Abstract
Objective
To describe the role of health economics (HE) in wound care in relation to coverage and reimbursement.
Approach
Narrative description of key concepts with supporting references.
Results
The process of approval or clearance of wound care products within the U.S. regulatory framework often causes lack of high level of evidence regarding clinical outcomes. There is also a paucity of HE information and great reluctance to use such information (when it is available) by insurers and Centers for Medicare and Medicaid, as well as other health-care agencies. Cost-effectiveness (CE) studies are the most common type of HE study in wound care, and the most common outcomes are incremental CE ratios (ICERs). Interpretation of ICERs requires considerable judgment when results are not obvious and is hampered by lack of contemporary and useful benchmarks. While many lessons have been learned in applying CE to coverage and reimbursement decisions in other western countries—including transparency of decision-making and involvement of patients—there is still a major aversion to using CE in the United States Applying CE to basic wound care and advanced therapeutics has the potential to decrease the costs of wound healing considerably.
Innovation and Conclusions
Many CE approaches, including modeling, provide sufficiently detailed information that decision-makers can make informed decisions about wound care products in regard to coverage and reimbursement. The reluctance to use CE information in the United States, however, is likely to contribute heavily to the ever-increasing costs in wound care.

Marissa Janine Carter, PhD, MA
Introduction
In wound care, as in other medical specialties, bringing a product or process to the market and being appropriately reimbursed for it is an expensive and difficult exercise for a manufacturer. Moreover, there is a not a single path; rather, there are several possible avenues, each with its own pros and cons.
In the United States, the first step is U.S. Food and Drug Administration (FDA) approval or clearance. Recent years have seen large changes occur at the agency as a result of a decision to classify a product based on its primary mode of action, and thus, many wound care products launched decades ago are now being reclassified as biologicals. For example, human cells, tissues, and cellular- and tissue-based products (HCT/Ps) that are more than minimally manipulated now require a biologics license application (BLA). The easiest process is the pathway of the medical device, which is classified into three levels based on the level of risk posed to the patient. Class 1 devices are exempt from premarket notification (PMN) 510 (k), whereas class 2 devices—substantial equivalence to a predicate device—require PMN 510 (k). A majority of class 3 devices demand the more onerous premarket approval (PMA), because they are new, although the real reason is that such devices may have unknown or higher risks to patients.1 The difference between PMN (clearance) and PMA (approval) is quite startling: PMN may involve few or no clinical trials, whereas the PMA typically involves clinical trials of designs that demonstrate safety and efficacy similar to drug approval processes.1 These rather dichotomous processes were the subject of a recent investigation by the Institute of Medicine (IOM), which was commissioned by the FDA to examine whether the current PMN was jeopardizing patient safety. The conclusions suggest that while public health is not at risk based on the existing PMN, “… it believes that the FDA's resources would be put to better use in obtaining information needed to develop a new regulatory framework for Class 2 medical devices and addressing problems with other components of the medical-device regulatory framework.”2 Despite this ongoing discussion, it should be noted that in wound care, published clinical trials are fairly common for class 2 devices, even though not required by the FDA.
The next hurdle is coverage of the wound care product. The FDA's mission is dissimilar to that of the Centers for Medicare & Medicaid Services (CMS) and private health insurance organizations with CMS’ coverage based on whether a medical product is reasonable and necessary to diagnose or treat illness or injury. If the product is biological or has followed the PMA route, considerable high level of evidence may be available for the efficacy of the product; this may not be true for a product following the PMN route, although some postmarketing studies may have partially remedied this deficiency. Convincing clinicians and health-care providers to use the product in their facilities, however, often depends on answering the questions whether the product will help patients in real-world practice and whether they will get reasonably paid for administering it. The problem is that most randomized controlled trials (RCTs) are not often generalizable to real-world practice3 and reimbursement figures for products do not tell the story of whether a product will save money in the long run given its effectiveness. A further disconnect in the United States is that the Agency for Healthcare Research and Quality (AHRQ) has put considerable effort into health economics (HE) research—what the cost of a product is in relation to its benefits—but the CMS has traditionally shunned such data when making coverage decisions.4 However, the current climate may soon change.
