Abstract
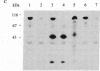
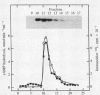
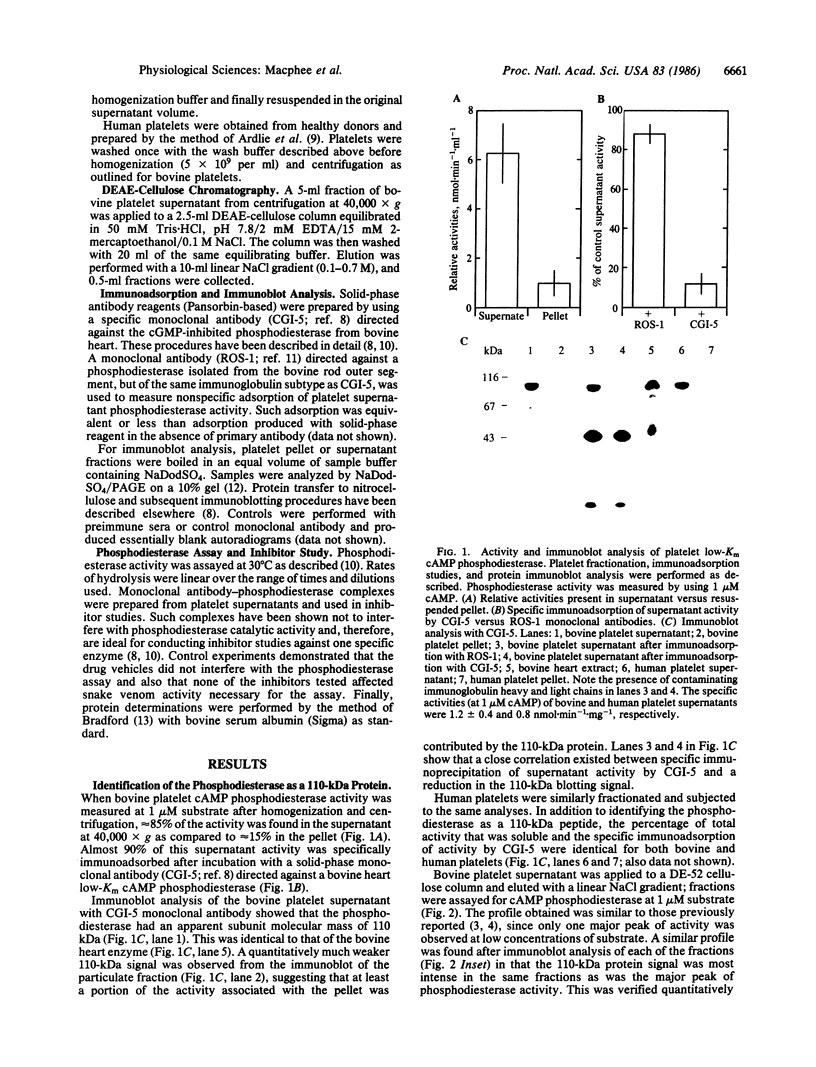
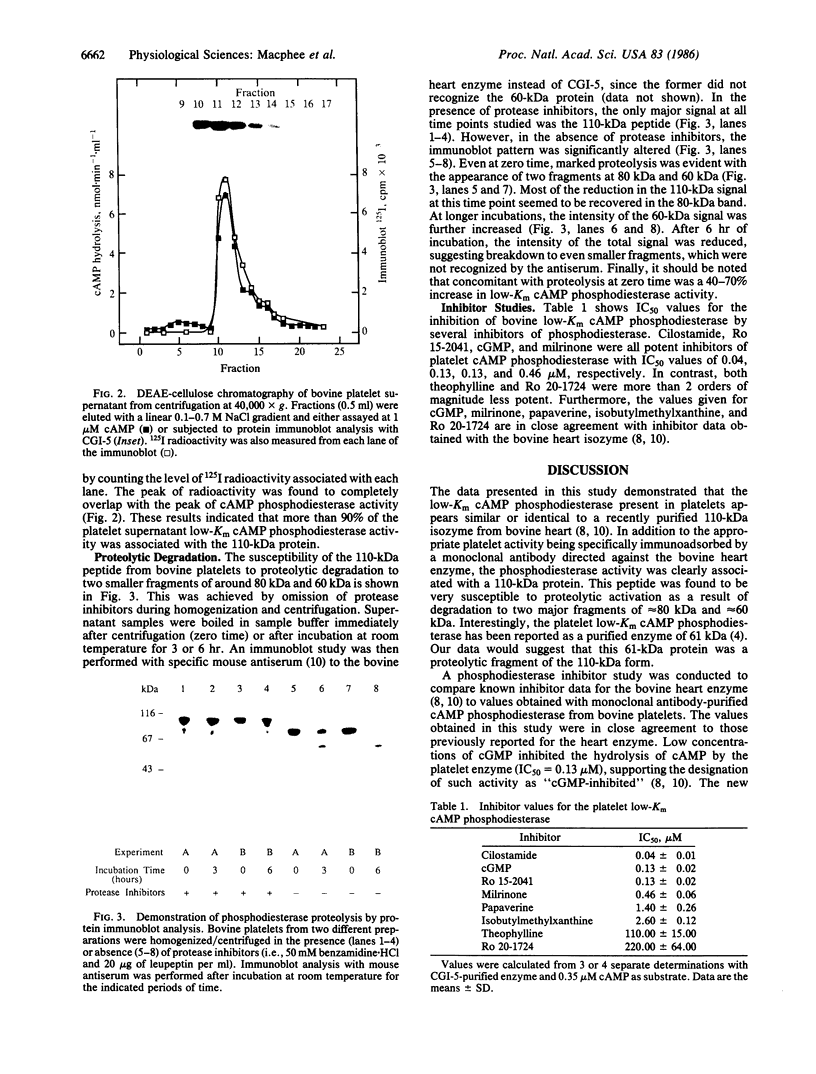
Immunoblot and enzyme-activity analyses, using specific immunological probes, indicated that more than 80% of the total low-Km cAMP phosphodiesterase activity present in bovine and human platelets resided in a single phosphodiesterase isozyme. In the presence of protease inhibitors, the platelet enzyme has an apparent subunit size of 110 kDa and appears immunologically and structurally indistinguishable from a recently purified bovine heart isozyme. When protease inhibitors were absent during homogenization and centrifugation, this platelet phosphodiesterase was susceptible to sequential proteolysis forming 80-kDa and 60-kDa peptides. As a previous report on the purification of the platelet low-Km cAMP phosphodiesterase described a 61-kDa protein, our data would suggest that this was a proteolytic fragment. Moreover, in our study a 40-70% increase in catalytic activity was associated with proteolysis. Further similarities between the platelet and heart phosphodiesterases were demonstrated by pharmacological studies that showed identical inhibitor profiles for both enzymes. Several known phosphodiesterase inhibitor compounds that have been found useful in inhibiting platelet aggregation also inhibited the platelet low-Km cAMP phosphodiesterase with potencies very similar to their antithrombotic effects. Cilostamide, Ro 15-2041, milrinone, papaverine, isobutylmethylxanthine, and theophylline inhibited the 110-kDa platelet enzyme with IC50 values of 0.04, 0.13, 0.46, 1.4, 2.6, and 110 microM, respectively.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez R., Taylor A., Fazzari J. J., Jacobs J. R. Regulation of cyclic AMP metabolism in human platelets. Sequential activation of adenylate cyclase and cyclic AMP phosphodiesterase by prostaglandins. Mol Pharmacol. 1981 Sep;20(2):302–309. [PubMed] [Google Scholar]
