Abstract
Several cytogenetic abnormalities are associated with poor outcomes in multiple myeloma (MM). We prospectively analyzed the impact of cytogenetic abnormalities on outcomes during the phase 2 PX-171-003-A1 study of single-agent carfilzomib for relapsed and refractory MM. In the response-evaluable population (257/266), fluorescence in situ hybridization (FISH)/conventional cytogenetic profiles were available for 229 patients; 62 (27.1%) had high-risk cytogenetics—del 17p13, t(4;14) or t(14;16) by interphase FISH or deletion 13 or hypodiploidy by metaphase cytogenetics—and 167 (72.9%) had standard-risk profiles. Generally, baseline characteristics were similar between the subgroups, but International Staging System stage III disease was more common in high- vs standard-risk patients (41.9% vs 27.5%) as was Eastern Cooperative Oncology Group performance status 1/2 (85.5% vs 68.3%). Overall response was comparable between the subgroups (25.8% vs 24.6%, respectively; P=0.85), while time-to-event end points showed a trend of shorter duration in high-risk patients, including median duration of response (5.6 months (95% confidence interval (CI) 3.7–7.8) vs 8.3 months (95% CI 5.6–12.3)) and overall survival (9.3 (95% CI 6.5–13.0) vs 19.0 months (95% CI 15.4–NE); P=0.0003). Taken together, these findings demonstrate that single-agent carfilzomib is efficacious and has the potential to at least partially overcome the impact of high-risk cytogenetics in heavily pre-treated patients with MM.
Keywords: multiple myeloma, cytogenetics, proteasome inhibitor, carfilzomib, relapsed, refractory
Introduction
Chromosomal aberrations are a hallmark of multiple myeloma (MM) and are often complex, involving changes in both the number and structure of the chromosomes.1, 2, 3, 4, 5 Generally, the primary lesions are reciprocal translocations (t) involving the immunoglobulin heavy chain gene locus on chromosome 14 and various partner loci.6 These include t(11;14), t(4;14), t(14;16) and t(14;20), with the latter three indicative of poorer prognosis.4 As the disease progresses, secondary aberrations are common and are associated with more aggressive disease. These include translocations of the MYC gene, the loss or deletion of chromosome 13, deletion of 17p13 and complex changes involving chromosome 1.2, 4, 7, 8 It is not uncommon for patients to acquire multiple karyotypic changes over the course of their disease, resulting in an increasingly complex and aggressive disease that proves difficult to treat.4
Chromosomal abnormalities have a variable impact on therapeutic response and time-to-event end points in patients with MM.9, 10, 11 Before the introduction of targeted therapy with proteasome inhibitors and immunomodulatory drugs, response rates were lower and duration of response (DOR) and event-free and overall survival (OS) shorter in patients with high-risk cytogenetic abnormalities compared with standard-risk patients.12 The proteasome inhibitor bortezomib and the immunomodulators thalidomide and lenalidomide have demonstrated some benefit in patients with high-risk cytogenetic characteristics, but outcomes can be variable and dependent on the specific marker and treatment regimen (for example, single agent vs multi-agent).11, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 In the case of bortezomib, a number of studies provide evidence that it can reduce or eliminate the impact of certain adverse cytogenetic markers.13, 14, 15, 16, 17, 18 For immunomodulatory drugs, the evidence is less established but lenalidomide and pomalidomide both appear to provide benefit in certain high-risk cytogenetic subgroups, whereas few studies have demonstrated a favorable outcome with thalidomide.11, 19, 20, 21, 22 Because of their impact on treatment, cytogenetic risk status has become a standard stratification factor in MM trials evaluating established and novel agents.2, 3, 23
Carfilzomib, a selective proteasome inhibitor, has demonstrated durable single-agent activity in patients with advanced MM.24, 25, 26, 27 Carfilzomib was recently approved in the United States for the treatment of relapsed and refractory MM (RRMM)28 based on efficacy and safety results from the pivotal phase 2 study PX-171-003-A1.24 In this trial, 266 patients with RRMM were enrolled with a median age of 63 years, a median of 5.4 years since diagnosis and a median of five previous treatment regimens for MM. Nearly all patients (99.6%) had received bortezomib before and 73% were refractory to bortezomib. The overall response rate (ORR) was 23.7% in the response-evaluable population (n=257) with a median DOR of 7.8 months, a median progression-free survival (PFS) of 3.7 months and a median OS of 15.6 months. Adverse events were manageable without cumulative toxicities. The most common events included fatigue (49%), anemia (46%), nausea (45%) and thrombocytopenia (39%); 12.4% of patients experienced Grade 1 or 2 peripheral neuropathy.
The primary objective of the present analysis was to prospectively evaluate the effect of cytogenetic abnormalities on the response rate from the PX-171-003-A1 study and secondarily to evaluate their impact on time-to-event end points, including DOR, time to progression (TTP), PFS and OS.