Clinical Problem Addressed
Health-care costs continue to spiral upward, and with the implementation of cuts across the board in Medicare (the sequestration), as well as the final provisions of the Patient Protection and Affordable Care Act (PPACA) in 2013,5–8 it is important that clinicians understand the cost-effectiveness (CE) of the care that they are giving to wound care patients so that treatments, which are expensive but do little to improve outcomes, are minimized.
This article is a perspective and educational piece, not a review or systematic review of the subject of HE.
Materials and Methods
For each section, the literature was searched for useful references to studies, legislation, or resources that might be helpful. Google was searched in April 2013 for references to legislation and other resources, and PubMed was searched from 1970 to April 2013, retrieving only references in English, using the following keywords: premarket notification 510 (k); premarket approval FDA; Agency for Healthcare Research and Quality and Health Economics Research; Affordable Health Care Act; cost-effectiveness; willingness to pay; incremental cost-effectiveness ratio; Markov modeling, discrete modeling, bootstrapping, direct costs, indirect costs, and societal costs in combination with cost-effectiveness; cost-effectiveness benchmarks; wound care reimbursement.
Results
Healthcare economics research
What is HE research? HE research has two major objectives: (a) to improve public health through rational decision-making and (b) to determine the relative values of alternative therapies.9 Consider the following three scenarios, which are all examples of HE research:
1. Do some wound care populations benefit more from negative pressure wound therapy given the same level of cost?
2. How much more does it cost to provide hyperbaric oxygen therapy (HBOT) to patients with Wagner 3 diabetic foot ulcers (DFUs) if treatment is started immediately versus waiting for a month or more?
3. What is the quality-of-life improvement over 1 year for patients with a venous leg ulcer if they are given an advanced therapeutic in addition to standard of care?
In each of these scenarios, specific research questions are posed that have to be translated into elements for which data can be collected and analyzed. Thus, for scenario (2), cost data would be needed on HBOT for a large number of patients, in which the time for start of HBOT relative to the first visit at a wound care facility is known. However, answering the question would also depend on the method of analysis. To begin with, a choice would need to be made whether means or medians should be used if cost is a non-normal (Gaussian) variable, meaning that the cost data do not exhibit a nice bell-shaped curve. Also, adjustments might need to be made to the simple analysis to account for other parameters, such as stratifying patients into high and low risks for lower extremity amputation (LEA), or the types of comorbidity the patients have, which might affect the healing of the DFU. Additionally, modelling the data may be a useful technique to examine how costs are affected in regard to start of HBOT with respect to time, patient characteristics, wound type, and events that occur in the course of care (such as healing and LEAs).
To facilitate HE research, several formal types of approaches are utilized: CE, cost consequence, cost utility, cost benefit, cost minimization, budget impact analysis or model, and summary of health economic analyses.9,10 However, CE will be focused upon as it is the most common type of HE research in wound care.
CE studies generally answer the question of how benefits can be maximized with finite resources.10 In wound care, the most commonly used benefit units are the quality-adjusted life-year (QALY), ulcer-free time, or amputation averted (limb preservation),11 but the choice of the unit depends considerably on the intervention/diagnostic being studied, the time horizon of the study (duration over which the benefit is being calculated), and the nature of the benefit conferred on the patient. The cost portion, specified in a particular currency, is calculated for a particular calendar year, and most commonly includes direct costs (typically those paid by a medical insurance company or Medicare/Medicaid in the United States), but may also include indirect costs, such as loss of patient's economic productivity or out-of-pocket costs or societal costs, which are less tangible hard-to-capture costs, but represent costs of care to society as a whole.12,13
There are also two very different approaches to calculating CE. The first captures actual costs prospectively or retrospectively in a given setting (such as a nursing home) and patient population (e.g., patients with stage III/IV pressure ulcers), as well as outcomes that will be utilized to determine benefits. The second uses modeling with input data taken from a variety of sources, either cyclically, such as the Markov model (week by week or month by month), discrete event simulation models, which track hypothetical patients through various health states and events over time, or bootstrapping, which is a mathematical way of calculating an incremental CE ratio (ICER) by resampling cost and effect pairs from original clinical trial data thousands of times.14–16 The ICER is the most common final output of CE calculations and represents the difference in costs and benefits, thus, (C1−C2)/(B1−B2). For example, if the respective direct costs of wound care products 1 and 2 were $1,500 and $900, respectively, and the benefits were 15 and 12 ulcer-free weeks, respectively, over a time horizon of 1 year, then the ICER would be $200 per ulcer-free week, meaning that it would cost an extra $200 to obtain 1 ulcer-free week using product 1 instead of product 2.
