast, the selenium spectra of whale and the fish samples are significantly different, as illustrated by the difference spectrum shown in Fig. 2.
Fig. 2.
Difference spectra for Hg LIII and Se K near-edges of whale and swordfish. In both plots the lower trace shows the difference, and this has been vertically displaced for clarity.
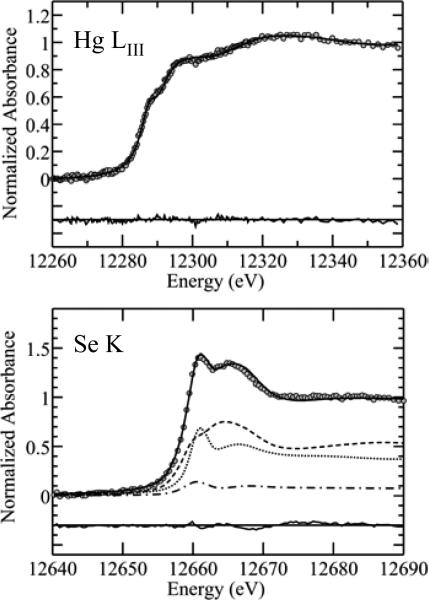
Fig. 3 shows quantitative analyses of the spectra of whale by fitting with a linear combination of standards. As expected from the similarity to the fish spectra,3,4 the Hg LIII is adequately fitted by using only a single component, which indicates the presence of methylmercury L-cysteinate or a chemically similar species in which mercury is coordinated by a methyl group and by an aliphatic thiolate.3,4 There are no indications of significant quantities of mercuric selenide or mercuric sulfide species that have been observed in other experiments.16,17 Our library of standard mercury LIII near-edge spectra currently contains some 52 different compounds; two-component fits of all combinations of these gave a majority component of methylmercury L-cysteinate as the best fit, and in all cases the second component was a small fraction which did not satisfy the criteria for inclusion in the fit. The analysis of the selenium K near-edge spectra is also shown in Fig. 3. Three significant components are indicated, with the greatest individual component being a selenocysteineate, and smaller contributions from species resembling selenomethionine and the selenyl-sulfide represented by Cys-S-Se-Cys. This is somewhat different than the analysis of both swordfish4 and Pacific bluefin tuna (Fig. 4), as summarized in Table 1, although as noted above, the mercury speciation was essentially identical in that it indicated only a single species – methylmercury-L-cysteinate. The identification of the minor component selenite in swordfish skeletal muscle, while somewhat unexpected, is apparently also found in mammalian blood plasma.32 As discussed above, near edge spectra cannot distinguish between species with similar atomic neighborhoods. Thus, mercury and selenium compounds with similar ligands have essentially indistinguishable spectra, and our analysis gives the types of compounds and not the exact species present.
Fig. 3.
Linear combination least-squares fitting analysis of the near-edge spectra of whale skeletal muscle. The Hg LIII near-edge spectrum is fitted by a single component of methylmercury L-cysteineate, while three components are needed for the selenium K near-edge spectrum. Individual components are shown; selenocysteinate (----), selenomethionine (—) and the selenylsulfide Cys-S-Se-Cys (–.–). In both Hg and Se plots the lower traces show the fit residual plus a zero line, and these have has been vertically displaced for clarity.
Fig. 4.
Linear combination least-squares fitting analysis of the spectra of bluefin tuna skeletal muscle. The Hg LIII near-edge spectrum is fitted by a single component of methylmercury L-cysteineate, while three components are needed for the selenium K near-edge spectrum. Individual components are shown; selenocysteinate (----), selenomethionine (—) and the selenylsulfide Cys-S-Se-Cys (–.–). In both Hg and Se plots the lower traces show the fit residual plus a zero line, and these have been vertically displaced for clarity.
Table 1.
