Abstract
Background: Many studies have examined the association between interleukin-6 (IL-6) gene polymorphisms and bone mineral density (BMD). However, the results remain conflicting. To assess the relationship more precisely, a meta-analysis was performed. Methods: The PubMed, Embase, Chinese BioMedical Literature (CBM), Wanfang, and China National Knowledge Infrastructure (CNKI) database were searched for relevant articles published up to March 2013. Weighted mean difference (WMD) and 95% confidence interval (95% CI) were calculated using a fixed-effects or random-effects model. Results: A total of 16 articles with 11,957 subjects were investigated in this meta-analysis. Overall, −634C/G polymorphism was significantly associated with BMD at the femoral neck (WMD, −0.016 g/cm2; 95% CI, −0.028 to −0.003 g/cm2), lumbar spine (WMD, −0.049 g/cm2; 95% CI, −0.069 to −0.030 g/cm2), and whole body (WMD, −0.023 g/cm2; 95% CI, −0.037 to −0.009 g/cm2) for GG versus CC+CG. In subgroup analyses stratified by ethnicity, individuals carrying −634GG genotype had a significantly lower mean BMD at any skeletal site examined, compared with individuals with −634CC or −634CG genotype in Asian populations. For −174G/C polymorphism, the BMD differences between CC+CG and GG genotype were 0.004 g/cm2 at the distal radius (95% CI, 0.004 to 0.005 g/cm2), 0.011 g/cm2 at the trochanter (95% CI, 0.002 to 0.020 g/cm2), and 0.017 g/cm2 at the Ward's triangle (95% CI, 0.003 to 0.032 g/cm2). No significant publication bias was observed in either the −634C/G or −174G/C polymorphism. Conclusions: This suggests that there are modest effects of the −634C/G and −174G/C polymorphisms on BMD. Large-scale and well-designed studies are required to further investigate gene–gene and gene–environment interactions on IL-6 polymorphisms and BMD in various populations.
Introduction
Osteoporosis is a major clinical and public health problem worldwide that results in increased individual morbidity, mortality, healthcare costs, need for hospital care, and dependency. Osteoporosis is defined to exist when bone mineral density (BMD) values at the lumbar spine or hip fall at least 2.5 standard deviation (SD) values below the population average in young healthy individuals (Holroyd et al., 2008). Although several environmental factors influence BMD, such as diet and lifestyle, genetic factors play critical roles in determining bone mass, accounting for 50%–80% of the interindividual variation in BMD (Pocock et al., 1987; Evans et al., 1988; Slemenda et al., 1991). During the past two decades, candidate gene association studies have explored the association between BMD and polymorphisms in candidate genes.
Interleukin-6 (IL-6) is a pleiotropic cytokine that plays a central role in immune, inflammatory and acute-phase responses, hematopoiesis, atherogenesis, and several endocrine and metabolic processes (Hirano, 1998). As IL-6 stimulates the development of osteoclasts and thereby the process of bone resorption, it is likely to be a pathogenic factor in bone loss, especially that triggered by estrogen deficiency (Manolagas and Jilka, 1995). Several polymorphisms have been identified in the IL-6 gene promoter region. Among them, the common −174G/C polymorphism (rs1800795, also known as −237G/C) involves a DNA-binding site for nuclear factor IL-6, a transcription factor that can interact with the estradiol/estrogen receptor complexes to regulate IL-6 gene expression (Fishman et al., 1998), whereas the −634C/G polymorphism (rs1800796, also known as −572G/C) does not have a strong homology to any known transcription factor-binding site, located in close vicinity to glucocorticoid response elements (Terry et al., 2000). For now, there are a large number of association studies conducted to evaluate the role of IL-6 polymorphisms in BMD. However, the association between IL-6 polymorphisms and BMD is still controversial.
Meta-analysis offers a powerful means of overcoming the problems associated with small sample sizes, and particularly, of overcoming the inadequate statistical powers of genetic studies on complex traits (Egger et al., 1997b). Therefore, in the present study, we performed a meta-analysis from all eligible studies to assess the association of IL-6 −174G/C and −634C/G polymorphisms with BMD.
Materials and Methods
Literature searching strategy
We conducted a computerized literature search of PubMed, Embase, Chinese BioMedical Literature (CBM), Wanfang, and China National Knowledge Infrastructure (CNKI) databases (from January 1991 to March 2013) using the following keywords and subject terms: “interleukin-6” or “IL-6,” “polymorphism” or “allele,” and “bone mineral density” or “BMD” or “bone density.” References of retrieved articles and review articles were also screened. The publication language was restricted to English or Chinese.
Inclusion criteria
Studies included in the meta-analysis had to meet all the following criteria: (1) evaluating the association between IL-6 −174G/C or −634C/G polymorphism and BMD; (2) using unrelated individuals; (3) BMD measured by dual-energy X-ray absorptiometry (DXA); (4) including a number of subjects, and the mean and SD of BMD for each genotype of the IL-6 gene; (5) subjects without a history of taking drugs, which affects bone metabolism, and without chronic diseases impacting BMD; (6) using a cohort, case–control, or cross-sectional design.
When a study reported results on different subpopulations based on age, gender, or menopausal status, we treated each subpopulation as a separate comparison. If more than one article was published using the same subjects, only the study with the largest sample size was selected.
Data extraction
Two investigators (Zhao Wang and Ke Yu) extracted data independently. When it came to conflicting evaluations, an agreement was reached after a discussion. Data were collected on the authors, journal, year of publication, ethnicity and country of study population, demographics, inclusion and exclusion criteria, characteristics of subjects, study design, study duration, type of polymorphisms (−174G/C or −634C/G), methods for genotyping, skeletal sites evaluated for BMD, instrument used for BMD measurement, number of subjects recruited and genotyped, mean and SD of BMD for each genotype of polymorphisms, frequency of the −174C and −634G alleles and of haplotypes (when available), and interactions between environment factors or genes.
Quality score assessment
Quality of studies was independently assessed by the same two investigators (Zhao Wang and Ke Yu). Quality assessment scores were modified from previous meta-analysis of observational studies (Thakkinstian et al., 2004). Total scores ranged from 0 (worst) to 9 (best) (Table 1). A study was considered low (or high) quality if scores<6 (or ≥6).
Table 1.
Criteria of Methodological Quality Assessment for Eligible Studies
| Criteria | Score |
|---|---|
| A. Representativeness of subjects | |
| Consecutive/randomly selected from population with clearly defined sampling frame | 2 |
| Consecutive/randomly selected from population without clearly defined sampling frame | 1 |
| Not described | 0 |
| B. Ascertainment of BMD measurement | |
| Clearly described standard method of measuring BMD, for example, using DXA, with details about calibration | 2 |
| Described standard method of measuring BMD, for example, using DXA, without details about calibration | 1 |
| Not described | 0 |
| C. Ascertainment of IL-6 genotype | |
| Genotyping done under blind conditions | 1 |
| Unblinded or not mentioned | 0 |
| D. Test for HWE | |
| HWE in study group | 2 |
| Hardy–Weinberg disequilibrium in study group | 1 |
| Insufficient data for test | 0 |
| E. Assessment of association | |
| Assessed association between genotypes and BMD with appropriate statistic and adjusting confounders | 2 |
| Assessed association between genotypes and BMD with appropriate statistic without adjusting confounders | 1 |
| Inappropriate statistic used | 0 |
| Total | 9 |
IL-6, interleukin-6; BMD, bone mineral density; DXA, dual-energy X-ray absorptiometry; HWE, Hardy–Weinberg equilibrium.
