Abstract
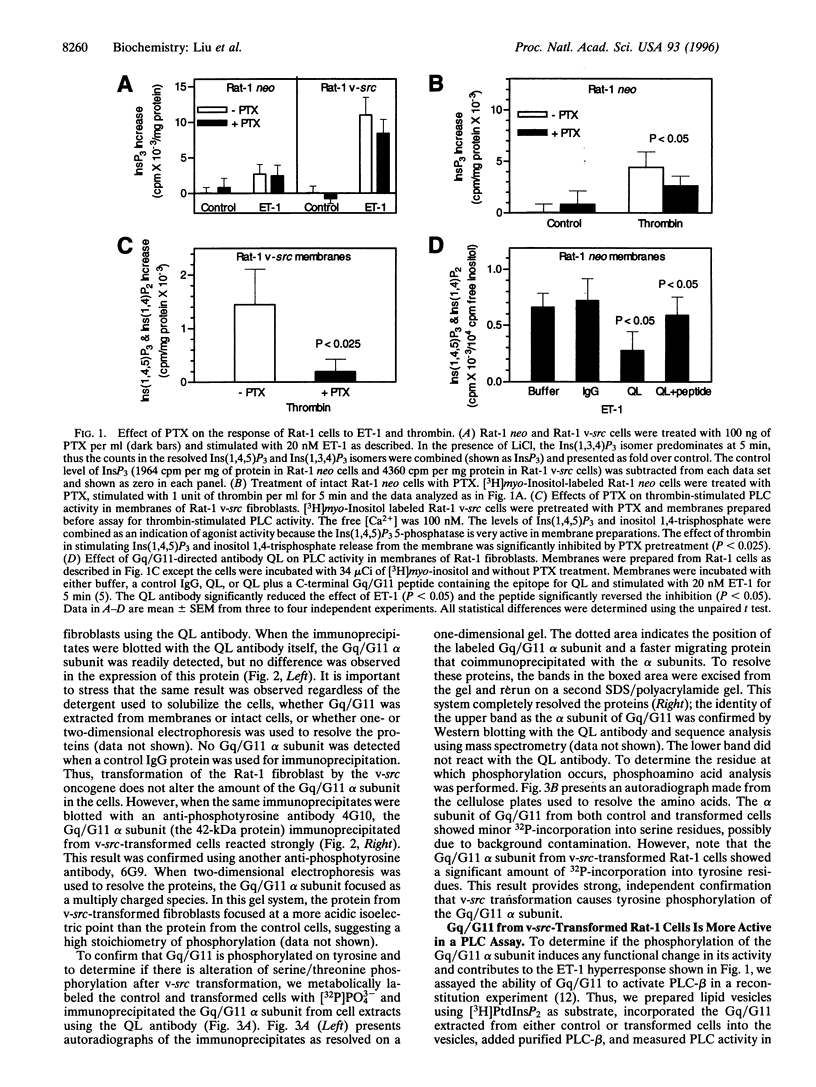
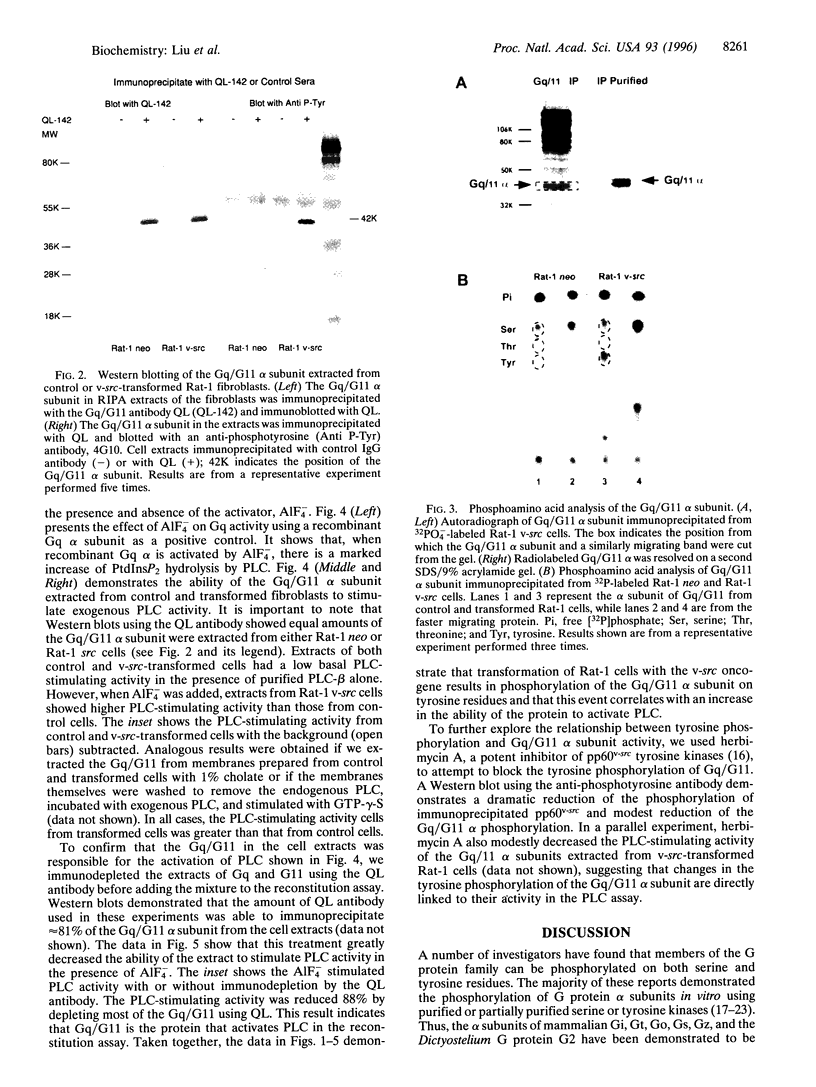
Two major intermediaries in signal transduction pathways are pp60v-sre family tyrosine kinases and heterotrimeric guanine nucleotide-binding proteins. In Rat-1 fibroblasts transformed by the v-src oncogene, endothelin-1 (ET-1)-induced inositol 1,4,5-trisphosphate accumulation is increased 6-fold, without any increases in the numbers of ET-1 receptors or in the response to another agonist, thrombin. This ET-1 hyperresponse can be inhibited by an antibody directed against the carboxyl terminus of the Gq/G11 alpha subunit, suggesting that the Gq/G11 protein couples ET-1 receptors to phospholipase C (PLC). While v-src transformation did not increase the expression of the Gq/G11 alpha subunit, immunoblotting with anti-phosphotyrosine antibodies and phosphoamino acid analysis demonstrated that the Gq/G11 alpha subunit becomes phosphorylated on tyrosine residues in v-src-transformed cells. Moreover, when the Gq/G11 protein was extracted from control and transformed cell lines and reconstituted with exogenous PLC, AIF*4-stimulated Gq/G11 activity was markedly increased in extracts from v-src-transformed cells. Our results demonstrate that the process of v-src transformation can increase the tyrosine phosphorylation state of the Gq/G11 alpha-subunit in intact cells and that the process causes an increase in the Gq/G11 alpha-subunit's ability to stimulate PLC following activation with AIF-4.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Growth factors and cancer. Science. 1991 Nov 22;254(5035):1146–1153. doi: 10.1126/science.1659742. [DOI] [PubMed] [Google Scholar]
- Baltensperger K., Karoor V., Paul H., Ruoho A., Czech M. P., Malbon C. C. The beta-adrenergic receptor is a substrate for the insulin receptor tyrosine kinase. J Biol Chem. 1996 Jan 12;271(2):1061–1064. doi: 10.1074/jbc.271.2.1061. [DOI] [PubMed] [Google Scholar]
- Boyer J. L., Waldo G. L., Harden T. K. Beta gamma-subunit activation of G-protein-regulated phospholipase C. J Biol Chem. 1992 Dec 15;267(35):25451–25456. [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Chen M. Y., Devreotes P. N., Gundersen R. E. Serine 113 is the site of receptor-mediated phosphorylation of the Dictyostelium G protein alpha-subunit G alpha 2. J Biol Chem. 1994 Aug 19;269(33):20925–20930. [PubMed] [Google Scholar]
- Daub H., Weiss F. U., Wallasch C., Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996 Feb 8;379(6565):557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- De Vivo M., Chen J., Codina J., Iyengar R. Enhanced phospholipase C stimulation and transformation in NIH-3T3 cells expressing Q209LGq-alpha-subunits. J Biol Chem. 1992 Sep 15;267(26):18263–18266. [PubMed] [Google Scholar]