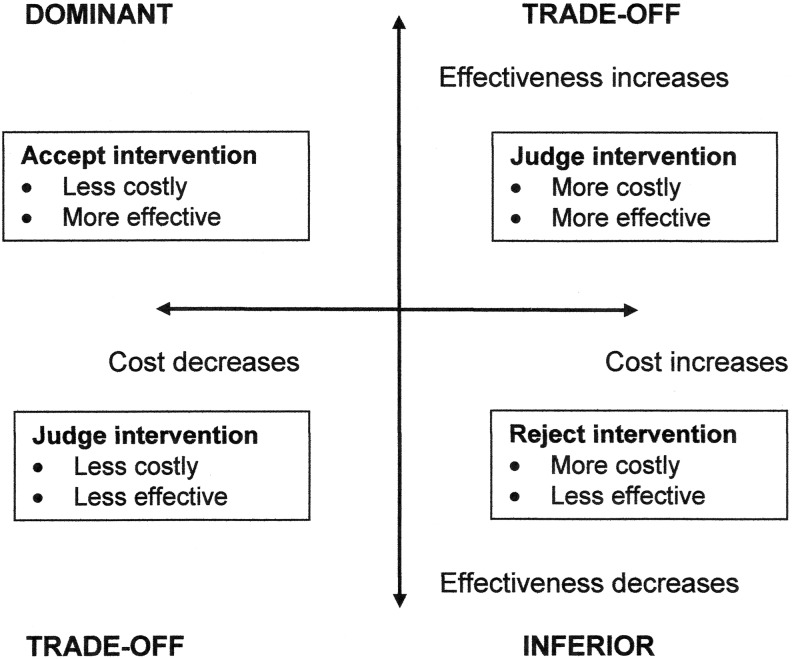
Unfortunately, interpretation of the ICER is not always easy (Fig. 1). In this quadrant model, there are two definitive situations: dominant, in which the intervention or diagnostic is less costly and more effective, and inferior, where the converse is true. It is easy to interpret these findings, but not so easy in the other two quadrants, in which judgment is required. To help in these situations, analysts often conduct bootstrapping and develop willingness to pay curves, which are graphic representations of incremental cost versus the probability that the intervention or diagnostic will be effective with 95% confidence intervals developed.17 However, decision-makers will always need to decide what is acceptable in their situation.
Figure 1.
The quadrant cost-effectiveness model and its interpretation.
Advantages and disadvantages of CE approaches in wound care
While ICERs can inform decision-makers about the costs and effectiveness or benefits when two or more products or processes are compared, dominance and inferiority excepted, there are no “gold standards” by which a situation can be judged. Well over 20 years ago, the state of Oregon in the United States developed a prioritized list of health-care services, ranging from the most important to the least important.18 Although costs were not involved, it ignited a major conflict between CE analysis and the will to rescue endangered life18 and a major discussion on the value of social worth when developing a comprehensive plan for allocating limited health resources.19 It was during this same decade (the 1990s) that development of benchmarks in cost–utility analysis occurred, in which $50,000 per QALY in the United States was seen as a threshold below which actions or interventions were judged as cost-effective, but above which were seen to be non–cost-effective. This arcane number was in fact based on a poorly conceived projection of end-stage renal patients by Medicare in 198020 that turned out to be wrong.21 During the same time period, others weighed in on the subject with benchmarks of $20,000, $50,000, or $100,000 per QALY,22–24 and the National Institute for Health and Clinical Excellence in the United Kingdom (NICE; equivalent to AHRQ in the United States) adopted a threshold of £30,000 per QALY gained for whether the National Health Service would pay for a given treatment.25 While cost–utility benchmarking may be more palatable in other Western countries, in the United States, cost–utility (and technically cost–benefit) research itself has been banned under the PPACA legislation for the Patient-Centered Outcomes Research Institute, the agency responsible for conducting comparative effectiveness research.26 Benchmarking may not be helpful for any number of reasons, but banning CE research—the one scientific methodology that provides specific cost and effectiveness metrics on medical devices, drugs, processes, and diagnostics—is equivalent to flying without any reference to instruments.
