Abstract
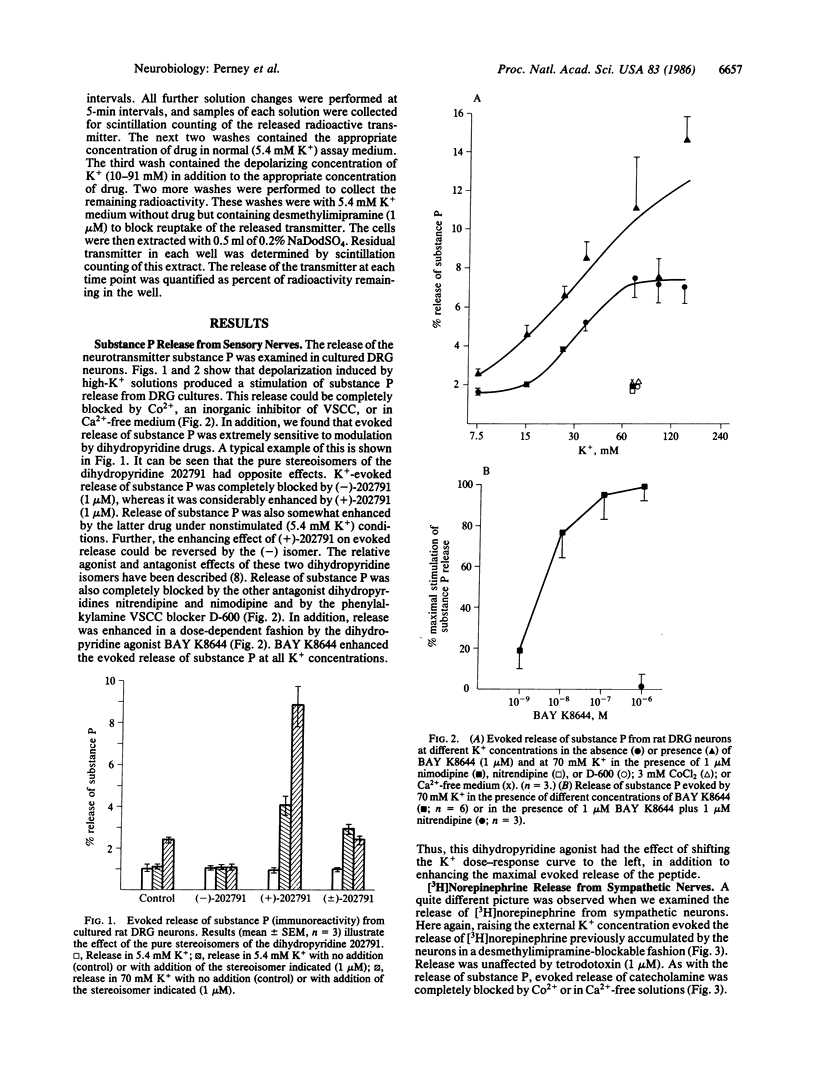
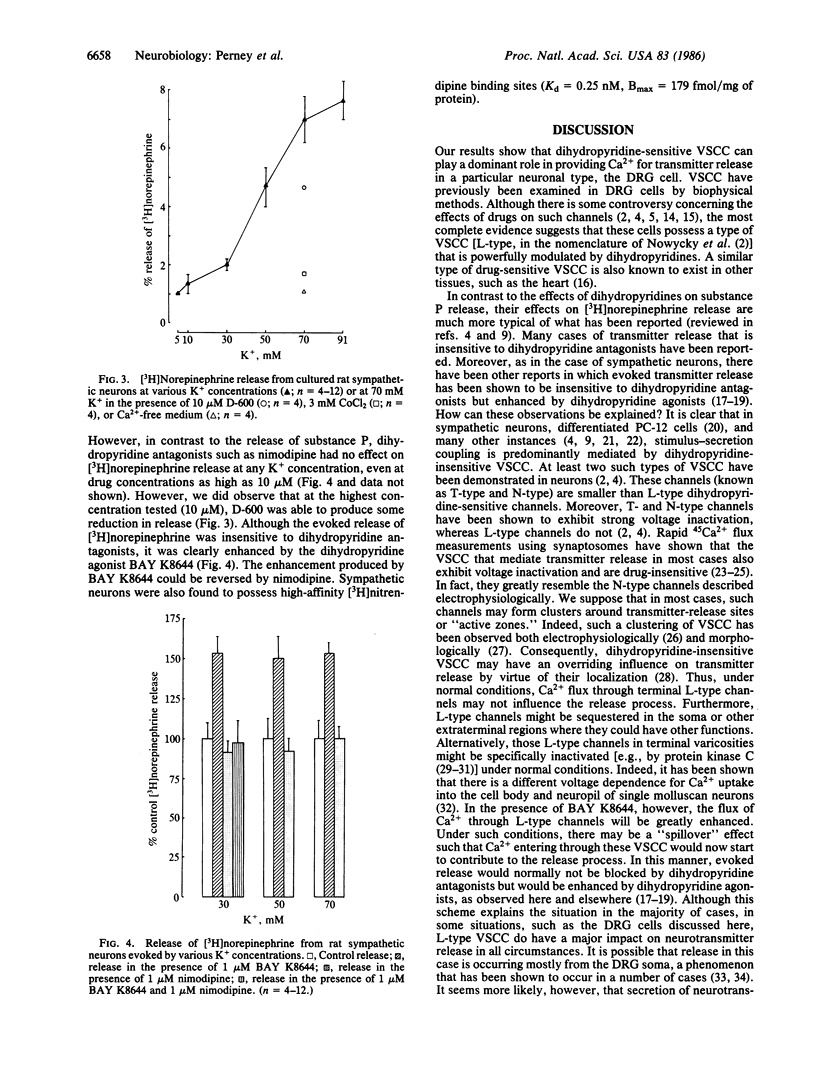
We examined the effects of dihydropyridine drugs on evoked neurotransmitter release from cultured neonatal rat sensory and sympathetic neurons. Depolarization with K+-rich solutions increased the release of substance P from cultured sensory neurons. This release was enhanced by BAY K8644 and (+)-202791 and was blocked by a variety of other dihydropyridines including (-)-202791, by Co2+, or in Ca2+-free solutions. K+-rich solutions also stimulated the release of [3H]norepinephrine from cultured sympathetic neurons. This release was also completely blocked by Co2+ or in Ca2+-free solution. In contrast to the situation in sensory neurons, however, the evoked release of [3H]norepinephrine was completely resistant to the blocking effects of dihydropyridine such as nimodipine. However, BAY K8644 was able to enhance the evoked release of [3H]norepinephrine, and this enhancement was blocked by nimodipine. These results are discussed in relation to the possible participation of multiple types of calcium channels in the release of neurotransmitters.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boll W., Lux H. D. Action of organic antagonists on neuronal calcium currents. Neurosci Lett. 1985 May 23;56(3):335–339. doi: 10.1016/0304-3940(85)90265-4. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984 Aug 9;310(5977):501–502. doi: 10.1038/310501a0. [DOI] [PubMed] [Google Scholar]
- Chow I., Poo M. M. Release of acetylcholine from embryonic neurons upon contact with muscle cell. J Neurosci. 1985 Apr;5(4):1076–1082. doi: 10.1523/JNEUROSCI.05-04-01076.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau P., Blaustein M. P. Initial release of [3H]dopamine from rat striatal synaptosomes: correlation with calcium entry. J Neurosci. 1983 Apr;3(4):703–713. doi: 10.1523/JNEUROSCI.03-04-00703.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedulova S. A., Kostyuk P. G., Veselovsky N. S. Two types of calcium channels in the somatic membrane of new-born rat dorsal root ganglion neurones. J Physiol. 1985 Feb;359:431–446. doi: 10.1113/jphysiol.1985.sp015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman S. B., Dawson G., Villereal M. L., Miller R. J. Identification and characterization of voltage-sensitive calcium channels in neuronal clonal cell lines. J Neurosci. 