Abstract
The aim of this article is to provide a better understanding of the roles of interstitial cells of Cajal (ICC) in regulating gastrointestinal motility by reviewing in vitro and in vivo physiological motility studies. Based on the in vitro studies, ICC are proposed to have the following functions: to generate slow waves, to mediate neurotransmission between the enteric nerves and the gastrointestinal muscles and to act as mechanoreceptors. However, there is limited evidence available for these hypotheses from the in vivo motility studies. In this review, we first introduce the major subtypes of ICC and their established functions. Three Kit mutant mouse and rodent models are presented and the loss of ICC subtypes in these mutants is reviewed. The physiological motility findings from various in vitroand in vivo experiments are discussed to give a critical review on the roles of ICC in generating slow waves, regulating gastrointestinal motility, mediating neural transmission and serving as mechanoreceptors. It is concluded that the role of ICC as pacemakers may be well established, but other cells may also be involved in the generation of slow waves; the theory that ICC are mediators of neurotransmission is challenged by the majority of the in vivo motility studies; the hypothesis that ICC are mechanoreceptors has not found supportive evidence from the in vivo studies yet. More studies are needed to explain discrepancies in motility findings between the in vitro and in vivo experiments.
Keywords: ICC, gastrointestinal motility, electrogastrography, neurotransmission, slow waves, emptying
-
Subtypes and functions of ICC in the gut
- Subtypes of ICC
- ICC along the gut
- Functions of ICC
Mutant animal models used in in vitro and in vivo studies
-
Roles of ICC in generating slow waves
- Gastrointestinal slow waves and their clinical
- significance
- In vitro studies
- In vivo studies
-
Roles of ICC in regulating gastrointestinal motility
- Gastrointestinal motility and roles of slow waves
- Roles of ICC in regulating peristaltic contractions
-
Roles of ICC in mediating neural transmission
-
- In vitro studies
- LOS and pylorus sphincter
- Stomach
- Small intestine
- Colon
-
- In vivo studies
- Lower oesophageal sphincter
- Stomach
- Colon
- Anal sphincter
-
Roles of ICC as mechanoreceptors
Conclusions
Subtypes and functions of ICC in the gut
Subtypes of ICC
The interstitial cells of Cajal (ICC) were first characterized by Cajal [1] and are now known to play an important role in gastrointestinal motility [2–5]. According to the location in the gut wall, ICC can be classified into following major subtypes: ICC-MY (ICC in the myen-teric plexus, also called ICC-AP or ICC-MP), ICC-IM (ICC within the circular and longitudinal layers of muscle), ICC-DMP (ICC in the deep muscular plexus) and ICC-SMP (ICC in the submuscular plexus).
ICC along the gut
In the oesophagus, ICC are in the smooth muscle of the oesophagus and within the lower oesophageal sphincter (LOS). The ICC are of the ICC-IM subtype [6, 7]. In the stomach, the ICC are more densely located in the corpus and antrum than in the fundus. In the antrum, both ICC-MY and ICC-IM are present, whereas in the fun-dus, only ICC-IM are found [8–10]. Although there are conflicting reports, in general, the distribution of ICC-MY in the stomach is in agreement with the in vivo electrophysiological recording of slow waves responsible for gastric contractions. In the pylorus, there are ICC-IM [11, 12]. In the small intestine, there are ICC-MY, ICC-IM and ICC-DMP [13, 14]. Compared to the small intestine, there are less ICC in the colon; the ICC in the colon include subtypes of ICC-MY, ICC-IM and ICC-SMP [15, 16]. In the anorectal region, spindle-shaped ICC are present in both muscle layers, parallel to the smooth muscle cells. ICC are abundant, surrounding the myenteric ganglia. ICC at the submuscular plexus are less dense [17–20].
Functions of ICC
Based on in vitro studies, ICC are theorized to have the following functions: (1) to pace the slow waves and regulate slow wave propagation. The involved subtypes of ICC for these functions are ICC-MY in the stomach and small intestine, and ICC-SMP in the colon [21–25]. (2) To mediate enteric neural signals to the smooth muscles. ICC-IM are considered to have this function [16, 26–28]. (3) To act as mechanosensors [29]. However, some of the above theories have been put into question by a number of in vivo studies that are reviewed in this article. The discrepancies between the in vitro and in vivo studies may suggest that the available mutant mice or rats have complicated physiologies than are usually assumed [30].
Mutant animal models used in in vitro and in vivo studies
Three mutant models, W/Wv mice [31–33], Sl/Sld, mice [34, 35], Ws/Ws rats [28, 36–41] have been used to investigate the roles of ICC in the regulation of gastrointestinal motility. Specific subtypes of ICC are obliterated, reduced in numbers or damaged at different locations in the gastrointestinal tract. Accordingly, these models provide unique opportunities for the investigation of the roles of various subtypes of ICC in different organs of the gut.
W/Wv mutants have been most frequently used in the investigation of the roles of ICC in regulating gastrointestinal motility. In the W/Wv mouse, there is a loss of ICC-IM in the LOS [42], an almost absence of ICC-IM in the stomach [43, 44], a loss of ICC-IM in the pylorus sphincter [42], an almost complete loss of ICC-MY in the small intestine [43, 44], a loss of ICC-MY in the middle and distal colon [45] and an absence of ICC-MY in the internal anal sphincter [17].
Similar to W/Wv mice, there is an almost complete loss of ICC-MY in the small intestine of Sl/Sld mice [34, 35]. The ICC-DMP in the small intestine was, however, found to be normal. It appears that the characteristics of ICC in Sl/Sld mice are similar to those of W/Wv mice, but few studies have been performed with the Sl/Sld mouse to investigate the roles of ICC in regulating gastrointestinal motility.
The Ws/Ws rat is also frequently used in the ICC studies. Since the rat is larger in size, certain in vivo studies are more feasible in the Ws/Ws rat than the W/Wv mouse [28, 36–41]. ICC-IM were found to be absent in the LOS [36], the antrum [37] and the pylorus [38]. The ICC-MY are present in the stomach [37]. In the small intestine, ICC-MY were reported to be absent, but ICC-DMP were present [39]. The density of Kit-positive cells in the DMP of Ws/Ws rats was similar to those in wild-type rats. ICC-DMP in the rat of both wild-type and Ws/Ws mutants were similar in structure to ICC-DMP of the mouse. In the colon, the loss of Kit-positive cells (all ICC subtypes) was found to be more than 90% in the middle and distal parts of the colon; the loss of ICC-SMP was almost complete and the loss of ICC-MY was about 50% in the proximal colon [28]. That is, the two most important subtypes of ICC in the colon, ICC-DMP and ICC-IM, are virtually completely absent.
In general, the loss of ICC subtypes in various locations along the gastrointestinal tract is comparable among these three mutant models of rats and mice.
Roles of ICC in generating slow waves
Gastrointestinal slow waves and their clinical significance
In most of the clinical studies investigating the role of slow waves in controlling gastrointestinal motility, the slow waves are recorded using bipolar electrodes attached to the serosa or mucosa of the stomach or the abdominal skin [46]. The myoelectrical activity of the gut is composed of slow waves and spikes [47, 48]. According to in vivo serosal recordings, the fundus is believed to be electrically quiescent (without slow waves) and the corpus is where the pacemaker is located. The gastric slow wave propagates distally with an increased amplitude and velocity. The frequency of the slow wave is 3 cycles/min. (cpm) in human beings and 5 cpm in dogs. Figure 1 shows typical gastric slow waves measured from eight pairs of serosal electrodes along the greater curvature in a dog. Normal dis-tally propagated slow waves are present in the top panels (A).
Fig. 1.
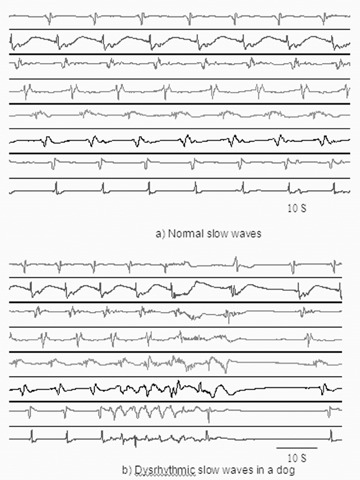
In vivo serosal recordings of normal gastric slow waves in dogs. The top tracing was from the electrodes 16 cm above the pylorus. The bottom tracing was from the electrodes 2 cm above the pylorus. The distance between the two channels was 2 cm. (A) Regular gastric slow waves and their distal propagations were observed. (B) Regular gastric slow waves and their distal propagations were observed at the beginning, and then, an ectopic pacemaker was present in the distal stomach, generating retrogradely propagated slow waves at a higher frequency.