Patients and methods
The methodology for PX-171-003-A1 has been described previously.24 Briefly, this was a multicenter, open-label, single-arm, phase 2 study (Clinicaltrials.gov NCT00511238) involving 31 centers in the United States and Canada. Patients ⩾18 years of age with measurable progressive MM were eligible for this trial if they had received ⩾2 previous regimens, including bortezomib, thalidomide or lenalidomide, an alkylating agent, or an anthracycline, unless contraindicated, and achieved a response to ⩾1 previous regimen. Patients had to be refractory to their most recent therapy (that is, ⩽25% response or progression during treatment or within 60 days of last dose), have an Eastern Cooperative Oncology Group (ECOG) performance score of 0–2 and adequate bone marrow (platelet count ⩾50 000/mm3), hepatic (for example, serum aspartate aminotransferase concentrations <3 times the upper limit of normal) and renal function (creatinine clearance >30 ml/min).
Single-agent carfilzomib was administered intravenously over 2–10 min on Days 1, 2, 8, 9, 15 and 16 of each 28-day cycle. The dose for Cycle 1 was 20 mg/m2, which was then escalated to 27 mg/m2 for all cycles thereafter up to a maximum of 12 cycles.24
The primary end point was ORR,29 and secondary efficacy end points included the clinical benefit rate (CBR=ORR+minimal response), DOR, TTP, PFS and OS. Responses were assessed on Day 15 of Cycle 1, Day 1 of subsequent cycles and at the end of the study according to the International Myeloma Working Group (IMWG) criteria modified to include minimal response as defined by the European Group for Blood and Marrow Transplantation.29, 30 Response and disease progression (when clinically possible) were confirmed by two consecutive assessments. Thus, the earliest confirmation could occur within Cycle 2. Of note, response by the cytogenetic risk subgroup (high-risk vs standard-risk) was a prospectively planned analysis.
Conventional metaphase cytogenetic and/or interphase fluorescence in situ hybridization (FISH) were conducted at screening by a local laboratory of the participating center per standard of practice at the time of the trial and included the use of unpurified bone marrow samples for most patients. Patients were classified as having standard-risk or high-risk cytogenetic abnormalities per the criteria of the IMWG.1, 2, 3 High-risk cytogenetic markers included del 13 or hypodiploidy by metaphase cytogenetic analysis and/or del 17p13, t(4;14), t(14;16) by interphase FISH. For this analysis, patients without these abnormal markers were considered standard risk. Of note, del 13q14 by FISH alone was not considered a high-risk marker.3, 31, 32
Statistical analysis
Analyses were conducted with the response-evaluable population, which was defined as patients who received at least one dose of carfilzomib and underwent baseline disease response assessments and at least one post-baseline disease assessment or patients who discontinued protocol treatment before the first day of Cycle 2 due to an adverse event that was considered to be possibly or probably related to carfilzomib. ORR, CBR rate, disease control rate (DCR=CBR+stable disease) and time-to-event end points (that is, DOR, TTP, PFS and OS) were determined by the status of cytogenetic abnormalities (high risk vs standard risk). In addition, response was assessed by the number of cytogenetic abnormalities (1 vs ⩾2) and for specific abnormalities—del 13, hypodiploidy, del 17p13, t(4;14) and t(14;16).
Categorical end points and continuous variables were summarized with descriptive statistics. For time-to-event end points, medians and 95% confidence intervals (CIs) were estimated by the Kaplan–Meier method. Comparisons between the high- and standard-risk subgroups were made using the Chi-square test for categorical end points and the Log-rank test for time-to-event end points. All statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC, USA).
Results
A total of 266 patients were enrolled in the PX-171-003-A1 study. Of the response-evaluable population (257 patients), metaphase cytogenetic and/or FISH data were available for 229 patients. These patients were the focus of this analysis, with 167 (72.9%) identified as standard risk and 62 (27.1%) identified as high risk. The incidence of a single high-risk abnormality was 18.8%, and 8.3% of patients had multiple (⩾2) high-risk abnormalities (Table 1). The most common cytogenetic abnormality was del 17p13 (13.1%), while t(14;16) was the least frequent (1.3%). Cytogenetic deletion 13 was observed in 14 patients (6.1%). In the standard-risk subgroup, del 13q14 by FISH was reported as a single abnormality in 9 patients (3.9%).
Table 1. Cytogenetic status in the response-evaluable population (n=229).
| Cytogenetics | FISH | n (%) | ||||
|---|---|---|---|---|---|---|
| |
Del 13 |
Hypodiploidy |
Del 17p13 |
t(4;14) |
t(14;16) |
|
| Standard risk | — | — | — | — | — | 167 (72.9) |
| High risk | 14 (6.1) | 23 (10.0) | 30 (13.1) | 18 (7.9) | 3 (1.3) | 62 (27.1) |
| Single abnormality | 43 (18.8) | |||||
| * | — | — | — | — | 2 (0.9) | |
| — | * | — | — | — | 11 (4.8) | |
| — | — | * | — | — | 17 (7.4) | |
| — | — | — | * | — | 11 (4.8) | |
| — | — | — | — | * | 2 (0.9) | |
| Multiple abnormalities | 19 (8.3) | |||||
| * | * | — | — | — | 5 (2.2) | |
| * | — | * | — | — | 1 (0.4) | |
| * | * | * | — | — | 4 (1.7) | |
| * | * | — | * | — | 1 (0.4) | |
| * | * | * | — | * | 1 (0.4) | |
| — | * | * | — | — | 1 (0.4) | |
| — | — | * | * | — | 6 (2.6) | |
Abbreviations: Del, deletion; FISH, fluorescence in situ hybridization; t, translocation. *Indicates abnormality present.