Statistical analysis
The main analysis addressed differences in BMD between different genotypes. Genotype contrasts were assessed as follows: CC versus GG, CC versus CG, CG versus GG, CC versus CG+GG, and CC+CG versus GG for −174G/C polymorphism; GG versus CC, GG versus CG, CG versus CC, GG versus CC+CG, and CG+GG versus CC for −634C/G polymorphism. Between-study heterogeneity was measured using a Q-statistic test (Lau et al., 1997) and an I2 statistic (Higgins et al., 2003). p<0.10 was considered representative of significant statistical heterogeneity because of the low power of the statistic. If the significant Q-statistic indicated heterogeneity across studies, the random-effects model (DerSimonian and Laird method) was used, otherwise the fixed-effects model (inverse variance method) was adopted (Petitti, 1994). The Z-test was used to assess the significance of the pooled weighted mean difference (WMD) and a p-value less than 0.05 was considered significant.
Separate analyses were conducted for BMD at each skeletal site because genetic factors contributing to BMD may be site specific (Gennari and Brandi, 2001; Yerges et al., 2009). Unadjusted values were used unless only adjusted estimates were available to achieve maximal consistency in the synthesized data. We only analyzed BMD for skeletal sites with more than 1000 subjects to avoid performing extensive multiple comparisons. Subgroup analyses were stratified by racial descent, menopausal status, gender, study design, study quality, and Hardy–Weinberg equilibrium (HWE), respectively. Furthermore, meta-regression analysis (Thompson and Sharp, 1999) was performed to investigate seven potential sources of heterogeneity, including ethnicity, menopausal status, gender, age, body mass index (BMI), study design, quality score, and DXA instrument. Statistical significance was defined as a p-value less than 0.10 because of the relatively weak statistical power.
Sensitivity analyses were performed by sequential omission of individual studies under various comparisons and by excluding data in which the HWE was violated to evaluate the stability of the results. Publication bias was investigated by the funnel plot. Funnel plot asymmetry was assessed by the method of Egger's linear regression test (Egger et al., 1997a).
HWE was tested by the χ2 test. Analyses were performed using the software Stata version 11.0 (StataCorp LP, College Station, TX). All p-values were two sided.
Results
Eligible studies
The literature search yielded a total of 504 potentially relevant records. After removing 141 duplications, 303 records were excluded because of obvious irrelevance to our study aim by checking the abstracts. Forty-four of the remaining 60 records were excluded based on selection criteria. The details of the selection process are presented in a flow chart in Figure 1. In addition, one duplicated study (Ferrari et al., 2003) and one study with a problematic BMD at the Ward's triangle (Ferrari et al., 2004) from two articles were further excluded. Therefore, 23 studies with 11,957 subjects from 16 articles were eligible in the BMD analysis (Lorentzon et al., 2000; Ferrari et al., 2001; Ota et al., 2001; Fontova et al., 2002; Garnero et al., 2002; Chung et al., 2003; Ferrari et al., 2003; Yamada et al., 2003; Ferrari et al., 2004; James et al., 2004; Moffett et al., 2004; Nordström et al., 2004; Li et al., 2008; Czerny et al., 2010; Deveci et al., 2012; Oishi et al., 2012).
FIG. 1.
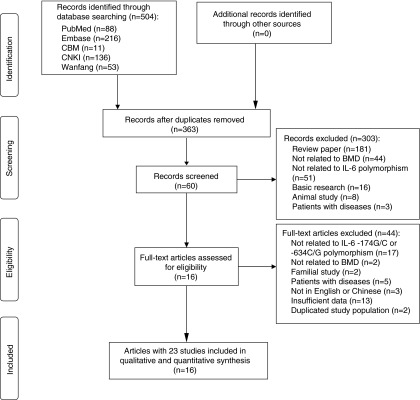
Flow diagram of the study selection process.
Characteristics of studies and subjects
The characteristics of selected studies are summarized in Table 2. The frequency of −174C allele (mean±SD) was 0.40±0.02 in European populations, and the −634G allele frequency was 0.29±0.06 in Asian populations. HWE of −174G/C polymorphism in two studies and of −634C/G polymorphism in one study was violated. Studies ranged from four to eight in quality score, with 83% (19 of 23) classified as high quality. Among the 16 eligible articles, 8 were population-based cohorts, 3 were population-based cross-sectional studies, 1 was a hospital-based cross-sectional study, 3 were hospital-based case–control studies, and 1 was cross-sectional on a randomized control trial. Overall, we analyzed BMD of the whole body, femoral neck, total hip, trochanter, Ward's triangle, lumbar spine, ultradistal radius, middle radius, and distal radius for −174G/C polymorphism, and BMD of the whole body, femoral neck, total hip, trochanter, Ward's triangle, and lumbar spine for −634C/G polymorphism.
Table 2.
Characteristics of the Eligible Studies
| Author | Year | Country | Ethnicity | Sex | Age (mean±SD) | Menopausal status | BMD instrument | Study design | No. of subjects | Polymorphism | BMD measurement | Covariates | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lorentzon et al. | 2000 | Sweden | European | Male | 16.9±0.3 | None | DPX-L | Population-based C | 90 | −174G/C | FN, LS, WB, Humerus | None | 6 |
| Ferrari et al. | 2001 | USA | Mixeda | Female | 71.9±5.6 | Postmenopausal | QDR-4500 | Population-based C | 434 | −174G/C | FN, TH, TR, 1/3R, MID, UDR | Age | 7 |
| Ota et al. | 2001 | Japan | Asian | Female | 73.2±5.8 | Postmenopausal | DPX-L | Population-based CS | 470 | −634C/G | Radial bone | Age, height, weight | 5 |
| Fontova et al. | 2002 | Spain | European | Female | 59.0±4.7 | Postmenopausal | DPX-L | Hospital-based CC | 94 | −174G/C | FN, TR, WT, LS | None | 6 |
| Garnero et al.-a | 2002 | France | European | Female | 40.0±6.2 | Premenopausal | QDR-2000 | Population-based C | 255 | −174G/C | FN, TH, TR, WT, LS, WB, DR, MID, UDR | None | 8 |
| Garnero et al.-b | 2002 | France | European | Female | 64.0±8.6 | Postmenopausal | QDR-2000 | Population-based C | 372 | −174G/C | FN, TH, TR, LS, WB, DR, MID, UDR | None | 8 |
| Chung et al. | 2003 | Korea | Asian | Female | 37.7±6.7 | Premenopausal | Lunar Pixi | Hospital-based CS | 331 | −634C/G | 1/3R | None | 7 |
| Ferrari et al. | 2003 | USA | Mixeda | Female | 71.9±5.9 | Postmenopausal | QDR-4500 | Population-based C | 451 | −174G/C | LS | Age | 6 |
| Yamada et al.-a | 2003 | Japan | Asian | Female | 46.2±5.0 | Premenopausal | DQR-4500, Desiscan 1000 | Population-based C | 279 | −634C/G | FN, TR, WT, LS, WB | Age, BMI | 8 |
| Yamada et al.-b | 2003 | Japan | Asian | Female | 64.0±8.6 | Postmenopausal | DQR-4500, Desiscan 1000 | Population-based C | 817 | −634C/G | FN, TR, WT, LS, WB | Age, BMI | 8 |
| Yamada et al.-c | 2003 | Japan | Asian | Male | 59.2±10.9 | None | DQR-4500, Desiscan 1000 | Population-based C | 1126 | −634C/G | FN, TR, WT, LS, WB | Age, BMI | 8 |
| Ferrari et al.-a | 2004 | USA | Mixeda | Female | 59.5±9.2 | Mixedb | DPX-L | Population-based C | 816 | −174G/C, −634C/G |
FN, TR, WT | None | 8 |
| Ferrari et al.-b | 2004 | USA | Mixeda | Male | 60.1±9.5 | None | DPX-L | Population-based C | 835 | −174G/C, −634C/G |
FN, TR, WT | None | 8 |
| James et al.-a | 2004 | UK | European | Female | 60.7±0.0 | Postmenopausal | QDR-4500 | Population-based CRT | 35 | −174G/C | TH, WT, LS | None | 7 |
| James et al.-b | 2004 | UK | European | Female | 60.7±0.0 | Postmenopausal | QDR-4500 | Population-based CRT | 30 | −174G/C | TH, WT, LS | None | 7 |
| Moffett et al. | 2004 | USA | European | Female | 73.0±5.0 | Postmenopausal | QDR-1000 | Population-based C | 3376 | −174G/C | FN, TH, DR, PR | Age, BMI, estrogen use, study centerc | 8 |
| Nordström et al. | 2004 | Sweden | European | Female | 75.0±0.0 | Postmenopausal | DPX-L | Population-based C | 964 | −174G/C | FN, LS, WB | None | 7 |
| Li et al. | 2008 | China | Asian | Female | 10.0±0.7 | Premenarche | QDR-4500 | Population-based CS | 176 | −634C/G | FN, TH, LS, WB | None | 7 |
| Czerny et al.-a | 2010 | Poland | European | Female | 63.3±5.1 | Postmenopausal | Lunar Densitometer | Hospital-based CC | 226 | −174G/C | FN, LS | None | 5 |
| Czerny et al.-b | 2010 | Poland | European | Female | 64.8±6.3 | Postmenopausal | Lunar Densitometer | Hospital-based CC | 224 | −174G/C | FN, LS | None | 6 |
| Deveci et al. | 2012 | Turkey | European | Female | 57.0±7.0 | Postmenopausal | NA | Hospital-based CC | 356 | −174G/C | TH, LS | None | 5 |
| Oishi et al.-a | 2012 | Japan | Asian | Female | 19.3±1.0 | Premenopausal | Prodigy Advance | Population-based CS | 100 | −634C/G | FN, LS, WB | None | 4 |
| Oishi et al.-b | 2012 | Japan | Asian | Female | 68.1±4.8 | Postmenopausal | QDR-A | Population-based CS | 100 | −634C/G | FN, LS, WB | Age, height, weight | 6 |
Mixed ethnicities, >94% Caucasians.
