Abstract
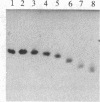
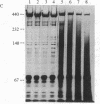
Low density lipoprotein (LDL) isolated from human plasma anticoagulated with EDTA (EDTA/LDL) was 4-fold more resistant to oxidation by reagent H2O2, as assayed by the thiobarbituric acid (TBA) assay, than LDL prepared from plasma anticoagulated with citrate (CDP/LDL). The LDLs required 1-3 mM H2O2 for maximal oxidation by this assay, and ED50S were 1.7 X 10(-3) M for EDTA/LDL and 4.5 X 10(-4) M for CDP/LDL. Oxidation was enhanced 2.3-fold by Cu2+ ions. Rabbit endothelial cell line monolayers released two orders of magnitude less H2O2 than was required to oxidize LDL and failed to induce TBA reactivity in either EDTA/LDL or CDP/LDL after a 24-hr coincubation. However, this LDL was subsequently degraded by mouse macrophages more rapidly than untreated LDL. Freshly isolated human monocytes (2 X 10(6) cells per ml), with or without phorbol myristate acetate (100 ng/ml) to trigger the respiratory burst, did not oxidize LDL in the TBA assay, despite producing large amounts of reactive oxygen intermediates. EDTA/LDL, CDP/LDL, and acetoacetylated LDL failed to trigger H2O2 release from human monocytes or macrophages. These results separate oxidation of LDL as measured by TBA assay from the modification of LDL by rabbit aortic endothelial cell line that leads to its subsequent enhanced degradation by macrophages.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrick B. A., Griffo W., Cohn Z., Nathan C. Hydrogen peroxide from cellular metabolism of cystine. A requirement for lysis of murine tumor cells by vernolepin, a glutathione-depleting antineoplastic. J Clin Invest. 1985 Aug;76(2):567–574. doi: 10.1172/JCI112008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Cathcart M. K., Morel D. W., Chisolm G. M., 3rd Monocytes and neutrophils oxidize low density lipoprotein making it cytotoxic. J Leukoc Biol. 1985 Aug;38(2):341–350. doi: 10.1002/jlb.38.2.341. [DOI] [PubMed] [Google Scholar]
- Chung B. H., Wilkinson T., Geer J. C., Segrest J. P. Preparative and quantitative isolation of plasma lipoproteins: rapid, single discontinuous density gradient ultracentrifugation in a vertical rotor. J Lipid Res. 1980 Mar;21(3):284–291. [PubMed] [Google Scholar]
- De la Harpe J., Nathan C. F. A semi-automated micro-assay for H2O2 release by human blood monocytes and mouse peritoneal macrophages. J Immunol Methods. 1985 Apr 22;78(2):323–336. doi: 10.1016/0022-1759(85)90089-4. [DOI] [PubMed] [Google Scholar]
- Demopoulos H. B. Control of free radicals in biologic systems. Fed Proc. 1973 Aug;32(8):1903–1908. [PubMed] [Google Scholar]
- Heinecke J. W., Baker L., Rosen H., Chait A. Superoxide-mediated modification of low density lipoprotein by arterial smooth muscle cells. J Clin Invest. 1986 Mar;77(3):757–761. doi: 10.1172/JCI112371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinecke J. W., Rosen H., Chait A. Iron and copper promote modification of low density lipoprotein by human arterial smooth muscle cells in culture. J Clin Invest. 1984 Nov;74(5):1890–1894. doi: 10.1172/JCI111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Enhanced macrophage degradation of biologically modified low density lipoprotein. Arteriosclerosis. 1983 Mar-Apr;3(2):149–159. doi: 10.1161/01.atv.3.2.149. [DOI] [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Enhanced macrophage degradation of low density lipoprotein previously incubated with cultured endothelial cells: recognition by receptors for acetylated low density lipoproteins. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6499–6503. doi: 10.1073/pnas.78.10.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff H. F., Gaubatz J. W. Isolation, purification, and characterization of a lipoprotein containing Apo B from the human aorta. Atherosclerosis. 1982 Apr;42(2-3):273–297. doi: 10.1016/0021-9150(82)90157-5. [DOI] [PubMed] [Google Scholar]
- Johnson W. J., Pizzo S. V., Imber M. J., Adams D. O. Receptors for maleylated proteins regulate secretion of neutral proteases by murine macrophages. Science. 1982 Nov 5;218(4572):574–576. doi: 10.1126/science.6289443. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morel D. W., DiCorleto P. E., Chisolm G. M. Endothelial and smooth muscle cells alter low density lipoprotein in vitro by free radical oxidation. Arteriosclerosis. 1984 Jul-Aug;4(4):357–364. doi: 10.1161/01.atv.4.4.357. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Carriero S. M., Harris A. M., Jaffee E. A. Human mononuclear phagocyte antiprotozoal mechanisms: oxygen-dependent vs oxygen-independent activity against intracellular Toxoplasma gondii. J Immunol. 1985 Mar;134(3):1982–1988. [PubMed] [Google Scholar]
- Nakagawara A., DeSantis N. M., Nogueira N., Nathan C. F. Lymphokines enhance the capacity of human monocytes to secret reactive oxygen intermediates. J Clin Invest. 1982 Nov;70(5):1042–1048. doi: 10.1172/JCI110691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Steinbrecher U. P., Barnett J., Witztum J. L., Steinberg D. Essential role of phospholipase A2 activity in endothelial cell-induced modification of low density lipoprotein. Proc Natl Acad Sci U S A. 1985 May;82(9):3000–3004. doi: 10.1073/pnas.82.9.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond T. L., Reynolds S. A. Lipoproteins of the extravascular space: alterations in low density lipoproteins of interstitial inflammatory fluid. J Lipid Res. 1983 Feb;24(2):113–119. [PubMed] [Google Scholar]
- Reed P. W. Glutathione and the hexose monophosphate shunt in phagocytizing and hydrogen peroxide-treated rat leukocytes. J Biol Chem. 1969 May 10;244(9):2459–2464. [PubMed] [Google Scholar]
- Schuh J., Fairclough G. F., Jr, Haschemeyer R. H. Oxygen-mediated heterogeneity of apo-low-density lipoprotein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3173–3177. doi: 10.1073/pnas.75.7.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Role of lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J Biol Chem. 1978 Dec 25;253(24):9053–9062. [PubMed] [Google Scholar]
- Weiss S. J., LoBuglio A. F. Phagocyte-generated oxygen metabolites and cellular injury. Lab Invest. 1982 Jul;47(1):5–18. [PubMed] [Google Scholar]