Baseline characteristics were generally comparable between the high- and standard-risk subgroups with some exceptions, most notably International Staging System (ISS) stage and ECOG performance status (Table 2). ISS stage III disease was more common in the high-risk than in the standard-risk subgroup (41.9% vs 27.5%), as was ECOG performance status of 1 or 2 (85.5% vs 68.3%).
Table 2. Baseline characteristics for response-evaluable patients by cytogenetic status (N=229).
| Characteristic | Standard risk (n=167) | High risk (n=62) |
|---|---|---|
| Age, years, median (range) | 65 (37–87) | 63 (39–84) |
| Male, n (%) | 91 (54.5) | 41 (66.1) |
| Time from diagnosis, years, median (range) | 5.6 (0.5–21.2) | 5.3 (1.3–22.3) |
| Plasma cells on bone marrow biopsy, median, n (%) | ||
| <50% | 96 (57.5) | 30 (48.4) |
| ⩾50% | 65 (38.9) | 29 (46.8) |
| Unknown/not specified | 6 (3.6) | 3 (4.8) |
| International Staging System stage, n (%) | ||
| I | 56 (33.5) | 10 (16.1) |
| II | 63 (37.7) | 24 (38.7) |
| III | 46 (27.5) | 26 (41.9) |
| Unknown/not specified | 2 (1.2) | 2 (3.2) |
| ECOG performance status, n (%) | ||
| 0 | 53 (31.7) | 9 (14.5) |
| 1 | 94 (56.3) | 45 (72.6) |
| 2 | 20 (12.0) | 8 (12.9) |
| Previous regimens, median (range) | 5 (1–20) | 5 (2–12) |
| ⩾4, n (%) | 133 (79.6) | 51 (82.3) |
| Refractory to last regimen, n (%) | ||
| Progressive disease on therapy | 118 (70.7) | 53 (85.5) |
| Progressive disease within 60 days | 23 (13.8) | 6 (9.7) |
| ⩽25% response | 13 (7.8) | 2 (3.2) |
| Previous agents, n (%) | ||
| Bortezomib | 166 (99.4) | 62 (100) |
| Lenalidomide or thalidomide | 167 (100) | 62 (100) |
| Corticosteroid | 163 (97.6) | 62 (100) |
| Alkylating agent | 153 (91.6) | 57 (91.9) |
| Stem cell transplant | 119 (71.3) | 45 (72.6) |
| Anthracycline | 107 (64.1) | 39 (62.9) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FISH, fluorescence in situ hybridization.
The ORR was similar between the high-risk and standard-risk subgroups (25.8% vs 24.6%, respectively), while the CBR was lower for the high-risk subgroup (30.7% vs 40.7%) (Table 3). The rate of greater than or equal to very good partial response was 0% and 8.4%, respectively. The incidence of progressive disease was comparable between the two subgroups (22.6% vs 27.5%, respectively), but the rate of treatment discontinuation due to progressive disease within the first two cycles was 29.0% (18/62) in the high-risk subgroup vs 20.4% (34/167) in the standard-risk subgroup.
Table 3. Response rates and time-to-event data by cytogenetic status in response-evaluable patientsa.
| Standard risk (n=167) | High risk (n=62) | P-value | |
|---|---|---|---|
| Best response | |||
| CR | 1 (0.6) | 0 (0) | |
| VGPR | 13 (7.8) | 0 (0) | |
| PR | 27 (16.2) | 16 (25.8) | |
| MR | 27 (16.2) | 3 (4.8) | |
| SD | 46 (27.5) | 25 (40.3) | |
| PD | 46 (27.5) | 14 (22.6) | |
| NE | 7 (4.2) | 4 (6.5) | |
| ORR (CR+VGPR+PR) | 41 (24.6) | 16 (25.8) | 0.85 |
| CBR (ORR+MR) | 68 (40.7) | 19 (30.7) | 0.16 |
| DCR (CBR+SD) | 114 (68.3) | 44 (71.0) | 0.69 |
| Time-to-event variable, median (95% CI), months | |||
| DORb | 8.3 (5.6–12.3) | 5.6 (3.7–7.8) | — |
| TTP | 4.6 (2.8–6.5) | 3.6 (2.3–4.6) | 0.10 |
| PFS | 4.6 (2.8–5.8) | 3.5 (2.1–4.5) | 0.06 |
| OS | 19.0 (15.4–NE) | 9.3 (6.5–13.0) | 0.0003 |
Abbreviations: CBR, clinical benefit rate; CI, confidence interval; CR, complete response; DCR, disease control rate; DOR, duration of response; MR, minimal response; NE, not estimable; ORR, overall response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease; TTP, time to progression; VGPR, very good partial response.