1984 Jun;4(6):1453–1467. doi: 10.1523/JNEUROSCI.04-06-01453.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman S. B., Miller R. J. Calcium channel activation: a different type of drug action. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5580–5583. doi: 10.1073/pnas.81.17.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graubard K., Ross W. N. Regional distribution of calcium influx into bursting neurons detected with arsenazo III. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5565–5569. doi: 10.1073/pnas.82.16.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. M., Kongsamut S., Miller R. J. Protein kinase C mediated regulation of calcium channels in PC-12 pheochromocytoma cells. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1298–1305. doi: 10.1016/0006-291x(86)90391-8. [DOI] [PubMed] [Google Scholar]
- Hawrot E., Patterson P. H. Long-term culture of dissociated sympathetic neurons. Methods Enzymol. 1979;58:574–584. doi: 10.1016/s0076-6879(79)58174-9. [DOI] [PubMed] [Google Scholar]
- Kongsamut S., Kamp T. J., Miller R. J., Sanguinetti M. C. Calcium channel agonist and antagonist effects of the stereoisomers of the dihydropyridine 202-791. Biochem Biophys Res Commun. 1985 Jul 16;130(1):141–148. doi: 10.1016/0006-291x(85)90393-6. [DOI] [PubMed] [Google Scholar]
- Kongsamut S., Miller R. J. Nerve growth factor modulates the drug sensitivity of neurotransmitter release from PC-12 cells. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2243–2247. doi: 10.1073/pnas.83.7.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemiss D. N., Spedding M. A functional correlate for the dihydropyridine binding site in rat brain. Nature. 1985 Mar 7;314(6006):94–96. doi: 10.1038/314094a0. [DOI] [PubMed] [Google Scholar]
- Miller R. J., Freedman S. B. Are dihydropyridine binding sites voltage sensitive calcium channels? Life Sci. 1984 Mar 26;34(13):1205–1221. doi: 10.1016/0024-3205(84)90543-5. [DOI] [PubMed] [Google Scholar]