According to the in vivo serosal recordings, the small intestinal slow waves originate from a region in the proximal 1 cm of the duodenum and propagate as an annular wavefront in an aborad direction [49]. It determines the frequency and the direction of propagation of intestinal contractions. In the dog, the proximal 10–30% of the small intestine (30–115 cm of the duodenum and jejunum) maintains the same slow wave frequency, 18–20 cpm, in a region called the ‘frequency plateau’[50]. Aborad to this point, there is a diminishing slow wave frequency gradient along the small bowel to a rate of 14 cpm in the distal ileum [50, 51]. In human beings, slow waves in the duodenum and proximal jejunum occur at about 12 cpm, with an aborad gradient to about 9 cpm in the terminal ileum [52, 53]. Whether a proximal plateau of identical frequencies is present in the human duodenum and proximal jejunum has not been clearly shown [52]. Transection and reanas-tomosis of the small bowel decrease the slow wave frequency in the distal segment in both dogs [54] and man [55]. In addition, at least in dogs, the propagation of slow waves in the distal segment becomes abnormal, with a high percentage of these slow waves propagating in an orad rather than an aborad direction [56].
The colonic slow waves in in vivo studies are not well characterized and have multiple frequencies without a dominant rhyth-micity. In a human study with bipolar serosal electrodes, it was reported that the ascending colon had a low level of signal that showed the simultaneous presence of variable and multiple frequency components in each of the two frequency ranges of 2–9 cpm and 9–13 cpm [57]. The slow wave in the transverse colon was characterized mostly by a single, stable frequency in the range of 9–13 cpm, with the presence of single or multiple frequencies in the range of 2–9 cpm. The means of slow wave frequencies were about 4 cpm and 11 cpm in the descending and sigmoid colon, respectively.
Slow wave dysrhythmias have been frequently reported in the stomach, but rarely in the small intestine and colon. The gastric slow waves and dysrhythmias can be detected using non-invasive methods of electrogastrography [46], whereas the measurement of intestinal and colonic slow waves in clinical settings is difficult since there is no non-invasive method. Gastric dysrhythmia includes bradygastria (slow wave frequency lower than normal), tachygastria (slow wave frequency higher than normal) and arrhythmia (no rhythmic slow waves). Numerous studies have shown that gastric dysrhythmia is associated with gastric motor disorders and/or gastrointestinal symptoms [46, 58–60]. A recent study in our laboratory has revealed that tachygastria is ectopic and of an antral origin [61]. In more than 80% of cases, tachygas-tria is located in the antrum and propagates retrogradely towards the pacemaker area of the proximal stomach. It may completely override normal distally propagating slow waves. However, most commonly, it does not completely override the normal gastric slow waves. In this case, there are two different slow wave activities: normal slow waves in the proximal stomach and tachygastrial slow waves in the distal stomach. A typical example is presented in Figure 1B: normal gastric slow waves propagating from the proximal stomach to the distal stomach were noted at the beginning of the tracing, and then, an ectopic pacemaker was present in the distal stomach firing slow waves at a higher frequency and propagating them orally to the proximal stomach. Close to the end of the tracings, the ectopic tachygastrial slow waves were overridden by the normal distally propagated slow waves. Tachygastria or tachyarrhythmia is associated with the absence or impairment of gastric contractions [60] and symptoms of nausea and vomiting [62, 63]. Unlike tachygastria, bradygastria is not ectopic and reflects purely a reduction in the frequency of normal pacemaking activity. That is, the entire stomach has one single frequency when bradygastria occurs [61]. Figure 2 illustrates a typical example: gastric slow waves were of a reduced frequency, but originated and propagated distally from the proximal stomach. It is seen that bradygastria originates in the corpus and propagates distally towards the pylorus. The gastric contractions may or may not be impaired with bradygastria [60].
Fig. 2.
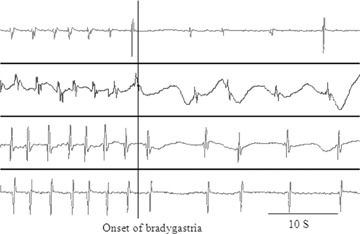
In vivo serosal recordings of normal and bradygastric slow waves in a dog. The top tracing was from the electrodes 16 cm above the pylorus. The bottom tracing was from the electrodes 2 cm above the pylorus. The distance between the two channels was 4 cm. Regular gastric slow waves and their distal propagations were observed at the beginning, followed with bradygastrial slow waves.
Little is known about the pathophysiology of the intestinal slow waves. A few studies reported intestinal slow wave dysrhythmia in clinical settings such as nausea and vomiting, intestinal pseudo-obstruction and intestinal ischemia [62, 64–68]. The abnormalities in the intestinal slow waves include dysrhythmia, reduced frequency and uncoordinated slow waves along the intestine and are associated with impaired intestinal contractions.
Nothing has been reported on the pathophysiology of the in vivo colonic slow waves, mainly attributed to the following factors: (1) the slow waves in the colon are not well characterized, and there is no means of differentiating dysrhythmic slow waves from normal slow waves in the colon and (2) there is a lack of in vivo measurement methods for the colonic slow waves that can be applied in clinical settings.
In vitro studies
A large number of in vitro studies have demonstrated the role of ICC as pacemakers with the evidence that can be summarized as follows: (1) ICC generate slow waves, (2) in the mutant animals where the ICC are absent, there are no slow waves and (3) in a specially prepared gastric tissue where ICC are obliterated by neutralizing antibody to Kit (activated Cdc42-associated kinase-2; ACK2), slow waves are absent [69]. A few studies from the groups of Huizinga and Ward performed with cultured ICC provided direct and strong evidence that the generation of pacemaker activity is an intrinsic property of the ICC [21, 70, 71]. Recordings from the intestinal muscles of the W/Wv mice in which ICC-MY are almost completely absent showed a complete loss of slow wave activity [72, 73]. Similar results were obtained from the muscles of Sl/Sl mice [34]. In order to investigate the role of ICC-MY in the generation of gastric slow waves, strips of gastric muscle tissues were incubated with the ACK2 for 31–50 days. This procedure obliterated all ICC, including ICC-MY, and abolished slow waves in the circular muscle cells [69]. Moreover, in the absence of ICC-MY, electrical field stimulation at a pulse width of about 122 msec. was not able to phase advance or pace gastric slow waves. These findings suggest that ICC-MY are pacemaker cells, and slow waves cannot be paced without ICC-MY.
In vivo studies
Conflicting results have been reported in a recent in vivo study suggesting that ICC may not be necessary for the generation of slow waves. In W/Wv mice (in which the pacemaking ICC, ICC-MY, are almost completely lost in the small intestine), gastrointestinal myoelectrical recordings were made from pairs of electrodes placed on the gastric and intestinal serosa. The slow waves were recorded in both anaesthetized and conscious W/Wv mice and they were not blocked by atropine or verapamil [74]. The in vivo slow waves recorded from the stomach of the W/Wv mice were identical to those from the stomach of the wild-type control mice. The in vivo slow waves from the small intestine of the W/Wv mice were, however, impaired compared to those recorded from the control mice, reflected as a decrease in the slow wave frequency (from 18.8 cpm to 10.7 cpm) and rhythmicity. Moreover, in comparison to the control rats, the W/Wv mice showed significantly reduced antegrade propagation and increased simultaneous and retrograde propagation. Various patterns of slow wave propagation are illustrated in Fig. 3. Similar findings were also reported in in vitro studies in W/Wv mice: the muscle strips taken from W/Wv mice in organ bath continued to generate slow waves and rhythmic phasic contractions, but both are more irregular in frequency and smaller in amplitude [72, 75–77]. Since the ICC-MY in the small intestine are almost completely lost in W/Wv mice, these findings suggest that ICC-MY may not be the sole pacemaker. Apparently, more studies, especially in vivostudies, are needed to explain the difference in the generation of slow waves between the in vitro studies and in vivorecordings.
Fig. 3.
