Abstract
Purpose
First-in-man study of KOS-1584, a second generation epothilone.
Methods
Patients with advanced solid malignancies received KOS-1584 every 3 weeks until disease progression. Using a modified Fibonacci dose escalation scheme, one patient was enrolled at each dose level until the first instance of grade 2 toxicity. Thereafter, a standard 3 + 3 design was utilized.
Results
Sixty-six patients in 14 cohorts were dosed from 0.8 to 48 mg/m2. Diarrhea, arthralgias, and encephalopathy were dose-limiting toxicities (DLTs) at doses ≥36 mg/m2. At the recommended phase II dose (RP2D), the most common adverse effects were peripheral neuropathy (low grade), fatigue, arthralgias/myalgias, and diarrhea (31, 6%). The incidence of neutropenia was low. The overall clearance, volume of distribution, and half-life of KOS-1584 were 11 ± 6.17 L/h/m2, 327 ± 161 L/m2, and 21.9 ± 8.75 h, respectively. The half-life for the seco-metabolite (KOS-1891) was 29.6 ± 13.8 h. KOS-1584 exhibited linear pharmacokinetics. A dose-dependent increase in microtubulin bundle formation was observed at doses ≥27 mg/m2. Two patients achieved partial responses and 24 patients had stable disease (SD).
Conclusions
The RP2D of KOS-1584 is 36 mg/m2. The lack of severe neurologic toxicity, diarrhea, neutropenia, or hypersensitivity reactions; favorable pharmacokinetic profile; and early evidence of activity support further evaluation.
Keywords: Epothilone, KOS-1584, Phase I, Solid tumors
Introduction
Microtubules play a pivotal role in cell division and are validated targets for cytotoxic agents. Vinca alkaloids and taxanes interfere with microtubule function and induce apoptotic cell death [1–3]. Despite efficacy in a variety of malignancies, these agents have limitations of multi-drug resistance (MDR), neutropenia, neurotoxicity, and hypersensitivity reactions [3–6]. The development of second generation agents has aimed to overcome these problems.
Epothilones, derived from the myxobacterium Sorangium cellulosum, are a novel class of microtubule interactive agents. Importantly, they do not exhibit cross-resistance to taxanes (due to poor susceptibility to p-glycoprotein (P-gp)-mediated drug efflux and high affinity to various β tubulin isoforms) and have a more favorable side effect profile [4, 7, 8]. Epothilones in recent development include ixabepilone, patupilone, sagopilone, and KOS-862 [9–14].
Figure 1 depicts the chemical structures of epothilone derivatives in recent development. Small chemical variations result in important differences in terms of susceptibility to P-gp, need for cremophor-based formulation, pharmacokinetics, toxicity profile, and in vivo anti-tumor activity [3]. The epoxide group at carbon 12–13 seen with ixabepilone and patupilone is highly reactive and contributes to epothilone toxicity. Desoxyepothilone B (epothilone D, KOS-862) was designed with the epoxide reduced to a double bond in an effort to give this compound less toxicity. KOS-862 was shown in vivo to have greater activity compared with ixabepilone and patupilone [11, 15]. However, phase II trials of KOS-862 have shown significant neurotoxicity, limiting further development [16–18].
Fig. 1.
Chemical structures of selected epothilones in development. The lactone is the primary site of metabolic attack and opening of the ring results in loss of cytocidal activity. The synthetic ixabepilone (BMS-247550, aza-epothilone B) has oxygen in the ring replaced by a nitrogen; the resultant lactam is more resistant to ring opening. Since the epoxide group is highly reactive and thought to contribute to epothilone toxicity, desoxyepothilone B (epothilone D, KOS-862) has the epoxide at carbon 12–13 reduced to a double bond in an effort to give this compound less toxicity. The second generation KOS-1584 makes only one alteration compared to KOS-862: an additional reduced double bond at carbon 9–10, flattening the 16-member ring
KOS-1584 [(E)-9,10-didehydroepothilone D] is a second generation epothilone specifically designed to have a longer elimination half-life, larger volume of distribution, less toxicity, more potency, and higher solubility (negating cremophor) compared with KOS-862 [3, 18–20]. Modifications included a reduced double bond at carbon 9–10 and flattening the 16-member ring [3]. In P-gp-overexpressing cell lines, KOS-1584 was more potent than paclitaxel. In paclitaxel-resistant xenografts, anti-tumor activity has been demonstrated. KOS-1584 is highly bound to plasma proteins (97%). The primary seco-metabolite (KOS-1891) was inactive in cytotoxicity assays up to 10 μM [18].
We performed this first-in-human, phase I dose escalation study of KOS-1584 in patients with advanced cancers. Primary objectives included determination of safety, DLTs, maximum tolerated dose (MTD), and recommended phase II dose (RP2D). Secondary objectives included evaluation of pharmacokinetics, pharmacodynamics, and anti-tumor activity.
Patients and methods
Eligibility criteria
Eligible patients had advanced or metastatic solid tumors measurable by Response Evaluation Criteria in Solid Tumors (RECIST, Version 1.0) [21]; Eastern Cooperative Oncology Group performance status ≤1; age ≥18 years; life expectancy ≥3 months; and adequate bone marrow (ANC ≥1.5 × 109/L, hemoglobin ≥8.5 g/dL, platelets ≥75 × 109/L), renal (Cr <1.5 ULN) and hepatic (AST ≤2.5 ULN, total bilirubin ≤1.5 ULN) function.