Linear combination analysis of near-edge spectraa
| Edge | Component | Whale | Swordfishb | Tuna |
|---|---|---|---|---|
| Hg LIII | Methylmercury-L-Cysteineate | 100(1) | 100(1) | 100(1) |
| Se K | Selenocysteinate | 50(2) | <1(1) | 29(2) |
| Selenomethionine | 42(3) | 52(3) | 51(2) | |
| Cys-S-Se-Cys | 8(2) | 38(3) | 16(2) | |
| Selenite | — | 10(1) | 4(1)c | |
values in parenthesis are the estimated standard deviations obtained from the diagonal elements of the covariance matrix.
values taken from ref. 4
the fitted value is very close to the rejection limit.
X-ray absorption spectroscopy has been used to determine the speciation of mercury in tissues taken from a number of different marine mammals.33 Many of these studies did not examine skeletal muscle tissue, possibly because it often has much lower mercury concentrations and consequently is more difficult to examine than other organs such as liver. Liver in particular has been shown to contain high levels of HgSe and possibly β-HgS in beluga whale16 and striped dolphin.17 HgSe has also been detected using other techniques.34 This chemistry is relevant to human toxicology as human brain samples from individuals exposed to organic mercury have recently been shown to contain nano-particulate HgSe.35 Both of these compounds are extremely insoluble, having molar solubility products Ksp ≈ 10−59 and 10−36, respectively,36 and can be considered as essentially bio-unavailable and therefore largely non-toxic forms. The fact that such compounds are relatively benign is illustrated by the fact that α-HgS is a component in many Chinese traditional medicines. The doses administered can be around 1.9 grams a day,37 although there is evidence that this use may not be completely without adverse effects.37,38 α-HgS will have a somewhat different toxicological profile than β-HgS and HgSe, but the presence of relatively small quantities of HgSe or β-HgS in whale muscle is not expected to have any significant health impacts upon human consumers.
Nakazawa et al.17 have used a combination of X-ray fluorescence mapping, micro-diffraction and XAS to examine various tissues sampled from a striped dolphin. Their study included muscle, liver, kidney, brain, lung, pancreas and spleen, finding a predominance of HgSe in all. The XAS analysis of Nakazawa et al.17 used material that had been prepared by protein denaturation, extraction with various solvents and centrifugation to yield what these workers referred to as a non-extractible fraction. This procedure would have most likely removed any organic mercury components. Their analysis was based upon comparison of the experimental spectra with the Hg LII edges of four model compounds, HgSe, HgO, α-HgS and β-HgS. Hg LII edges are governed by similar selection rules to Hg LIII edges, and their spectra and ours are thus to a first approximation comparable, although of substantially poorer energy resolution (e.g. compare the specta of HgO). Nevertheless, the identification of HgSe in the non-extractible fraction seems unequivocal,17 although how relevant this finding is to human exposure is not clear. The study presented herein indicates that the samples of whale muscle investigated contain significant levels of mercury in a chemical form that strongly resembles methylmercury L-cysteinate. We observe no detectible contributions from inorganic forms such as HgSe16,17 or possibly β-HgS.16 The reason for this difference may well be the extraction procedures used by Nakazawa and co-workers.17 Huggins and co-workers studied lyophilized beluga tissues and found HgSe and β-HgS, but did not examine muscle.16 The whale muscle consumed in the Faroes is that of the long-finned pilot whale, Globicephala melas. Like the long-finned pilot whale, beluga whale (Delphinapterus leucas),16 striped dolphin (Stenella coeruleoalba)17 and the pygmy sperm whale studied herein are all toothed whales and thus reasonably closely related. Despite this, the species of whale may contribute to mercury speciation, along with the age and sex of the individual animals concerned. Diet could also be a factor. Pilot whale, striped dolphin and pygmy sperm whales feed predominantly on fish and squid although pygmy sperm whales also bottom feed, and beluga whales feed predominantly at the bottom of shallow waters.39
The presence of an organo-mercury species in whale muscle similar to that observed in fish muscle suggests that the direct effects of consuming a similar dose of organic mercury from either whale or fish should be similar. However, as discussed above there are potential adverse effects due to non-mercury components in consumed whale tissues which are not addressed in the work reported here. Moreover, it has also been suggested that the beneficial effects of essential unsaturated fatty acids that are abundant in ocean fish may partly counteract adverse effects of methyl mercury compounds.40 These chemical species are less abundant in whale muscle and blubber and this too may contribute to the comparative impacts of consuming whale versus fish. Moreover, while blubber generally has about 1/5th of the mercury content than skeletal muscle,41 making its study by XAS somewhat challenging, the selenium content is relatively higher at about half that of muscle.41 Because skeletal muscle and blubber are generally consumed at the same meal by the Faroese the chemical nature of the mercury and selenium in blubber may also be important and we plan to address this in a subsequent study.