Eighty-five percent were postmenopausal women.
Unadjusted BMD was also available.
C, cohort; CS, cross-sectional study; CC, case–control study; CRT, cross-sectional on randomized control trial; FN, femoral neck; LS, lumbar spine; WB, whole body; TH, total hip; TR, trochanter; 1/3R, one-third distal radius; MID, mid-distal radius; UDR, ultradistal radius; WT, Ward's triangle; DR, distal radius; PR, proximal radius; NA, not available; SD, standard deviation; BMI, body mass index.
IL-6 −634C/G polymorphism and BMD
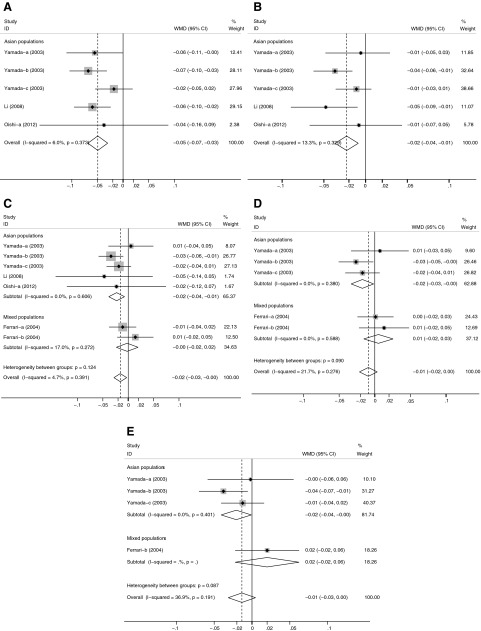
Table 3 indicates the associations between −634C/G polymorphism and BMD. Overall, −634C/G polymorphism was significantly associated with BMD at each skeletal site analyzed. At the lumbar spine, individuals carrying the GG genotype had a significantly lower mean BMD compared with individuals with the CC+CG genotype (WMD, −0.049 g/cm2; 95% confidence interval [95% CI], −0.069 to −0.030 g/cm2; p<0.001; I2=6.0%; pheterogeneity=0.373) in Asian populations (Fig. 2A). Similarly, significant associations were found at the femoral neck, whole body, trochanter, and Ward's triangle in the Asian populations (Fig. 2). In all populations, the association remained significant at the femoral neck, but became insignificant at the trochanter and Ward's triangle for GG versus CC+CG.
Table 3.
WMDs (95% CIs) and I2 for Various Contrasts of IL-6 −634C/G Polymorphism and Bone Mineral Density
| |
|
|
GG vs. CC |
CG vs. CC |
GG vs. CG |
CG+GG vs. CC |
GG vs. CC+CG |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMD | Populationa | No. of studies (sample size) | WMD (95% CI) | I2(%) | WMD (95% CI) | I2(%) | WMD (95% CI) | I2(%) | WMD (95% CI) | I2(%) | WMD (95% CI) | I2(%) |
| Femoral neckb,c | ||||||||||||
| Overall | 8 (4249) | −0.023 (−0.039 to −0.007)d | 0.0 | −0.002 (−0.014 to 0.011) | 54.7e | −0.015 (−0.028 to −0.002)d | 0.0 | −0.004 (−0.016 to 0.009) | 56.6e | −0.016 (−0.028 to −0.003)d | 4.7 | |
| Subgroup | Asian | 6 (2598) | −0.023 (−0.039 to −0.007)d | 0.0 | −0.002 (−0.014 to 0.011) | 54.7e | −0.022 (−0.039 to −0.006)d | 0.0 | −0.004 (−0.016 to 0.009) | 56.6e | −0.023 (−0.038 to −0.007)d | 0.0 |
| Japanese | 5 (2422) | −0.022 (−0.038 to −0.006)d | 0.0 | 0.001 (−0.007 to 0.009) | 0.0 | −0.022 (−0.039 to −0.005)d | 0.0 | −0.002 (−0.010 to 0.006) | 13.1 | −0.022 (−0.038 to −0.006)d | 0.0 | |
| Female | 6 (2288) | −0.025 (−0.046 to −0.005)d | 2.4 | 0.000 (−0.018 to 0.019) | 63.6e | −0.020 (−0.037 to −0.004)d | 0.0 | −0.001 (−0.020 to 0.017) | 65.2e | −0.020 (−0.037 to −0.004)d | 0.0 | |
| High quality | 7 (4149) | −0.024 (−0.040 to −0.007)d | 2.4 | −0.004 (−0.017 to 0.008) | 53.8e | −0.014 (−0.027 to −0.001)d | 5.9 | −0.006 (−0.018 to 0.006) | 55.3e | −0.015 (−0.028 to −0.003)d | 20.3 | |
| Lumbar spineb | ||||||||||||
| Overall | 6 (2598) | −0.050 (−0.070 to −0.030)d | 12.8 | −0.000 (−0.010 to 0.010) | 31.3 | −0.048 (−0.069 to −0.028)d | 0.5 | −0.006 (−0.016 to 0.004) | 29.2 | −0.049 (−0.069 to −0.030)d | 6.0 | |
| Subgroup | Japanese | 5 (2422) | −0.044 (−0.067 to −0.020)d | 18.2 | 0.003 (−0.008 to 0.015) | 29.5 | −0.047 (−0.071 to −0.023)d | 24.4 | −0.003 (−0.014 to 0.008) | 33.6 | −0.045 (−0.068 to −0.022)d | 20.8 |
| Female | 5 (1472) | −0.062 (−0.085 to −0.039)d | 0.0 | 0.001 (−0.011 to 0.013) | 44.2 | −0.061 (−0.085 to −0.036)d | 0.0 | −0.006 (−0.018 to 0.006) | 43.4 | −0.062 (−0.084 to −0.039)d | 0.0 | |
| High quality | 5 (2498) | −0.051 (−0.071 to −0.031)d | 28.6 | −0.003 (−0.013 to 0.008) | 0.0 | −0.048 (−0.068 to −0.027)d | 24.2 | −0.009 (−0.019 to 0.002) | 0.0 | −0.050 (−0.069 to −0.030)d | 28.9 | |
| Whole bodyb | ||||||||||||
| Overall | 6 (2598) | −0.024 (−0.038 to −0.010)d | 39.9 | −0.002 (−0.012 to 0.009) | 51.2e | −0.021 (−0.035 to −0.006)d | 0.0 | −0.004 (−0.015 to 0.007) | 57.9e | −0.023 (−0.037 to −0.009)d | 13.3 | |
| Subgroup | Japanese | 5 (2422) | −0.020 (−0.035 to −0.005)d | 27.7 | 0.000 (−0.007 to 0.008) | 16.1 | −0.019 (−0.034 to −0.003)d | 0.0 | −0.003 (−0.010 to 0.005) | 38.5 | −0.020 (−0.034 to −0.005)d | 2.2 |
| Female | 5 (1472) | −0.032 (−0.050 to −0.014)d | 35.4 | −0.001 (−0.015 to 0.013) | 59.2e | −0.027 (−0.045 to −0.008)d | 0.0 | −0.004 (−0.018 to 0.011) | 64.0e | −0.030 (−0.048 to −0.012)d | 0.0 | |
| High quality | 5 (2498) | −0.026 (−0.040 to −0.011)d | 49.3 | −0.005 (−0.013 to 0.002) | 43.8 | −0.021 (−0.036 to −0.006)d | 0.0 | −0.007 (−0.017 to 0.003) | 50.8e | −0.024 (−0.038 to −0.009)d | 31.1 | |
| Trochanterc | ||||||||||||
| Overall | 5 (3873) | −0.018 (−0.034 to −0.002)d | 21.4 | −0.001 (−0.009 to 0.007) | 0.0 | −0.008 (−0.021 to 0.005) | 0.0 | −0.003 (−0.011 to 0.004) | 19.0 | −0.009 (−0.022 to 0.004) | 21.7 | |