- Ardlie N. G., Packham M. A., Mustard J. F. Adenosine diphosphate-induced platelet aggregation in suspensions of washed rabbit platelets. Br J Haematol. 1970 Jul;19(1):7–17. doi: 10.1111/j.1365-2141.1970.tb01596.x. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Hansen R. S., Harrison S. A., Hurwitz R. L., Martins T. J., Mumby M. C. Identification and properties of cyclic nucleotide phosphodiesterases. Mol Cell Endocrinol. 1982 Nov-Dec;28(3):387–410. doi: 10.1016/0303-7207(82)90135-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bushfield M., McNicol A., MacIntyre D. E. Inhibition of platelet-activating-factor-induced human platelet activation by prostaglandin D2. Differential sensitivity of platelet transduction processes and functional responses to inhibition by cyclic AMP. Biochem J. 1985 Nov 15;232(1):267–271. doi: 10.1042/bj2320267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elks M. L., Manganiello V. C. Antilipolytic action of insulin: role of cAMP phosphodiesterase activation. Endocrinology. 1985 May;116(5):2119–2121. doi: 10.1210/endo-116-5-2119. [DOI] [PubMed] [Google Scholar]
- Endoh M., Satoh K., Yamashita S. Inhibition of cyclic AMP phosphodiesterase activity and myocardial contractility: effects of cilostamide, a novel PDE inhibitor, and methylsiobutylxanthine on rabbit and canine ventricular muscle. Eur J Pharmacol. 1980 Aug 22;66(1):43–52. doi: 10.1016/0014-2999(80)90293-9. [DOI] [PubMed] [Google Scholar]
- Farah A. E., Alousi A. A., Schwarz R. P., Jr Positive inotropic agents. Annu Rev Pharmacol Toxicol. 1984;24:275–328. doi: 10.1146/annurev.pa.24.040184.001423. [DOI] [PubMed] [Google Scholar]
- Grant P. G., Colman R. W. Purification and characterization of a human platelet cyclic nucleotide phosphodiesterase. Biochemistry. 1984 Apr 10;23(8):1801–1807. doi: 10.1021/bi00303a034. [DOI] [PubMed] [Google Scholar]
- Hamet P., Franks D. J., Tremblay J., Coquil J. F. Rapid activation of cAMP phosphodiesterase in rat platelets. Can J Biochem Cell Biol. 1983 Nov;61(11):1158–1165. doi: 10.1139/o83-149. [DOI] [PubMed] [Google Scholar]
- Harrison S. A., Reifsnyder D. H., Gallis B., Cadd G. G., Beavo J. A. Isolation and characterization of bovine cardiac muscle cGMP-inhibited phosphodiesterase: a receptor for new cardiotonic drugs. Mol Pharmacol. 1986 May;29(5):506–514. [PubMed] [Google Scholar]