Is there an advantage to using cost–utility versus cost–benefit research in wound care? While we have some knowledge of utility values for various conditions commonly encountered in wound care, such as the healing of a DFU, such values can be inaccurate for patients with multiple comorbidities and certain age groups.10 Moreover, measurements of utility values for given settings and populations may be needed. Cost–benefit analyses ignore these kind of problems, but benefit units are often awkward and will apply only to certain problems; for example, ulcer-free weeks to healing of an ulcer or amputations averted. In other words, there is no universal benefit unit.
The decision on what kind of approach to take in CE research depends, in part, on the goal of the research. Using actual costs incurred for patients, whether those costs are collected prospectively or retrospectively, does decrease the error of uncertainty regarding costs, but those data may be limited in generalizability depending on the setting used. Similarly, effectiveness in such studies employs outcomes obtained from the same set of patients, which may also limit generalizability. Conversely, modeling can be far more versatile when exploring factors that affect CE particularly when the cost and effectiveness input data are less definitive or fuzzy. However, modeling in general requires far more specialist knowledge and can be time-consuming, particularly for discrete event simulation. In wound care, modeling is limited by the dearth of well-conducted observational studies with time horizons up to 1 year or more that have detailed outcomes, and the fact that most RCTs have relatively short time horizons or 12–16 weeks. Moreover, assembling the model requires the following careful thoughts:
• Should only wound-related costs be included?
• Should patient deductibles be included?
• Should infection be treated as a health state or health states, or an event with costs associated with it?
• Should an LEA be considered a terminal, but healed state?
• Should ulcer recurrence be included, and if so, how should it be modeled?
Again, due to lack of gold standards, it should be incumbent on researchers to clearly define the goals of their CE research and their rationale for including or not including certain elements.
Why should HE research be an important factor in reimbursement decision-making?
In wound care, “doing the right thing”—carrying out good basic wound care—is challenging and comes down to three issues: complexity, cognitive effort, and compensation.27 Complexity is the difficulty in carrying out a task, such as adequate compression bandaging of a venous leg ulcer, whereas cognitive effort involves remembering and correctly applying knowledge related to the evaluation and treatment of wounds. Compensation is the amount of money the physician and facility receives for performing these tasks.
The more complex a task is, in basic wound care, the less likely it will be carried out, properly or even not at all: applying a dressing in a minute is easy, but performing total contact casting (TCC) on a DFU can be challenging for a nonexpert, although instant TCC may ameliorate the problem to some extent.28 Likewise, clinical practice guidelines—the cornerstone of wound care evaluation—still have a way to go so that clinicians can apply them with immediacy to their work.29 Finally, inadequate reimbursement has consequences, as noted by Bolton et al.: “Sufficient evidence supports improved venous ulcer care in the U.S. but inadequate and/or inconsistent reimbursement policies impede quality evidence-based venous ulcer practice, delaying healing and increasing the burden of venous ulcers on society.”30 These authors are not talking about advanced therapeutics, but basic wound care.
If clinicians and facilities have not received adequate returns on investment for conducting basic wound care, how have they survived financially? One theory is that for hospital-based outpatient wound care departments, there has been the development of an “… uncontrolled system in which ever-increasing numbers of procedures can occur without regard to patient-centered outcomes.”31 Those procedures include debridement and surgical procedures, HBOT, and application of cellular- and/or tissue-based products for wounds. The conclusion is not that these procedures are without merit, but rather that wound care clinics must utilize them to stay afloat financially and compensate for the lack of income regarding basic wound care. In other words, clinicians are incentivized to use more remunerative procedures regardless of their benefit to the patient.31
Since the 1990s, an increasing number of advanced therapeutics has been introduced into the wound care marketplace, aimed at chronic wounds—those wounds that have stalled in regard to normal healing. However, for those products for which RCTs have been conducted, either as a part of the FDA approval process, as a postmarketing clinical trial, or by independent researchers, the vast majority have excluded patients with serious comorbidities and severe wounds.3 Indeed, a systematic review recently conducted by Greer et al. on behalf of the Department of Veterans Affairs noted that there was “… insufficient evidence to guide clinicians and policy makers regarding whether efficacy differs according to patient demographics, comorbid conditions, treatment compliance, or activity level.”32 This a key point. What is known with even less certainty is how such advanced therapeutics fare in real-world wound care populations due to a lack of well-conducted cohort clinical trials or level II evidence studies.