| Subgroup | Asian | 3 (2222) | −0.018 (−0.034 to −0.002)d | 21.4 | −0.001 (−0.009 to 0.007) | 0.0 | −0.017 (−0.034 to −0.000)d | 0.0 | −0.003 (−0.011 to 0.004) | 19.0 | −0.017 (−0.033 to −0.002)d | 0.0 |
| Female | 3 (1912) | −0.018 (−0.039 to 0.004) | 60.7 | −0.000 (−0.011 to 0.011) | 29.8 | −0.010 (−0.026 to 0.007) | 3.9 | −0.002 (−0.013 to 0.008) | 58.6 | −0.010 (−0.026 to 0.006) | 36.4 | |
| Ward's trianglec | ||||||||||||
| Overall | 4 (3057) | −0.022 (−0.043 to −0.002)d | 0.0 | −0.004 (−0.014 to 0.007) | 0.0 | −0.011 (−0.030 to 0.008) | 28.2 | −0.006 (−0.016 to 0.004) | 0.0 | −0.013 (−0.031 to 0.005) | 36.9 | |
| Subgroup | Asian | 3 (2222) | −0.022 (−0.043 to −0.002)d | 0.0 | −0.004 (−0.014 to 0.007) | 0.0 | −0.019 (−0.040 to 0.003) | 0.0 | −0.006 (−0.016 to 0.004) | 0.0 | −0.021 (−0.041 to −0.001)d | 0.0 |
| Female | 2 (1096) | −0.029 (−0.058 to −0.001)d | 28.3 | −0.000 (−0.015 to 0.014) | 0.0 | −0.029 (−0.059 to 0.001) | 0.0 | −0.005 (−0.019 to 0.009) | 0.0 | −0.029 (−0.057 to −0.001)d | 14.1 | |
All population-based studies at all skeletal sites analyzed; all studies recruited Asian populations at the lumbar spine and whole body; all Asian studies recruited Japanese populations at the trochanter and Ward's triangle; all studies were classified as high quality at the trochanter and Ward's triangle.
Because there was only one subject with GG in one study (Oishi et al.-b, 2012), the difference for GG versus CC, GG versus CG, and GG versus CC/CG could not be included in the calculations.
Because there was only one subject with CC in two studies (Ferrari et al.-a, 2004; Ferrari et al.-b, 2004), the difference for GG versus CC, CG versus CC, and CG/GG versus CC could not be included in the calculations.
Significant results, p-value<0.05.
Significant between-study heterogeneity (p<0.10), random-effects model was used.
WMD, weighted mean difference; 95% CI, 95% confidence interval.
FIG. 2.
Meta-analysis of the association between interleukin-6 (IL-6) −634C/G polymorphism and bone mineral density (BMD) stratified according to different ethnicities (GG vs. CC+CG): (A) lumbar spine, (B) whole body, (C) femoral neck, (D) trochanter, (E) Ward's triangle. For each study, the estimate of mean BMD difference and its 95% confidence interval (95% CI) is plotted with a diamond (♦) and a horizontal line. The size of a box (■) is proportional to the weight that the study has in calculating the summary effect estimate (⋄). The center of the diamond indicates the weighted mean difference (WMD) and the ends of the diamond correspond to the 95% CI.
BMD differences of genotype contrasts and between-study heterogeneity were largely similar when subgroup analyses were stratified by menopausal status, gender, study design, study quality, and HWE (Table 3). At the whole body and femoral neck, significant heterogeneity was observed for comparisons of CG versus CC and CG+GG versus CC. When analyses were limited to Japanese populations, the heterogeneity was insignificant for these comparisons. There was no evidence for heterogeneity at any other skeletal site. Furthermore, meta-regression identified that only BMI significantly contributed to the heterogeneity for CG versus CC at the femoral neck.
IL-6 −174G/C polymorphism and BMD
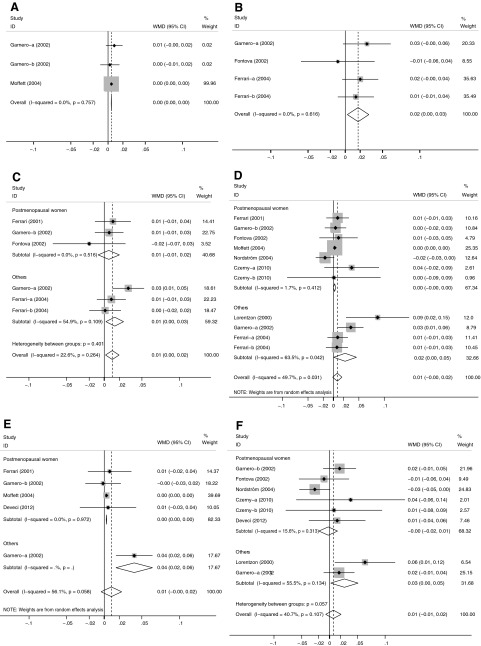
The results are summarized in Table 4. At the distal radius, −174G/C polymorphism was significantly associated with BMD regardless of the genetic contrasts. The CC homozygotes had higher distal radius BMD values than either CG or GG subjects (Fig. 3A). At the Ward's triangle, individuals with CC or CG genotype had a significantly elevated mean BMD than subjects with GG genotype (WMD, 0.017 g/cm2; 95% CI, 0.003 to 0.032 g/cm2; p=0.019; I2=0.0%; pheterogeneity=0.616) (Fig. 3B). A similar result was obtained for CC+CG versus GG at the trochanter (WMD, 0.011 g/cm2; 95% CI, 0.002 to 0.020 g/cm2; p=0.021; I2=22.6%; pheterogeneity=0.264) (Fig. 3C). At the femoral neck, the BMD difference between CC and GG was 0.001 g/cm2 (95% CI, 0.001 to 0.001 g/cm2; p<0.001; I2=0.0%; pheterogeneity=0.202), whereas the BMD difference between CC and CG was −0.002 g/cm2 (95% CI, −0.002 to −0.002 g/cm2, p<0.001; I2=0.0%; pheterogeneity=0.851). Similarly, WMDs of the total hip were 0.004 g/cm2 (95% CI, 0.004 to 0.004 g/cm2, p<0.001; I2=47.5%; pheterogeneity=0.107), −0.002 g/cm2 (95% CI, −0.003 to −0.002 g/cm2; p<0.001; I2=0.0%; pheterogeneity=0.496), and −0.004 g/cm2 (95% CI, −0.004 to −0.004 g/cm2; p<0.001; I2=0.0%; pheterogeneity=0.752) for CG versus GG, CC versus CG+GG, and CC versus CG, respectively. No association was found between −174G/C polymorphism and BMD at other skeletal sites (Table 4; Fig. 3).