- Mudge A. W., Leeman S. E., Fischbach G. D. Enkephalin inhibits release of substance P from sensory neurons in culture and decreases action potential duration. Proc Natl Acad Sci U S A. 1979 Jan;76(1):526–530. doi: 10.1073/pnas.76.1.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachshen D. A., Blaustein M. P. Some properties of potassium-stimulated calcium influx in presynaptic nerve endings. J Gen Physiol. 1980 Dec;76(6):709–728. doi: 10.1085/jgp.76.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachshen D. A., Blaustein M. P. The effects of some organic "calcium antagonists" on calcium influx in presynaptic nerve terminals. Mol Pharmacol. 1979 Sep;16(2):576–586. [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Long-opening mode of gating of neuronal calcium channels and its promotion by the dihydropyridine calcium agonist Bay K 8644. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2178–2182. doi: 10.1073/pnas.82.7.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Pumplin D. W., Reese T. S., Llinás R. Are the presynaptic membrane particles the calcium channels? Proc Natl Acad Sci U S A. 1981 Nov;78(11):7210–7213. doi: 10.1073/pnas.78.11.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane S. G., Dunlap K. Kinase C activator 1,2-oleoylacetylglycerol attenuates voltage-dependent calcium current in sensory neurons. Proc Natl Acad Sci U S A. 1986 Jan;83(1):184–188. doi: 10.1073/pnas.83.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. M., Llinás R. R. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985 Sep;48(3):485–498. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetake K., Kojima H., Inanaga K., Koketsu K. Catecholamine is released from non-synaptic cell-soma membrane: histochemical evidence in bullfrog sympathetic ganglion cells. Brain Res. 1981 Feb 2;205(2):436–440. doi: 10.1016/0006-8993(81)90357-7. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Ogura A. Dihydropyridines as potent calcium channel blockers in neuronal cells. FEBS Lett. 1983 Feb 21;152(2):191–194. doi: 10.1016/0014-5793(83)80377-9. [DOI] [PubMed] [Google Scholar]
- Turner T. J., Goldin S. M. Calcium channels in rat brain synaptosomes: identification and pharmacological characterization. High affinity blockade by organic Ca2+ channel blockers. J Neurosci. 1985 Mar;5(3):841–849. doi: 10.1523/JNEUROSCI.05-03-00841.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Steinbusch H. W., Jessell T. M. Differentiated properties of identified serotonin neurons in dissociated cultures of embryonic rat brain stem. J Cell Biol. 1981 Oct;91(1):142–152. doi: 10.1083/jcb.91.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]