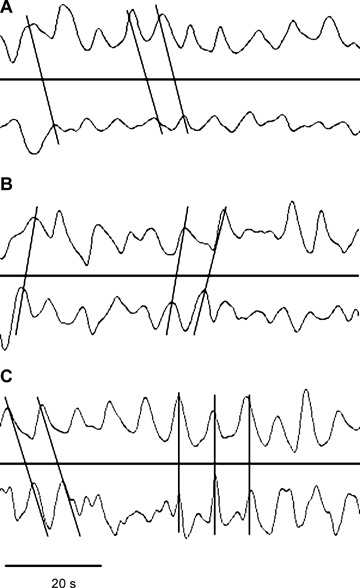
A 1-min. recording of intestinal slow waves in a conscious W/Wv mouse. The electrical signals were recorded from two pairs of electrodes placed on the serosal surface of the duodenum at an interval of 5 cm. Top channel in each panel was from the proximal pair of electrodes, whereas the bottom channel was from the distal pair of electrodes. (A) Antegrade propagation. (B) Retrograde propagation. (C) Antegrade propagation followed by an absence of propagation.
Another in vivo study has suggested that ICC may not be needed for pacing slow waves [78]. In the W/Wv mice without ICC-MY in the small intestine, a complete entrainment of the intestinal slow waves was achieved in the same way as that in the control mice. The required stimulation pulse width was about 50 msec. and was the same for both control and W/Wv mice [78]. The finding of the study demonstrates that exogenous pacing can be achieved with the absence of ICC-MY. Similar findings are also reported in in vitro studies [79, 80]: in the intestine of W/Wv mice, there is a pacing system in the longitudinal muscle that does not require ICC-MY, and the absence of a normal network of ICC-MY does not abolish regular pacing activity. However, these studies were done to measure the contractile activity without actually recording the electrical pacemaker activity.
Roles of ICC in regulating gastrointestinal motility
Gastrointestinal motility and roles of slow waves
Gastrointestinal motility is important in the transportation of ingested food and absorption of nutrients along the gut. Upon food intake, the gastric fundus relaxes to accommodate the ingested food. Peristalsis (distally propagated contractions) generates in the proximal antrum and propagates distally to the pylorus. Before the antral contractile front reaches, the pylorus relaxes or opens, and the gastric chyme expels out to the small intestine through the pylorus. In the small intestine, mixed contractile patterns (antegrade, simultaneous and retrograde) are present in the post-prandial state. These mixed patterns of contractions are necessary for the absorption of nutrients and transport of the chyme along the small intestine. The contractile patterns in the colon are more complex and have not been well defined. The only well-defined pattern of colonic contractions is the giant migrating contractions that are known to transport faeces from the proximal colon to the distal colon.
In addition to the rhythmic contractions or peristalsis mentioned above, the gut also generates and maintains tone. The tone or resting pressure of various sphincters along the gut also plays important roles in the transport of the ingested food along the gut. The gut sphincters include the LOS, pylorus and anal sphincter. A decrease in LOS may lead to gastro-oesophageal reflux, whereas an impaired relaxation of LOS during swallows is one of major causes of dysphagia. Impaired pyloric relaxation may lead to pyloric stenosis or delayed gastric emptying. In the anus, the weakness of the anal sphincter is related to faecal incontinence, and the failure in the relaxation of the anal sphincter is associated with outlet-obstructive constipation.
Although numerous studies have reported the absence of ICC in various patient populations [81–89], the roles of ICC or slow waves in regulating gastrointestinal motility should not be exaggerated. Slow waves are known to determine the time, frequency and propagation of contractions. However, they do notdirectly initiate effective gastrointestinal contractions. The binding of acetylcholine to muscarinic receptors during slow wave depolarization is essential for the initiation of rhythmic contractions [90, 91]. Accordingly, the roles of ICC in the regulation of rhythmic contractions are, somehow, limited: when ICC are normally distributed and present, a gastrointestinal organ may still have impaired motility due to electromechanical uncoupling (presence of ICC but lack of contractions); when ICC are damaged or lost, the gastrointestinal organ is expected to have impaired motility. However, it has not been established as to what extent ICC have to be damaged in order for the organ to exhibit impaired motility. The ICC do not directly control the gastrointestinal tone. However, recent in vitro studies have suggested that certain subtypes of ICC are involved in the neurotransmission between the enteric neurons and the smooth muscles, and therefore, play important roles in the regulation of the gastrointestinal tone since the tone is largely regulated by the enteric neurons via certain neurotransmitters such as nitric oxide (NO). A detailed discussion on the roles of ICC in neurotransmission is presented in the following sections.
Roles of ICC in regulating peristaltic contractions
A number of studies (mostly in vivo) have investigated the roles of ICC in regulating peristaltic contractions along the gut. Impaired gastrointestinal contractions have been frequently reported in all three Kit-mutant models of rats and mice discussed previously [16, 28, 34, 36–41, 92].
In an in vivo study performed in Huizinga's lab, movement of barium sulfate in the small intestine of W/Wv mice was monitored using radiography. Regular peristaltic waves were observed and distal movement of intestinal contents was noted in the control mice, but was absent in the W/Wv mice [75]. The action potentials and contractions appeared random, the contents of the small intestine moved back and forth in an irregular manner in the W/Wv mice and the net propulsive effect of the contractile activity in the W/Wv mice was much weaker than that in the control mice. However, no difference was observed in the small intestinal transit between control mice and W/Wv mice in a study performed in our lab in which movement of a non-nutrient dye (phenol red) through the entire small intestine was assessed quantitatively [78]. Transit studies with various nutrient-rich materials are yet to be performed. The role of ICC in the regulation of intestinal contractions was studied in an in vivo study in Ws/Ws rats using strain gauge transducers. Compared to the control rats, the ICC-deficient (a 95% loss of ICC-MY) Ws/Ws rats showed a reduced small intestinal contractile force in the fed state and an absence of regular migrating motor complex in the fasting state. A reduced contractile force and an impaired propagation of contractions in the ileum were reported in an in vitro study in W/Wv mice [93].
In the colon, impaired colonic contractions were recorded in Ws/Ws rats in which the density of Kit-positive cells was markedly reduced. The wild-type, but not Ws/Ws, rats showed low- and high-frequency cyclic depolarization that was associated with highly regular myogenic motor patterns at the same frequencies. In Ws/Ws rats, irregular patterns of action potentials triggered irregular muscle contractions, occurring within a band width of 10–20 cpm. Spontaneous activity of the nitrergic nerves caused a sustained inhibition of the muscle activity in both wild-type and Ws/Ws rats [28]. In another in vivo study, the number of contractions in both the ascending and sigmoid colon in Ws/Ws rats was found to be significantly lower than that in the control rats [94]. An in vitro muscle strip study, however, showed unaltered colonic contractions in the proximal colon of Ws/Ws mutant rats [41].
In summary, ample data exist in the literature showing motility abnormalities with the loss of ICC [95], and these findings are consistent with the physiological findings in patients: abnormalities in slow waves lead to impaired motility of the gut [60]. There is no doubt that ICC play an important role in controlling gastrointestinal motility.
Roles of ICC in mediating neural transmission
While slow waves determine the timing, frequency and propagation of gastrointestinal contractions, and an impairment in the slow waves leads to a disturbed gastrointestinal motility, there is no one-to-one correlation between the slow waves and contractions. The excitation and inhibition of gastrointestinal muscles are achieved by neural inputs from the enteric nerves mainly via the cholinergic and nitrergic pathways. Accordingly, a comprehensive control of gastrointestinal motility by ICC cannot be achieved unless ICC are also involved in the transmission of neural transmitters from the enteric nerves to the gut muscles. A number of recent in vitro studies have indeed suggested such a pivotal role of ICC in neurotrans-mission. However, conflicting results have been reported in a number of in vivo studies, which is the subject of this section.
In vitro studies
A number of in vitro studies have suggested that ICC-IM in the stomach and ICC-DMP in the small intestine mediate the enteric neural input to the gastrointestinal muscle cells.