Table 4.
WMDS (95% CIs) and I2 for Various Contrasts of IL-6 −174G/C Polymorphism and Bone Mineral Density
| |
|
|
CC vs. GG |
CG vs. GG |
CC vs. CG |
CC+CG vs. GG |
CC vs. CG+GG |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMD | Populationa | No. of studies (sample size) | WMD (95% CI) | I2(%) | WMD (95% CI) | I2(%) | WMD (95% CI) | I2(%) | WMD (95% CI) | I2(%) | WMD (95% CI) | I2(%) |
| Femoral neckb | ||||||||||||
| Overall | 11 (7596) | 0.001 (0.001 to 0.001)c | 26.2 | 0.007 (−0.003 to 0.017) | 50.4d | −0.002 (−0.002 to −0.002)c | 0.0 | 0.008 (−0.002 to 0.017) | 49.7d | −0.001 (−0.001 to −0.000)c | 0.0 | |
| Subgroup | European | 8 (5601) | 0.001 (0.001 to 0.001)c | 41.3 | 0.010 (−0.006 to 0.026) | 65.7d | −0.002 (−0.002 to −0.002)c | 0.0 | 0.010 (−0.004 to 0.024) | 63.4d | −0.001 (−0.001 to −0.000)c | 0.0 |
| Female | 9 (6761) | 0.001 (0.001 to 0.001)c | 0.0 | 0.005 (−0.005 to 0.014) | 42.8d | −0.002 (−0.002 to −0.002)c | 0.0 | 0.002 (0.002 to 0.003)c | 36.5 | −0.001 (−0.001 to −0.000)c | 0.0 | |
| Postmenopausal | 7 (5690) | 0.001 (0.001 to 0.001)c | 0.0 | 0.003 (0.003 to 0.003)c | 28.2 | −0.002 (−0.002 to −0.002)c | 0.0 | 0.002 (0.002 to 0.003)c | 1.7 | −0.001 (−0.001 to −0.000)c | 0.0 | |
| High quality | 10 (7370) | 0.001 (0.001 to 0.001)c | 32.7 | 0.006 (−0.004 to 0.016) | 51.7d | −0.002 (−0.002 to −0.002)c | 0.0 | 0.007 (−0.002 to 0.016) | 51.0d | −0.001 (−0.001 to −0.000)c | 0.0 | |
| Lumbar spineb,e | ||||||||||||
| Overall | 9 (3032) | 0.011 (−0.009 to 0.031) | 39.8 | 0.007 (−0.009 to 0.022) | 37.9 | 0.007 (−0.011 to 0.026) | 0.0 | 0.007 (−0.007 to 0.021) | 40.7 | 0.015 (−0.001 to 0.031) | 0.0 | |
| Subgroup | European | 8 (2581) | 0.011 (−0.009 to 0.031) | 39.8 | 0.007 (−0.009 to 0.022) | 37.9 | 0.007 (−0.011 to 0.026) | 0.0 | 0.007 (−0.007 to 0.021) | 40.7 | 0.010 (−0.008 to 0.027) | 0.0 |
| Female | 8 (2942) | 0.003 (−0.018 to 0.024) | 0.0 | 0.003 (−0.013 to 0.019) | 32.5 | 0.004 (−0.016 to 0.023) | 0.0 | 0.003 (−0.011 to 0.017) | 17.9 | 0.011 (−0.006 to 0.028) | 0.0 | |
| Postmenopausal | 7 (2687) | −0.003 (−0.027 to 0.020) | 0.0 | −0.002 (−0.021 to 0.018) | 39.5 | 0.001 (−0.021 to 0.023) | 0.0 | −0.002 (−0.019 to 0.014) | 15.6 | 0.009 (−0.010 to 0.027) | 4.7 | |
| High quality | 7 (2450) | 0.017 (−0.020 to 0.053) | 58.2d | 0.010 (−0.017 to 0.037) | 56.6d | 0.007 (−0.013 to 0.027) | 0.0 | 0.008 (−0.015 to 0.032) | 55.9d | 0.014 (−0.003 to 0.032) | 37.8 | |
| Trochanterb | ||||||||||||
| Overall | 6 (2716) | 0.013 (−0.001 to 0.026) | 34.4 | 0.012 (0.002 to 0.021)c | 0.0 | 0.001 (−0.012 to 0.014) | 0.0 | 0.011 (0.002 to 0.020)c | 22.6 | 0.006 (−0.006 to 0.018) | 19.4 | |
| Subgroup | European | 3 (721) | 0.018 (−0.003 to 0.039) | 58.6 | 0.018 (0.003 to 0.033)c | 55.8 | −0.001 (−0.021 to 0.019) | 0.0 | 0.012 (−0.013 to 0.036) | 62.0d | 0.007 (−0.012 to 0.026) | 3.2 |
| Female | 5 (1971) | 0.019 (0.004 to 0.034)c | 0.0 | 0.013 (0.002 to 0.024)c | 8.7 | 0.006 (−0.009 to 0.020) | 0.0 | 0.013 (0.003 to 0.023)c | 27.2 | 0.012 (−0.002 to 0.025) | 0.0 | |
| Postmenopausal | 3 (900) | 0.013 (−0.009 to 0.035) | 24.2 | 0.007 (−0.008 to 0.023) | 0.0 | 0.006 (−0.015 to 0.027) | 40.6 | 0.006 (−0.008 to 0.020) | 0.0 | 0.009 (−0.011 to 0.029) | 43.4 | |
| Total hip | ||||||||||||
| Overall | 5 (4793) | 0.000 (−0.000 to 0.000) | 33.4 | 0.004 (0.004 to 0.004)c | 47.5 | −0.004 (−0.004 to −0.004)c | 0.0 | 0.009 (−0.004 to 0.023) | 56.1d | −0.002 (−0.003 to −0.002)c | 0.0 | |
| Subgroup | European | 4 (4359) | 0.000 (−0.000 to 0.000) | 40.9 | 0.010 (−0.007 to 0.027) | 60.6d | −0.004 (−0.004 to −0.004)c | 0.0 | 0.010 (−0.007 to 0.028) | 66.8d | −0.002 (−0.003 to −0.002)c | 0.0 |
| Postmenopausal | 4 (4538) | 0.000 (−0.000 to 0.000) | 0.0 | 0.004 (0.004 to 0.004)c | 0.0 | −0.004 (−0.004 to −0.004)c | 0.0 | 0.003 (0.003 to 0.003)c | 0.0 | −0.002 (−0.003 to −0.002)c | 0.0 | |
| High quality | 4 (4437) | 0.000 (−0.000 to 0.000) | 48.2 | 0.009 (−0.006 to 0.025) | 60.5d | −0.004 (−0.004 to −0.004)c | 0.0 | 0.010 (−0.006 to 0.027) | 67.0d | −0.002 (−0.003 to −0.002)c | 1.5 | |
| Whole body | ||||||||||||
| Overall | 4 (1681) | 0.015 (−0.005 to 0.035) | 55.4d | 0.012 (−0.008 to 0.032) | 70.8d | 0.006 (−0.006 to 0.018) | 0.0 | 0.013 (−0.006 to 0.032) | 71.6d | 0.008 (−0.003 to 0.019) | 0.0 | |
| Subgroup | Female | 3 (1591) | 0.007 (−0.006 to 0.019) | 42.1 | 0.007 (−0.013 to 0.028) | 73.6d | 0.006 (−0.007 to 0.018) | 0.0 | 0.008 (−0.010 to 0.027) | 71.9d | 0.006 (−0.005 to 0.017) | 0.0 |
| Postmenopausal | 2 (1336) | 0.035 (0.012 to 0.059)c | 0.0 | 0.024 (0.007 to 0.042)c | 0.0 | 0.009 (−0.012 to 0.031) | 0.0 | 0.027 (0.010 to 0.043)c | 0.0 | 0.020 (−0.000 to 0.041) | 0.0 | |
| Ward's triangleb | ||||||||||||
| Overall | 4 (1910) | 0.020 (−0.002 to 0.042) | 32.5 | 0.020 (0.004 to 0.035)c | 0.0 | −0.000 (−0.021 to 0.021) | 32.4 | 0.017 (0.003 to 0.032)c | 0.0 | 0.008 (−0.012 to 0.029) | 42.0 | |
