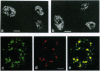
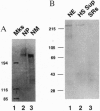
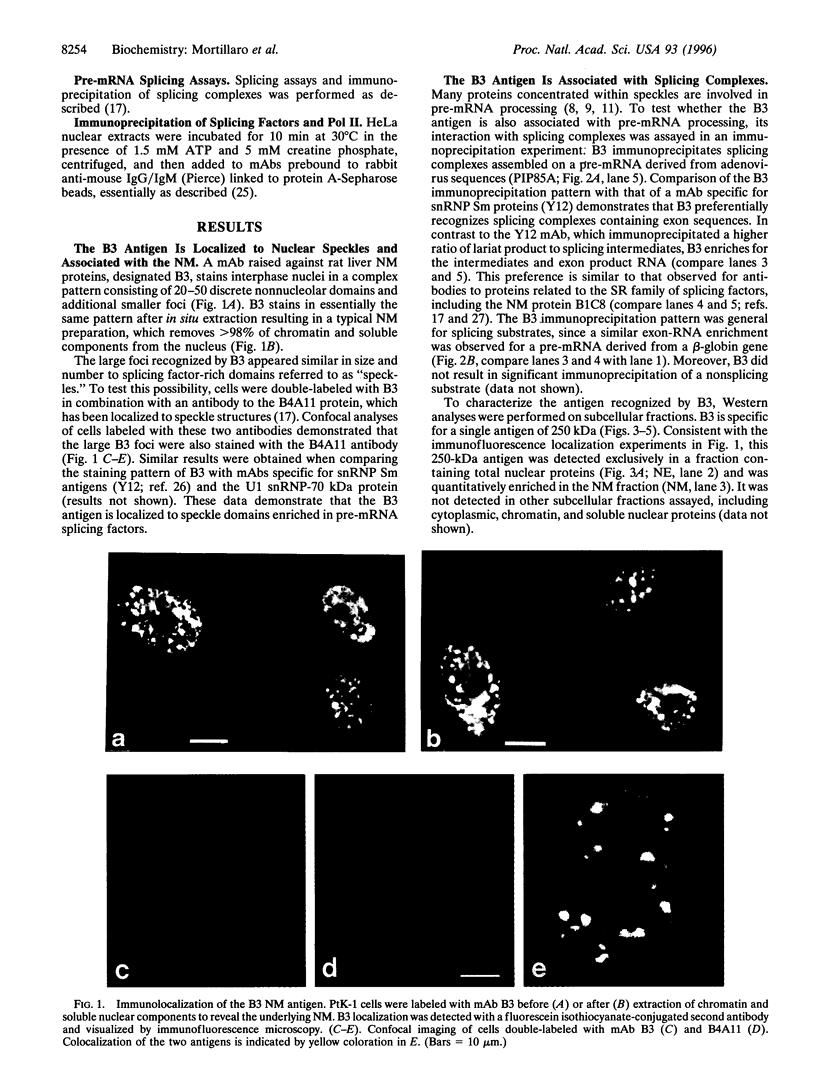
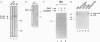Abstract
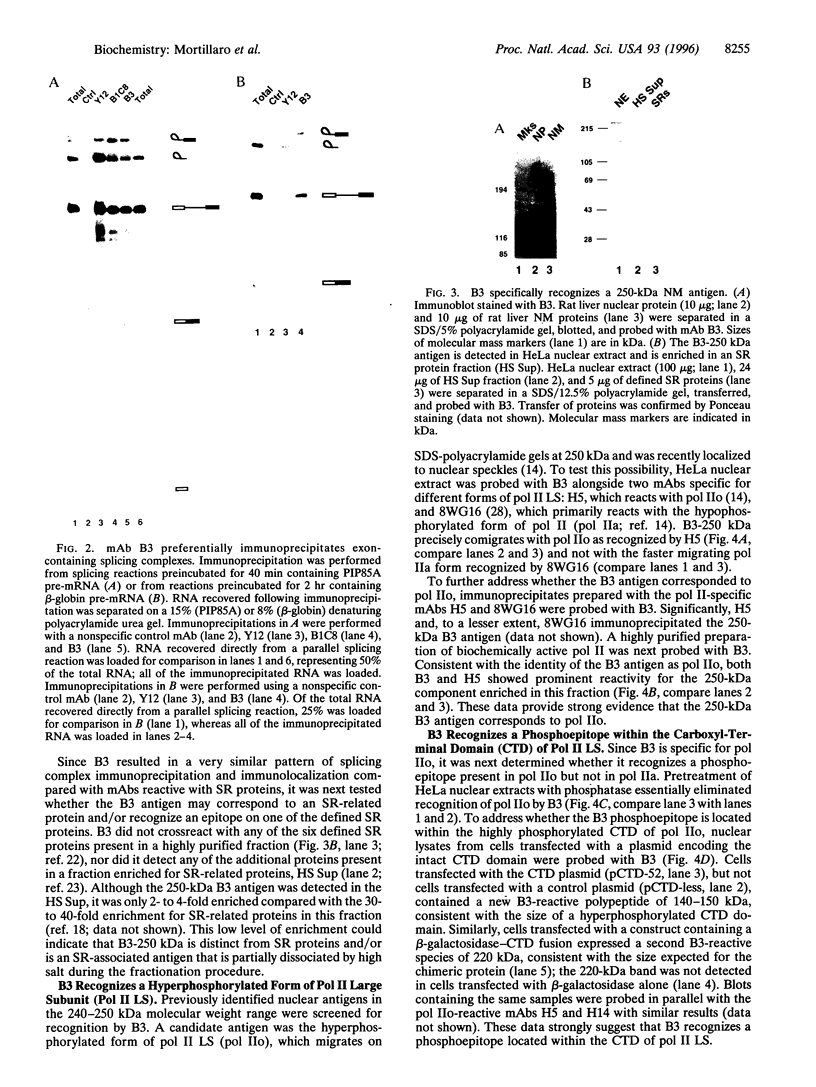
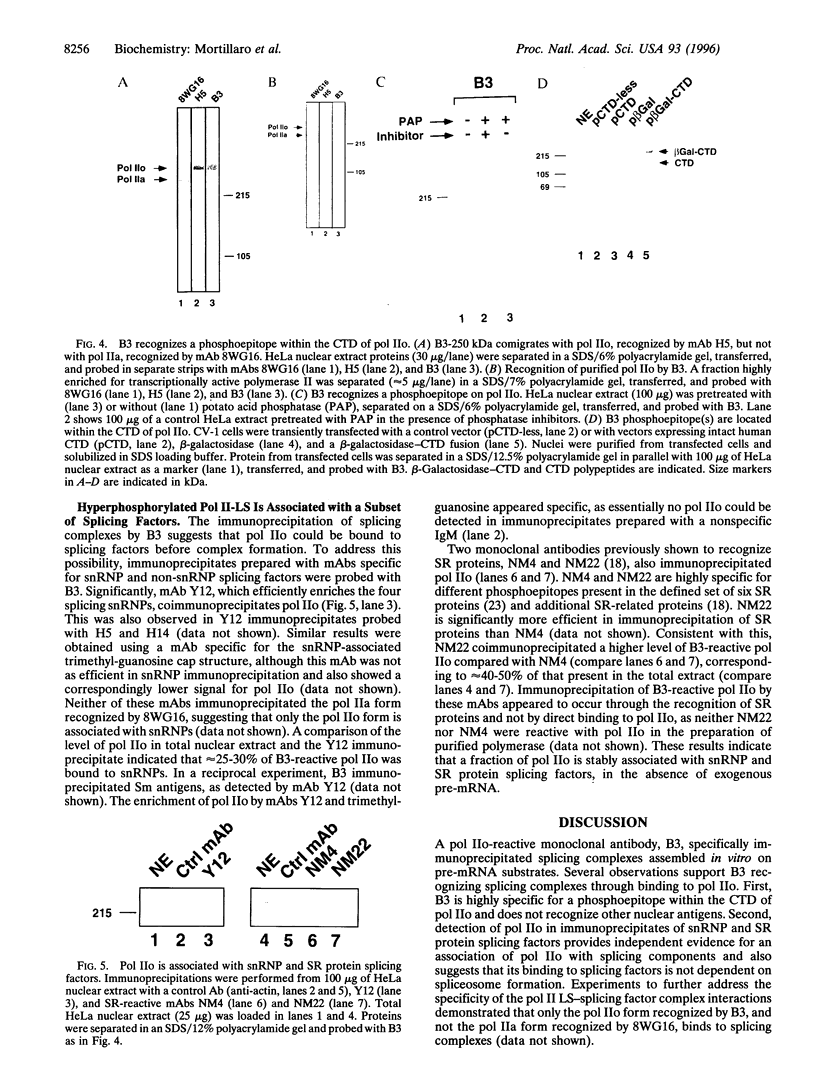
A hyperphosphorylated form of the largest subunit of RNA polymerase II (pol IIo) is associated with the pre-mRNA splicing process. Pol IIo was detected in association with a subset of small nuclear ribonucleoprotein particle and Ser-Arg protein splicing factors and also with pre-mRNA splicing complexes assembled in vitro. A subpopulation of pol IIo was localized to nuclear "speckle" domains enriched in splicing factors, indicating that it may also be associated with RNA processing in vivo. Moreover, pol IIo was retained in a similar pattern following in situ extraction of cells and was quantitatively recovered in the nuclear matrix fraction. The results implicate nuclear matrix-associated hyperphosphorylated pol IIo as a possible link in the coordination of transcription and splicing processes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adami G., Babiss L. E. DNA template effect on RNA splicing: two copies of the same gene in the same nucleus are processed differently. EMBO J. 1991 Nov;10(11):3457–3465. doi: 10.1002/j.1460-2075.1991.tb04910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baurén G., Wieslander L. Splicing of Balbiani ring 1 gene pre-mRNA occurs simultaneously with transcription. Cell. 1994 Jan 14;76(1):183–192. doi: 10.1016/0092-8674(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Belgrader P., Dey R., Berezney R. Molecular cloning of matrin 3. A 125-kilodalton protein of the nuclear matrix contains an extensive acidic domain. J Biol Chem. 1991 May 25;266(15):9893–9899. [PubMed] [Google Scholar]
- Bergers G., Reikerstorfer A., Braselmann S., Graninger P., Busslinger M. Alternative promoter usage of the Fos-responsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO J. 1994 Mar 1;13(5):1176–1188. doi: 10.1002/j.1460-2075.1994.tb06367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer A. L., Osheim Y. N. Splice site selection, rate of splicing, and alternative splicing on nascent transcripts. Genes Dev. 1988 Jun;2(6):754–765. doi: 10.1101/gad.2.6.754. [DOI] [PubMed] [Google Scholar]