Based on enrolled population.
Includes only patients who achieved PR or better; presented descriptively because of the limited number of responders for statistical comparison.
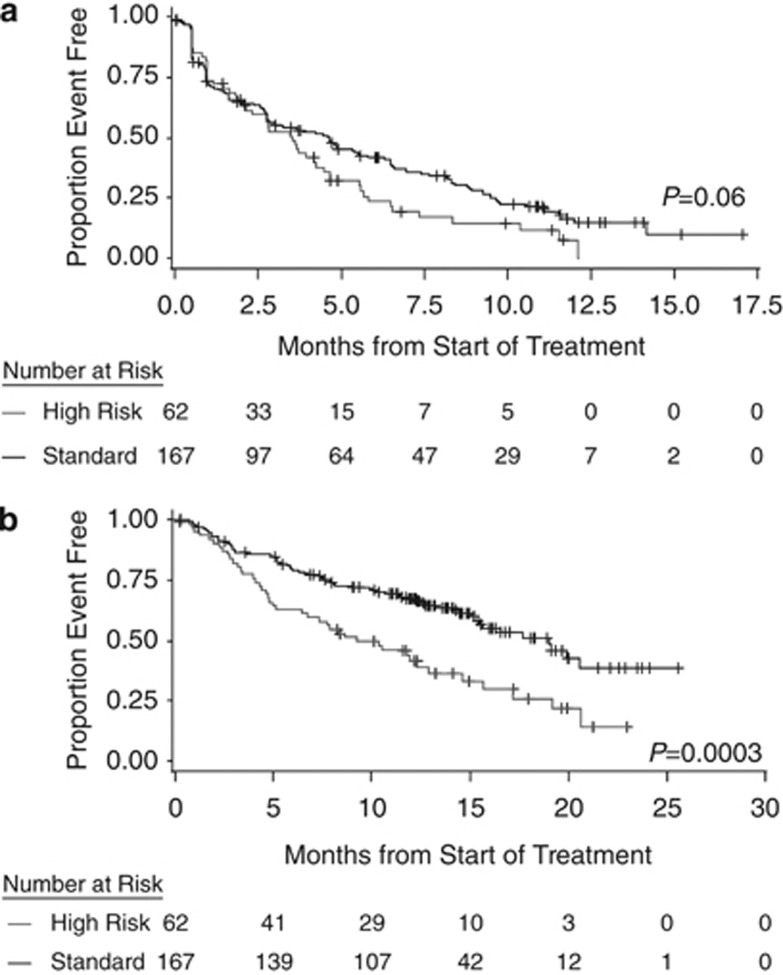
With respect to time-to-event end points, there was a general trend of shorter duration in the high-risk subgroup compared with the standard-risk subgroup, including median DOR (5.6 vs 8.3 months). For survival outcomes, median PFS was 3.5 vs 4.6 months (P=0.06), respectively, and median OS was 9.3 vs 19.0 months, respectively (P=0.0003) (Figure 1).
Figure 1.
Kaplan–Meier survival curves by cytogenetic status in response-evaluable patients: PFS (a) and OS (b).
Analysis of outcomes by specific abnormalities (see Supplementary Table) showed that patients with t(4;14) had the highest ORR (38.9%), whereas patients with del 17p13 had the lowest (16.7%). Furthermore, patients with t(4;14) had the longest median OS at 11.8 months (95% CI 3.1–NE), whereas those with del 17p13 had the shortest at 7.0 months (95% CI 4.0–20.7). It is important to note that these subgroups are not independent of each other because patients with ⩾2 abnormalities were counted in multiple subgroups. An analysis of the high-risk subgroup suggested differences in outcomes based on the number of abnormalities present (1 vs ⩾2). The ORR in patients with one abnormality was 30.2% compared with 15.8% for those having ⩾2 abnormalities, the median PFS was 3.6 months (95% CI 2.8–4.6) vs 2.1 months (95% CI 0.8–4.6) and the median OS was 10.6 months (95% CI 4.9–20.7) vs 8.4 months (95% CI 3.1–14.7). In patients with isolated t(4;14) as a single abnormality, the ORR was 63.6% with a median PFS of 4.5 months (95% CI 2.8–11.5) and a median OS of 15.8 months (95% CI 4.2–NE).