LOS and pylorus sphincter
In the LOS and pyloric sphincter, ICC-IM was reported to mediate nitrergic neurotransmission between the enteric nerves and muscles [42]. The LOS and pylorus sphincter contain spindle-shaped ICC-IM that form close relationships with the NO synthase-containing nerve fibers. The pylorus contains ICC within the myenteric plexus and c-Kit immunopositive cells along the submucosal surface of the circular muscle. In W/Wv mice, ICC-IM was reported to be absent in the LOS and pylorus, but the distribution of inhibitory nerves was found to be normal. An in vitro study showed that NO-dependent inhibitory neurotransmission was reduced and hyperpolarizations to sodium nitroprusside were also attenuated in W/Wv mice. The authors concluded that ICC-IM play an important role in NO-dependent neurotransmission in the LOS and pylorus, and the loss of ICC-IM may interfere with relaxations and normal motility in these sphincters [42].
Stomach
Similar to the LOS and pylorus sphincter, there is a loss of ICC-IM but normal distribution of inhibitory nerves in the stomach. However, the NO-dependent inhibitory neuroregulation was reported to be greatly reduced, and smooth muscle tissues relaxed in response to exogenous sodium nitroprusside, whereas the membrane potential effects of sodium nitroprusside were attenuated [26]. These data suggested that ICC-IM in the stomach play a critical role in NO-dependent neurotransmission in the stomach. A similar role of ICC-IM in cholinergic excitatory neurotransmission was also reported [96]: in W/Wv mice, there was a loss of neural responses in the smooth muscles to cholinergic stimulation [97].
Small intestine
In the small intestine, ICC-IM are replaced by a dense network of ICC located at the level of deep muscular plexus; ICC-DMP are intimately associated with the enteric nerve terminals [98]. The enteric nerve terminals appear to form synapses preferentially with ICC-DMP rather than the smooth muscle cells [99]. In an ultrastructure study, it was found that ICC-DMP were innervated by both cholinergic and nitrergic nerves and were the only cells to possess specialized synapse-like junctions with nerve varicosities and gap junction contacts with the smooth muscle cells [100]. The functional role of ICC-DMP is difficult to prove since they persist in the small intestine of W/Wv and Sl/Sld mutants. However, the loss of ICC-DMP by blocking Kit was shown to cause loss of cholinergic and nitrergic neural responses [101], suggesting that in the small intestine, ICC-DMP play a critical role in the cholinergic and nitrergic neurotransmission.
Colon
In the colon, the findings of an in vitro study do not seem to support the role of ICC in the nitrergic neurotransmission [28]. In Ws/Ws rats, all subtypes of ICC were found to be lost by more than 90% in the middle and distal colon. Compared to the wild-type, the muscle strips of Ws/Ws rats showed irregular muscle contractions. However, spontaneous activity of nitrergic nerves caused a sustained inhibition of the muscle activity in both wild-type and Ws/Ws rats. Electrical field stimulation of enteric nerves, after blockade of cholinergic and adrenergic activity, elicited inhibition of the mechanical activity and biphasic inhibitory junction potentials in both wild-type and Ws/Ws rats [28]. These data suggest that ICC are not essential for the nitrergic neural transmission.
In vivo studies
On the contrary, most of the in vivo studies do not seem to support the theory that ICC-IM or ICC-DMP are critical for neuro-transmission. The findings of these in vivo studies are discussed in this section.
Lower oesophageal sphincter
In the LOS, ICC-IM do not seem to play a role in the nitrergic neurotransmission. The LOS mains a basal tone and relaxes with swallowing that is caused by the activation of inhibitory nonadrenerignoncholingergic nerves. NO has been shown to be a major inhibitory neurotransmitter. In mice with neuronal NO synthase gene disruption (nNOS−/–), W/Wv mice lacking ICC-IM and control mice, an intraluminal manometry was performed under anaesthesia [102]. The LOS in the nNOS−/– mice was significantly hypertensive and its relaxation to swallowing and efferent vagal stimulation was markedly impaired. In contract, the LOS in the W/Wv mice is hypotensive and relaxes normally to swallowing and efferent vagal activation. Similar normal relaxation to electrical stimulation was also noted in the LOS circular muscle strip of W/Wv mice [103], suggesting that despite the absence of c-Kit-positive ICC, a nerve–muscle interaction can be accomplished likely by the diffusion of neurotransmitters to the smooth muscle cells.
Stomach
The role of ICC-IM in the mediation of neurotransmission in the stomach was denied in a recent in vivo study investigating gastric adaptive relaxation in W/Wv mice [36]. Gastric accommodation refers to an in vivo reflex that causes relaxation of the stomach to avoid undue intraluminal pressure due to filling of the stomach. This reflex involves the vagal and intrinsic enteric nerves stimulated by the distention of the stomach. Upon gastric distention, the vagal nerves are activated and an inhibitory neurotransmitter, NO, is released and the stomach is relaxed. In the recent in vivo study, gastric accommodation was assessed in the isolated whole stomach of wild-type and W/Wv mice [103]. It was reported that despite the absence of ICC-IM, normal gastric adaptive relaxation occurred in the W/Wv mice stomach, suggesting that ICC-IM are not essential for the activation of NO-mediated relaxation. Similar to the in vivo study of the LOS [102], the basal tone of the stomach in the W/Wv mice was found to be lower than the control mice. The discrepancy in the findings between the in vitro and in vivo studies is explained in a recent study [104]. In a carefully designed in vitro study, Huizinga et al. examined the responses of muscle strips taken from the fundus of Ws/Ws rats to electrical field stimulation and found that the lack of inhibitory responses to the electrical field stimulation was not due to the inability of neurotransmissions to reach the smooth muscle cells but rather due to the lack of an active tone, which was also reported in the LOS [102]. In addition, nerve stimulation excited the noncholinergic excitatory nerves that masked the effect of the inhibitory nerves [104].
Colon
Peristaltic reflex is a well-known phenomenon in the small intestine and colon, mediated by the cholinergic excitation and nitrergic relaxation. One in vivo study in W/Wv mice has demonstrated that ICC may have no important role in this neural peristaltic reflex [45]. In the intestine, local distention by either inflation of a small balloon or mechanical distention induces contraction (ascending contraction) and relaxation (descending relaxation) at the regions oral and anal to the distended region, respectively. Such neural reflexes are of great importance since they move the intestinal bolus from the proximal gut to the distal gut. The ascending contraction is mediated by the cholinger-gic or tachykinin pathway, whereas the descending relaxation is achieved via the nitrergic mechanism. The segment of distal colon taken from the W/Wv mice showed a normal ascending contraction and a descending contraction in response to balloon distention of the segment [45].
Anal sphincter
In the anal sphincter, in vivo studies reported similar findings as in the stomach and colon, suggesting limited or no roles of ICC in the nitrergic relaxation of the anal sphincter [17, 105]. In one in vivo study, the anal sphincter of W/Wv mice showed a reduced basal pressure and normal relaxation to rectal distention, a phenomenon called rectoanal inhibitory reflex (RAIR) mediated by the inhibitory nitrergic pathway [105]. In another in vivo study, distention of the rectum elicited a volume-dependent relaxation of the anal sphincter in W/Wv mice; the degree of relaxation was lower than that in the control mice at low distention volume but comparable to the control nice at high distention volume [17]. The in vitro experiment of the same study also failed to confirm the role of ICC-IM in neurotransmission. Electrical stimulation of the internal anal sphincter of W/Wv mice relaxed to the same extent as those from controls. In addition, blockade of NO biosynthesis greatly reduced these relaxations, indicating that nitrergic innervation was intact. The latter was further confirmed by immunohistochemical staining. Since ICC-IM was lacking in the W/Wv mice, these results argue against a role for ICC as an intermediate between the nitrergic nerves and smooth muscle cells [17].
Roles of ICC as mechanoreceptors
The theory that ICC act as mechanoreceptors in the gut is based on morphological studies and one single physiological study [29, 106, 107]. Based on an in vitro study, it was theorized as follows: the ingestion of a meal stretches the antrum; the ICC-IM in the antrum sense this stretch, and the sensing mechanisms in the ICC-IM transiently depolarize the smooth muscle cells to cause an increase in the slow wave frequency and a decrease in its amplitude. This hypothesis is based on the finding that the antral muscle strips from W/Wv mice lacking ICC-IM do not exhibit the transient increase in the slow wave frequency and decrease in its amplitude [29]. While not much information is available in the literature to support or dispute the above hypothesis, in vivo physiological data seem to be against the notion that the gastric slow wave frequency increases transiently after the ingestion of a meal or when the stomach is distended. A number of in vivo physiological studies have actually shown the opposite: there is a transient decrease in the slow wave frequency upon food ingestion, measured by serosal bipolar electrodes as well as cutaneous abdominal electrodes, a method called electrogastrography [46, 108–111]. In an in vivo canine study with the gastric slow waves measured from a pair of electrodes placed on the gastric serosa, it was found that the frequency of the gastric slow waves decreased linearly with greater initial meal volume. A transient frequency dip in the gastric slow waves after a meal has been frequently and consistently reported in electrogastrographic studies [109–114]. Figure 4 shows a typical decrease in the slow wave frequency measured in a conscious dog using a pair of gastric serosal electrodes when the stomach was distended by an intra-gastric balloon; the frequency of the gastric slow wave was about 6 cpm before gastric distention and reduced to about 5 cpm during gastric distention.