| Subgroup | Female | 3 (1165) | 0.035 (0.006 to 0.064)c | 0.0 | 0.020 (−0.001 to 0.040) | 0.0 | 0.015 (−0.013 to 0.043) | 0.0 | 0.019 (0.001 to 0.037)c | 0.0 | 0.023 (−0.003 to 0.050) | 0.0 |
| Distal radius | ||||||||||||
| Overall | 3 (4003) | 0.008 (0.008 to 0.008)c | 0.0 | 0.003 (0.003 to 0.003)c | 0.0 | 0.005 (0.005 to 0.005)c | 0.0 | 0.004 (0.004 to 0.005)c | 0.0 | 0.006 (0.006 to 0.006)c | 0.0 | |
| Middle radius | ||||||||||||
| Overall | 3 (1061) | 0.007 (−0.004 to 0.018) | 0.0 | 0.006 (−0.002 to 0.014) | 0.0 | 0.001 (−0.010 to 0.011) | 0.0 | 0.007 (−0.001 to 0.014) | 0.0 | 0.003 (−0.007 to 0.013) | 0.0 | |
| Ultradistal radius | ||||||||||||
| Overall | 3 (1061) | 0.005 (−0.006 to 0.016) | 0.0 | 0.004 (−0.004 to 0.012) | 0.0 | 0.002 (−0.009 to 0.012) | 0.0 | 0.004 (−0.003 to 0.012) | 0.0 | 0.003 (−0.007 to 0.013) | 0.0 | |
All studies recruited European populations at whole body and distal radius; all subjects were women at the total hip, distal radius, middle radius, and ultradistal radius; all studies were classified as high quality at the trochanter, whole body, Ward's triangle, distal radius, middle radius, and ultradistal radius.
Only the difference for CC/CG versus GG was included in one study (Fontova et al., 2002).
Significant results, p-value<0.05.
Significant between-study heterogeneity (p<0.10), random-effects model was used.
Only the difference for CC versus CG/GG was included in one study (Ferrari et al., 2003).
FIG. 3.
Meta-analysis of the association between IL-6 −174G/C polymorphism and BMD stratified according to menopausal status (CC+CG vs. GG): (A) distal radius, (B) Ward's triangle, (C) trochanter, (D) femoral neck, (E) total hip, (F) lumbar spine. For each study, the estimate of mean BMD difference and its 95% CI is plotted with a diamond (♦) and a horizontal line. The size of a box (■) is proportional to the weight that the study has in calculating the summary effect estimate (⋄). The center of the diamond indicates the WMD and the ends of the diamond correspond to the 95% CI.
BMD differences of genotype contrasts and between-study heterogeneity were largely similar when analyses were limited to women, European populations, population-based studies, studies with high quality, and studies in HWE (Table 4). There was significant heterogeneity for comparisons of CC versus GG, CG versus GG, and CC+CG versus GG at the whole body, CG versus GG and CC+CG versus GG at the femoral neck, and CC+CG versus GG at the total hip. However, among the postmenopausal women, the heterogeneity was not observed in these comparisons, and the pooled results reached significance (Table 4, Fig. 3). Additionally, meta-regression indicated that both age and menopausal status significantly contributed to the heterogeneity.
Sensitivity analyses and publication bias diagnostics
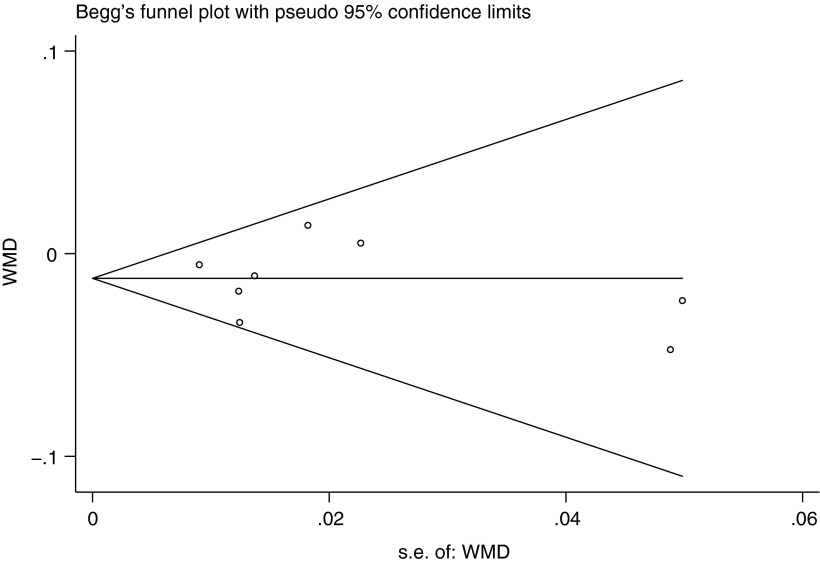
Sensitivity analysis confirmed the stability of the association between −634C/G polymorphism and BMD at the lumbar spine. However, the significant associations between −174G/C polymorphism and BMD at any analyzed skeletal sites were unstable for various comparisons. No publication bias was detected in the included studies for IL-6 −174G/C or −634C/G polymorphism. An example of a funnel plot for the femoral neck BMD is shown in Figure 4, demonstrating symmetry in GG versus CC+CG genotype comparison for −634C/G polymorphism (t=−0.13, p=0.903).
FIG. 4.
Begg's funnel plot of the Egger's test for publication bias of IL-6 −634C/G polymorphism and BMD at the femoral neck (GG vs. CC+CG). The horizontal line in the funnel plot indicates the fixed-effects summary estimate, whereas the sloping lines indicate the expected 95% CIs for a given standard error (s.e.).
Discussion
This article investigated the relationship between IL-6 polymorphisms and BMD in 11,957 subjects. Meta-analysis showed that −634C/G polymorphism was significantly associated with BMD at all examined skeletal sites. The −634GG homozygotes had a lower BMD than CC homozygotes and CG heterozygotes. This finding was consistently observed in subgroup analyses stratified by racial descent, menopausal status, gender, study design, study quality, and HWE. Sensitivity analysis indicated robustness of our results at the lumbar spine. For −174G/C polymorphism, there were significant associations at the distal radius, femoral neck, total hip, trochanter, and Ward's triangle, but not at the whole body, lumbar spine, ultradistal radius, and middle radius. However, sensitivity analysis showed that these associations were unstable. The significant associations at the femoral neck, total hip, and distal radius were mostly decided by a single study (Moffett et al., 2004) that contributed more than 99% weight for the pooled results. By omitting this study, the pooled differences became insignificant. Therefore, the relationship between −174G/C polymorphism and BMD should be interpreted with caution.