- Fields T. A., Casey P. J. Phosphorylation of Gz alpha by protein kinase C blocks interaction with the beta gamma complex. J Biol Chem. 1995 Sep 29;270(39):23119–23125. doi: 10.1074/jbc.270.39.23119. [DOI] [PubMed] [Google Scholar]
- Fukami K., Matsuoka K., Nakanishi O., Yamakawa A., Kawai S., Takenawa T. Antibody to phosphatidylinositol 4,5-bisphosphate inhibits oncogene-induced mitogenesis. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9057–9061. doi: 10.1073/pnas.85.23.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. M., Macara I. G. Serum-stimulated phosphatidylinositol turnover is enhanced in 3T3 cells with active pp60v-src. Oncogene. 1989 Oct;4(10):1213–1217. [PubMed] [Google Scholar]
- Gutowski S., Smrcka A., Nowak L., Wu D. G., Simon M., Sternweis P. C. Antibodies to the alpha q subfamily of guanine nucleotide-binding regulatory protein alpha subunits attenuate activation of phosphatidylinositol 4,5-bisphosphate hydrolysis by hormones. J Biol Chem. 1991 Oct 25;266(30):20519–20524. [PubMed] [Google Scholar]
- Hadcock J. R., Port J. D., Gelman M. S., Malbon C. C. Cross-talk between tyrosine kinase and G-protein-linked receptors. Phosphorylation of beta 2-adrenergic receptors in response to insulin. J Biol Chem. 1992 Dec 25;267(36):26017–26022. [PubMed] [Google Scholar]
- Hausdorff W. P., Pitcher J. A., Luttrell D. K., Linder M. E., Kurose H., Parsons S. J., Caron M. G., Lefkowitz R. J. Tyrosine phosphorylation of G protein alpha subunits by pp60c-src. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5720–5724. doi: 10.1073/pnas.89.13.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek T., Weber M. J. Optimization of the resolution of phosphoamino acids by one-dimensional thin-layer electrophoresis. Biotechniques. 1993 Oct;15(4):628–630. [PubMed] [Google Scholar]
- Johnson R. M., Wasilenko W. J., Mattingly R. R., Weber M. J., Garrison J. C. Fibroblasts transformed with v-src show enhanced formation of an inositol tetrakisphosphate. Science. 1989 Oct 6;246(4926):121–124. doi: 10.1126/science.2506643. [DOI] [PubMed] [Google Scholar]
- Jouneaux C., Mallat A., Serradeil-Le Gal C., Goldsmith P., Hanoune J., Lotersztajn S. Coupling of endothelin B receptors to the calcium pump and phospholipase C via Gs and Gq in rat liver. J Biol Chem. 1994 Jan 21;269(3):1845–1851. [PubMed] [Google Scholar]