We know that as patients have increasing numbers of comorbidities, their chronic wounds cost much more to heal and costs accrue in an almost linear-like fashion for longstanding chronic wounds that do not heal.33 If good basic wound care does not help these kinds of wounds, alternate therapies, even expensive treatments, may lower costs in the long run, provided they are reasonably effective. However, when clinicians use wound care products that are costly and add little or no benefit, the end result is higher cost to treat a wound, whether it heals or not. Evidence-based medicine can inform whether a product is efficacious or effective in practice, assuming data are available, but it says nothing about the cost of using the product.34 Without well-conducted HE studies on these products, we do not know the financial impact of using them.
Discussion
Why are we reluctant to use HE data in wound care in the United States? Is it because there is a lack of good data to use as inputs to such studies, or are we afraid to utilize HE data to make decisions? The answer is probably yes to both. For example, several august bodies have considered the evidence for efficacy of advanced therapeutics used in the treatment of DFUs and their conclusions are similar: that with the exception of HBOT and perhaps negative pressure wound therapy, there is little published evidence to justify the use of more recent therapies.35,36 Organizations have also published position articles to explain why wound care research is difficult and what can be done for the future to improve the nature of that research so that the evidence level is higher for studies.37,38 These works certainly suggest that there is a paucity of good data from which to draw to create viable models, which hampers the creation of HE studies to inform decision-makers.
In the United States, the public has discovered that the country does not have infinite resources and money to save lives, and provides all possible means to treat disease, and although the Oregon scenario is one that the public is desperate to avoid, the fact is that that health care is already being rationed via the existence of health maintenance organizations, the high cost of health-insurance premiums, and rationing by individual physicians at the point of care.39 The latter point is a scenario that should be avoided as physicians are usually ill-equipped to handle such decisions.40 Lessons that have been learned in the past several years include transparency and accountability as far as decision-making in health-care provision is concerned (the case of Viagra coverage in Australia, which was seen as closed-doors deal-making at its worst), and the success of the United Kingdom's NICE program, in which rationales for decisions are publicly posted, as well as the participation of laypeople on boards.39,41
The United States already has an agency (AHRQ) that has considerable expertise in HE research, and with ongoing discussions regarding particular issues of wound care research with AHRQ and stakeholders, it only remains for the United States to get into lockstep with other nations by nominating this agency as its public research vehicle of CE analysis that can be used to help make decision-making in health care. For wound care stakeholders, in particular, users and manufacturers of particular devices and drugs, the lessons of the past should be clear: provide high evidence level clinical study data, as well as high-level HE studies to demonstrate that products provide obvious benefits with costs plainly stated. Table 1 shows a checklist of criteria that such studies should follow. Equally, for all agencies and insurers that provide reimbursement and coverage determinations, not including relevant HE studies as part of the decision-making process will only lead to many products being covered that have no value and add cost alongside products that do add value, without any distinction between either.
Table 1.
Criteria that should be met in clinical studies and health economics studies that can be used to inform health-care decision-making
| Element | Clinical Studies | HE Studies |
|---|---|---|
| Design | RCT or well-conducted comparative cohort | Prefer CE or CB, whether modeling employed or not; if other type, rationale clearly stated |
| Outcomes | Outcomes match the goals of the study, but are also rational and relevant | Outcomes match study goals; appropriate selection of benefit units, if used |
| Costs | Not applicable unless an HE study is piggybacked on to a clinical study | Clearly state which costs are included, with rationale; describe source costs and assumptions about costs |
| Analysis | Methods to obtain unadjusted and adjusted results clearly stated | Methods clearly stated; sensitivity analysis conducted for modeling studies |
| Reporting | Follows CONSORT, STARD, or STROBE guidelines as appropriate | Detailed reporting of data sources, modeling, calculations, and resource utilization |
| Sponsor involvement | Clearly stated | Clearly stated |
HE, health economics; CE, cost-effectiveness; RCT, randomized controlled trial; CB, cost–benefit; CONSORT, Consolidated Standards of Reporting Trials; STARD, Standards for the Reporting of Diagnostic Accuracy Studies; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology.