Fig. 4.
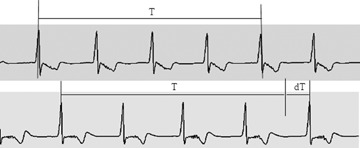
Effects of gastric distention on gastric slow wave frequencies in a dog. Top: 80-sec. slow wave recordings from a pair of serosal electrodes at the baseline. Bottom: 80-sec. slow wave recordings from the same pair of serosal electrodes when the stomach was distended by an intragastric balloon with a pressure of 40 mmHg. An obvious decrease in slow wave frequency can be appreciated by comparing the time intervals covering four cycles of slow waves at the baseline (T) and during distention (T + dT): the longer time period for the four slow waves during distention is indicative of a decrease in the slow wave frequency.
However, there is lack of in vivo studies to test the hypothesis that ICC serve as mechanoreceptors.
Conclusions
To better understand the roles of ICC in regulating gastrointestinal motility, the in vitro and in vivo physiological motility studies and their findings are discussed in this article. Based on morphological and in vitrophysiological studies, ICC are believed to be pacemaker cells generating slow waves to serve as mediators in neural transmission and may also act as mechanoreceptors.
The theory that ICC-MY are pacemaker cells seems well established based on numerous in vitro studies. However, pacemaker activity as observed in vivo may be complex and involve smooth muscle cells. (1) A recent in vivo study demonstrated the presence of slow waves in the small intestine of W/Wv mice with the absence of ICC-MY, (2) some in vitro studies also showed rhythmic electrical activities called action potentials and suggested that these electrical activities may be generated by smooth muscles and (3) although motor abnormalities in vivo are associated with the loss of ICC, further in vivo evidence is needed.
The role of ICC-IM or ICC-DMP in mediating neural transmission between the enteric nerves and the gut muscles is established according to the majority of in vitro studies. However, findings in vivo motility studies do not seem to support the role of ICC-IM as neural mediators, and recent in vitro studies have demonstrated alternative interpretations of previous findings. Accordingly, one has to exercise caution in interpreting in vitro and in vivo data regarding the role of ICC-IM or ICC-DMP in neural transmission.
The hypothesis that ICC-IM may also act as mechanoreceptors needs further investigation. However, in vivo physiological findings thus far have not provided evidence.
The clinical significance of ICC is dependent on the roles of ICC in regulating gastrointestinal motility. There is no doubt that the damage or loss of ICC results in dysmotility in the gut. However, future studies are needed to establish a quantitative correlation between the loss of ICC and the severity of motility disorders. If this can be established, ICC may be used as biomarkers of gastrointestinal motility disorders. More studies are needed to confirm the role of ICC as mediators of neurotransmission. If ICC are truly involved in neurotransmission, they will be of great clinical significance.
Acknowledgments
This work is partially supported by grants from the NIH (DK063733, DK055437 and DK075155).
References
- 1.Cajal. Sur les ganglions et plexus nerveux d’intestin. C R Soc Biol. 1893;5:217–23. [Google Scholar]
- 2.Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neu-rotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- 3.Huizinga JD, Thuneberg L, Vanderwinden JM, Rumessen JJ. Interstitial cells of Cajal as targets for pharmacological intervention in gastrointestinal motor disorders. Trends Pharmacol Sci. 1997;18:393–403. doi: 10.1016/s0165-6147(97)01108-5. [DOI] [PubMed] [Google Scholar]
- 4.Vanderwinden JM, Rumessen JJ. Interstitial cells of Cajal in human gut and gastrointestinal disease. Microsc Res Tech. 1999;47:344–60. doi: 10.1002/(SICI)1097-0029(19991201)47:5<344::AID-JEMT6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Streutker CJ, Huizinga JD, Driman DK, Riddell RH. Interstitial cells of Cajal in health and disease. Part I: normal ICC structure and function with associated motility disorders. Histopathology. 2007;50:176–89. doi: 10.1111/j.1365-2559.2006.02493.x. [DOI] [PubMed] [Google Scholar]
- 6.Faussone-Pellegrini MS, Cortesini C. Ultrastructural features and localization of the interstitial cells of Cajal in the smooth muscle coat of human esophagus. J. Submicrosc Cytol. 1985;17:187–97. [PubMed] [Google Scholar]
- 7.Torihashi S, Horisawa M, Watanabe Y. c-Kit immunoreactive interstitial cells in the human gastrointestinal tract. J Auton Nerv Syst. 1999;75:38–50. doi: 10.1016/s0165-1838(98)00174-x. [DOI] [PubMed] [Google Scholar]
- 8.Mazet B, Raynier C. Interstitial cells of Cajal in the guinea pig gastric antrum: distribution and regional density. Cell Tissue Res. 2004;316:23–34. doi: 10.1007/s00441-003-0835-9. [DOI] [PubMed] [Google Scholar]
- 9.Hirst GD, Beckett EA, Sanders KM, Ward SM. Regional variation in contribution of myenteric and intramuscular interstitial cells of Cajal to generation of slow waves in mouse gastric antrum. J Physiol. 2002;540:1003–012. doi: 10.1113/jphysiol.2001.013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen J, Rick GA, Lowe LS. Distributions of interstitial cells of Cajal in stomach and colon of cat, dog, ferret, opossum, rat, guinea pig and rabbit. J Auton Nerv Syst. 1992;37:47–56. doi: 10.1016/0165-1838(92)90144-6. [DOI] [PubMed] [Google Scholar]
- 11.Vanderwinden JM, Liu H, De Laet MH, Vanderhaeghen JJ. Study of the interstitial cells of Cajal in infantile hypertrophic pyloric stenosis. Gastroenterology. 1996;111:279–88. doi: 10.1053/gast.1996.v111.pm8690192. [DOI] [PubMed] [Google Scholar]
- 12.Langer JC, Berezin I, Daniel EE. Hypertrophic pyloric stenosis: ultrastruc-tural abnormalities of enteric nerves and the interstitial cells of Cajal. J Pediatr Surg. 1995;30:1535–43. doi: 10.1016/0022-3468(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 13.Rumessen JJ, Mikkelsen HB, Qvortrup K, Thuneberg L. Ultrastructure of interstitial cells of Cajal in circular muscle of human small intestine. Gastroenterology. 1993;104:343–50. doi: 10.1016/0016-5085(93)90400-7. [DOI] [PubMed] [Google Scholar]
- 14.Rumessen JJ, Thuneberg L. Interstitial cells of Cajal in human small intestine. Ultrastructural identification and organization between the main smooth muscle layers. Gastroenterology. 1991;100:1417–31. [PubMed] [Google Scholar]
- 15.Torihashi S, Horisawa M, Watanabe Y. c-Kit immunoreactive interstitial cells in the human gastrointestinal tract. J Auton Nerv Syst. 1999;75:38–50. doi: 10.1016/s0165-1838(98)00174-x. [DOI] [PubMed] [Google Scholar]
- 16.Sanders KM, Ward SM. Kit mutants and gastrointestinal physiology. J Physiol. 2007;578:33–42. doi: 10.1113/jphysiol.2006.122473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Lorijn F, de Jonge WJ, Wedel T, Vanderwinden JM, Benninga MA, Boeckxstaens GE. Interstitial cells of Cajal are involved in the afferent limb of the rectoanal inhibitory reflex. Gut. 2005;54:1107–13. doi: 10.1136/gut.2004.051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horiguchi K, Keef KD, Ward SM. Distribution of interstitial cells of Cajal in tunica muscularis of the canine rectoanal region. Am J Physiol Gastrointest Liver Physiol. 2003;284:G756–67. doi: 10.1152/ajpgi.00294.2002. [DOI] [PubMed] [Google Scholar]