Exclusion criteria included uncontrolled or hemorrhagic diarrhea; active peptic ulcer disease; grade ≥2 neurological symptoms; hypersensitivity reaction to hydroxypropyl-β-cyclodextrin, ethanol, or propylene glycol; intracranial metastasis; pregnancy/lactation; clinically significant cardiac disease; dementia or altered mental status; and other conditions interfering with study participation.
All patients gave written informed consent. Approval was obtained from the institutional review boards at both institutions.
Dosage, dose escalation, and drug administration
The starting dose was 0.8 mg/m2 (one-sixth the MTD in dogs) [18] given intravenously over 3 h, every 3 weeks. Dose escalation followed a modified Fibonacci scheme [22]. Prophylactic anti-emetics and premedications were not routinely administered; however, anti-emetics were allowed at the investigator’s discretion after documented nausea during a previous infusion. DLT was defined as any first cycle grade 4 neutropenia for ≥7 consecutive days or febrile neutropenia; grade 4 thrombocytopenia or bleeding episode requiring platelet transfusion; grade ≥3 nausea and/or vomiting despite maximal medical intervention; all other grade ≥3 non-hematological toxicity; or delay ≥4 weeks from the time of scheduled retreatment, if due to delayed recovery of drug-related toxicity. One patient per cohort was enrolled until the first instance of grade ≥2 drug-related toxicity (except nausea, vomiting, fatigue, anorexia, or alopecia) or upon observation of cumulative toxicity (meeting the definition of DLT in cycle 2 and above). Thereafter, a standard 3 + 3 design was utilized.
Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, v3.0 [23]. Dose reduction by one level was allowed for patients who experienced DLT. The MTD was defined as the highest dose at which no more than one of six patients in the cohort developed DLT. Once the MTD was defined, an additional 10 patients were enrolled to further define the RP2D and to characterize toxicity, pharmacokinetics, and pharmacodynamic relationships.
KOS-1584 was supplied by Kosan Biosciences, Inc (Hayward, CA) and diluted with KOS-1584 diluent (133 mg/mL hydroxypropyl-β-cyclodextrin in water for injection) and the appropriate amount of drug was withdrawn and mixed with saline (0.9% w/v sodium chloride) to achieve a final concentration of 0.05 or 0.1 mg/mL.
Safety and efficacy assessments
A medical history and physical history with detailed neurological examination, vital signs, performance status assessment, and laboratory determinations including pregnancy test, prothrombin time, activated partial thromboplastin time, and urinalysis were obtained at baseline and at the beginning of each cycle. Complete blood count and serum chemistries were obtained weekly. Three serial 12-lead electrocardiograms (ECGs) were obtained prior to and 30 min after treatment during cycles 1 and 2. The QT interval was determined using automated readings and corrected for heart rate according to Bazette’s formula (QTcB) [24]. Fecal occult blood tests (FOBT) were performed at the pretreatment evaluation and on day 3 of the first two cycles. RECIST response evaluation was performed every two cycles.
Pharmacokinetics
Blood specimens were collected preinfusion, just prior to the end of infusion, 5 min and 0.5, 1, 2, 3, 5, 8, 10, 24, 48, and 72 h after the end of infusion during cycles 1 and 2. Blood was collected into EDTA-containing tubes, placed on ice, and plasma was separated from whole blood and stored at −70°C until analysis.
At the MTD level, urine specimens were collected at baseline and during the intervals of 0–5 and 5–24 h following the start of the infusion of the first two cycles and stored at 2–8°C. The total volume was recorded, and three 30-mL aliquots from each interval were frozen at −70°C until analysis. Completeness of the 24-h collection was determined by a 24-h urinary creatinine. A proprietary LC/MS/MS method developed by Kosan Biosciences, Inc was used to identify urinary KOS-1584 and metabolites over time.
Tubulin polymerization in PBMCs
Blood specimens were collected into heparin-CPT tubes prior to treatment, at the end of infusion, and 1, 3, and 24 h following the end of the first two infusions. Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation, and red blood cells were removed by brief hypotonic lysis. PBMC slides were prepared in a cytospin (Thermo Shandon, Inc., Pittsburgh, PA) and sent to Kosan Biosciences, Inc. for immunohistochemistry and quantitation of cells exhibiting tubulin bundle formation using a previously described method [25].
Statistical analysis
Tumor response and safety data were reported by descriptive statistics. Plasma concentration data were analyzed by non-compartmental methods [26] using Kinetica™, Version 4.4.1 (Thermo-Fisher Scientific Corporation, Philadelphia). The ratio of KOS-1891 AUC0–∞ to KOS-1584 AUC0–∞ was used as a measure of the extent of metabolism of KOS-1584 to KOS-1891. KOS-1584 and its metabolites were profiled in the urine. A sigmoidal maximum effect pharmacodynamic model was fit to describe the dose–response relationship between plasma concentration of KOS-1584 and percent microtubule bundle formation. The maximum effect (Emax), dose at which 50% of the maximum is produced (ED50), and steepness factor (γ) were estimated using WinNonlin Version 1.5 (Pharsight, Cary, North Carolina).