- Blencowe B. J., Carmo-Fonseca M., Behrens S. E., Lührmann R., Lamond A. I. Interaction of the human autoantigen p150 with splicing snRNPs. J Cell Sci. 1993 Jul;105(Pt 3):685–697. doi: 10.1242/jcs.105.3.685. [DOI] [PubMed] [Google Scholar]
- Blencowe B. J., Issner R., Kim J., Mccaw P., Sharp P. A. New proteins related to the Ser-Arg family of splicing factors. RNA. 1995 Oct;1(8):852–865. [PMC free article] [PubMed] [Google Scholar]
- Blencowe B. J., Nickerson J. A., Issner R., Penman S., Sharp P. A. Association of nuclear matrix antigens with exon-containing splicing complexes. J Cell Biol. 1994 Nov;127(3):593–607. doi: 10.1083/jcb.127.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman D. B., Du L., van der Zee S., Warren S. L. Transcription-dependent redistribution of the large subunit of RNA polymerase II to discrete nuclear domains. J Cell Biol. 1995 Apr;129(2):287–298. doi: 10.1083/jcb.129.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew R. W., Samuels M., Sharp P. A. Formation of transcription preinitiation complexes with an amanitin-resistant RNA polymerase II. J Biol Chem. 1988 Nov 15;263(32):17128–17135. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. D. The superfamily of arginine/serine-rich splicing factors. RNA. 1995 Sep;1(7):663–680. [PMC free article] [PubMed] [Google Scholar]
- Greenleaf A. L. Positive patches and negative noodles: linking RNA processing to transcription? Trends Biochem Sci. 1993 Apr;18(4):117–119. doi: 10.1016/0968-0004(93)90016-g. [DOI] [PubMed] [Google Scholar]
- Huang S., Spector D. L. Nascent pre-mRNA transcripts are associated with nuclear regions enriched in splicing factors. Genes Dev. 1991 Dec;5(12A):2288–2302. doi: 10.1101/gad.5.12a.2288. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Cook P. R. Transcription occurs at a nucleoskeleton. EMBO J. 1985 Apr;4(4):919–925. doi: 10.1002/j.1460-2075.1985.tb03719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. A., Hassan A. B., Errington R. J., Cook P. R. Visualization of focal sites of transcription within human nuclei. EMBO J. 1993 Mar;12(3):1059–1065. doi: 10.1002/j.1460-2075.1993.tb05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-García L. F., Spector D. L. In vivo evidence that transcription and splicing are coordinated by a recruiting mechanism. Cell. 1993 Apr 9;73(1):47–59. doi: 10.1016/0092-8674(93)90159-n. [DOI] [PubMed] [Google Scholar]