- 19.Hagger R, Gharaie S, Finlayson C, Kumar D. Distribution of the interstitial cells of Cajal in the human anorectum. J Auton Nerv Syst. 1998;73:75–9. doi: 10.1016/s0165-1838(98)00038-1. [DOI] [PubMed] [Google Scholar]
- 20.Hagger R, Gharaie S, Finlayson C, Kumar D. Regional and transmural density of interstitial cells of Cajal in human colon and rectum. Am J Physiol Gastrointest Liver Physiol. 1998;275:G1309–16. doi: 10.1152/ajpgi.1998.275.6.G1309. [DOI] [PubMed] [Google Scholar]
- 21.Lee JC, Thuneberg L, Berezin I, Huizinga JD. Generation of slow waves in membrane potential is an intrinsic property of interstitial cells of Cajal. Am J Physiol. 1999;277:G409–23. doi: 10.1152/ajpgi.1999.277.2.G409. [DOI] [PubMed] [Google Scholar]
- 22.Komuro T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J Physiol. 2006;576:653–8. doi: 10.1113/jphysiol.2006.116624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirst GD, Edwards FR. Role of interstitial cells of Cajal in the control of gastric motility. J Pharmacol Sci. 2004;96:1–10. doi: 10.1254/jphs.crj04002x. [DOI] [PubMed] [Google Scholar]
- 24.Liu LW, Daniel EE, Huizinga JD. Excitability of canine colon circular muscle disconnected from the network of interstitial cells of Cajal. Can J Physiol Pharmacol. 1992;70:289–95. doi: 10.1139/y92-036. [DOI] [PubMed] [Google Scholar]
- 25.Liu LW, Huizinga JD. Electrical coupling of circular muscle to longitudinal muscle and interstitial cells of Cajal in canine colon. J Physiol. 1993;470:445–61. doi: 10.1113/jphysiol.1993.sp019868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci USA. 1996;93:12008–13. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki H, Ward SM, Bayguinov YR, Edwards FR, Hirst GD. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J Physiol. 2003;546:751–63. doi: 10.1113/jphysiol.2002.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albertí E, Mikkelsen HB, Wang XY, Díaz M, Larsen JO, Huizinga JD, Jiménez M. Pacemaker activity and inhibitory neuro-transmission in the colon of Ws/Ws mutant rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1499–510. doi: 10.1152/ajpgi.00136.2006. [DOI] [PubMed] [Google Scholar]
- 29.Won KJ, Sanders KM, Ward SM. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci USA. 2005;102:14913–8. doi: 10.1073/pnas.0503628102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarna SK. Are interstitial cells of Cajal plu-rifunction cells in the gut? Am J Physiol Gastrointest Liver Physiol. 2008;294:G372–90. doi: 10.1152/ajpgi.00344.2007. [DOI] [PubMed] [Google Scholar]
- 31.Besmer P, Manova K, Duttlinger R, Huang EJ, Packer A, Gyssler C, Bachvarova RF. The kit ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogene-sis. Dev Suppl. 1993:125–37. [PubMed] [Google Scholar]
- 32.Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-onco-gene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–9. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 33.Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K, Nishikawa S. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–75. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- 34.Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995;269:C1577–85. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- 35.Mikkelsen HB, Malysz J, Huizinga JD, Thuneberg L. Action potential generation, Kit receptor immunohistochemistry and morphology of steel-Dickie (Sl/Sld) mutant mouse small intestine. Neurogastroenterol Motil. 1998;10:11–26. doi: 10.1046/j.1365-2982.1998.00082.x. [DOI] [PubMed] [Google Scholar]
- 36.Farré R, Wang XY, Vidal E, Domènech A, Pumarola M, Clave P, Huizinga JD, Jiménez M. Interstitial cells of Cajal and neuromuscular transmission in the rat lower oesophageal sphincter. Neurogastroenterol Motil. 2007;19:484–96. doi: 10.1111/j.1365-2982.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- 37.Mitsui R, Komuro T. Distribution and ultrastructure of interstitial cells of Cajal in the gastric antrum of wild-type and Ws/Ws rats. Anat Embryol. 2003;206:453–60. doi: 10.1007/s00429-003-0323-8. [DOI] [PubMed] [Google Scholar]
- 38.Nakama A, Hirota S, Okazaki T, Nagano K, Kawano S, Hori M, Kitamura Y. Disturbed pyloric motility in Ws/Ws mutant rats due to deficiency of c-kit-expressing interstitial cells of Cajal. Pathol Int. 1998;48:843–97. doi: 10.1111/j.1440-1827.1998.tb03850.x. [DOI] [PubMed] [Google Scholar]
- 39.Takeda M, Takayama I, Terada N, Baba T, Ward SM, Ohno S, Fujino MA. Immunoelectron-microscopic study of Kit-expressing cells in the jejunum of wild-type and Ws/Ws rats. Cell Tissue Res. 2001;304:21–30. doi: 10.1007/s004410000333. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi A, Tomomasa T, Kaneko H, Hatori R, Ishige T, Suzuki M, Mochiki E, Morikawa A, Kuwano H. In vivo recording of colonic motility in conscious rats with deficiency of interstitial cells of Cajal, with special reference to the effects of nitric oxide on colonic motility. J Gastroenterol. 2005;40:1043–8. doi: 10.1007/s00535-005-1688-7. [DOI] [PubMed] [Google Scholar]
- 41.Yoneda S, Kadowaki M, Sugimori S, Sekiguchi F, Sunano S, Fukui H, Takaki M. Rhythmic spontaneous contractions in the rat proximal colon. Jpn J Physiol. 2001;51:717–23. doi: 10.2170/jjphysiol.51.717. [DOI] [PubMed] [Google Scholar]
- 42.Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmis-sion in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–29. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]
- 43.Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995;269:C1577–85. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- 44.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–7. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okishio Y, Takeuchi T, Fujita A, Suenaga K, Fujinami K, Munakata S, Takewaki T, Hata F. Ascending contraction and descending relaxation in the distal colon of mice lacking interstitial cells of Cajal. J Smooth Muscle Res. 2005;41:163–74. doi: 10.1540/jsmr.41.163. [DOI] [PubMed] [Google Scholar]
- 46.Chen JDZ, McCallum RW. Electrogastrography: principles and applications. New York: Raven Press; 1994. [Google Scholar]
- 47.Sarna SK, Daniel EE. Gastrointestinal electrical activity: terminology. Gastroenterology. 1975;68:1631–5. [PubMed] [Google Scholar]
- 48.Hinder RA, Kelly KA. Human gastric pacemaker potential: site of origin, spread, and response to gastric transaction and proximal gastric vagotomy. Am J Surg. 1977;133:29–33. doi: 10.1016/0002-9610(77)90187-8. [DOI] [PubMed] [Google Scholar]
- 49.Hermon-Taylor J, Code CF. Localization of the duodenal pacemaker and its role in the organization of duodenal myoelectric activity. Gut. 1971;12:40–7. doi: 10.1136/gut.12.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szurszewski JH, Elveback LR, Code CF. Configuration and frequency gradient of electric slow wave over canine small bowel. Am J Physiol. 1970;218:1468–73. doi: 10.1152/ajplegacy.1970.218.5.1468. [DOI] [PubMed] [Google Scholar]
- 51.Bunker CE, Johnson LP, Nelsen S. Chronic in situ studies of the electrical activity of the small intestine. Arch Surg. 1967;95:259–68. doi: 10.1001/archsurg.1967.01330140097023. [DOI] [PubMed] [Google Scholar]