In conclusion, when high evidence-level data are available regarding efficacy and CE of drugs and devices in wound care, informed decisions can be made about the wisdom of using them. Without these kind of data, and the will to use them, the cost of providing wound care can only continue to spiral upward with no end in sight.
Innovation
Clinical studies of drugs and devices in wound care are often poorly conducted. Nevertheless, CE studies can shed light on whether wound care products do add value when used as adjunct treatments. The corollary is that failure to use CE studies when making coverage and reimbursement decisions will lead to higher costs. Practice in the United States regarding CE must change and follow examples from other Western countries if progress is to be made in reducing costs in wound care and improving outcomes.
Key Findings.
• There is a paucity of high-level clinical and CE studies in wound care.
• Not considering CE studies in reimbursement and coverage decisions will always lead to higher costs.
• CE studies can provide informative data for decision-making when such studies are well conducted and transparently reported.
Abbreviations and Acronyms
- AHRQ
Agency for Healthcare Research and Quality
- BLA
biologics license application
- CE
cost-effectiveness
- CMS
Centers for Medicare & Medicaid Services
- DFU
diabetic foot ulcer
- FDA
Food and Drug Administration
- HBOT
hyperbaric oxygen therapy
- HCT/Ps
human cells, tissues, and cellular and tissue-based products
- HE
health economics
- ICER
incremental CE ratio
- IOM
Institute of Medicine
- LEA
lower extremity amputation
- NICE
National Institute for Health and Clinical Excellence
- PMA
premarket approval
- PMN
premarket notification
- PPACA
Patient Protection and Affordable Care Act
- QALY
quality-adjusted life-year
- RCT
randomized controlled trial
- TCC
total contact casting
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the author listed. No ghostwriters were used to write this article.
About The Author
Marissa Janine Carter is the President of Strategic Solutions, Inc., and has an extremely broad science background, which includes work in several medical fields, as well as physical and engineering disciplines. Her expertise includes evidence-based medicine, clinical practice guidelines, cost-effectiveness and health economics, biostatistics, and clinical trial design and analysis. Dr. Carter holds an MA in biochemistry from Oxford University and a PhD in chemistry from Brandeis University. She is the author or coauthor of over 75 peer-reviewed articles and book chapters in medicine and chemistry and was recently recognized as a major contributor to the community of wound care through the award of the MAPWCA.
References
- 1.Sweet BV. Schwemm AK. Parsons DM. Review of the processes for FDA oversight of drugs, medical devices, and combination products. J Manag Care Pharm. 2011;17:40. doi: 10.18553/jmcp.2011.17.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IOM (Institute of Medicine) Medical Devices and the Public's Health: The FDA 510(k) Clearance Process at 35 Years. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 3.Carter MJ. Fife CE. Thomson B. Walker D. Estimating the applicability of wound care randomized controlled trials to general wound-care populations by estimating the percentage of individuals excluded from a typical wound-care population in such trials. Adv Skin Wound Care. 2009;22:316. doi: 10.1097/01.ASW.0000305486.06358.e0. [DOI] [PubMed] [Google Scholar]
- 4.Kolber MS. Opacity and cost effectiveness analysis in Medicare coverage decisions: health policy encounters administrative law. Food Drug Law J. 2009;64:515. [PubMed] [Google Scholar]