Finally, we turn to the potential protective effects of selenium.16-23 The chemical form of selenium is different in the fish and whale samples studied and selenium may be involved in the de-methylation of methylmercury species.35,42,43,40 Whether or not this contributes to comparative adverse effects of consuming whale and fish muscle remains a possibility, but the fact that similar selenium species are present in tuna and whale muscle tissues may argue against this.
Conclusions
We have shown that the predominant chemical form of mercury in skeletal muscle from a pygmy sperm whale is methylmercury L-cysteineate, closely resembling the chemical form of mercury found in fish. No involvement of selenium in coordination of mercury is indicated, and no significant inorganic HgSe or HgS type phases were detected. These results suggest that for a given dose any direct detrimental effects from consuming mercury-containing skeletal muscle from whale and fish should be similar. This conclusion does not take into account possible interactions with other elements, including selenium, either in the gastrointestinal tract4 or within the tissues of in the individual consuming the meat.18,30 Moreover, the complexity biochemical interplay of toxic metal species and essential elements such as selenium is only now beginning to be understood.4,18,30,44 At present, sweeping conclusions about the relative safety of consuming whale versus fish would be premature and more work on the chemical speciation at the in-situ level is needed.
Acknowledgements
We thank Dr. Blair Maise-Guthrie and Dr. Ruth Ewing of NOAA for providing the sample of whale skeletal muscle and members of the George and Pickering research groups for their assistance with data acquisition. This work was supported by the Canadian Institutes for Health Research, Natural Sciences and Engineering Research Council (Canada), and by the National Institute of Environmental Health Sciences (Environmental Health Sciences Center Grant P30-ES01247). GNG and IJP are Canada Research Chairs and TCM is a CIHR-THRUST fellow. Portions of this work were carried out at the Stanford Synchrotron Radiation Lightsource, a Directorate of the SLAC National Accelerator Laboratory and an Office of Science User Facility operated by the U.S. Department of Energy Office of Sciences by Stanford University. The Structural Molecular Biology program is funded by the U.S. Department of Energy, Office of Biological and Environmental Sciences and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program.
References
- 1. http://www.fao.org/
- 2. http://www.cfsan.fda.gov/~dms/admehg3.html.
- 3.Harris HH, Pickering IJ, George GN. Science. 2003;301:1203. doi: 10.1126/science.1085941. [DOI] [PubMed] [Google Scholar]
- 4.George GN, Singh SP, Prince RC, Pickering IJ. Chem. Res. Toxicol. 2008;21:2106–2110. doi: 10.1021/tx800176g. [DOI] [PubMed] [Google Scholar]
- 5.Choi AL, Grandjean P. Environ. Chem. 2008;5:112–120. [Google Scholar]
- 6.Myers GJ, Thurston SW, Pearson AT, Davidson PW, Cox C, Shamlaye CF, Cernichiari E, Clarkson TW. NeuroToxicol. 2009;30:338–349. doi: 10.1016/j.neuro.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.See: Goyer RA, Aposhian HV, Arab L, Bellinger DC, Burbacher TM, Burke TA, Jacobson JL, Knobeloch LM, Ryan LM, Stern AH, (Committee on the Toxicological Effects of Methylmercury) National Research Council, Toxicological Effects of Methylmercury. National Academy Press; Washington, DC: 2000.