Results
General
Between November 2004 and August 2007, sixty-six patients were treated across 14 cohorts, at doses ranging from 0.8 to 48 mg/m2. A total of 49 cycles (median 2, range 1–8) were given to the 16 patients at 36 mg/m2 (MTD) cohort. Patients received a median of four prior chemotherapy regimens, including taxanes (62%), platinums (81%), or both (49%). Thirty-one patients (47%) had baseline grade 1 sensory neuropathy. Patient demographics and characteristics are listed in Table 1.
Table 1.
Patient characteristics
| Characteristics | No. of patients |
|---|---|
| No. of patients | N = 66 |
| Age (years) | |
| Median | 60 |
| Range | 33–84 |
| Gender | |
| Male | 30 |
| Female | 36 |
| Race | |
| Caucasian | 43 |
| African American | 15 |
| Other | 8 |
| ECOG PS | |
| 0 | 18 |
| 1 | 48 |
| Primary diagnosis | |
| Lung | 16 |
| Ovary/uterine | 16 |
| Colorectal | 7 |
| Breast | 4 |
| Esophagus/stomach | 4 |
| Melanoma | 3 |
| Pancreas | 3 |
| Unknown primary | 1 |
| Liver | 1 |
| Prostate | 1 |
| Kidney | 1 |
| Sarcoma | 1 |
| Other | 8 |
| Prior chemotherapy regimens | |
| Median | 4 |
| Range | 0–14 |
| Prior taxane therapy | |
| Yes | 41 (62%) |
| No | 25 (38%) |
Safety and tolerability
Common treatment-related adverse events (AEs) are listed in Table 2. Six DLTs were observed. The third patient treated at 36 mg/m2 experienced a DLT of arthralgia, after which three additional patients were treated at this dose level without DLT. Two patients were then treated at 48 mg/m2; one developed a DLT of arthralgia and the other (a patient with extensive stage small cell lung cancer) developed a DLT of encephalopathy in the setting of dehydration and hyponatremia. Two of six patients subsequently treated at 42 mg/m2 developed DLT of diarrhea. Therefore, the MTD was defined as 36 mg/m2, and the cohort was expanded with an additional ten patients treated, one of whom developed a grade 3 diarrhea. At dose level 6.5 mg/m2, one patient had G3 dyspnea, occurring in cycle 2 and was not considered DLT. Another patient died of streptococcus pneumonia and sepsis, and although this was subsequently felt not to be related to drug, the cohort was expanded to 6 patients to further explore toxicity at this dose level.
Table 2.
Common treatment-related adverse events by dose level (no. of patients)
| 0.8 (n
= 1) |
1.5 (n
= 4) |
2.5 (n
= 4) |
3.7 (n
= 3) |
5 (n =
4) |
6.5 (n
= 6) |
8.5 (n
= 3) |
11.3 (n
= 4) |
15 (n
= 4) |
20 (n
= 5) |
27 (n
= 4) |
36π (n =
16) |
42 (n
= 6) |
48 (n
= 2) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Grade | Grade | Grade | Grade | Grade | Grade | Grade | Grade | Grade | Grade | Grade | Grade | Grade | |||||||||||||||
| Adverse event | 1/2 | 3 | 1/2 | 3 | 1/2 | 3 | 1/2 | 3 | 1/2 | 3 | 1/2 | 3 | 1/2 | 3 | 1/2 | 3 | 1/2 | 3 | 1/2 | 3 | 1/2 | 3 | 1/2 | 3 | 1/2 | 3 | 1/2 | 3 |
| Hematologic | ||||||||||||||||||||||||||||
| Neutropenia | 1 | |||||||||||||||||||||||||||
| Lymphopenia | 1 | 1 | 1 | |||||||||||||||||||||||||
| Anemia | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | ||||||||||||||||||||
| Thrombocytopenia | 1 | 1 | ||||||||||||||||||||||||||
| Non-hematologic | ||||||||||||||||||||||||||||
| Asthenia/fatigue | 1 | 4 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 4 | 9 | 1 | 3 | 1 | 1 | |||||||||||||
| Anorexia | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 3 | 2 | 1 | ||||||||||||||||||
| Constipation | 1 | 2 | 1 | 1 | 1 | 1 | 1 | |||||||||||||||||||||
| Diarrhea | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 4 | 1 | 3 | 2 | ||||||||||||||||
| Nausea/vomiting | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 7 | 3 | 1 | |||||||||||||||
| Abdominal pain | 2 | 1 | 1 | 2 | ||||||||||||||||||||||||
| Dizziness | 1 | 1 | 2 | 1 | 2 | |||||||||||||||||||||||
| Headache | 1 | 3 | 1 | |||||||||||||||||||||||||