- Kalinec G., Nazarali A. J., Hermouet S., Xu N., Gutkind J. S. Mutated alpha subunit of the Gq protein induces malignant transformation in NIH 3T3 cells. Mol Cell Biol. 1992 Oct;12(10):4687–4693. doi: 10.1128/mcb.12.10.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Krupinski J., Rajaram R., Lakonishok M., Benovic J. L., Cerione R. A. Insulin-dependent phosphorylation of GTP-binding proteins in phospholipid vesicles. J Biol Chem. 1988 Sep 5;263(25):12333–12341. [PubMed] [Google Scholar]
- Levistre R., Berguerand M., Bereziat G., Masliah J. The cross-regulation of Gi-protein by cholera toxin involves a phosphorylation by protein kinase A. Biochem J. 1995 Mar 15;306(Pt 3):765–769. doi: 10.1042/bj3060765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounsbury K. M., Casey P. J., Brass L. F., Manning D. R. Phosphorylation of Gz in human platelets. Selectivity and site of modification. J Biol Chem. 1991 Nov 15;266(32):22051–22056. [PubMed] [Google Scholar]
- Lounsbury K. M., Schlegel B., Poncz M., Brass L. F., Manning D. R. Analysis of Gz alpha by site-directed mutagenesis. Sites and specificity of protein kinase C-dependent phosphorylation. J Biol Chem. 1993 Feb 15;268(5):3494–3498. [PubMed] [Google Scholar]
- MacNulty E. E., Plevin R., Wakelam M. J. Stimulation of the hydrolysis of phosphatidylinositol 4,5-bisphosphate and phosphatidylcholine by endothelin, a complete mitogen for Rat-1 fibroblasts. Biochem J. 1990 Dec 15;272(3):761–766. doi: 10.1042/bj2720761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly R. R., Stephens L. R., Irvine R. F., Garrison J. C. Effects of transformation with the v-src oncogene on inositol phosphate metabolism in rat-1 fibroblasts. D-myo-inositol 1,4,5,6-tetrakisphosphate is increased in v-src-transformed rat-1 fibroblasts and can be synthesized from D-myo-inositol 1,3,4-trisphosphate in cytosolic extracts. J Biol Chem. 1991 Aug 15;266(23):15144–15153. [PubMed] [Google Scholar]
- Mattingly R. R., Wasilenko W. J., Woodring P. J., Garrison J. C. Selective amplification of endothelin-stimulated inositol 1,4,5-trisphosphate and calcium signaling by v-src transformation of rat-1 fibroblasts. J Biol Chem. 1992 Apr 15;267(11):7470–7477. [PubMed] [Google Scholar]
- Moyers J. S., Linder M. E., Shannon J. D., Parsons S. J. Identification of the in vitro phosphorylation sites on Gs alpha mediated by pp60c-src. Biochem J. 1995 Jan 15;305(Pt 2):411–417. doi: 10.1042/bj3050411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer E. J. Heterotrimeric G proteins: organizers of transmembrane signals. Cell. 1995 Jan 27;80(2):249–257. doi: 10.1016/0092-8674(95)90407-7. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Weber M. J. Genetics of src: structure and functional organization of a protein tyrosine kinase. Curr Top Microbiol Immunol. 1989;147:79–127. doi: 10.1007/978-3-642-74697-0_3. [DOI] [PubMed] [Google Scholar]
