Abstract
Objective
This study was aimed to assess pancreas beta cell activity using 99mTc-diethyleneaminepentaacetic acid-glipizide (DTPA-GLP), a sulfonylurea receptor agent. The effect of DTPA-GLP on the blood glucose level in rats was also evaluated.
Methods
DTPA dianhydride was conjugated with GLP in the presence of sodium amide, yielding 60%. Biodistribution and planar images were obtained at 30–120 min after injection of 99mTc-DTPA-GLP (1 mg/rat, 0.74 and 11.1 MBq per rat, respectively) in normal female Fischer 344 rats. The control group was given 99mTc-DTPA. To demonstrate pancreas beta cell uptake of 99mTc-DTPA-GLP via a receptor-mediated process, a group of rats was pretreated with streptozotocin (a beta cell toxin, 55 mg/kg, i.v.) and the images were acquired at immediately—65 min on day 5 post-treatment. The effect on the glucose levels after a single administration (ip) of DTPA-GLP was compared to glipizide (GLP) for up to 6 h.
Results
The structure of DTPA-GLP was confirmed by NMR, mass spectrometry and HPLC. Radiochemical purity assessed by ITLC was >96%. 99mTc-DTPA-GLP showed increased pancreas-to-muscle ratios, whereas 99mTc-DTPA showed decreased ratios at various time points. Pancreas could be visualized with 99mTc-DTPA-GLP in normal rat, however, 99mTc-DTPA has poor uptake suggesting the specificity of 99mTc-DTPA-GLP. Pancreas beta cell uptake could be blocked by pre-treatment with streptozotocin. DTPA-GLP showed an equal or better response in lowering the glucose levels compared to the existing GLP drug.
Conclusions
It is feasible to use 99mTc-DTPA-GLP to assess pancreas beta cell receptor recognition. 99mTc-DTPA-GLP may be helpful in evaluating patients with diabetes, pancreatitis and pancreatic tumors.
Keywords: 99mTc-DTPA-glipizide, Sulfonylurea receptor, Imaging, Pancreas
Introduction
Diabetes mellitus results from an absolute or relative reduction in pancreatic beta cell mass leading to insufficient secretion and hypoglycemia. Both type 1 and type 2 diabetes are characterized by deficits in beta cell mass: around 99% deficit in long-standing type 1 diabetes [1, 2], and 65% deficit in long-standing type 2 diabetes [3]. Type 1 diabetes results from a selective destruction of the beta cells by a T-cell mediated autoimmune process [4]. Type 2 diabetes begins with insulin resistance in the peripheral tissues, a gradual increase in circulating blood glucose that triggers beta cells to release even more insulin, and subsequent failure of beta cells to maintain normal glucose homeostasis [5]. Recent study suggested that the frequency of beta cell apoptosis is also significantly increased type 2 diabetes [3]. Treatments for diabetes mellitus range from drugs that increase insulin secretion and sensitivity, to insulin administration, to pancreatic islet cell transplantation [6]. Because of the anatomical inaccessibility of the human pancreas for biopsy, noninvasive procedures are needed to quantify beta cell mass in vivo for monitoring the onset, progression and responses to therapy of type 1 and 2 diabetes, and to detect rejection in pancreatic islet transplant patients. Two different approaches have been used to determine the functional beta cell mass in living humans. Information of beta cell mass currently can be deduced from functional tests of insulin secretion, but it has not been shown to correlate well with the dysfunction of beta cells that result from morphological and biochemical changes in the pancreas [3, 7].
During regenerative processes following experimental reduction of beta cell mass such as partial pancreatectomy and streptozotocin injection, beta cell mass increase is not associated with a corresponding improvement of beta cell function, thus indicating that regenerative beta cells did not achieve functional maturity [8]. These studies indicated that anatomical beta cell mass could not always predict functional capacity of the beta cell tissue. Thus, the non-invasive imaging techniques such as MRI, PET and SPECT could be useful to quantify the pancreatic beta cell mass [9].
Insulin secretion is regulated by the membrane potential of the beta cell, which depends on the activity of ATP-sensitive K+ channels (KATP channels) in the plasma membrane. The KATP channel is a hetero-octameric complex of two different types of protein subunits: an inwardly rectifying K+ Channel (Kir6.x) and a sulfonylurea receptor. More than one isoform exists for both Kir6.x (Kir6.1, Kir6.2) and sulfonylurea receptors [10]. Sulfonylurea receptor agents stimulate insulin release from pancreatic beta cells, which improves overall glycemia control in patients with type 2 diabetes [11]. While sulfonylurea receptor-1 is expressed in a very high density of at the internal face of the plasma membrane of the pancreatic islet cells but not in the exocrine cells, the sulfonylurea receptor-2 is predominant in the cardiac, skeletal, and smooth muscles cells [11, 12]. Tolbutamide, chlorprop-amide, gliclazide, and glipizide are specific for the pancreatic β-cells sulfonylurea receptor-1, and thus their effect is largely on potentiating insulin secretion [13]. Therefore, these sulfonylurea receptor agents labeled with radionuclide could be a feasible tracer for visualizing the beta cell mass in humans by accumulation at the internal face of the plasma membrane of the pancreatic islet cells by binding to sulfonylurea receptor-1. Recently, glibenclamide, a sulfonylurea receptor agent, was used as a compound candidate to image the pancreatic beta cell in humans. However, they could not obtain a reliable assessment and visualization of the pancreatic islet cell mass due to overall radiochemical yields, low binding affinity, and longer deposition time at the receptor [14, 15]. Schneider et al. suggested that 99mTc-labeled glibenclamide derivative with a lower lipophilicity and different in vivo behavior may be possible as compound for noninvasive imaging of the pancreatic islet cell mass [16].