| Peripheral neuropathy | 2 | 1 | 1 | 1 | 1 | 2 | 4 | 11 | 4 | 2 | 1 | |||||||||||||||||
| Arthralgia/myalgia | 2 | 1 | 1 | 2 | 7 | 1 | 1 | |||||||||||||||||||||
| Back pain | 2 | 1 | 1 | 1 | 1 | |||||||||||||||||||||||
| Edema | 1 | 1 | 1 | 2 | ||||||||||||||||||||||||
| Chest pain | 1 | 2 | 1 | |||||||||||||||||||||||||
| Dyspnea∞ | 1 | 1 | 1 | 1 | ||||||||||||||||||||||||
| Pleural effusion | 1 | 1 | ||||||||||||||||||||||||||
| Alopecia | 1 | 1 | 4 | 2 | ||||||||||||||||||||||||
| Dry skin/rash | 1 | 1 | 1 | |||||||||||||||||||||||||
There were no treatment-related grade 4 toxicities. Doses are mg/m2. N number of patients
At the 36 mg/m2 dose level, three patients had G3 toxicity; one patient had three separate G3 toxicities
Grade 3 dyspnea was reported in three patients: one each at 0.8 mg/m2 (renal cell carcinoma patient with mediastinal lymph node metastasis, during cycle 4, lasting>1 month), 6.5 mg/m2 (melanoma patient with lung metastasis, during cycle 2, lasting 2 days), and 27 mg/m2 (pancreas cancer patient with liver metastasis, unclear etiology, but transient-only lasted 1 day). These events were listed as remotely related to study drug, but in retrospect may be more likely related to disease progression
Most AEs, including sensory neuropathy, were generally low grade and transient. Sensory neuropathy occurred more frequently at doses ≥20 mg/m2 with onset during the first two cycles. Patients who developed either new or worse grade neuropathy generally had symptom resolution or return to baseline shortly after drug discontinuation. Similarly, diarrhea and arthralgia had early onset, were not cumulative, and were managed effectively with anti-diarrheal and anti-inflammatory agents, respectively. Five patients had positive FOBT or hematochezia. There were no cases of bowel perforation, necrosis, or significant GI bleeding. Additional grade 3 toxicities not listed in Table 2 occurred at doses generally higher than RP2D and included dehydration (42 mg/m2), hypocalcemia (42 mg/m2), hypoalbuminemia (27 mg/m2), tremor (42 mg/m2), hypotension (42 mg/m2), encephalopathy (48 mg/m2), and painful respiration (36 mg/m2). One episode of grade 3 small bowel obstruction was observed during the second week of cycle 1 in a patient with metastatic non-small cell lung cancer treated at the 42 mg/m2 dose level, after having been treated with loperamide for grade 3 diarrhea in the first week. Comparisons of baseline or pre- and post-infusion ECGs did not show any effects of KOS-1584 on QTcB interval.
Five patients discontinued therapy due to adverse effects from KOS-1584: one patient each at 27 mg/m2 (grade 1 nausea, vomiting, and neuropathy), 36 mg/m2 (grade 2 neuropathic pain), and 48 mg/m2 (encephalopathy); and two patients at 42 mg/m2 (grade 2 neuropathy; fatigue and grade 3 neuropathy). Two patients died within 4 weeks of receiving study drug: one at 6.5 mg/m2 due to streptococcal pneumonia with septicemia occurring on day 3 of the first cycle, and one at 20 mg/m2 due to complications from a malignant pleural effusion.
Pharmacokinetics
Concentration–time profiles of KOS-1584 were available for all 66 patients treated. KOS-1584 concentrations reached a maximum at the end of the infusion and declined biexponentially. Plasma profiles for the seco-metabolite, KOS-1891, demonstrated much lower concentrations but were similar in shape for cohorts dosed at 6.5 mg/m2 and higher.
Table 3 summarizes the PK parameters of KOS-1584 and KOS-1891 for cycle 1. The mean overall clearance, volume of distribution, and half-life of KOS-1584 were 11.0 ± 6.17 L/h/m2, 327 ± 161 L/m2, and 21.9 ± 8.75 h, respectively. The half-life for KOS-1891 was 29.6 ± 13.8 h, and the KOS-1891/KOS-1584 AUC ratio was 9.43 ± 4.86%. There appears to be no dose dependency in clearance values, consistent with linear kinetics.
Table 3.