- Hidaka H., Asano T. Human blood platelet 3': 5'-cyclic nucleotide phosphodiesterase. Isolation of low-Km and high-Km phosphodiesterase. Biochim Biophys Acta. 1976 Apr 8;429(2):485–497. doi: 10.1016/0005-2744(76)90296-5. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Hayashi H., Kohri H., Kimura Y., Hosokawa T., Igawa T., Saitoh Y. Selective inhibitor of platelet cyclic adenosine monophosphate phosphodiesterase, cilostamide, inhibits platelet aggregation. J Pharmacol Exp Ther. 1979 Oct;211(1):26–30. [PubMed] [Google Scholar]
- Hurwitz R. L., Bunt-Milam A. H., Beavo J. A. Immunologic characterization of the photoreceptor outer segment cyclic GMP phosphodiesterase. J Biol Chem. 1984 Jul 10;259(13):8612–8618. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam S. C., Guccione M. A., Packham M. A., Mustard J. F. Effect of cAMP phosphodiesterase inhibitors on ADP-induced shape change, cAMP and nucleoside diphosphokinase activity of rabbit platelets. Thromb Haemost. 1982 Apr 30;47(2):90–95. [PubMed] [Google Scholar]
- Lugnier C., Stierlé A., Beretz A., Schoeffter P., Lebec A., Wermuth C. G., Cazenave J. P., Stoclet J. C. Tissue and substrate specificity of inhibition by alkoxy-aryl-lactams of platelet and arterial smooth muscle cyclic nucleotide phosphodiesterases relationship to pharmacological activity. Biochem Biophys Res Commun. 1983 Jun 29;113(3):954–959. doi: 10.1016/0006-291x(83)91091-4. [DOI] [PubMed] [Google Scholar]
- Muggli R., Tschopp T. B., Mittelholzer E., Baumgartner H. R. 7-Bromo-1,5-dihydro-3,6-dimethylimidazo[2,1-b]quinazolin-2(3H)- one (Ro 15-2041), a potent antithrombotic agent that selectively inhibits platelet cyclic AMP-phosphodiesterase. J Pharmacol Exp Ther. 1985 Oct;235(1):212–219. [PubMed] [Google Scholar]
- Numano F. Cyclic nucleotides, prostaglandins, and ischemic heart disease. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:661–670. [PubMed] [Google Scholar]
- Tremblay J., Lachance B., Hamet P. Activation of cyclic GMP-binding and cyclic AMP-specific phosphodiesterases of rat platelets by a mechanism involving cyclic AMP-dependent phosphorylation. J Cyclic Nucleotide Protein Phosphor Res. 1985;10(4):397–411. [PubMed] [Google Scholar]