Discussion
This report represents the first prospective analysis to evaluate the impact of cytogenetics on treatment outcomes with single-agent carfilzomib. In multiple studies, high-risk cytogenetic abnormalities, including del 13, hypodiploidy, del 17p13, t(4;14) and t(14;16), have been associated with poor response and shorter DOR, PFS and/or OS.10, 12, 33, 34 Del 17p13 assessed by FISH is usually a late event in the evolution of MM. It is indicative of more aggressive disease2, 8 and has consistently been shown to be one of the more significant prognostic factors among high-risk cytogenetic abnormalities regardless of the treatment strategy, including conventional chemotherapy or targeted therapies.12, 15, 21, 31 Although t(4;14) is also associated with poor prognosis, its impact appears to be less pronounced than del 17p13.15, 21 Del 13 by cytogenetics can be a primary or secondary aberration and is indicative of a highly proliferative disease state. The prognostic impact of del 13 may be related to its association with del 17p13 and t(4;14).12
The primary analysis of PX-171-003-A1 demonstrated that single-agent carfilzomib was clinically efficacious with a favorable safety profile in patients with RRMM who were heavily pretreated and had limited treatment options.24 Overall, patients were able to achieve clinically meaningful and durable responses. In this prospectively planned subgroup analysis, single-agent carfilzomib was efficacious in patients with high-risk and standard-risk cytogenetics. Response rates were comparable between these subgroups and consistent with the overall study results.24 There was, however, a general trend in the time-to-event end points that suggested a shorter duration in the high-risk subgroup for DOR, PFS and OS. The presence of del 17p13 appeared to have the greatest impact on response and OS, whereas t(4:14) did not. Taken together, our findings suggest that carfilzomib as a single agent has the potential to, at least partially, overcome the adverse impact of certain cytogenetic abnormalities.
In terms of putting these findings into context with historical data, as noted earlier, studies have shown variable activity for bortezomib15, 16, 17 and lenalidomide in patients with high-risk cytogenetics11, 21, 22 either alone or in combination with dexamethasone. In the SUMMIT study, a single-arm, phase 2 trial of bortezomib±dexamethasone for RRMM,16 there was a trend of lower ORR (24% vs 33% P=NS (not significant)) and shorter OS (median, 10 vs 15 months; P=0.14) in patients with del 13 by metaphase cytogenetics compared with those without del 13, with matched-pairs analysis showing a similar trend. In a matched-pairs analysis of patients enrolled in the APEX study, a phase 3 trial in relapsed MM that compared bortezomib with high-dose dexamethasone, there was a similar trend in patients with and without del 13 by metaphase cytogenetics with respect to ORR (25% vs 35% P=NS) and OS (median 12.5 months vs NR (not reported); P=0.79).16 In the high-dose dexamethasone arm, there was no statistical difference for ORR (9% vs 26%, respectively), but OS was significantly shorter for patients with del 13 (median 3.3 months vs NR; P<0.002). Additionally, a retrospective study in 65 patients with relapsed or refractory MM treated with bortezomib showed no statistical difference in outcomes between patients with and without t(4:14) by FISH for ORR (67% vs 56%), PFS (median 10.5 vs 6.8 months) and OS (median 15.1 vs 12.3 months).17 In a separate prospective study, Dimopoulos et al.10 examined the impact of high-risk cytogenetics (defined similarly as in our study) in 99 patients with relapsed/refractory MM treated with lenalidomide and dexamethasone (RD, n=50) or bortezomib with RD (VRD, n=49). High-risk cytogenetics was associated with a lower response rate compared with standard-risk cytogenetics (44% vs 74% P=0.007) and shorter PFS (10.1 vs 5.6 months; P=0.003) in the overall population. In the subgroup receiving RD, the ORR was also lower in patients with high-risk cytogenetics (31% vs 74% P=0.01), with a similar trend in the subgroup receiving VRD (55% vs 74% P=0.22). With respect to individual abnormalities, only del 17p was retained as a significant predictor of response in multivariate analysis (odds ratio 43.91; 95% CI 3.92–490.96; P=0.002), which has also been observed in other studies with bortezomib and lenalidomide.15, 21, 22
The trends in the results reported herein are generally consistent with these historical studies, particularly for t(4;14), and del 17p appeared to be associated with greater risk than other markers. For del 17p, outcomes may be improved with complex multidrug treatments,10, 13, 14, 18, 35 but fully overcoming its negative impact will likely require novel approaches or combinations that do not act via apoptosis induction.36 It should be noted that there are a number of important differences between PX-171-003-A1 and these historical studies, making comparisons to historical data difficult. In particular, the PX-171-003-A1 study population differs from that of many earlier studies in which previous exposure to bortezomib was less common and patients were less likely to be refractory to novel agents and had fewer lines and types of previous therapy.24 Furthermore, cytogenetic stratification criteria have evolved over time and therefore are not consistent across studies. For instance, many historical studies considered del 13q assessed by FISH as a high-risk marker,17 whereas current guidelines indicate that del 13q by FISH is not prognostically important unless accompanied by del 13 by metaphase cytogenetics or another high-risk marker.3 Despite their inclusion in the most recent IMWG criteria,3 del 13 and hypodiploidy by metaphase cytogenetics and t(4;14) as markers of risk is also evolving. These abnormalities are currently considered intermediate markers of risk in Mayo Clinic Guidelines.23
Potential limitations specific to the data reported here should be acknowledged, including the single-arm phase 2 study design, imbalances between the high- and standard-risk subgroups in other baseline prognostic factors (for example, ISS stage III disease and ECOG performance status)37 and the small patient numbers, which preclude the use of robust multivariate analyses. In addition, a greater proportion of high-risk patients discontinued treatment for progressive disease within the first two cycles based on a protocol-defined early response assessment on Day 15 before full doses were administered, which may have impacted outcomes.