Glipizide (GLP) is a potent sulfonylurea receptor agent and is a second generation oral hypoglycaemic agent similar in potency to glibenclamide [17–19]. The efficacy, safety, and dose–response characteristics of glipizide using the gastrointestinal therapeutic system on plasma glucose, glycosylated hemoglobin (HbA1c), and insulin secretion to a liquid-mixed meal in diabetic patients were evaluated [18]. In this study, glipizide is effective in improving glycemic indices by numerous methods including HbA1c, meal tolerance, and intravenous glucose tolerance testing and there are no significant adverse effects with therapy except for mild to moderate hypoglycemia.
Due to favorable physical characteristics and its low cost, 99mTc has been preferred for labeling radiopharmaceuticals. Diethylenetriaminepentaacetic acid (DTPA) can be labeled with 99mTc easily and efficiently with high radiochemical purity and stability and is excreted through kidney [20]. Thus, we developed a noninvasive method to image the beta cell mass by labeling a sulfonylurea receptor agent, GLP with 99mTc using DTPA as a chelator. In this paper, we report the synthesis, biodistribution and imaging study in normal rats using 99mTc-DTPA-glipizide (DTPA-GLP). The effect of DTPA-GLP on the blood glucose level in rats was also evaluated.
Materials and methods
Chemicals and analysis
Mass spectral analyses were conducted at The University of Texas Health Science Center (Houston, TX, USA). The mass data were obtained by fast atom bombardment on a Kratos MS 50 instrument (Kratos Instrument, Ltd., Manchester, UK). 1H- and 13C-NMR studies were performed on Bruker 300 MHz spectrometer at the NMR core facility at MD Anderson Cancer Center (Houston, TX, USA). Glipizide is commercially obtained from Acros Organics (New Jersey, USA). All other chemicals were purchased from Aldrich Chemical Company (Milwaukee, WI, USA). Silica gel-coated thin-layer chromatography (TLC) plates were purchased from Whatman (Clifton, NJ, USA).
Synthesis of DTPA-GLP
The synthetic scheme of DTPA-GLP is shown in Fig. 1. 85.8 mg (2.2 mol) of NaNH2 was added to the solution of 445.5 mg (1.0 mol) of glipizide in 5 mL of dried DMSO. The apparatus was attached with a nitrogen balloon. After stirring for 30 min, 357.3 mg (1.0 mol) of diethylenetriaminepentaacetic acid (DTPA)-dianhydride was placed into the reaction mixture. After stirring over-night at room temperature, 160.5 mg (3.0 mol) of NH4Cl and 54 μL (3.0 mol) of water was added to the mixture. After stirring for 1 h, the mixture of 1.5 mL of 2 N Na2CO3 and 20 mL of water was slowly added into the reaction mixture inside of an ice bath. The pH of the solution was around 7.6 upon removal. This solution was then dialyzed using a membrane with the cut off at MW <1,000 for 5 h. The remaining solution was dried using a lyophilizer. The crude product was isolated through a column packed with Sephadex G-50 and eluted with water. The yield for desired product was 507 mg (61.7%). The structure of DTPA-GLP was confirmed by 1H- and 13C-NMR and mass spectrometry. High-performance liquid chromatography (HPLC), equipped with a UV detector (220 nm), is performed on a C-18 reverse phase column (Biosep SEC-S3000, 7.8 × 300 mm, Phenomenex, Torrance CA) using a flow rate of 0.5 mL/min. The eluant was 5 mM ammonium phosphate/acetonitrile (50/50, pH = 8.1).
Fig. 1.
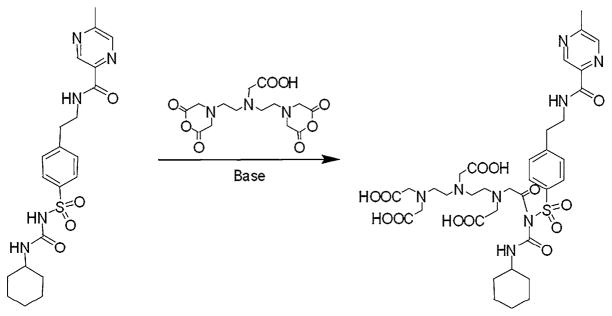
Synthetic scheme of DTPA-GLP
Radiosynthesis of 99mTc-DTPA-GLP conjugate (99mTc-DTPA-GLP)
Radiosynthesis of 99mTc-DTPA-GLP was achieved through dissolving DTPA-GLP (0.1 mg) in water (0.2 mL), followed by adding tin (II) chloride (0.1 mg, 0.1 mL). Sodium pertechnetate (NaTcO4) (37 MBq) was then added. The final concentration was 370 MBq/μmol. Radiochemical purity was assessed by using a radio-TLC scanner (Bio-scan, Inc., Washington, DC) using saline or acetone as an eluant. The chromatographic paper used was Whatman #1 (Fischer Scientific, Houston, TX).
Biodistribution studies
The animals were housed in The University of Texas M.D. Anderson Cancer Center facility. All protocols involving animals were approved by the University of Texas M.D. Anderson Institutional Animal Use and Care Committee (IACUC). Fischer 344 rats (150 ± 30 g) (Harlan Sprague–Dawley, Indianapolis, IN, USA) (n = 18) were injected intravenously with 740 ± 19 KBq of 99mTc-DTPA-GLP or 99mTc-DTPA. The injected mass of 99mTc-DTPA-GLP was 0.1 mg/rat. Separate biodistribution studies using 99mTc-DTPA-GLP (Study 1, nine rats) and 99mTc-DTPA (Study 2, nine rats) were conducted. For each compound, the rats were divided into three groups for three time intervals (30, 60 and 180 min, n = 3/time point). At 30, 60 and 180 min following administration of the radiotracers, the rats were killed by cervical dislocation. Samples of the blood and selected tissues were obtained, weighed, and subjected to counting along with a standard of the injected dose in a gamma counter (Packard Cobra Auto-Gamma Counter, Packard Instruments, Downers Grove, IL, USA). The bio-distribution of tracer in each sample was calculated as a percentage of the injected dose per gram of tissue wet weight (%ID/g). Pancreas to non-target tissue count density ratios were calculated from the corresponding %ID/g values.