KOS-1584 & KOS-1891 pharmacokinetic parameters (mean ± SD) following KOS-1584 infusion (3 h) on cycle 1
| COHORT | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Starting dose (mg/m2) | 0.8 | 1.5 | 2.5 | 3.7 | 5.0 | 6.5 | 8.5 | 11.3 | 15.0 | 20.0 | 27 | 36 | 42 | 48 |
| N | 1 | 4 | 4 | 3 | 4 | 6 | 3 | 4 | 4 | 5 | 4 | 16 | 6 | 2 |
| KOS-1584 | ||||||||||||||
| Tmax (ng/mL) | 3.0 | 2.0 ± 1.2 | 3.0 ± 0.02 | 3.0 ± 0.06 | 2.61 ± 0.74 | 2.49 ± 0.76 | 2.96 ± 0.03 | 2.60 ± 0.68 | 2.97 ± 0.03 | 2.38 ± 0.80 | 3.10 ± 0.27 | 2.67 ± 0.58 | 2.95 ± 0.04 | 2.92 ± 0.0 |
| Cmax (ng/mL) | 5.73 | 9.29 ± 4.16 | 18.0 ± 5.24 | 32.9 ± 10.8 | 41.9 ± 13.4 | 41.9 ± 11.3 | 78.4 ± 28.8 | 140 ± 41.9 | 220 ± 84.7 | 284 ± 153 | 259 ± 110 | 701 ± 425 | 1,066 ± 513 | 769 ± 42.4 |
| AUC (ng h/mL) | 26.7 | 64.9 ± 16.3 | 170 ± 2.21 | 359 ± 40.7 | 468 ± 140 | 566 ± 159 | 620 ± 317 | 2,206 ± 994 | 2,204 ± 946 | 2,440 ± 945 | 3,190 ± 1,490 | 5,267 ± 3,190 | 7,355 ± 4,718 | 4,727 ± 167 |
| CL (L/h/m2) | 30.0 | 24.6 ± 7.63 | 14.9 ± 1.95 | 10.4 ± 1.27 | 11.3 ± 2.84 | 12.4 ± 2.91 | 15.9 ± 6.34 | 6.28 ± 3.56 | 7.69 ± 2.89 | 9.28 ± 3.56 | 10.1 ± 5.00 | 8.98 ± 4.59 | 8.52 ± 5.43 | 10.1 ± 0.27 |
| Vλ (L/m2) | 541 | 553 ± 146 | 385 ± 109 | 279 ± 60.1 | 269 ± 104 | 348 ± 79.9 | 327 ± 190 | 267 ± 197 | 263 ± 113 | 288 ± 165 | 434 ± 339 | 329 ± 134 | 277 ± 165 | 349 ± 205 |
| t1/2 (h) | 12.5 | 16.0 ± 3.96 | 18.2 ± 6.37 | 18.7 ± 3.64 | 16.0 ± 2.53 | 19.9 ± 4.68 | 13.7 ± 4.57 | 28.5 ± 6.77 | 23.5 ± 1.77 | 20.2 ± 8.15 | 27.8 ± 9.08 | 27.9 ± 10.6 | 24.1 ± 9.37 | 24.1 ± 14.7 |
| KOS-1891 | ||||||||||||||
| tmax (h) | ND | ND | ND | ND | ND | 3.58 ± 0.80 | 3.17 ± 0.31 | 3.25 ± 0.51 | 3.30 ± 0.47 | 3.05 ± 0.08 | 3.65 ± 0.4 | 3.38 ± 0.48 | 3.34 ± 0.27 | 3.55 ± 0.64 |
| Cmax (ng/mL) | ND | ND | ND | ND | ND | 1.93 ± 0.73 | 2.27 ± 1.03 | 5.85 ± 1.72 | 8.05 ± 2.08 | 11.8 ± 9.15 | 14.3 ± 3.64 | 35.6 ± 24.0 | 30.1 ± 15.4 | 46.2 ± 1.84 |
| AUC (ng h/mL) | ND | ND | ND | ND | ND | 55.0 ± 27.1 | 39.5 ± 16.3 | 204 ± 88.7 | 226 ± 165 | 233 ± 156 | 319 ± 106 | 562 ± 446 | 527 ± 461 | 484 ± 17.0 |
| t1/2 (h) | ND | ND | ND | ND | ND | 27.5 ± 11.0 | 19.2 ± 2.69 | 44.0 ± 15.9 | 25.7 ± 4.09 | 27.0 ± 9.93 | 34.6 ± 17.0 | 31.0 ± 16.0 | 24.1 ± 16.2 | 31.3 ± 9.76 |
| KOS-1891/KOS-1584 AUC Ratio (%) | ND | ND | ND | ND | ND | 9.27 ± 2.52 | 6.62 ± 1.69 | 11.2 ± 8.71 | 9.75 ± 2.55 | 9.65 ± 3.97 | 10.7 ± 2.87 | 9.76 ± 6.76 | 7.39 ± 2.01 | 9.16 ± 0.84 |
Urinary excretion of unchanged KOS-1584 represented <10% of drug-related materials. In addition to KOS-1891, three glucuronide metabolites and seven oxidative metabolites were excreted in urine, suggesting metabolism of KOS-1584 by multiple pathways.
Pharmacodynamics
The degree of microtubulin bundle formation (MBF) was assessed as a surrogate marker of binding of KOS-1584 to tubulin in PBMCs. For all cohorts, the percentage of PBMCs with microtubule bundles increased to a maximum at the end of drug infusion and then decreased by 24 h after drug infusion (Fig. 2a). A dose-dependent increase in MBF was observed with a plateau of maximal effect between 50 and 60% at the 3–4 highest dose levels (27–48 mg/m2). The pattern of MBF was similar across cycles 1 and 2.
Fig. 2.
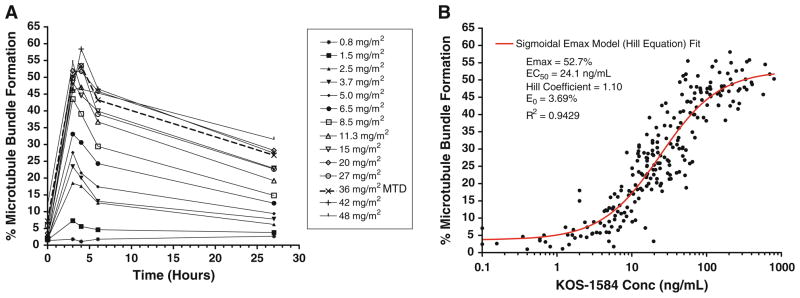
Degree of microtubule bundle formation. a Dose-dependent effects of KOS-1584 on median microtubule bundle formation in PBMCs following a 3-h infusion of KOS-1584 (0.8–48 mg/m2) on day 1. b Percent microtubulin bundle formation as a function of KOS-1584 plasma concentration. Closed circles represent observed values; the solid line represents the model fit
Figure 2b describes the relationship between the percentage of PBMCs with MBF and KOS-1584 concentration in plasma. A sigmoidal maximum effect pharmacodynamic model (R2 = 0.943) fits this relationship with an EC50 = 24.1 ng/mL, Emax = 52.7%, slope = 1.10, and E0 = 3.69%.