Given these considerations, the response rates in this high-risk patient population are particularly encouraging and warrant additional exploration of carfilzomib and cytogenetic characteristics in various treatment settings and, importantly, in the randomized setting. Preliminary results of a phase 2 study assessing carfilzomib in combination with lenalidomide and low-dose dexamethasone (CRd) in patients with newly diagnosed MM (N=53) suggest that outcomes are comparable between high-risk and standard-risk patients regardless of age.38, 39 It is also of clinical interest to differentiate carfilzomib activity in specific abnormalities, particularly in view of the potential for improved outcomes in patients with t(4;14) suggested by the data reported here. To this, a number of single-arm as well as phase 3 studies with carfilzomib, as a single agent or as part of multidrug combinations, have been initiated and should provide more robust data on the impact of high-risk abnormalities on carfilzomib activity and outcomes. These include the FOCUS study (carfilzomib vs corticosteroid±cyclophosphamide in RRMM, NCT01302392),40 the ASPIRE study (CRd vs Rd in relapsed MM, NCT01080391), the ENDEAVOR study (carfilzomib plus dexamethasone vs bortezomib plus dexamethasone in relapsed MM, NCT01568866) and CLARION (carfilzomib, melphalan, prednisone vs bortezomib, melphalan, prednisone in newly diagnosed MM, NCT01818752).
In conclusion, single-agent carfilzomib showed encouraging activity in heavily pretreated patients with high-risk cytogenetic abnormalities who were enrolled in this prospective analysis from the PX-171-003-A1 study. The observation that response rates were similar for high-risk and standard-risk patients and durable across a range of cytogenetic profiles suggests that carfilzomib may be beneficial in patients with high-risk disease. The achievement of a more definitive benefit with regard to time-to-event end points may require combining carfilzomib with other agents. The findings from this study serve as the basis for further evaluation in ongoing and future clinical studies as to the role of high-risk cytogenetic characteristics in the broader context of the clinical development program for carfilzomib.
Acknowledgments
We thank all patients who contributed to this study and their families. Thanks are also due to the investigators and staff from the additional participating study sites. We would like to acknowledge all the participating research nurses and data coordinators. The clinical operations lead for the study was Jessica Taylor (Onyx Pharmaceuticals, Inc.), statistical support was provided by Yu-Lin Chang, Sandra Dixon, MS and Mei Huang, MS (Onyx Pharmaceuticals, Inc) and critical review of the manuscript for scientific accuracy was undertaken by Thomas Renau, PhD, A. Peter Morello III, PhD, CMPP and Edward Kavalerchik, MD (Onyx Pharmaceuticals, Inc.). Medical writing and editing services were provided by Brian E. Szente, PhD and Michael Raffin from Fishawack Communications (North Wales, PA) and supported by funding from Onyx Pharmaceuticals, Inc. and the Multiple Myeloma Research Consortium.
AJJ: Consultant and Advisory Boards for Celgene, Millennium, Onyx Pharmaceuticals and Bristol-Myers Squibb; honoraria from Celgene, Millennium, Bristol-Myers Squibb; speakers bureau for Celgene. DSS: Consultant, honoraria, and Board of Directors or advisory committee membership for Millennium and Celgene. TM: Honoraria from Celgene; consultant for Onyx Pharmaceuticals. MW: Research funding from Onyx Pharmaceuticals. RV: Consultant for and research funding from Onyx Pharmaceuticals. SL: Consultant for Millennium, Celgene, Novartis, Bristol-Myers Squibb, Onyx Pharmaceuticals and Merck. VK: Honoraria from Celgene, Janssen Ortho and Roche. NB: Honoraria from and speakers bureau for Celgene. MA: Consultant for Millennium and Novartis; Board of Directors or advisory committee membership for Millennium and Novartis; research funding from Millennium and Allergan. FJR: Research funding from Celgene and speakers bureau for Onyx Pharmaceuticals. GS: Advisory committee for Onyx Pharmaceuticals. JZ: Consultant for Millennium and Amgen; speakers bureau for Millennium and Celgene; research funding from Millennium; honoraria from Medtronics. AKS: Consultant for and research funding from Celgene, Millennium, Novartis, Bristol-Myers Squibb and Onyx Pharmaceuticals. ES: Consultant and speakers bureau for Celgene and Millennium. AFW: Employed by and equity ownership in Onyx Pharmaceuticals. RZO: Honoraria from and Board of Directors or advisory committee membership for Onyx Pharmaceuticals, Amgen, Array Biopharma, Bristol-Myers Squibb, Celgene, Janssen Pharmaceuticals, Merck and Millennium Pharmaceuticals and research funding from Bristol-Myers Squibb, Celgene and Millennium Pharmaceuticals. SJ: Honoraria for Millennium, Celgene, Onyx Pharmaceuticals and Merck; Board of Directors or advisory committee membership for Ortho Biotech, Imedex, Medicom World Wide, OptumHealth Education and PER Group. The other authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- Fonseca R, Barlogie B, Bataille R, Bastard C, Bergsagel PL, Chesi M, et al. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 2004;64:1546–1558. doi: 10.1158/0008-5472.can-03-2876. [DOI] [PubMed] [Google Scholar]
- Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23:2210–2221. doi: 10.1038/leu.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi NC, Anderson KC, Bergsagel PL, Shaughnessy J, Palumbo A, Durie B, et al. Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. Blood. 2011;117:4696–4700. doi: 10.1182/blood-2010-10-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer JR. The prognostic significance of cytogenetics and molecular profiling in multiple myeloma. Cancer Genet. 2011;204:3–12. doi: 10.1016/j.cancergencyto.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Morgan GJ, Walker BA, Davies FE. The genetic architecture of multiple myeloma. Nat Rev Cancer. 2012;12:335–348. doi: 10.1038/nrc3257. [DOI] [PubMed] [Google Scholar]
- Corre J, Avet-Loiseau H. The impact of genomics on the management of myeloma. J Natl Compr Canc Netw. 2011;9:1200–1206. doi: 10.6004/jnccn.2011.0097. [DOI] [PubMed] [Google Scholar]
- Lonial S. Presentation and risk stratification—improving prognosis for patients with multiple myeloma. Cancer Treat Rev. 2010;36 (Suppl 2:S12–S17. doi: 10.1016/S0305-7372(10)70007-4. [DOI] [PubMed] [Google Scholar]
- Herve AL, Florence M, Philippe M, Michel A, Thierry F, Kenneth A, et al. Molecular heterogeneity of multiple myeloma: pathogenesis, prognosis, and therapeutic implications. J Clin Oncol. 2011;29:1893–1897. doi: 10.1200/JCO.2010.32.8435. [DOI] [PubMed] [Google Scholar]
- Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23:3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos MA, Kastritis E, Christoulas D, Migkou M, Gavriatopoulou M, Gkotzamanidou M, et al. Treatment of patients with relapsed/refractory multiple myeloma with lenalidomide and dexamethasone with or without bortezomib: prospective evaluation of the impact of cytogenetic abnormalities and of previous therapies. Leukemia. 2010;24:1769–1778. doi: 10.1038/leu.2010.175. [DOI] [PubMed] [Google Scholar]
- Avet-Loiseau H, Soulier J, Fermand JP, Yakoub-Agha I, Attal M, Hulin C, et al. Impact of high-risk cytogenetics and prior therapy on outcomes in patients with advanced relapsed or refractory multiple myeloma treated with lenalidomide plus dexamethasone. Leukemia. 2010;24:623–628. doi: 10.1038/leu.2009.273. [DOI] [PubMed] [Google Scholar]
- Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109:3489–3495. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]
- Neben K, Lokhorst HM, Jauch A, Bertsch U, Hielscher T, van der Holt B, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119:940–948. doi: 10.1182/blood-2011-09-379164. [DOI] [PubMed] [Google Scholar]
- Sonneveld P, Schmidt-Wolf IG, van der Holt B, El Jarari L, Bertsch U, Salwender H, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol. 2012;30:2946–2955. doi: 10.1200/JCO.2011.39.6820. [DOI] [PubMed] [Google Scholar]
- Avet-Loiseau H, Leleu X, Roussel M, Moreau P, Guerin-Charbonnel C, Caillot D, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p) J Clin Oncol. 2010;28:4630–4634. doi: 10.1200/JCO.2010.28.3945. [DOI] [PubMed] [Google Scholar]
- Jagannath S, Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, et al. Bortezomib appears to overcome the poor prognosis conferred by chromosome 13 deletion in phase 2 and 3 trials. Leukemia. 2007;21:151–157. doi: 10.1038/sj.leu.2404442. [DOI] [PubMed] [Google Scholar]
- Chang H, Trieu Y, Qi X, Xu W, Stewart KA, Reece D. Bortezomib therapy response is independent of cytogenetic abnormalities in relapsed/refractory multiple myeloma. Leuk Res. 2007;31:779–782. doi: 10.1016/j.leukres.2006.08.002. [DOI] [PubMed] [Google Scholar]
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- Barlogie B, Pineda-Roman M, van Rhee F, Haessler J, Anaissie E, Hollmig K, et al. Thalidomide arm of Total Therapy 2 improves complete remission duration and survival in myeloma patients with metaphase cytogenetic abnormalities. Blood. 2008;112:3115–3121. doi: 10.1182/blood-2008-03-145235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy MQ, Hayman SR, Gertz MA, Dispenzieri A, Buadi F, Kumar S, et al. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol. 2009;27:5008–5014. doi: 10.1200/JCO.2009.23.6802. [DOI] [PubMed] [Google Scholar]