Planar scintigraphic imaging studies
Scintigraphic images were obtained using an M-camera from Siemens Medical Systems (Hoffman Estates, IL, USA). The camera was equipped with a low-energy parallel-hole collimator. The field of view was 53.3 × 38.7 cm. The intrinsic spatial resolution was 3.2 mm. The matrix is 64 × 64 and the pixel size was 19.18 mm (32 × 32, zoom = 1) to 0.187 mm (1,024 × 1,024, zoom = 3.2). With a low-energy, high-resolution collimator, the system has a resultant sensitivity of 172 counts/min (cpm)/μCi and spatial resolution of 4 mm. Scintigraphic images were obtained at 45 min after intravenous injection of 300 μCi (11.1 MBq/rat) of 99mTc-DTPA-GLP and 99mTc-DTPA as control in normal healthy female Fischer 344 rats (150–175 g) (Harlan Sprague–Dawley, Inc., Indianapolis, IN, USA) via the tail vein, respectively. Scintigraphic images were acquired 1 × 106 counts. To ascertain whether the pancreas beta cell uptake with 99mTc-DTPA-GLP was related to sulfonylurea receptors, we performed a blocking study. A group of rats were pretreated with streptozotocin (a beta cell toxin, 55 mg/kg, i.v.) and the images were acquired at 5, 25, and 45 min after intravenous injection of 11.1 MBq/rat of 99mTc-DTPA-GLP on day 5 post-treatment.
Pharmacological effects of DTPA-GLP on the blood glucose level
To demonstrate the effectiveness of DTPA-GLP in lowering the blood glucose level in Fischer 344 rats, 4 groups of Fischer 344 rats received 300 or 760 mg GLP/kg, 300 or 760 mg DTPA-GLP/kg in single treatment (ip). GLP was dissolved in the cotton seed oil, whereas DTPA-GLP was dissolved in saline. The concentration prepared for both agents was 100 mg/mL in animal studies. The blood glucose levels were measured with the tail-bleeding technique [21] using a blood glucose meter (Bayer’s Breeze®, Bayer HealthCare, NY, USA) immediate before injection, 5 min to 60 min with an interval of 5, 90, 120, 180, 240, and 360 min post-injection.
Statistical analysis
The in vivo percentage of injected dose per gram of wet tissue weight (%ID/g), pancreas-to-normal muscle and pancreas-to-blood count ratios were presented as means ± standard errors of the means. To compare differences of %ID/g and ratios between control and DTPA-GLP groups, the Student t test was used. All analyses were 2-tailed and considered as type 2. A p value <0.05 was considered statistically significant. All statistical computations were processed by using a computer software program (Excel, Microsoft, Redmond, WA, USA).
Results
Chemistry and radiochemistry
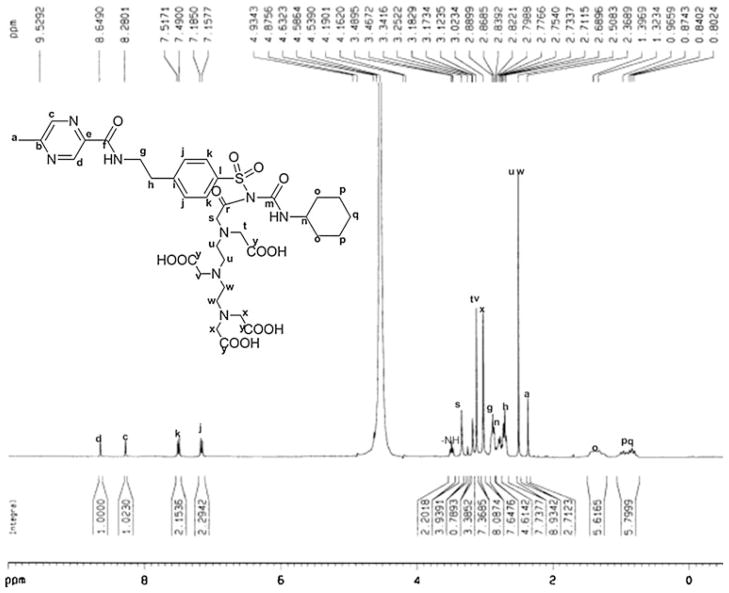
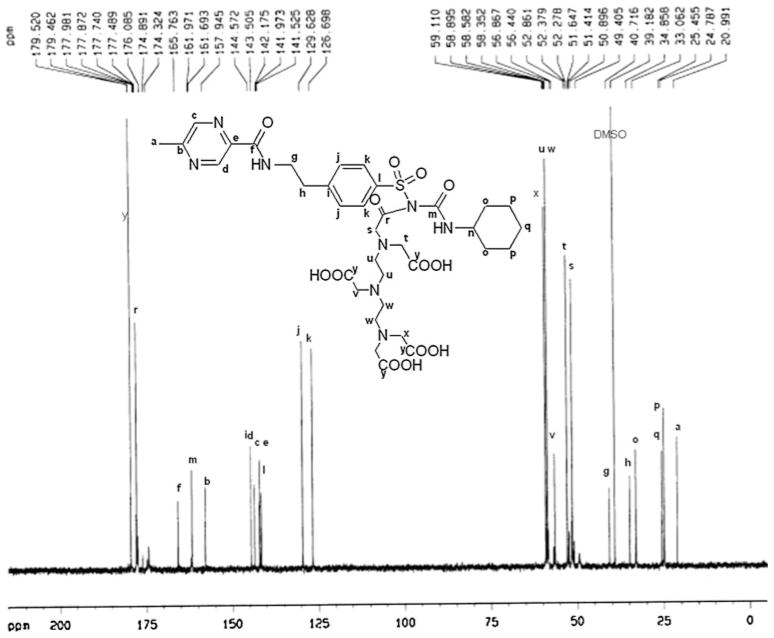
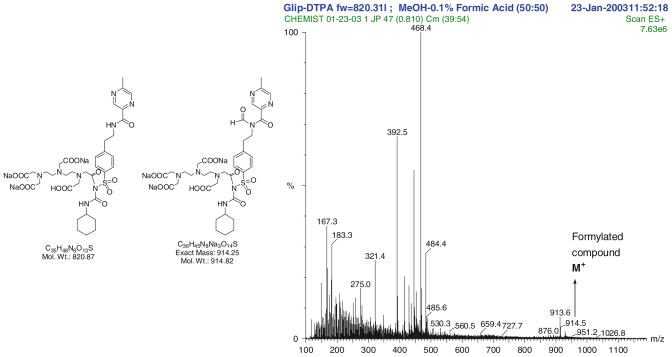