It is possible that the effect sizes of genetic factors related to BMD are different across various ethnic populations (Wang et al., 2007; Li et al., 2012). By analyzing the included studies, the present study found that BMD in European populations were significantly different from those in Asian populations (p<0.001), which was similar to some previous studies (Bhudhikanok et al., 1996; Barrett-Connor et al., 2005). Besides, the frequencies of −634G and −174C were much lower in Asians than Europeans, according to the HapMap data and our data. Moreover, nearly all subjects included for −174G/C polymorphism were Europeans due to the absence of the −174C allele in Asian populations. For −634G/C polymorphism, most eligible studies were performed on Asian populations, except for two studies with mixed populations. Consequently, the included subjects may not be representative of the general population. Our results of −634G/C polymorphism may not be applied to European populations. No obvious discrepancy found in different ethnic populations may be due to insufficient data. Furthermore, all Asian studies were based on Japanese populations except for one Chinese study. Geographical discrepancy should be considered in the analyses. Meta-analysis of these Japanese studies showed consistent results with those from Asian studies. Therefore, −634G/C polymorphism may be associated with BMD in Asian populations, especially in the Japanese population, and hence, more well-designed European studies should be performed to find out the relationship in the European populations.
The age, BMI, and menopause status are all important determinants of BMD. Although subgroup analysis showed a similar trend in the association between −174G/C or −634C/G polymorphism and BMD, meta-regression identified both age and menopausal status for −174G/C polymorphism and BMI for −634G/C polymorphism as potential sources of between-study heterogeneity. Therefore, different characteristics of subjects might be responsible for the discrepancy in overall estimates among meta-analyses. However, most eligible studies did not adjust for such potential confounders, nor did they provide the data of individuals. We can only conduct meta-analysis based on adjusted effect estimates for −634C/G polymorphism due to insufficient data for −174G/C polymorphism. The results were consistent with those from unadjusted BMD. Therefore, potential confounding bias in the original studies might not contribute to the between-study heterogeneity for −634C/G polymorphism. For −634C/G polymorphism, the significant heterogeneity disappeared when a Chinese study was omitted (Li et al., 2008). Different from other studies, this study only recruited premenarche Chinese girls aged between 9 and 11 years. Thus, it may be a potential cause for the heterogeneity. For IL-6 −174G/C polymorphism, among the postmenopausal women, the significant heterogeneity was not observed and the pooled results reached significance at the femoral neck, total hip, and whole body.
In bone, produced by osteoblasts, monocytes, and T cells, IL-6 is involved in the regulation of bone metabolism through induction of osteoclastogenesis and osteoclast activity, and therefore plays a prominent role in bone loss. Clinical studies have shown that IL-6 mRNA expression in bone is enhanced in 95% of patients with osteoporotic vertebral fracture, but in only 50% of postmenopausal controls (Ralston, 1994). In vitro studies of −174G/C polymorphism indicate that constructs containing the C allele have about 40% lower promoter activity than those with the G allele, and subjects with the CC genotype have lower levels of plasma IL-6 than those with the other IL-6 genotypes (Fishman et al., 1998; Terry et al., 2000). For −634C/G polymorphism, functional analysis revealed that the −634G allele is associated with an elevated production and secretion of the IL-6 protein by peripheral blood mononuclear cells in vitro (Kitamura et al., 2002). Our meta-analysis shows that the IL-6 gene −634C allele is associated with higher BMD at all skeletal sites in Asian populations, and −174C allele is associated with higher BMD at some skeletal sites. Nevertheless, it is possible that the main effect of IL-6 gene polymorphisms on BMD is modest, and it is also equally possible that polymorphisms may confer a greater or smaller effect when they interact with an environmental exposure or with other genes. As the biological roles of IL-6 gene polymorphisms are not quite clear now, it is difficult to interpret how IL-6 gene polymorphisms affect BMD. Further function studies are required to investigate the effect of IL-6 gene on BMD and fracture.
The current meta-analysis has several limitations that should be considered. First, because only published studies were retrieved in the meta-analysis, publication bias might be possible, even though the statistical test did not show it. Second, study designs varied across different studies. Some studies were population based, while others were hospital based. Patients from hospital-based case–control studies were all osteoporotic women, who may not represent the general population. Third, only a few studies investigated interaction effects of the IL-6 gene and environmental or genetic factors such as diet, physical activity, smoking, alcohol consumption, and the osteocalcin gene. Due to limited data and different study designs, the effects of gene–gene and gene–environment interactions were not addressed in this meta-analysis. Fourth, multiple testing problems are inevitable since analyses were performed according to different skeletal sites under five different genetic models. To reduce the type I error induced by multiple tests, adjusted p-values of the Z-test were calculated by p×k (the number of groups). This adjustment did not change conclusions at the whole body and lumbar spine for −634C/G polymorphism. Fifth, there were only four case–control studies, which investigated the relationship between IL-6 gene polymorphisms and osteoporosis risk, three for IL-6 −174G/C and one for −634C/G. Because of small sample size and insufficient data, the role of IL-6 gene polymorphisms in osteoporosis risk was not assessed in this meta-analysis. Finally, the number of included studies was small in this meta-analysis. Therefore, the analyses may not have sufficient statistical power to identify the association between IL-6 polymorphisms and BMD. The results of subgroup analyses should be interpreted with caution because of limited data.
Despite these limitations, this meta-analysis suggests that IL-6 gene −634C/G polymorphism is significantly associated with BMD in Asian populations, especially in Japanese populations. In particular, individuals carrying the −634GG genotype may have a lower BMD compared with those with the CC+CG, CC, and CG genotype. However, the association between −174G/C polymorphism and BMD should be interpreted with caution because it may be site specific, and may not be so reliable due to instable results. More epidemiological and mechanistic studies are needed to further investigate the role of IL-6 gene polymorphisms in regulating BMD and osteoporosis in the future.
Acknowledgments
This study was supported by the Shaoxin Major Scientific and Technological Project (2011A11013) and National Natural Science Foundation of China (Grant no. 81000804).
Author Disclosure Statement
The authors have declared that no competing financial interests exist.