Anti-tumor activity
Two patients experienced objective partial responses (PR). The first patient, treated at the 6.5 mg/m2 dose level, had advanced ovarian cancer and was heavily pretreated with 6 prior regimens including paclitaxel and docetaxel. She had PR (−31%) of target retroperitoneal lymph nodes; however, a confirmatory scan was not performed. The second patient, treated at the 36 mg/m2 dose level, had metastatic pancreatic cancer that was previously treated with four chemotherapy regimens including paclitaxel. He had confirmed PR, determined by CR of his target retroperitoneal lymphadenopathy, and SD of non-target, subcentimeter pulmonary nodules and hilar lymphadenopathy. Stable disease (SD) was observed in 24 patients, 13 of whom had durable SD of ≥3 months. Among these 24 patients with SD, the median number of prior regimens was 4.5 and the best response to their most recent previous therapy included progressive disease (PD) in 15, SD in 5, PR in 1, and not evaluable/data not available in 3 patients.
Discussion
Epothilones are microtubule-active agents with potential to overcome the issues of MDR, myelosuppression, neurotoxicity, and hypersensitivity reactions associated with the currently available agents. Ixabepilone, currently the only FDA-approved epothilone, has DLTs of neutropenia, neuropathy, and abdominal pain/nausea [9, 10, 27–29]. It is formulated with a cremophor base; therefore, similar to taxanes, hypersensitivity has been observed and premedication is required. Other epothilones currently under investigation include patupilone, which has DLTs of diarrhea, peripheral neuropathy, and fatigue [11, 12, 30, 31], and sagopilone, with DLTs of peripheral neuropathy, infection, hyponatremia, diarrhea, and central ataxia [11, 13, 14]. KOS-1584, specifically designed to have more favorable pharmacokinetics and less toxicity compared with first-generation epothilone D derivatives, has demonstrated activity in paclitaxel-resistant xenografts and in cell lines with MDR due to overexpression of P-gp.
In this first-in-man, phase I dose escalation study, we evaluated the safety, tolerability, and biologic activity of KOS-1584. The MTD and RP2D are 36 mg/m2 each. We treated patients on 14 cohorts and saw a 45-fold difference between the starting dose and RP2D, illustrating the shortcomings of dose translation from animal to human studies as previously documented by others [32–34]. Observed DLTs included diarrhea, arthralgias, and encephalopathy. In contrast to a paralleled conducted study of KOS-1584, given on two weekly dosing schedules where diarrhea was increasingly more severe after successive infusions despite maximal supportive care [35], diarrhea was managed effectively with loperamide or diphenoxylate/atropine in our trial. Arthralgias also were effectively treated and subsequently prevented, with anti-inflammatory agents or corticosteroids.
It is unclear at this point whether the DLT of encephalopathy was related to drug penetration into the blood–brain barrier (BBB). Evaluation of penetration of KOS-1584 across the intact BBB has not been evaluated in humans. However, in tissue distribution studies performed in mice, KOS-1584 did penetrate the BBB [18]. Continued vigilance for central nervous system toxicity will be required in future studies of this drug.
Peripheral neuropathy was common, although grade ≥3 peripheral neuropathy occurred only at the two highest dose levels (above the MTD). This observation was very encouraging given that most of our patients were previously treated with taxanes and/or platinums, and many had grade 1 neuropathy at study entry. Generally, new or worsening neuropathy occurred during the first two cycles, persisted at the same severity, and resolved upon drug discontinuation. At the RP2D, the frequency of all-grade and grade ≥3 peripheral neuropathy was 69 and 0%, respectively. Peripheral neuropathy is also a common adverse effect of ixabepilone [36] and was a DLT for sagopilone [13] and patupilone [12, 31]. KOS-1584 was not associated with any hypersensitivity reactions and had a very low incidence of myelosuppression, even among this group of heavily pretreated patients. KOS-1584 exhibited linear pharmacokinetics, with no accumulation of KOS-1584 or its secometabolite. As KOS-1584 is a potent inhibitor of CYP3A4/5 in vitro, potential interactions with CYP3A4/5 substrates should be evaluated in future trials [18]. There were no discernable differences in the pharmacokinetics of KOS-1584 in patients who experienced grade ≥3 drug-related toxicities compared to those who did not.
Similar to ixabepilone, a dose-dependent increase in PBMC MBF was observed. At the RP2D, the mean percentages of MBF were 50 and 26% at 1 h and 24 h after infusion, respectively. Ixabepilone at the RP2D of 40 mg/m2 (using this same assay) demonstrated mean MBF of 63% and 16–23% (n = 27) at 1 h and 24 h after infusion, respectively [10, 25]. An association between the maximal percentage of PBMC MBF and severity of neutropenia has been previously reported with ixabepilone [10, 37]. In contrast, we did not observe any correlation between the maximal MBF of KOS-1584 and any grade neutropenia or grade ≥3 toxicity.
The disease control rate (CR + PR + SD) was 39%. MBF was not an accurate predictor of clinical benefit among evaluable patients; however, the effects of KOS-1584 on this target were not measured in pre- and post-exposure tumor samples.
The RP2D of KOS-1584 is 36 mg/m2 administered intravenously every 3 weeks. The lack of prohibitive severe neurologic toxicity, neutropenia, or hypersensitivity reactions; favorable pharmacokinetic profile; and early evidence of activity support further evaluation.