- 52.Soper NJ, Sarr MG, Kelly KA. Human duodenal myoelectric activity after operation and with pacing. Surgery. 1990;107:63–8. [PubMed] [Google Scholar]
- 53.Christensen J, Schedl HP, Clifton JA. The small intestine basic electrical rhythm (slow wave) frequency gradient in normal men and in patients with a variety of diseases. Gastroenterology. 1966;50:309–15. [PubMed] [Google Scholar]
- 54.Akwari OE, Kelly KA, Steinbach JH, Code CF. Electric pacing of intact and transected canine small intestine and its computer model. Am J Physiol. 1975;229:1188–97. doi: 10.1152/ajplegacy.1975.229.5.1188. [DOI] [PubMed] [Google Scholar]
- 55.Richter HM, III, Kelly KA. Effect of transec-tion and pacing on human jejunal pacesetter potentials. Gastroenterology. 1986;91:1380–5. doi: 10.1016/0016-5085(86)90190-3. [DOI] [PubMed] [Google Scholar]
- 56.Karistrom L, Kelly KA. Ectopic jejunal pacemakers and gastric emptying after Roux gastractomy: effect of intestinal pacing. Surgery. 1989;106:867–71. [PubMed] [Google Scholar]
- 57.Sarna SK, Bardakjian BL, Waterfall WE, Lind JF. Human colonic electrical control activity (ECA) Gastroenterology. 1980;78:1526–36. [PubMed] [Google Scholar]
- 58.Camilleri M, Hasler W, Parkman HP, Quigley EMM, Soffer E. Measurement of gastroduodenal motility in the GI laboratory. Gastroenterology. 1998;115:747–62. doi: 10.1016/s0016-5085(98)70155-6. [DOI] [PubMed] [Google Scholar]
- 59.Abell TL, Malagelada J-R. Glucagonevoked gastric dysrhythmias in humans shown by an improved electrogastrographic technique. Gastroenterology. 1985;88:1932–40. doi: 10.1016/0016-5085(85)90022-8. [DOI] [PubMed] [Google Scholar]
- 60.Chen JDZ, Pan J, McCallum RW. Clinical significance of gastric myoelectrical dysrhythmas. Dig Dis. 1995;13:275–90. doi: 10.1159/000171508. [DOI] [PubMed] [Google Scholar]
- 61.Qian LW, Pasricha PJ, Chen JDZ. Origin and patterns of spontaneous and drug-induced canine gastric myoelectrical dys-rhythmias. Dig Dis Sci. 2003;48:508–15. doi: 10.1023/a:1022532515172. [DOI] [PubMed] [Google Scholar]
- 62.You CH, Chey WY, Lee KY, Menguy R, Bortoff A. Gastric and small intestinal myoelectrical dysrhythmia associated with chronic intractable nausea and vomiting. Ann Int Med. 1981;95:449–51. doi: 10.7326/0003-4819-95-4-449. [DOI] [PubMed] [Google Scholar]
- 63.Telander RL, Morgan KG, Kreulen DL, Schemalz PF, Kelly KA, Szurszewki JH. Human gastric atony with tachygastria and gastric retention. Gastroenterology. 1978;75:495–501. [PubMed] [Google Scholar]
- 64.Abell TL, Kim CH, Malagelada J-R. Idiopathic cyclic nausea and vomiting – a disorder of gastrointestinal motility? Mayo Clin Proc. 1988;63:1169–75. doi: 10.1016/s0025-6196(12)65401-9. [DOI] [PubMed] [Google Scholar]
- 65.Sullivan MA, Snape WJ, Jr, Matarazzo SA, Ptrokubi RJ, Jeffries G, Cohen S. Gastrointestinal myoelectrical activity in idiopathic intestinal pseudoobstruction. N Engl J Med. 1977;297:233–8. doi: 10.1056/NEJM197708042970501. [DOI] [PubMed] [Google Scholar]
- 66.Schuffler MD. Chronic intestinal pseudoobstruction syndrome. Med Clin North Am. 1981;65:1331–58. doi: 10.1016/s0025-7125(16)31475-4. [DOI] [PubMed] [Google Scholar]
- 67.Ladipo JK, Bradshaw LA, Halter S, Richards WO. Intestinal tachyarrhythmias during small bowel ischemia. Am J Physiol. 1999;277:G993–9. doi: 10.1152/ajpgi.1999.277.5.G993. [DOI] [PubMed] [Google Scholar]
- 68.Golzarian J, Staton DJ, Wikswo JP, Jr, Friedman RN, Richards WO. Diagnosing intestinal ischemia using a noncontact superconducting quantum interference device. Am J Surg. 1994;167:586–92. doi: 10.1016/0002-9610(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 69.Ordog T, Ward SM, Sanders KM. Interstitial cells of Cajal generate electrical slow waves in the murine stomach. J Physiol. 1999;518:257–69. doi: 10.1111/j.1469-7793.1999.0257r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J Physiol. 1998;513:203–13. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomsen L, Robinson TL, Lee JF, Farraway LA, Hughes MJ, Andrews DW, Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med. 1998;4:848–51. doi: 10.1038/nm0798-848. [DOI] [PubMed] [Google Scholar]
- 72.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–7. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–9. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 74.Hou XH, Yin J, Liu JS, Pasricha PJ, Chen JDZ. In vivo gastric and intestinal slow waves in W/Wv mice. Dig Dis Sci. 2005;50:1335–41. doi: 10.1007/s10620-005-2783-6. [DOI] [PubMed] [Google Scholar]
- 75.Der-Silaphet T, Malysz J, Hagel S, Larry Arsenault A, Huizinga JD. Interstitial cells of Cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology. 1998;114:724–36. doi: 10.1016/s0016-5085(98)70586-4. [DOI] [PubMed] [Google Scholar]
- 76.Huizinga JD, Ambrous K, Der-Silaphet T. Co-operation between neural and myogenic mechanisms in the control of distension-induced peristalsis in the mouse small intestine. J Physiol. 1998;506:843–56. doi: 10.1111/j.1469-7793.1998.843bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Malysz J, Thuneberg L, Mikkelsen HB, Huizinga JD. Action potential generation in the small intestine of W mutant mice that lack interstitial cells of Cajal. Am J Physiol. 1996;271:G387–99. doi: 10.1152/ajpgi.1996.271.3.G387. [DOI] [PubMed] [Google Scholar]
- 78.Yin J, Hou XH, Chen JDZ. Roles of interstitial cells of Cajal in intestinal transit and exogenous electrical pacing. DigDis Sci. 2006;51:1818–23. doi: 10.1007/s10620-006-9313-z. [DOI] [PubMed] [Google Scholar]
- 79.Daniel EE, Willis A, Cho WJ, Boddy GL. Comparisons of neural and pacing activities in intestinal segment for W/W++ and W/Wv mice. Neurogastroenterol Motil. 2005;17:355–65. doi: 10.1111/j.1365-2982.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 80.Boddy G, Daniel EE. Role of L-Ca2+ channels in intestinal pacing in wild-type and W/Wv mice. Am J Physiol. 2005;288:G439–46. doi: 10.1152/ajpgi.00255.2004. [DOI] [PubMed] [Google Scholar]
- 81.Kubota M, Ito Y, Ikeda K. Membrane properties and innervation of smooth muscle cells in Hirschsprung's disease. Am J Physiol. 1983;244:G406–15. doi: 10.1152/ajpgi.1983.244.4.G406. [DOI] [PubMed] [Google Scholar]
- 82.Yu CS, Kim HC, Hong HK, Chung DH, Kim HJ, Kang GH, Kim JC. Evaluation of myenteric ganglion cells and interstitial cells of Cajal in patients with chronic idiopathic constipation. Int J Colorectal Dis. 2002;17:253–8. doi: 10.1007/s00384-001-0380-5. [DOI] [PubMed] [Google Scholar]
- 83.Wedel T, Spiegler J, Soellner S, Roblick UJ, Schiedeck TH, Bruch HP, Krammer HJ. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology. 2002;123:1459–67. doi: 10.1053/gast.2002.36600. [DOI] [PubMed] [Google Scholar]
- 84.Zarate N, Mearin F, Wang XY, Hewlett B, Huizinga JD, Malagelada JR. Severe idiopathic gastroparesis due to neuronal and interstitial cells of Cajal degeneration: pathological findings and management. Gut. 2003;52:966–70. doi: 10.1136/gut.52.7.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakahara M, Isozaki K, Hirota S, Vanderwinden JM, Takakura R, Kinoshita K, Miyagawa J, Chen H, Miyazaki Y, Kiyohara T, Shinomura Y, Matsuzawa Y. Deficiency of KIT-positive cells in the colon of patients with diabetes mellitus. J Gastroenterol Hepatol. 2002;17:666–70. doi: 10.1046/j.1440-1746.2002.02756.x. [DOI] [PubMed] [Google Scholar]