- 8.Needham LL, Grandjean P, Heinzow B, Jørgensen PJ, Nielsen F, Patterson DG, Jr., Sjdin A, Turner WE, Weih P. Environ. Sci. Technol. 2011;45:1121–1126. doi: 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moon H-B, Kannan K, Choi H-G, An Y-R, Choi S-G, Park J-Y, Kim Z-G. Chemosphere. 2010;79:733–739. doi: 10.1016/j.chemosphere.2010.02.043. [DOI] [PubMed] [Google Scholar]
- 10.Hoekstra PF, O'Hara TM, Backus SM, Hanns C, Muir DCG. Environ. Res. 2005;98:329–340. doi: 10.1016/j.envres.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Noëla M, Barrett-Lennard L, Guinet C, Dangerfield N, Ross PS. Mar. Environ. Res. 2009;68:196–202. doi: 10.1016/j.marenvres.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Andersen A, Julshamn K, Ringdal O, Moerkoere J. Sci. Tot. Environ. 1987;65:63–68. doi: 10.1016/0048-9697(87)90161-6. [DOI] [PubMed] [Google Scholar]
- 13.Clarkson TW, Magos L. Crit. Rev. Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 14.George GN, Pickering IJ, Doonan CJ, Korbas M, Singh SP, Hoffmeyer R. Adv. Mol. Toxicol. 2008;2:125–155. [Google Scholar]
- 15.Clarkson TW, Strain JJ. J. Nutr. 2003;133(5 Suppl. 1):1539S–543S. doi: 10.1093/jn/133.5.1539S. [DOI] [PubMed] [Google Scholar]
- 16.Huggins FE, Raverty SA, Nielsen OS, Sharp NS, Robertson JD, Ralston NVC. Environ. Bioindic. 2009;4:291–302. [Google Scholar]
- 17.Nakazawa E, Ikemoto T, Hokura A, Tereda Y, Kunito T, Tanabe S, Nakai I. Metallomics. 2011 doi: 10.1039/c0mt00106f. Advance Article, DOI: 10.1039/C0MT00106F. [DOI] [PubMed] [Google Scholar]
- 18.Gailer J, George GN, Pickering IJ, Madden S, Prince RC, Yu EY, Denton MB, Younis HS, Aposhian HV. Chem. Res. Toxicol. 2000;13:1135–1142. doi: 10.1021/tx000050h. [DOI] [PubMed] [Google Scholar]
- 19.Ganther HE, Goudie C, Sunde ML, Kopecky MJ, Wagner P. Science. 1972;175:1122–1124. doi: 10.1126/science.175.4026.1122. [DOI] [PubMed] [Google Scholar]
- 20.Prohaska JR, Ganther HE. Chem.-Biol. Interact. 1977;16:155–167. doi: 10.1016/0009-2797(77)90125-9. [DOI] [PubMed] [Google Scholar]
- 21.Chang LW, Dudley Jr AW, Dudley MA, Ganther HE, Sunde ML. Neurotoxicol. 1977;1:275–282. [Google Scholar]
- 22.Ganther HE, Sunde ML. Biol. Trace Elem. Res. 2007;119:221–233. doi: 10.1007/s12011-007-8006-6. [DOI] [PubMed] [Google Scholar]
- 23.Ralston NVC, Blackwell III JL, Raymond LJ. Biol. Trace Elem. Res. 2007;119:255–268. doi: 10.1007/s12011-007-8005-7. [DOI] [PubMed] [Google Scholar]
- 24.Kaneko JJ, Ralston NVC. Biol. Trace Elem. Res. 2007;119:242–254. doi: 10.1007/s12011-007-8004-8. [DOI] [PubMed] [Google Scholar]
- 25.Weber DN, Connaughton VP, Dellinger JA, Klemer D, Udvadia A, Carvan MJ., III Physiol. Behav. 2008;93:250–260. doi: 10.1016/j.physbeh.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cramer SP, Tench O, Yocum M, George GN. Nucl. Instrum. Meth. 1998:A266, 586–591. [Google Scholar]
- 27. http://ssrl.slac.stanford.edu/exafspak.html.