HPLC analysis showed the retention time of DTPA-GLP was 5.738 min with purity greater than 93%. 1H-NMR data of DTPA-GLP is 1H-NMR (300 MHz, D2O) δ ppm 8.65 (s, 1H), 8.28 (s, 1H), 7.50 (d, 2H, J = 8.2 Hz), 7.17 (d, 2H, J = 8.2 Hz), 3.34 (s, 2H), 3.12 (s, 4H), 3.02 (s, 4H), 2.89 (t, 2H, J = 6.5 Hz), 2.75–2.83 (m, 1H), 2.71 (t, 2H, J = 6.5 Hz), 2.51 (s, 8H), 2.36 (s, 3H), 1.30–1.40 (m, 4H), 0.77–1.01 (m, 6H) (Fig. 2). 13C-NMR (75 MHz, D2O) δ ppm 179.5, 177.9, 165.8, 161.7, 157.9, 144.6, 143.5, 142.2, 142.0, 141.5, 129.6, 126.7, 59.1, 58.6, 56.4, 52.9, 51.4, 49.4, 40.7, 39.2, 34.9, 33.1, 25.5, 24.8, 21.0 (Fig. 3). The molecular weight of DTPA-GLP is 820.87. The mass spectrum of DTPA-GLP was confirmed by formylated DTPA-GLP (Fig. 4). The radiochemical purity of 99mTc-DTPA-GLP was 97.2% (eluted with saline or acetone) determined by radio-instant thin-layer chromatogram.
Fig. 2.
1H-NMR spectrum of DTPA-GLP
Fig. 3.
13C-NMR spectrum of DTPA-GLP
Fig. 4.
Mass spectrum of DTPA-GLP
Biodistribution studies
The biodistribution of 99mTc-DTPA and 99mTc-DTPA-GLP in normal Fischer 344 rats is shown in the Tables 1 and 2, respectively. The uptake of 99mTc-DTPA-GLP in the kidneys was high, indicating that the radiotracer was excreted mainly through the urine. Pancreatic tissue displayed a stable accumulation of ≈0.25 and 0.21% of the injected 99mTc-DTPA-GLP dose at 30 and 60 min, respectively. Pancreas uptake (% ID/g) in 99mTc-DTPA-GLP was significantly higher than 99mTc-DTPA at 1 and 3 h (0.21 ± 0.00 vs. 0.08 ± 0.01, 0.15 ± 0.01 vs. 0.05 ± 0.01, p <0.05, respectively). Pancreas-to-muscle ratios were higher in 99mTc-DTPA-GLP group than in 99mTc-DTPA groups. At 2 h, pancreas-to-muscle ratio 99mTc-DTPA-GLP was significantly higher than 99mTc-DTPA (2.76 ± 0.07 vs. 1.74 ± 0.23, p <0.05). Generally, blood, heart, and lung uptakes in 99mTc-DTPA-GLP groups were higher than 99mTc-DTPA groups. In contrast, tracer uptake in the kidney was lower in 99mTc-DTPA-GLP group than in 99mTc-DTPA group. There was virtually no retention of radioactivity in the brain during the observation period.
Table 1.
Biodistribution of 99mTc-DTPA in normal healthy F-344 rats
| 30 min | 60 min | 180 min | |
|---|---|---|---|
| Blood | 1.08 ± 0.21 | 0.37 ± 0.04 | 0.19 ± 0.01 |
| Heart | 0.27 ± 0.04 | 0.10 ± 0.01 | 0.06 ± 0.01 |
| Lung | 0.56 ± 0.08 | 0.24 ± 0.02 | 0.14 ± 0.01 |
| Thyroid | 0.54 ± 0.18 | 0.21 ± 0.02 | 0.19 ± 0.03 |
| Pancreas | 0.20 ± 0.00 | 0.08 ± 0.01 | 0.05 ± 0.01 |
| Liver | 0.80 ± 0.20 | 0.76 ± 0.04 | 0.52 ± 0.09 |
| Spleen | 0.75 ± 0.11 | 0.97 ± 0.13 | 0.76 ± 0.16 |
| Kidney | 8.38 ± 1.17 | 11.33 ± 0.49 | 10.20 ± 1.17 |
| Stomach | 0.39 ± 0.06 | 0.20 ± 0.05 | 0.14 ± 0.02 |
| Intestine | 0.38 ± 0.00 | 0.24 ± 0.07 | 0.12 ± 0.02 |
| Brain | 0.03 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Muscle | 0.12 ± 0.01 | 0.04 ± 0.01 | 0.02 ± 0.00 |
| Bone | 0.27 ± 0.04 | 0.15 ± 0.02 | 0.08 ± 0.01 |
| P/blood | 0.19 ± 0.04 | 0.20 ± 0.01 | 0.25 ± 0.02 |
| P/muscle | 1.72 ± 0.10 | 1.74 ± 0.23 | 1.92 ± 0.19 |
Values are % of injected dose per gram of tissue weight and represent the mean ± standard errors of the mean of data from 3 animals per time interval
P pancreas
Table 2.