Acknowledgments
The authors thank the patients who participated in this trial; the nurses and nurse practitioners who cared for these patients at The Ohio State University and Montefiore-Einstein Cancer Center; and the staff of the OSU Clinical Trials Processing Laboratory who processed the correlative specimens. This work was supported in part by the P30 CA016058-34 grant (PI: Michael Caligiuri, 12/01/2004—11/30/2010) and by funding from Kosan Biosciences, Inc. MV and SM received research funding from Kosan Biosciences, Inc.
Footnotes
Preliminary results of this study were presented in part at the 42nd annual American Society of Clinical Oncology meeting in an oral presentation and general poster session, Atlanta, Georgia, June 2–6, 2006 and at the 18th EORTC-NCI-AACR Symposium on “Molecular Targets and Cancer Therapeutics” as a poster, Prague, Czech Republic, November 7–10, 2006.
Conflict of interest Employment or leadership position: RJ was CEO of Kosan Biosciences, Inc. Consultant or advisory role: GC, AH, and YZ were previously employed by or were paid consultants for Kosan Biosciences, Inc. Stock ownership: RJ previously held stock in Kosan Biosciences, Inc. Honoraria: None. Other remuneration: Kosan Biosciences, Inc. has been acquired by Bristol Myers Squibb.
Contributor Information
Elaine T. Lam, The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA
Sanjay Goel, Montefiore-Einstein Center for Cancer Care, Bronx, NY, USA.
Larry J. Schaaf, The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA
Gillian F. Cropp, Kosan Biosciences, Inc., Hayward, CA, USA
Alison L. Hannah, Kosan Biosciences, Inc., Hayward, CA, USA
Yiqing Zhou, Kosan Biosciences, Inc., Hayward, CA, USA.
Barbara McCracken, The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA.
Brandi I. Haley, The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA
Robert G. Johnson, Kosan Biosciences, Inc., Hayward, CA, USA
Sridhar Mani, Montefiore-Einstein Center for Cancer Care, Bronx, NY, USA.
Miguel A. Villalona-Calero, Email: Miguel.Villalona@osumc.edu, The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA. Dorothy M. Davis Chair in Cancer Research, Internal Medicine and Pharmacology, Division of Medical Oncology, The Ohio State University, B406 Starling-Loving Hall, 320 West 10th Avenue, Columbus, OH 43210-1240, USA
References
- 1.Morris PG, Fornier MN. Microtubule active agents: beyond the taxane frontier. Clin Cancer Res. 2008;14:7167–7172. doi: 10.1158/1078-0432.CCR-08-0169. [DOI] [PubMed] [Google Scholar]
- 2.Perez EA. Microtubule inhibitors: differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol Cancer Ther. 2009;8:2086–2095. doi: 10.1158/1535-7163.MCT-09-0366. [DOI] [PubMed] [Google Scholar]
- 3.Villalona-Calero MA, Schaaf L, Turowski R. Antimicrobule agents. In: Dai W, editor. Cancer drug discovery and development: checkpoint responses in cancer therapy. Humana Press; Tuxedo: 2008. pp. 177–206. [Google Scholar]
- 4.Fojo T, Menefee M. Mechanisms of multidrug resistance: the potential role of microtubule-stabilizing agents. Ann Oncol. 2007;18:v3–v8. doi: 10.1093/annonc/mdm172. [DOI] [PubMed] [Google Scholar]
- 5.Rowinsky EK, Cazenave LA, Donehower RC. Taxol: a novel investigational antimicrotubule agent. J Natl Cancer Inst. 1990;82:1247–1259. doi: 10.1093/jnci/82.15.1247. [DOI] [PubMed] [Google Scholar]
- 6.Rowinsky EK. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med. 1997;48:353–374. doi: 10.1146/annurev.med.48.1.353. [DOI] [PubMed] [Google Scholar]
- 7.Cheng KL, Bradley T, Budman DR. Novel microtubule-targeting agents—the epothilones. Biologics. 2008;2:789–811. doi: 10.2147/btt.s3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodin S, Kane MP, Rubin EH. Epothilones: mechanism of action and biologic activity. J Clin Oncol. 2004;22:2015–2025. doi: 10.1200/JCO.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Puhalla S, Brufsky A. Ixabepilone: a new chemotherapeutic option for refractory metastatic breast cancer. Biologics. 2008;2:505–515. doi: 10.2147/btt.s3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mani S, McDaid HM, Grossman A, et al. Peripheral blood mononuclear and tumor cell pharmacodynamics of the novel epothilone B analogue, ixabepilone. Ann Oncol. 2007;18:190–195. doi: 10.1093/annonc/mdl315. [DOI] [PubMed] [Google Scholar]
- 11.Fumoleau P, Coudert B, Isambert N, Ferrant E. Novel tubulin-targeting agents: anticancer activity and pharmacologic profile of epothilones and related analogues. Ann Oncol. 2007;18(Suppl 5):v9–15. doi: 10.1093/annonc/mdm173. [DOI] [PubMed] [Google Scholar]
- 12.Rubin EH, Rothermel J, Tesfaye F, et al. Phase I dose-finding study of weekly single-agent patupilone in patients with advanced solid tumors. J Clin Oncol. 2005;23:9120–9129. doi: 10.1200/JCO.2005.03.0981. [DOI] [PubMed] [Google Scholar]