- 5.HealthCare.gov. Read the law. www.healthcare.gov/law/full/index.html. [Apr 11;2013 ]. www.healthcare.gov/law/full/index.html
- 6.Mortimer D. Peacock S. Social welfare and the Affordable Care Act: is it ever optimal to set aside comparative cost? Soc Sci Med. 2012;75:1156. doi: 10.1016/j.socscimed.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Miller EA. The affordable care act and long-term care: comprehensive reform or just tinkering around the edges? J Aging Soc Policy. 2012;24:101. doi: 10.1080/08959420.2012.659912. [DOI] [PubMed] [Google Scholar]
- 8.Lavarreda SA. Brown ER. Bolduc CD. Underinsurance in the United States: an interaction of costs to consumers, benefit design, and access to care. Annu Rev Public Health. 2011;32:471. doi: 10.1146/annurev.publhealth.012809.103655. [DOI] [PubMed] [Google Scholar]
- 9.Whittington R. Introduction to Health Economics: A Beginners Guide. Flintshire: United Kingdom: Greenflint Ltd.; 2008. [Google Scholar]
- 10.Carter MJ. Cost-effectiveness research in wound care: definitions, approaches, and limitations. Ostomy Wound Manage. 2010;56:48. [PubMed] [Google Scholar]
- 11.Driver VR. Health economics of wound care and limb preservation: beyond clinical evidence. Diabet Microvasc Complications Today. 2006;4:29. [Google Scholar]
- 12.Ernst R. Indirect costs and cost-effectiveness analysis. Value Health. 2006;9:253. doi: 10.1111/j.1524-4733.2006.00114.x. [DOI] [PubMed] [Google Scholar]
- 13.Goossens ME. Rutten-van Mölken MP. Vlaeyen JW. van der Linden SM. The cost diary: a method to measure direct and indirect costs in cost-effectiveness research. J Clin Epidemiol. 2000;53:688. doi: 10.1016/s0895-4356(99)00177-8. [DOI] [PubMed] [Google Scholar]
- 14.Campbell MK. Torgerson DJ. Bootstrapping: estimating confidence intervals for cost-effectiveness ratios. QJM. 1999;92:177. doi: 10.1093/qjmed/92.3.177. [DOI] [PubMed] [Google Scholar]
- 15.Le Lay A. Despiegel N. François C. Duru G. Can discrete event simulation be of use in modelling major depression? Cost Eff Resour Alloc. 2006;4:19. doi: 10.1186/1478-7547-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullins CD. Weisman CS. A simplified approach to teaching Markov models. Am J Pharm Educ. 1996;60:42. [Google Scholar]
- 17.Weintraub WS. Cohen DJ. The limits of cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2009;2:55. doi: 10.1161/CIRCOUTCOMES.108.812321. [DOI] [PubMed] [Google Scholar]
- 18.Hadorn DC. Setting health care priorities in Oregon: cost-effectiveness meets the rule of rescue. JAMA. 1991;265:2218. [PubMed] [Google Scholar]
- 19.Borna S. Sundaram S. An approach to allocating limited health resources. J Health Soc Policy. 1999;11:85. doi: 10.1300/J045v11n02_07. [DOI] [PubMed] [Google Scholar]
- 20.Rettig RA. Marks EL. Implementing the End-Stage Renal Disease Program of Medicare. Rand Publication No. R-2505-HCFA/HEW. Santa Monica, CA: The Rand Corp.; 1980. [Google Scholar]
- 21.Diamond GA. Kaul S. Cost, effectiveness, and cost-effectiveness. Circ Cardiovasc Qual Outcomes. 2009;2:49. doi: 10.1161/CIRCOUTCOMES.108.793406. [DOI] [PubMed] [Google Scholar]
- 22.Laupacis A. Feeny D. Detsky AS. Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992;146:473. [PMC free article] [PubMed] [Google Scholar]
- 23.Laupacis A. Feeny D. Detsky AS. Tugwell PX. Tentative guidelines for using clinical and economic evaluations revisited. CMAJ. 1993;148:927. [PMC free article] [PubMed] [Google Scholar]
- 24.Heudebert GR. Centor RM. Klapow JC. Marks R. Johnson L. Wilcox CM. What is heartburn worth? A cost-utility analysis of management strategies. J Gen Intern Med. 2000;15:175. doi: 10.1046/j.1525-1497.2000.02639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Institute for Health and Clinical Excellence. Citizens Council report on departing from the threshold. www.nice.org.uk/newsroom/features/CitizensCouncilReport.jsp. [Apr 15;2013 ]. www.nice.org.uk/newsroom/features/CitizensCouncilReport.jsp