Biodistribution of 99mTc-DTPA-glipizide in normal healthy F-344 rats
| 30 min | 60 min | 180 min | |
|---|---|---|---|
| Blood | 2.08 ± 0.11* | 1.66 ± 0.04* | 1.25 ± 0.04* |
| Heart | 0.49 ± 0.04* | 0.33 ± 0.02* | 0.29 ± 0.03* |
| Lung | 0.84 ± 0.01* | 0.72 ± 0.05* | 0.52 ± 0.02* |
| Thyroid | 0.70 ± 0.14 | 0.54 ± 0.02* | 0.41 ± 0.01* |
| Pancreas | 0.25 ± 0.03 | 0.21 ± 0.00* | 0.15 ± 0.01* |
| Liver | 1.10 ± 0.04 | 1.01 ± 0.05* | 0.78 ± 0.00* |
| Spleen | 0.72 ± 0.03 | 0.74 ± 0.04 | 0.60 ± 0.02* |
| Kidney | 5.78 ± 0.30 | 7.52 ± 0.33* | 7.38 ± 0.29 |
| Stomach | 0.25 ± 0.01 | 0.20 ± 0.00 | 0.16 ± 0.01 |
| Intestine | 0.59 ± 0.11 | 1.66 ± 0.04 | 0.25± 0.01* |
| Brain | 0.06 ± 0.01 | 0.04 ± 0.01* | 0.04 ± 0.00* |
| Muscle | 0.10 ± 0.00 | 0.08 ± 0.00* | 0.06 ± 0.00* |
| Bone | 0.20 ± 0.03 | 0.11 ± 0.01 | 0.14 ± 0.02* |
| P/blood | 0.12 ± 0.01 | 0.13 ± 0.01* | 0.12 ± 0.00* |
| P/muscle | 2.54 ± 0.26 | 2.76 ± 0.07* | 2.50 ± 0.18 |
Values are % of injected dose per gram of tissue weight and represent the mean ± standard errors of the mean of data from 3 animals per time interval
p <0.05 vs. 99mTc-DTPA
Scintigraphic imaging
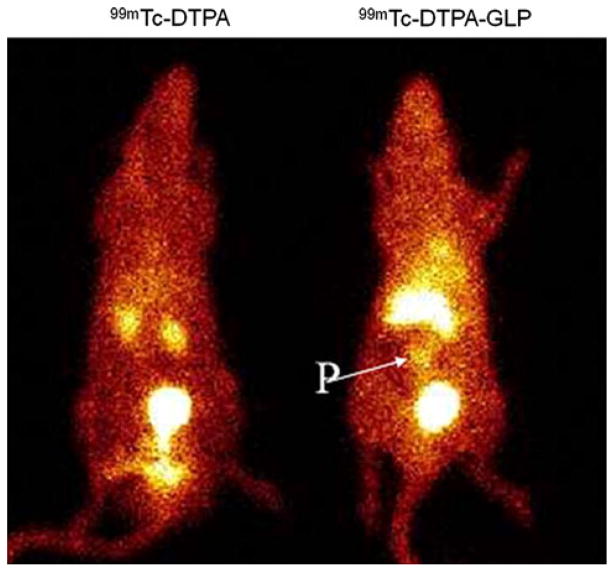
Planar images of the rats injected with two different compounds, 99mTc-DTPA and 9mTc-DTPA-GLP are shown in Fig. 3. Because thyroid and stomach activities were not detected on the planar images, it was considered to signify the in vivo stability of the radiopharmaceuticals. The pancreas could be visualized with 99mTc-DTPA-GLP in normal rats; however, 99mTc-DTPA has no definite, pancreas uptake suggesting the specificity of 99mTc-DTPA-GLP (Fig. 5). The surrounding organs (liver, spleen and intestine) around the pancreas showed faint uptakes of 99mTc-DTPA-GLP. In contrast, because DTPA was used as chelator, radioactivities in both the kidneys and urinary bladder were higher compared to the pancreatic uptake. In blocking studies with streptozotocin, pancreas beta cell uptake could be blocked by pre-treatment with streptozotocin; therefore, the pancreas could not be visualized in rats pretreated with streptozotocin in comparison with the untreated rat (Fig. 6). The finding indicated that pancreas beta cell uptake of 99mTc-DTPA-GLP is via a sulfonylurea receptor-mediated process.
Fig. 5.
Planar scintigraphy of 99mTc-DTPA and 99mTc-DTPA-GLP in rats (11.1 MBq/rat, i.v. injection) at 45 min. Pancreas (P) can be visualized by 99mTc-DTPA-GLP
Fig. 6.