- Shenker A., Goldsmith P., Unson C. G., Spiegel A. M. The G protein coupled to the thromboxane A2 receptor in human platelets is a member of the novel Gq family. J Biol Chem. 1991 May 15;266(14):9309–9313. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Strassheim D., Malbon C. C. Phosphorylation of Gi alpha 2 attenuates inhibitory adenylyl cyclase in neuroblastoma/glioma hybrid (NG-108-15) cells. J Biol Chem. 1994 May 13;269(19):14307–14313. [PubMed] [Google Scholar]
- Thomas G. MAP kinase by any other name smells just as sweet. Cell. 1992 Jan 10;68(1):3–6. doi: 10.1016/0092-8674(92)90199-m. [DOI] [PubMed] [Google Scholar]
- Uehara Y., Murakami Y., Sugimoto Y., Mizuno S. Mechanism of reversion of Rous sarcoma virus transformation by herbimycin A: reduction of total phosphotyrosine levels due to reduced kinase activity and increased turnover of p60v-src1. Cancer Res. 1989 Feb 15;49(4):780–785. [PubMed] [Google Scholar]
- Wasilenko W. J., Payne D. M., Fitzgerald D. L., Weber M. J. Phosphorylation and activation of epidermal growth factor receptors in cells transformed by the src oncogene. Mol Cell Biol. 1991 Jan;11(1):309–321. doi: 10.1128/mcb.11.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm B., Siess W. Activation of the cloned platelet thrombin receptor decreases the pertussis-toxin-dependent ADP-ribosylation of the membrane and soluble inhibitory guanine-nucleotide-binding-alpha proteins. Inhibition by the prostacyclin analog, iloprost. Eur J Biochem. 1993 Aug 15;216(1):81–88. doi: 10.1111/j.1432-1033.1993.tb18119.x. [DOI] [PubMed] [Google Scholar]
- Yatomi Y., Arata Y., Tada S., Kume S., Ui M. Phosphorylation of the inhibitory guanine-nucleotide-binding protein as a possible mechanism of inhibition by protein kinase C of agonist-induced Ca2+ mobilization in human platelet. Eur J Biochem. 1992 May 1;205(3):1003–1009. doi: 10.1111/j.1432-1033.1992.tb16867.x. [DOI] [PubMed] [Google Scholar]
- van Corven E. J., Groenink A., Jalink K., Eichholtz T., Moolenaar W. H. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989 Oct 6;59(1):45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by electrophoresis and chromatography on thin-layer cellulose plates. Electrophoresis. 1994 Mar-Apr;15(3-4):544–554. doi: 10.1002/elps.1150150173. [DOI] [PubMed] [Google Scholar]