- Larsson S. H., Charlieu J. P., Miyagawa K., Engelkamp D., Rassoulzadegan M., Ross A., Cuzin F., van Heyningen V., Hastie N. D. Subnuclear localization of WT1 in splicing or transcription factor domains is regulated by alternative splicing. Cell. 1995 May 5;81(3):391–401. doi: 10.1016/0092-8674(95)90392-5. [DOI] [PubMed] [Google Scholar]
- Lerner E. A., Lerner M. R., Janeway C. A., Jr, Steitz J. A. Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proc Natl Acad Sci U S A. 1981 May;78(5):2737–2741. doi: 10.1073/pnas.78.5.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. D., Lebkowski J. S., Daly A. K., Laemmli U. K. Interphase nuclear matrix and metaphase scaffolding structures. J Cell Sci Suppl. 1984;1:103–122. doi: 10.1242/jcs.1984.supplement_1.8. [DOI] [PubMed] [Google Scholar]
- Nakayasu H., Berezney R. Nuclear matrins: identification of the major nuclear matrix proteins. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10312–10316. doi: 10.1073/pnas.88.22.10312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson J. A., Blencowe B. J., Penman S. The architectural organization of nuclear metabolism. Int Rev Cytol. 1995;162A:67–123. doi: 10.1016/s0074-7696(08)61229-2. [DOI] [PubMed] [Google Scholar]
- O'Brien T., Hardin S., Greenleaf A., Lis J. T. Phosphorylation of RNA polymerase II C-terminal domain and transcriptional elongation. Nature. 1994 Jul 7;370(6484):75–77. doi: 10.1038/370075a0. [DOI] [PubMed] [Google Scholar]
- Payne J. M., Laybourn P. J., Dahmus M. E. The transition of RNA polymerase II from initiation to elongation is associated with phosphorylation of the carboxyl-terminal domain of subunit IIa. J Biol Chem. 1989 Nov 25;264(33):19621–19629. [PubMed] [Google Scholar]
- Spector D. L. Macromolecular domains within the cell nucleus. Annu Rev Cell Biol. 1993;9:265–315. doi: 10.1146/annurev.cb.09.110193.001405. [DOI] [PubMed] [Google Scholar]
- Spector D. L., Watt R. A., Sullivan N. F. The v- and c-myc oncogene proteins colocalize in situ with small nuclear ribonucleoprotein particles. Oncogene. 1987 Mar;1(1):5–12. [PubMed] [Google Scholar]
- Thompson N. E., Steinberg T. H., Aronson D. B., Burgess R. R. Inhibition of in vivo and in vitro transcription by monoclonal antibodies prepared against wheat germ RNA polymerase II that react with the heptapeptide repeat of eukaryotic RNA polymerase II. J Biol Chem. 1989 Jul 5;264(19):11511–11520. [PubMed] [Google Scholar]