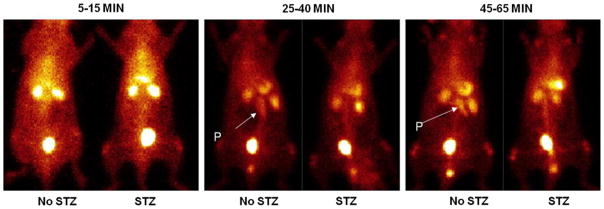
Planar scintigraphy image after administration of 99mTc-DTPA-GLP in rats (11.1 MBq/rat, i.v. injection). The first rat received 55 mg/kg streptozotocin (STZ) 5 days prior to injection. It showed the pancreas could be well visualized 25–65 min post-injection only in the rat which did not receive STZ. Scintigraphic images were acquired 1 × 106 counts, P pancreas
Pharmacological effects of DTPA-GLP on the blood glucose level
Figure 7 illustrates the effect of GLP and DTPA-GLP on the blood glucose levels in Fischer 344 rats. Rats died at 760 mg GLP/kg within 40 min after injection suggesting maximum tolerated dose achieved, whereas rats survived at 760 mg DTPA-GLP/kg in single treatment. The blood glucose levels were decreased by GLP and DTPA-GLP at the dosage tested. DTPA-GLP had faster onset and was more steady than GLP.
Fig. 7.
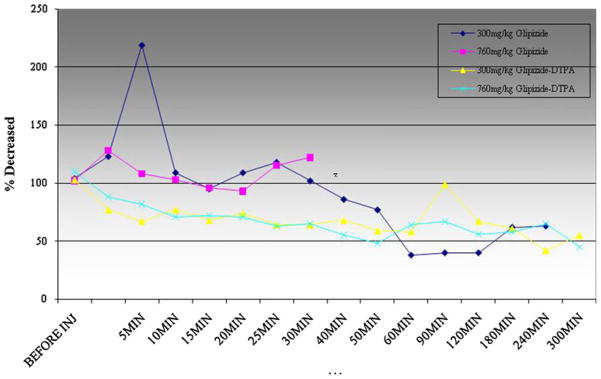
Pharmacological Effect of DTPA-GLP on the blood glucose level in Fischer-344 rats. The blood glucose levels were decreased by GLP and DTPA-GLP at the dosage tested. DTPA-GLP had faster onset and more steady than glipizide. Rats died at 760 mg GLP/kg within 40 min after injection suggesting maximum tolerated dose achieved, whereas rats survived at 760 mg DTPA-GLP/kg in single treatment
Discussion
In this paper, 99mTc-DTPA-GLP was synthesized for a novel agent to visualize the pancreatic beta cell mass. 99mTc-DTPA-GLP accumulated specifically in the pancreatic islet cell in the rats. To our knowledge, glipizide was labeled for the first time with 99mTc. We labeled SUR agent, GLP with 99mTc using DTPA as a chelator. Radio-TLC results with 99mTc-DTPA-GLP confirm the high radiochemical purity and stability. Due to favorable physical characteristics and its low cost, 99mTc has been chosen as a radionuclide instead of positron emitters (18F or 11C) used in other studies [14, 15, 22, 23]. Because DTPA can be labeled with 99mTc easily and efficiently with high radiochemical purity and stability and is also excreted through the kidney [20], DTPA was selected to synthesize a new 99mTc-DTPA-GLP as a chelator. Several compounds have been labeled with 99mTc using DTPA as a chelator [24–26].
Our biodistribution studies with Fischer 344 rat administrated 99mTc-DTPA-GLP revealed that a large portion of the injected dose (%ID/g) was rapidly accumulated by the kidneys, and pancreatic tissue displayed a stable accumulation of ≈0.25 and 0.21% of the injected 99mTc-DTPA-GLP dose at 30 and 60 min, respectively. Direct comparison of the biodistribution of GLP is difficult because there are no reports on the beta cell imaging with GLP labeled with gamma or positron emitters. There are two studies with 18F-glyburide [14], and 2-[18F]fluoroethoxy-5-bromogli-bendamide and 99mTc-glibenclamide, as the second generation sulfonylurea like GLP [14].
Tissue distribution of 2-[18F]fluoroethoxy-5-bromogli-benclamide in rats showed that pancreatic tissue displayed a weak but near constant accumulation of the tracer with 0.084 ± 0.01 %ID/g, and the tracer accumulation in the liver was approximately 100-fold higher compared to the pancreas uptake. In contrast, the tissue distribution of 99mTc-glibenclamide showed that the pancreas uptake was only reduced (tenfold) when compared with 2-[18F]fluoroethoxy-5-bromoglibenclamide at 1 h after tracer application. Schmitz et al. [15] reported similar results of tissue distribution with 18F-glyburide similar to previously mentioned study with 2-[18F]fluoroethoxy-5-bromoglibenclamide and 99mTc-glibenclamide. Our data showed that tracer accumulation in the pancreas was approximately 30-fold higher compared to that of these data described above.
Unlike studies with 18F-glyburide [15], and 2-[18F]fluoroethoxy-5-bromoglibendamide and 99mTc-glibenclamide [16], we found lower 99mTc-DTPA-GLP uptake in the organs (the liver and intestine) around the pancreas, which is an another advantage of 99mTc-DTPA-GLP because of the proximity of these organs to the pancreas.
In our study, biodistribution findings were comparable to scintigraphic imaging (Figs. 5, 6). We did not find thyroid and stomach activities on the planar images, strongly indicating the in vivo stability of the radiopharmaceuticals. The pancreas could be visualized with 99mTc-DTPA-GLP in normal rats. Because we selected DTPA as chelator to accelerate urinary excretion of the tracer, radioactivities in both the kidneys and urinary bladder were higher compared to the pancreatic uptake. The optimal time to image pancreas of 99mTc-DTPA-GLP was at 45–65 min. Heart and muscle had poor uptake at 45–65 min suggesting 99mTc-DTPA-GLP is for the pancreatic β-cells sulfonylurea receptor-1.
Streptozotocin is widely used to induce diabetic animal model [15, 27, 28]. Administration of a beta cell-specific toxin streptozotocin could induce diabetes in the F-344 rats and we have used the same animal model in our studies. The dosage to create the animal model is known [28]. Also, streptozotocin is a therapeutic agent in the treatment of patients with pancreatic neuroendocrine tumors. Streptozotocin destroyed beta cells to avoid pancreatic neuroendocrine tumor adherence [29]. Thus, streptozotocin was selected instead of sulfonylurea receptor agents in our blocking studies.