- 86.Lu G, Qian X, Berezin I, Telford GL, Huizinga JD, Sarna SK. Inflammation modulates in vitro colonic myoelectric and contractile activity and interstitial cells of Cajal. Am J Physiol. 1997;273:G1233–45. doi: 10.1152/ajpgi.1997.273.6.G1233. [DOI] [PubMed] [Google Scholar]
- 87.Vanderwinden JM, Liu H, De Laet MH, Vanderhaeghen JJ. Study of the interstitial cells of Cajal in infantile hypertrophic pyloric stenosis. Gastroenterology. 1996;111:279–88. doi: 10.1053/gast.1996.v111.pm8690192. [DOI] [PubMed] [Google Scholar]
- 88.Gerl A, Storck M, Schalhorn A, Muller-Hocker J, Jauch KW, Schildberg FW, Wilmanns W. Paraneoplastic chronic intestinal pseudoobstruction as a rare complication of bronchial carcinoid. Gut. 1992;33:1000–3. doi: 10.1136/gut.33.7.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jain D, Moussa K, Tandon M, Culpepper-Morgan J, Proctor DD. Role of interstitial cells of Cajal in motility disorders of the bowel. Am J Gastroenterol. 2003;98:618–24. doi: 10.1111/j.1572-0241.2003.07295.x. [DOI] [PubMed] [Google Scholar]
- 90.Sarna SK. Molecular, Functional and pharmacological targets for the development of gut promotility drugs. Am J Physiol. 2006;291:G545–5. doi: 10.1152/ajpgi.00122.2006. [DOI] [PubMed] [Google Scholar]
- 91.Sarna SK, Shi X-Z. In: Function and regulation of colonic contractions in health and disease. In: Physiology of the gastrointestinal tract. 4th ed. Johnson LR, Barret KE, Gishan FK, Merchant JL, Said H, Wood JD, editors. San Diego, CA: Elsevier; 2006. pp. 965–93. [Google Scholar]
- 92.Ordög T. Interstitial cells of Cajal in diabetic gastroenteropathy. Neurogastroenterol Motil. 2008;20:8–18. doi: 10.1111/j.1365-2982.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 93.Nakagawa T, Misawa H, Nakajima Y, Takaki M. Absence of peristalsis in the ileum of W/W(V) mutant mice that are selectively deficient in myenteric interstitial cells of Cajal. J Smooth Muscle Res. 2005;41:141–51. doi: 10.1540/jsmr.41.141. [DOI] [PubMed] [Google Scholar]
- 94.Takahashi A, Tomomasa T, Kaneko H, Hatori R, Ishige T, Suzuki M, Mochiki E, Morikawa A, Kuwano H. In vivo recording of colonic motility in conscious rats with deficiency of interstitial cells of Cajal, with special reference to the effects of nitric oxide on colonic motility. J Gastroenterol. 2005;40:1043–8. doi: 10.1007/s00535-005-1688-7. [DOI] [PubMed] [Google Scholar]
- 95.Huizinga JD. Neural injury, repair, and adaptation in the GI tract. IV. Pathophysiology of GI motility related to interstitial cells of Cajal. Am J Physiol. 1998;275:G381–6. doi: 10.1152/ajpgi.1998.275.3.G381. [DOI] [PubMed] [Google Scholar]
- 96.Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmis-sion from enteric motor neurons. J Neurosci. 2000;20:1393–403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suzuki H, Ward SM, Bayguinov YR, Edwards FR, Hirst GD. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J Physiol. 2003;546:751–63. doi: 10.1113/jphysiol.2002.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Torihashi S, Kobayashi S, Gerthoffer WT, Sanders KM. Interstitial cells in deep muscular plexus of canine small intestine may be specialized smooth muscle cells. Am J Physiol. 1993;265:G638–45. doi: 10.1152/ajpgi.1993.265.4.G638. [DOI] [PubMed] [Google Scholar]
- 99.Wang XY, Sanders KM, Ward SM. Intimate relationship between interstitial cells of Cajal and enteric nerves in the guinea-pig small intestine. Cell Tissue Res. 1999;295:247–56. doi: 10.1007/s004410051231. [DOI] [PubMed] [Google Scholar]
- 100.Wang XY, Paterson C, Huizinga JD. Cholinergic and nitrergic innervation of ICC-DMP and ICC-IM in the human small intestine. Neurogastroenterol Motil. 2003;15:531–43. doi: 10.1046/j.1365-2982.2003.00429.x. [DOI] [PubMed] [Google Scholar]
- 101.Ward SM, McLaren GJ, Sanders KM. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neuro-transmission in the mouse small intestine. J Physiol. 2006;573:147–59. doi: 10.1113/jphysiol.2006.105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sivarao DV, Mashimo HL, Thatte HS, Goyal RK. Lower esophageal sphincter is achalasic in nNOS(–/–) and hypotensive in W/W(v) mutant mice. Gastroenterology. 2001;121:34–42. doi: 10.1053/gast.2001.25541. [DOI] [PubMed] [Google Scholar]
- 103.Dixit D, Zarate N, Liu LW, Boreham DR, Huizinga JD. Interstitial cells of Cajal and adaptive relaxation in the mouse stomach. Am J Physiol. 2006;291:G1129–36. doi: 10.1152/ajpgi.00518.2005. [DOI] [PubMed] [Google Scholar]
- 104.Huizinga JD, Liu LW, Fitzpatrick A, White E, Gill S, Wang XY, Zarate N, Krebs L, Choi C, Starret T, Dixit D, Ye J. Deficiency of intramuscular ICC increases fundic muscle excitability but does not impede nitrergic innervation. Am J Physiol. 2008;294:G589–94. doi: 10.1152/ajpgi.00130.2007. [DOI] [PubMed] [Google Scholar]
- 105.Terauchi A, Kobayashi D, Mashimo H. Distinct roles of nitric oxide synthases and interstitial cells of Cajal in rectoanal relaxation. Am J Physiol. 2005;289:G291–9. doi: 10.1152/ajpgi.00005.2005. [DOI] [PubMed] [Google Scholar]
- 106.Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol. 1982;71:1–130. [PubMed] [Google Scholar]
- 107.Thuneberg L. One hundred years of interstitial cells of Cajal. Microsc Res Tech. 1999;47:223–38. doi: 10.1002/(SICI)1097-0029(19991115)47:4<223::AID-JEMT2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 108.Lin HC, Zhao XT, Chung B, GU YG, Elashoff JD. Frequency of gastric pacesetter potential depends on volume and site of distension. Am J Physiol. 1996;270:G470–5. doi: 10.1152/ajpgi.1996.270.3.G470. [DOI] [PubMed] [Google Scholar]
- 109.Geldof H, Van Der Schee EJ, van Blankenstein M, Smout AJ, Akkermans LM. Effects of highly selective vagotomy on gastric myoelectrical activity. An electrogastrographic study. Dig Dis Sci. 1990;35:969–75. doi: 10.1007/BF01537245. [DOI] [PubMed] [Google Scholar]
- 110.Kamiya T, Kobayashi Y, Hirako M, Misu N, Nagao T, Hara M, Matsuhisa E, Ando T, Adachi H, Sakuma N, Kimura G. Gastric motility in patients with recurrent gastric ulcers. J Smooth Muscle Res. 2002;38:1–9. doi: 10.1540/jsmr.38.1. [DOI] [PubMed] [Google Scholar]
- 111.Misu N, Kamiya T, Kobayashi Y, Hirako M, Nagao T, Shikano M, Matsuhisa E, Ando T, Adachi H, Kimura G. Effects of oral glucose intake on gastric myoelectrical activity and gastric emptying. J Smooth Muscle Res. 2004;40:169–76. doi: 10.1540/jsmr.40.169. [DOI] [PubMed] [Google Scholar]
- 112.Faussone-Pellegrini MS. Relationships between neurokinin receptor-expressing interstitial cells of Cajal and tachykininergic nerves in the gut. J Cell Mol Med. 2006;10:20–32. doi: 10.1111/j.1582-4934.2006.tb00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Popescu LM, Vidulescu C, Curici A, Caravia L, Simionescu AA, Ciontea SM, Simion S. Imatinib inhibits spontaneous rhythmic contractions of human uterus and intestine. Eur J Pharmacol. 2006;546:177–81. doi: 10.1016/j.ejphar.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 114.Shimojima N, Nakaki T, Morikawa Y, Hoshino K, Kitajima M. Imatinib blocks spontaneous mechanical activities in the adult mouse small intestine: possible inhibition of c-Kit signaling. Pharmacology. 2005;74:95–9. doi: 10.1159/000084021. [DOI] [PubMed] [Google Scholar]