References
- Barrett-Connor E. Siris ES. Wehren LE, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20:185–194. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- Bhudhikanok GS. Wang MC. Eckert K, et al. Differences in bone mineral in young Asian and Caucasian Americans may reflect differences in bone size. J Bone Miner Res. 1996;11:1545–1556. doi: 10.1002/jbmr.5650111023. [DOI] [PubMed] [Google Scholar]
- Chung HW. Seo JS. Hur SE, et al. Association of interleukin-6 promoter variant with bone mineral density in pre-menopausal women. J Hum Genet. 2003;48:243–248. doi: 10.1007/s10038-003-0020-8. [DOI] [PubMed] [Google Scholar]
- Czerny B. Kaminski A. Kurzawski M, et al. The association of IL-1beta, IL-2, and IL-6 gene polymorphisms with bone mineral density and osteoporosis in postmenopausal women. Eur J Obstet Gynecol Reprod Biol. 2010;149:82–85. doi: 10.1016/j.ejogrb.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Deveci D. Ozkan ZS. Yuce H. Is there any relation between IL-6 gene −174 G>C polymorphism and postmenopausal osteoporosis? Eur J Obstet Gynecol Reprod Biol. 2012;164:98–101. doi: 10.1016/j.ejogrb.2012.05.026. [DOI] [PubMed] [Google Scholar]
- Egger M. Davey Smith G. Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997a;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M. Smith GD. Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997b;315:1533–1537. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RA. Marel GM. Lancaster EK, et al. Bone mass is low in relatives of osteoporotic patients. Ann Intern Med. 1988;109:870–873. doi: 10.7326/0003-4819-109-11-870. [DOI] [PubMed] [Google Scholar]
- Ferrari SL. Ahn-Luong L. Garnero P, et al. Two promoter polymorphisms regulating interleukin-6 gene expression are associated with circulating levels of C-reactive protein and markers of bone resorption in postmenopausal women. J Clin Endocrinol Metab. 2003;88:255–259. doi: 10.1210/jc.2002-020092. [DOI] [PubMed] [Google Scholar]
- Ferrari SL. Garnero P. Emond S, et al. A functional polymorphic variant in the interleukin-6 gene promoter associated with low bone resorption in postmenopausal women. Arthritis Rheum. 2001;44:196–201. doi: 10.1002/1529-0131(200101)44:1<196::AID-ANR26>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Ferrari SL. Karasik D. Liu J, et al. Interactions of interleukin-6 promoter polymorphisms with dietary and lifestyle factors and their association with bone mass in men and women from the Framingham Osteoporosis Study. J Bone Miner Res. 2004;19:552–559. doi: 10.1359/JBMR.040103. [DOI] [PubMed] [Google Scholar]
- Fishman D. Faulds G. Jeffery R, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontova R. Gutierrez C. Vendrell J, et al. Bone mineral mass is associated with interleukin 1 receptor autoantigen and TNF-alpha gene polymorphisms in post-menopausal Mediterranean women. J Endocrinol Invest. 2002;25:684–690. doi: 10.1007/BF03345101. [DOI] [PubMed] [Google Scholar]
- Garnero P. Borel O. Sornay-Rendu E, et al. Association between a functional interleukin-6 gene polymorphism and peak bone mineral density and postmenopausal bone loss in women: the OFELY study. Bone. 2002;31:43–50. doi: 10.1016/s8756-3282(02)00810-4. [DOI] [PubMed] [Google Scholar]
- Gennari L. Brandi ML. Genetics of male osteoporosis. Calcif Tissue Int. 2001;69:200–204. doi: 10.1007/s00223-001-1049-3. [DOI] [PubMed] [Google Scholar]
- Higgins JP. Thompson SG. Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T. Interleukin 6 and its receptor: ten years later. Int Rev Immunol. 1998;16:249–284. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- Holroyd C. Cooper C. Dennison E. Epidemiology of osteoporosis. Best Pract Res Clin Endocrinol Metab. 2008;22:671–685. doi: 10.1016/j.beem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- James L. Onambele G. Woledge R, et al. IL-6-174G/C genotype is associated with the bone mineral density response to oestrogen replacement therapy in post-menopausal women. Eur J Appl Physiol. 2004;92:227–230. doi: 10.1007/s00421-004-1092-7. [DOI] [PubMed] [Google Scholar]
- Kitamura A. Hasegawa G. Obayashi H, et al. Interleukin-6 polymorphism (-634C/G) in the promotor region and the progression of diabetic nephropathy in type 2 diabetes. Diabet Med. 2002;19:1000–1005. doi: 10.1046/j.1464-5491.2002.00844.x. [DOI] [PubMed] [Google Scholar]
- Lau J. Ioannidis JP. Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- Li X. He GP. Zhang B, et al. Interactions of interleukin-6 gene polymorphisms with calcium intake and physical activity on bone mass in pre-menarche Chinese girls. Osteoporos Int. 2008;19:1629–1637. doi: 10.1007/s00198-008-0613-3. [DOI] [PubMed] [Google Scholar]
- Li Y. Xi B. Li K, et al. Association between vitamin D receptor gene polymorphisms and bone mineral density in Chinese women. Mol Biol Rep. 2012;39:5709–5717. doi: 10.1007/s11033-011-1380-3. [DOI] [PubMed] [Google Scholar]
- Lorentzon M. Lorentzon R. Nordström P. Interleukin-6 gene polymorphism is related to bone mineral density during and after puberty in healthy white males: a cross-sectional and longitudinal study. J Bone Miner Res. 2000;15:1944–1949. doi: 10.1359/jbmr.2000.15.10.1944. [DOI] [PubMed] [Google Scholar]
- Manolagas SC. Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332:305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- Moffett SP. Zmuda JM. Cauley JA, et al. Association of the G-174C variant in the interleukin-6 promoter region with bone loss and fracture risk in older women. J Bone Miner Res. 2004;19:1612–1618. doi: 10.1359/JBMR.040707. [DOI] [PubMed] [Google Scholar]
- Nordström A. Gerdhem P. Brandstrom H, et al. Interleukin-6 promoter polymorphism is associated with bone quality assessed by calcaneus ultrasound and previous fractures in a cohort of 75-year-old women. Osteoporos Int. 2004;15:820–826. doi: 10.1007/s00198-004-1610-9. [DOI] [PubMed] [Google Scholar]
- Oishi Y. Watanabe Y. Shinoda S, et al. The IL6 gene polymorphism −634C>G and IL17F gene polymorphism 7488T>C influence bone mineral density in young and elderly Japanese women. Gene. 2012;504:75–83. doi: 10.1016/j.gene.2012.04.054. [DOI] [PubMed] [Google Scholar]
- Ota N. Nakajima T. Nakazawa I, et al. A nucleotide variant in the promoter region of the interleukin-6 gene associated with decreased bone mineral density. J Hum Genet. 2001;46:267–272. doi: 10.1007/s100380170077. [DOI] [PubMed] [Google Scholar]
- Petitti DB. Oxford University Press; New York: 1994. Meta-analysis, decision analysis, and cost-effectiveness analysis: Methods for quantitative synthesis in medicine. [Google Scholar]
- Pocock NA. Eisman JA. Hopper JL, et al. Genetic determinants of bone mass in adults. A twin study. J Clin Invest. 1987;80:706–710. doi: 10.1172/JCI113125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston SH. Analysis of gene expression in human bone biopsies by polymerase chain reaction: evidence for enhanced cytokine expression in postmenopausal osteoporosis. J Bone Miner Res. 1994;9:883–890. doi: 10.1002/jbmr.5650090614. [DOI] [PubMed] [Google Scholar]
- Slemenda CW. Christian JC. Williams CJ, et al. Genetic determinants of bone mass in adult women: a reevaluation of the twin model and the potential importance of gene interaction on heritability estimates. J Bone Miner Res. 1991;6:561–567. doi: 10.1002/jbmr.5650060606. [DOI] [PubMed] [Google Scholar]
- Terry CF. Loukaci V. Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275:18138–18144. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- Thakkinstian A. D'Este C. Eisman J, et al. Meta-analysis of molecular association studies: vitamin D receptor gene polymorphisms and BMD as a case study. J Bone Miner Res. 2004;19:419–428. doi: 10.1359/JBMR.0301265. [DOI] [PubMed] [Google Scholar]
- Thompson SG. Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18:2693–2708. doi: 10.1002/(sici)1097-0258(19991030)18:20<2693::aid-sim235>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Wang CL. Tang XY. Chen WQ, et al. Association of estrogen receptor alpha gene polymorphisms with bone mineral density in Chinese women: a meta-analysis. Osteoporos Int. 2007;18:295–305. doi: 10.1007/s00198-006-0239-2. [DOI] [PubMed] [Google Scholar]
- Yamada Y. Ando F. Niino N, et al. Association of polymorphisms of interleukin-6, osteocalcin, and vitamin D receptor genes, alone or in combination, with bone mineral density in community-dwelling Japanese women and men. J Clin Endocrinol Metab. 2003;88:3372–3378. doi: 10.1210/jc.2002-021449. [DOI] [PubMed] [Google Scholar]
- Yerges LM. Klei L. Cauley JA, et al. High-density association study of 383 candidate genes for volumetric BMD at the femoral neck and lumbar spine among older men. J Bone Miner Res. 2009;24:2039–2049. doi: 10.1359/JBMR.090524. [DOI] [PMC free article] [PubMed] [Google Scholar]