Streptozotocin enters the beta cell via a glucose transporter-2 and causes alkylation of DNA. DNA damage induces activation of poly ADP-ribosylation, which leads to depletion of cellular NAD+ and ATP. Enhanced ATP dephosphorylation after streptozotocin treatment supplies a substrate for xanthine oxidase resulting in the formation of superoxide radicals. Furthermore, streptozotocin liberates toxic amounts of nitric oxide that inhibits aconitase activity and participates in DNA damage. As a result of the streptozotocin action, beta cells undergo the destruction by necrosis [30]. In this study, scintigraphic imaging of the streptozotocin-treated rat showed no uptake of 99mTc-DTPA-GLP in the pancreas compared to baseline studies. The finding indicated that pancreas beta cell uptake of 99mTc-DTPA-GLP is via a sulfonylurea receptor-mediated process.
Our study showed that DTPA-GLP have a similar ability as GLP itself to decrease the blood glucose levels. This indicates that DTPA-GLP binds to sulfonylurea receptors and then stimulates the insulin secretion from pancreatic beta cells in rat. The physical amount of DTPA-GLP will be 3–10 mg/70 kg. Pharmacological effect of glipizide is at the dosage level of 300 mg/kg (i.v.). Thus, the proposed dosage is 2,100–7,000 times less than pharmacological dose.
In conclusion, DTPA-GLP was synthesized and labeled with 99mTc easily and efficiently, with high radiochemical purity and cost-effectiveness. We found that the use of SPECT and 99mTc-DTPA-GLP provides a means to visualize the pancreatic beta cell mass and to discriminate differences in its uptakes among normal healthy rodents and those with streptozotocin-induced diabetes. Therefore, 99mTc-DTPA-GLP may be helpful to predict and select the patient candidate who may respond to beta cell therapy.
Acknowledgments
This work was supported in part by MDA Sponsored Research Grant (SR 2002-00007147SM, MDACC), by Cell>Point LLC Biotechnology (Englewood, CO), by a grant of ICSR (CNUHRICM-U-200518, Korea) and the John S. Dunn Foundation. The chemistry and animal research are supported by M.D. Anderson Cancer Center (CORE) Grant NIH CA-16672.
Contributor Information
Chang-Sok Oh, Division of Diagnostic Imaging, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Unit 59, Houston, TX 77030, USA.
Saady Kohanim, Division of Diagnostic Imaging, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Unit 59, Houston, TX 77030, USA.
Fan-Lin Kong, Division of Diagnostic Imaging, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Unit 59, Houston, TX 77030, USA.
Ho-Chun Song, Department of Nuclear Medicine, Chonnam National University Medical School and Hospital, 671 Jebongno, Donggu, Gwangju 501-757, South Korea.
Nathan Huynh, Division of Diagnostic Imaging, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Unit 59, Houston, TX 77030, USA.
Richard Mendez, Division of Diagnostic Imaging, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Unit 59, Houston, TX 77030, USA.
Mithu Chanda, Division of Diagnostic Imaging, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Unit 59, Houston, TX 77030, USA.
E. Edmund Kim, Division of Diagnostic Imaging, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Unit 59, Houston, TX 77030, USA.
David J. Yang, Email: dyang@mdanderson.org, Division of Diagnostic Imaging, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Unit 59, Houston, TX 77030, USA
References
- 1.Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14(10):619–33. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- 2.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia. 2005;48(11):2221–8. doi: 10.1007/s00125-005-1949-2. [DOI] [PubMed] [Google Scholar]
- 3.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–10. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 4.Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes. 2004;53(1):250–64. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- 5.Dwarakanathan A. Diabetes update. J Insur Med. 2006;38(1):20–30. [PubMed] [Google Scholar]
- 6.Robertson C, Drexler AJ, Vernillo AT. Update on diabetes diagnosis and management. J Am Dent Assoc. 2003;134(Spec No):16S–23S. doi: 10.14219/jada.archive.2003.0368. [DOI] [PubMed] [Google Scholar]
- 7.Polonsky KS, Given BD, Hirsch L, Shapiro ET, Tillil H, Beebe C, et al. Quantitative study of insulin secretion and clearance in normal and obese subjects. J Clin Investig. 1988;81(2):435–41. doi: 10.1172/JCI113338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kargar C, Ktorza A. Anatomical versus functional beta-cell mass in experimental diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):43–53. doi: 10.1111/j.1463-1326.2008.00940.x. [DOI] [PubMed] [Google Scholar]
- 9.Singhal T, Ding YS, Weinzimmer D, Normandin MD, Labaree D, Ropchan J, et al. Pancreatic beta cell mass PET imaging and quantification with [(11)C]DTBZ and [(18)F]FP-(+)-DTBZ in rodent models of diabetes. Mol Imaging Biol. 2011;13(5):973–84. doi: 10.1007/s11307-010-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguilar-Bryan L, Clement JPt, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Toward understanding the assembly and structure of KATP channels. Physiol Rev. 1998;78(1):227–45. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- 11.Proks P, Reimann F, Green N, Gribble F, Ashcroft F. Sulfonylurea stimulation of insulin secretion. Diabetes. 2002;51(Suppl 3):S368–76. doi: 10.2337/diabetes.51.2007.s368. [DOI] [PubMed] [Google Scholar]