- Wan K. M., Nickerson J. A., Krockmalnic G., Penman S. The B1C8 protein is in the dense assemblies of the nuclear matrix and relocates to the spindle and pericentriolar filaments at mitosis. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):594–598. doi: 10.1073/pnas.91.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansink D. G., Schul W., van der Kraan I., van Steensel B., van Driel R., de Jong L. Fluorescent labeling of nascent RNA reveals transcription by RNA polymerase II in domains scattered throughout the nucleus. J Cell Biol. 1993 Jul;122(2):283–293. doi: 10.1083/jcb.122.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Johnson C. V., Dobner P. R., Lawrence J. B. Higher level organization of individual gene transcription and RNA splicing. Science. 1993 Feb 26;259(5099):1326–1330. doi: 10.1126/science.8446901. [DOI] [PubMed] [Google Scholar]
- Xing Y., Johnson C. V., Moen P. T., Jr, McNeil J. A., Lawrence J. Nonrandom gene organization: structural arrangements of specific pre-mRNA transcription and splicing with SC-35 domains. J Cell Biol. 1995 Dec;131(6 Pt 2):1635–1647. doi: 10.1083/jcb.131.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Lawrence J. B. Nuclear RNA tracks: structural basis for transcription and splicing? Trends Cell Biol. 1993 Oct;3(10):346–353. doi: 10.1016/0962-8924(93)90105-a. [DOI] [PubMed] [Google Scholar]
- Yuryev A., Patturajan M., Litingtung Y., Joshi R. V., Gentile C., Gebara M., Corden J. L. The C-terminal domain of the largest subunit of RNA polymerase II interacts with a novel set of serine/arginine-rich proteins. Proc Natl Acad Sci U S A. 1996 Jul 9;93(14):6975–6980. doi: 10.1073/pnas.93.14.6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahler A. M., Lane W. S., Stolk J. A., Roth M. B. SR proteins: a conserved family of pre-mRNA splicing factors. Genes Dev. 1992 May;6(5):837–847. doi: 10.1101/gad.6.5.837. [DOI] [PubMed] [Google Scholar]
- Zhang G., Taneja K. L., Singer R. H., Green M. R. Localization of pre-mRNA splicing in mammalian nuclei. Nature. 1994 Dec 22;372(6508):809–812. doi: 10.1038/372809a0. [DOI] [PubMed] [Google Scholar]
- van Driel R., Wansink D. G., van Steensel B., Grande M. A., Schul W., de Jong L. Nuclear domains and the nuclear matrix. Int Rev Cytol. 1995;162A:151–189. doi: 10.1016/s0074-7696(08)61231-0. [DOI] [PubMed] [Google Scholar]