- 28.Pickering IJ, George GN, van Fleet-Stalder V, Chasteen TC, Prince RC. J. Biol. Inorg. Chem. 1999;4:791–794. doi: 10.1007/s007750050352. [DOI] [PubMed] [Google Scholar]
- 29.George GN, Pickering IJ. In: Brilliant Light in Life and Materials Sciences. Tsakanov V, Wiedemann H, editors. Springer; Dordrecht, NL: 2007. pp. 97–119. [Google Scholar]
- 30.Korbas M, O'Donoghue JL, Watson GE, Pickering IJ, Singh SP, Myers GJ, Clarkson TW, George GN. ACS Chem. Neurosci. 2010;1:810–881. doi: 10.1021/cn1000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Misra S, Peak D, Niyogi S. Metallomics. 2010;2:710–717. doi: 10.1039/c0mt00008f. [DOI] [PubMed] [Google Scholar]
- 32.Michalke B. In: Moderne Techniken der Ionenanalyse. Jensen K.F.u.D., editor. ECOMED Verlagsgesellschaft AG+Co, KG; Landsberg, Germany: 2002. p. 50. [Google Scholar]
- 33.Ng P-S, Li H, Matsumoto K, Yamazaki S, Kogure T, Tagai T, Nagasawa H. Proc. Japan Acad., Ser. B: Phys. Biol. Sci. 2001;77:178–183. [Google Scholar]
- 34.Martoja R, Berry JP. Vie Milieu. 1980;30:7–10. [Google Scholar]
- 35.Korbas M, O'Donoghue JL, Watson GE, Pickering IJ, Singh SP, Myers GJ, Clarkson TW, George GN. ACS Chem. Neurosci. 2010;1:810–881. doi: 10.1021/cn1000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martell AE, Smith RM, Motekaitis RJ. NIST Critically Selected Stability Constants of Metal Complexes Data Base; NIST Stand. Ref. Database no. 46. Department of Commerce; Gaithersburg, MD: 1998. [Google Scholar]
- 37.Yen C-C, Huang C-F, Lee W-J, Hsu M-J, Liu S-H, Lin-Shiau S-Y. Toxicol. Environ. Chem. 2008;90:181–201. [Google Scholar]
- 38.Yen C-C, Liu S-H, Chen W-K, Lin R-H, Lin-Shiau S-Y. J. Anal. Toxicol. 2002;26:286–295. doi: 10.1093/jat/26.5.286. [DOI] [PubMed] [Google Scholar]
- 39. http://www.nmfs.noaa.gov/pr/species/mammals/cetaceans.
- 40.Davidson PW, Leste A, Benstrong E, Burns CM, Valentin J, Sloane-Reeves J, Huang LS, Miller WA, Gunzler D, van Wijngaarden E, Watson GE, Zareba G, Shamlaye CF, Myers GJ. Neurotoxicology. 2010;31:439–447. doi: 10.1016/j.neuro.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Julshamn K, Andersen A, Ringdal O, Mørkøre J. Sci. Tot. Environ. 1987;65:53–62. doi: 10.1016/0048-9697(87)90160-4. [DOI] [PubMed] [Google Scholar]
- 42.Kahn MAK, Wang F. Chem. Res. Toxicol. 2010;23:1202–1206. doi: 10.1021/tx100080s. [DOI] [PubMed] [Google Scholar]
- 43.Naganuma A, Imura N. Chemosphere. 1981;10:441–443. [Google Scholar]
- 44.Gailer J. Coord. Chem. Rev. 2006;251:234–254. [Google Scholar]