- Reece D, Song KW, Fu T, Roland B, Chang H, Horsman DE, et al. Influence of cytogenetics in patients with relapsed or refractory multiple myeloma treated with lenalidomide plus dexamethasone: adverse effect of deletion 17p13. Blood. 2009;114:522–525. doi: 10.1182/blood-2008-12-193458. [DOI] [PubMed] [Google Scholar]
- Klein U, Jauch A, Hielscher T, Hillengass J, Raab MS, Seckinger A, et al. Chromosomal aberrations +1q21 and del(17p13) predict survival in patients with recurrent multiple myeloma treated with lenalidomide and dexamethasone. Cancer. 2011;117:2136–2144. doi: 10.1002/cncr.25775. [DOI] [PubMed] [Google Scholar]
- Mikhael JR, Dingli D, Roy V, Reeder CB, Buadi FK, Hayman SR, et al. Management of newly diagnosed symptomatic multiple myeloma: Updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) Consensus Guidelines 2013. Mayo Clin Proc 2013. 88:360–376. doi: 10.1016/j.mayocp.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–2825. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vij R, Siegel DS, Jagannath S, Jakubowiak AJ, Stewart AK, McDonagh K, et al. An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. Br J Haematol. 2012;158:739–748. doi: 10.1111/j.1365-2141.2012.09232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vij R, Wang M, Kaufman JL, Lonial S, Jakubowiak AJ, Stewart AK, et al. An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood. 2012;119:5661–5670. doi: 10.1182/blood-2012-03-414359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badros AZ, Vij R, Martin T, Zonder JA, Kunkel L, Wang Z, et al. Carfilzomib in multiple myeloma patients with renal impairment: pharmacokinetics and safety Leukemia 2013. e-pub ahead of print 1 March 2013;doi: 10.1038/leu.2013.29 [DOI] [PMC free article] [PubMed]
- Kyprolis® Prescribing Information. Onyx Pharmaceuticals, Inc.: South San Fransisco, CA, USA; 2012. [Google Scholar]
- Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- Avet-Loiseau H. Role of genetics in prognostication in myeloma. Best Pract Res Clin Haematol. 2007;20:625–635. doi: 10.1016/j.beha.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Chiecchio L, Protheroe RK, Ibrahim AH, Cheung KL, Rudduck C, Dagrada GP, et al. Deletion of chromosome 13 detected by conventional cytogenetics is a critical prognostic factor in myeloma. Leukemia. 2006;20:1610–1617. doi: 10.1038/sj.leu.2404304. [DOI] [PubMed] [Google Scholar]
- Avet-Loiseau H, Attal M, Campion L, Caillot D, Hulin C, Marit G, et al. Long-term analysis of the IFM 99 trials for myeloma: cytogenetic abnormalities [t(4;14), del(17p), 1q gains] play a major role in defining long-term survival. J Clin Oncol. 2012;30:1949–1952. doi: 10.1200/JCO.2011.36.5726. [DOI] [PubMed] [Google Scholar]
- Kapoor P, Kumar S, Fonseca R, Lacy MQ, Witzig TE, Hayman SR, et al. Impact of risk stratification on outcome among patients with multiple myeloma receiving initial therapy with lenalidomide and dexamethasone. Blood. 2009;114:518–521. doi: 10.1182/blood-2009-01-202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaughnessy JD, Zhou Y, Haessler J, van Rhee F, Anaissie E, Nair B, et al. TP53 deletion is not an adverse feature in multiple myeloma treated with total therapy 3. Br J Haematol. 2009;147:347–351. doi: 10.1111/j.1365-2141.2009.07864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunthararajah Y, Triozzi P, Rini B, Singh A, Radivoyevitch T, Sekeres M, et al. p53-Independent, normal stem cell sparing epigenetic differentiation therapy for myeloid and other malignancies. Semin Oncol. 2012;39:97–108. doi: 10.1053/j.seminoncol.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avet-Loiseau H, Durie BG, Cavo M, Attal M, Gutierrez N, Haessler J, et al. Combining fluorescent in situ hybridization data with ISS staging improves risk assessment in myeloma: an International Myeloma Working Group collaborative project. Leukemia. 2013;27:711–717. doi: 10.1038/leu.2012.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowiak AJ, Dytfeld D, Griffith KA, Lebovic D, Vesole DH, Jagannath S, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120:1801–1809. doi: 10.1182/blood-2012-04-422683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowiak A, Dytfeld D, Jasielec J, Griffith K, Lebovic D, Vesole D, et al. Carfilzomib, lenalidomide, low-dose dexamethasone (CRd) in elderly patients with newly diagnosed multiple myeloma (NDMM) Clin Lymphoma Myeloma 201313(Suppl 1)): S1-S260 (Abstract O-10). [Google Scholar]
- Hajek R, Bryce R, Ro S, Klencke B, Ludwig H. Design and rationale of FOCUS (PX-171-011): a randomized, open-label, phase 3 study of carfilzomib versus best supportive care regimen in patients with relapsed and refractory multiple myeloma (R/R MM) BMC Cancer. 2012;12:415. doi: 10.1186/1471-2407-12-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.