- 13.Schmid P, Kiewe P, Possinger K, et al. Phase I study of the novel, fully synthetic epothilone sagopilone (ZK-EPO) in patients with solid tumors. Ann Oncol. 2010;21:633–639. doi: 10.1093/annonc/mdp491. [DOI] [PubMed] [Google Scholar]
- 14.Arnold D, Voigt W, Kiewe P, et al. Weekly administration of sagopilone (ZK-EPO), a fully synthetic epothilone, in patients with refractory solid tumours: results of a phase I trial. Br J Cancer. 2009;101:1241–1247. doi: 10.1038/sj.bjc.6605327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou TC, O’Connor OA, Tong WP, et al. The synthesis, discovery, and development of a highly promising class of microtubule stabilization agents: curative effects of desoxyepothilones B and F against human tumor xenografts in nude mice. Proc Natl Acad Sci USA. 2001;98:8113–8118. doi: 10.1073/pnas.131153098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beer TM, Higano CS, Saleh M, et al. Phase II study of KOS-862 in patients with metastatic androgen independent prostate cancer previously treated with docetaxel. Invest New Drugs. 2007;25:565–570. doi: 10.1007/s10637-007-9068-1. [DOI] [PubMed] [Google Scholar]
- 17.Overmoyer B, Waintraub S, Kaufman P. Phase II trial of KOS-862 (epothilone D) in anthracycline and taxane pretreated metastatic breast cancer. J Clin Oncol 2005 ASCOAnnu Meet Proc. 2005;23:778. [Google Scholar]
- 18.Kosan Biosciences I. KOS-1584 investigator’s brochure. 2004. [Google Scholar]
- 19.Chou TC, Zhang X, Zhong ZY, et al. Therapeutic effect against human xenograft tumors in nude mice by the third generation microtubule stabilizing epothilones. Proc Natl Acad Sci USA. 2008;105:13157–13162. doi: 10.1073/pnas.0804773105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou y, Zhong Z, Liu F, et al. KOS-1584: a rationally designed epothilone D analog with improved potency and pharmacokinetic (PK) properties. Proc Amer Assoc Cancer Res. 2005;46:Abstract #2535. [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, O’Dwyer PJ, Christian M, Humphrey JS. Phase I clinical trial design in cancer drug development. J Clin Oncol. 2000;18:684–692. doi: 10.1200/JCO.2000.18.3.684. [DOI] [PubMed] [Google Scholar]
- 23.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, Version 3.0. DCTD, NCI, NIH, DHHS; 2006. [March 31, 2003]. [Accessed 9 Aug 2006]. http://ctep.cancer.gov. [Google Scholar]
- 24.Bazette H. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- 25.McDaid HM, Mani S, Shen HJ, et al. Validation of the pharmacodynamics of BMS-247550, an analogue of epothilone B, during a phase I clinical study. Clin Cancer Res. 2002;8:2035–2043. [PubMed] [Google Scholar]
- 26.Gibaldi M, Perrier D. Pharmacokinetics. 2. Marcel Dekker; New York: 1982. [Google Scholar]
- 27.Mani S, McDaid H, Hamilton A, et al. Phase I clinical and pharmacokinetic study of BMS-247550, a novel derivative of epothilone B, in solid tumors. Clin Cancer Res. 2004;10:1289–1298. doi: 10.1158/1078-0432.ccr-0919-03. [DOI] [PubMed] [Google Scholar]
- 28.Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25:5210–5217. doi: 10.1200/JCO.2007.12.6557. [DOI] [PubMed] [Google Scholar]
- 29.Sparano JA, Vrdoljak E, Rixe O, et al. Randomized phase III Trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2010;28:3256–3263. doi: 10.1200/JCO.2009.24.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. [Accessed 31 May 2010]; http://www.clinicaltrials.gov. In Edition.
- 31.Ten Bokkel Huinink WW, Sufliarsky J, Smit WM, et al. Safety and efficacy of patupilone in patients with advanced ovarian, primary fallopian, or primary peritoneal cancer: a phase I, open-label, dose-escalation study. J Clin Oncol. 2009;27:3097–3103. doi: 10.1200/JCO.2008.20.4826. [DOI] [PubMed] [Google Scholar]
- 32.Dixon RL. Problems in extrapolating toxicity data for laboratory animals to man. Environ Health Perspect. 1976;13:43–50. doi: 10.1289/ehp.761343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahmood I, Green MD, Fisher JE. Selection of the first-time dose in humans: comparison of different approaches based on interspecies scaling of clearance. J Clin Pharmacol. 2003;43:692–697. [PubMed] [Google Scholar]
- 34.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 35.Stopeck A, Moulder S, Jones S, et al. Phase I trial of KOS-1584 (a novel epothilone) using two weekly dosing schedules. J Clin Oncol. 2007;24 (Abstract 2571). ASCO Annual Meeting Proceedings Part I. [Google Scholar]
- 36.Ixabepilone (Ixempra) Package Insert. Bristol-Myers Squibb; 2010. [Google Scholar]
- 37.Goel S, Cohen M, Comezoglu SN, et al. The effect of ketoconazole on the pharmacokinetics and pharmacodynamics of ixabepilone: a first in class epothilone B analogue in late-phase clinical development. Clin Cancer Res. 2008;14:2701–2709. doi: 10.1158/1078-0432.CCR-07-4151. [DOI] [PubMed] [Google Scholar]