- 26.Neumann PJ. Weinstein MC. Legislating against use of cost-effectiveness information. N Engl J Med. 2010;363:1495. doi: 10.1056/NEJMp1007168. [DOI] [PubMed] [Google Scholar]
- 27.Fife CE. Carter MJ. Walker D. Why is it so hard to do the right thing in wound care? Wound Repair Regen. 2010;18:154. doi: 10.1111/j.1524-475X.2010.00571.x. [DOI] [PubMed] [Google Scholar]
- 28.Piaggesi A. Macchiarini S. Rizzo L. Palumbo F. Tedeschi A. Nobili LA. Leporati E. Scire V. Teobaldi I. Del Prato S. An off-the-shelf instant contact casting device for the management of diabetic foot ulcers: a randomized prospective trial versus traditional fiberglass cast. Diabetes Care. 2007;30:586. doi: 10.2337/dc06-1750. [DOI] [PubMed] [Google Scholar]
- 29.Warriner RA., 3rd Carter MJ. The current state of evidence-based protocols in wound care. Plast Reconstr Surg. 2011;127(Suppl 1):144S. doi: 10.1097/PRS.0b013e31820023dc. [DOI] [PubMed] [Google Scholar]
- 30.Bolton L. Corbett L. Bernato L. Dotson P. Laraus S. Merkle D. Patterson G. Phillips T. McNees P. Riedesel PP. Sheehan P. Development of a content-validated venous ulcer guideline. Ostomy Wound Manage. 2006;52:32. [PubMed] [Google Scholar]
- 31.Fife CE. Wall V. Carter MJ. Walker D. Thomson B. Turner T. Revenue in U.S. hospital based outpatient wound centers; implications for creating accountable care organizations. J Hosp Admin. 2013;2:38. [Google Scholar]
- 32.Greer N. Foman N. Dorrian J. Fitzgerald P. MacDonald R. Rutks I. Wilt T. Advanced Wound Care Therapies for Non-Healing Diabetic, Venous, Arterial Ulcers: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012. [Aug 14;2013 ]. VA-ESP Project No. 09-009. [PubMed] [Google Scholar]
- 33.Fife CE. Carter MJ. Walker D. Thomson B. Wound care outcomes and associated cost among patients treated in US outpatient wound centers: data from the US Wound Registry. Wounds. 2012;24:10. [PubMed] [Google Scholar]
- 34.Carter MJ. Evidence-based medicine: an overview of key concepts. Ostomy Wound Manage. 2010;56:68. [PubMed] [Google Scholar]
- 35.Game FL. Hinchliffe RJ. Apelqvist J. Armstrong DG. Bakker K. Hartemann A. Löndahl M. Price PE. Jeffcoate WJ. A systematic review of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev. 2012;28(Suppl 1):119. doi: 10.1002/dmrr.2246. [DOI] [PubMed] [Google Scholar]
- 36.National Institute for Health and Care Excellence: CG119 Diabetic foot problems—inpatient management: full guideline. http://guidance.nice.org.uk/CG119/Guidance. [Apr 16;2013 ]. http://guidance.nice.org.uk/CG119/Guidance
- 37.Gottrup F. Apelqvist J. Price P. Outcomes in controlled and comparative studies on non-healing wounds: recommendations to improve the quality of evidence in wound management. J Wound Care. 2010;19:237. doi: 10.12968/jowc.2010.19.6.48471. [DOI] [PubMed] [Google Scholar]
- 38.Serena T. Bates-Jensen B. Carter MJ. Cordrey R. Driver V. Fife CE. Haser PB. Krasner D. Nusgart M. Smith AP. Snyder RJ. Consensus principles for wound care research obtained using a Delphi process. Wound Repair Regen. 2012;20:284. doi: 10.1111/j.1524-475X.2012.00790.x. [DOI] [PubMed] [Google Scholar]
- 39.Dranove D. What's Your Life Worth? Health Care Rationing…Who Lives? Who Dies? And Who Decides? Upper Saddle River, NJ: Pearson Education, Inc.; 2003. [Google Scholar]
- 40.Ubel PA. Why it's Time for Health Care Rationing. Cambridge, MA: MIT Press; 1999. Pricing Life. [Google Scholar]
- 41.Neumann PJ. Using Cost-effectiveness Analysis to Improve Health Care: Opportunities, Barriers. New York: Oxford University Press; 2005. [Google Scholar]