- 12.Schwanstecher M, Schwanstecher C, Dickel C, Chudziak F, Moshiri A, Panten U. Location of the sulphonylurea receptor at the cytoplasmic face of the beta-cell membrane. Br J Pharmacol. 1994;113(3):903–11. doi: 10.1111/j.1476-5381.1994.tb17078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pantalone KM, Kattan MW, Yu C, Wells BJ, Arrigain S, Jain A, Atreja A, Zimmerman RS. The risk of overall mortality in patients with type 2 diabetes receiving glipizide, glyburide, or glimepiride monotherapy: a retrospective analysis. Diabetes Care. 2010;33(6):1224–9. doi: 10.2337/dc10-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider S, Feilen PJ, Schreckenberger M, Schwanstecher M, Schwanstecher C, Buchholz HG, et al. In vitro and in vivo evaluation of novel glibenclamide derivatives as imaging agents for the non-invasive assessment of the pancreatic islet cell mass in animals and humans. Exp Clin Endocrinol Diabetes. 2005;113(7):388–95. doi: 10.1055/s-2005-865711. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz A, Shiue CY, Feng Q, Shiue GG, Deng S, Pourdehnad MT, et al. Synthesis and evaluation of fluorine-18 labeled glyburide analogs as beta-cell imaging agents. Nucl Med Biol. 2004;31(4):483–91. doi: 10.1016/j.nucmedbio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Schneider S, Ueberberg S, Korobeynikov A, Schechinger W, Schwanstecher C, Schwanstecher M, et al. Synthesis and evaluation of a glibenclamide glucose-conjugate: a potential new lead compound for substituted glibenclamide derivatives as islet imaging agents. Regul Pept. 2007;139(1–3):122–7. doi: 10.1016/j.regpep.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Portha B, Tourrel-Cuzin C, Movassat J. Activation of the GLP-1 receptor signalling pathway: a relevant strategy to repair a deficient beta-cell mass. Exp Diabetes Res. 2011;2011:376509. doi: 10.1155/2011/376509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitabchi AE, Kaminska E, Fisher JN, Sherman A, Pitts K, Bush A, et al. Comparative efficacy and potency of long-term therapy with glipizide or glyburide in patients with type 2 diabetes mellitus. Am J Med Sci. 2000;319(3):143–8. doi: 10.1097/00000441-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Prendergast BD. Glyburide and glipizide, second-generation oral sulfonylurea hypoglycemic agents. Clin Pharm. 1984;3(5):473–85. [PubMed] [Google Scholar]
- 20.Assadi M, Eftekhari M, Hozhabrosadati M, Saghari M, Ebrahimi A, Nabipour I, et al. Comparison of methods for determination of glomerular filtration rate: low and high-dose Tc-99m-DTPA renography, predicted creatinine clearance method, and plasma sample method. Int Urol Nephrol. 2008;40(4):1059–65. doi: 10.1007/s11255-008-9446-4. [DOI] [PubMed] [Google Scholar]
- 21.Hui YH, Huang NH, Ebbert L, Bina H, Chiang A, Maples C, et al. Pharmacokinetic comparisons of tail-bleeding with cannula-or retro-orbital bleeding techniques in rats using six marketed drugs. J Pharmacol Toxicol Methods. 2007;56(2):256–64. doi: 10.1016/j.vascn.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Wangler B, Beck C, Shiue CY, Schneider S, Schwanstecher C, Schwanstecher M, et al. Synthesis and in vitro evaluation of (S)-2-([11C]methoxy)-4-[3-methyl-1-(2-piperidine-1-yl-phenyl)-butyl-carbam oyl]-benzoic acid ([11C]methoxy-repaglinide): a potential beta-cell imaging agent. Bioorg Med Chem Lett. 2004;14(20):5205–9. doi: 10.1016/j.bmcl.2004.07.059. [DOI] [PubMed] [Google Scholar]
- 23.Wangler B, Schneider S, Thews O, Schirrmacher E, Comagic S, Feilen P, et al. Synthesis and evaluation of (S)-2-(2-[18F]fluoroethoxy)-4-([3-methyl-1-(2-piperidin-1-yl-phenyl)-butyl-carbamoyl]-methyl)-benzoic acid ([18F]repaglinide): a promising radioligand for quantification of pancreatic beta-cell mass with positron emission tomography (PET) Nucl Med Biol. 2004;31(5):639–47. doi: 10.1016/j.nucmedbio.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Mishra AK, Hazari PP, Shukla G, Goel V, Chuttani K, Kumar N, et al. Synthesis of specific SPECT-radiopharmaceutical for tumor imaging based on methionine: (99m)Tc-DTPA-bis(methionine) Bioconjug Chem. 2010;21(2):229–39. doi: 10.1021/bc900197n. [DOI] [PubMed] [Google Scholar]
- 25.Mishra AK, Kakkar D, Tiwari AK, Chuttani K, Kaul A, Singh H. Comparative evaluation of glutamate-sensitive radiopharmaceuticals: technetium-99m-glutamic acid and technetium-99m-diethylenetriaminepentaacetic acid-bis(glutamate) conjugate for tumor imaging. Cancer Biother Radiopharm. 2010;25(6):645–55. doi: 10.1089/cbr.2010.0848. [DOI] [PubMed] [Google Scholar]
- 26.Hossain GA, Moinul Islam SM, Mahmood S, Khan N, Chakrabarty RK. Tc-99m DTPA scintigraphy in soft tissue tumor. Mymensingh Med J. 2005;14(2):185–8. [PubMed] [Google Scholar]
- 27.Simpson NR, Souza F, Witkowski P, Maffei A, Raffo A, Herron A, et al. Visualizing pancreatic beta-cell mass with [11C]DTBZ. Nucl Med Biol. 2006;33(7):855–64. doi: 10.1016/j.nucmedbio.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christ GJ, Day N, Santizo C, Sato Y, Zhao W, Sclafani T, Bakal R, Salman M, Davies K, Melman A. Intracorporal injection of hSlo cDNA restores erectile capacity in STZ-diabetic F-344 rats in vivo. Am J Physiol Heart Circ Physiol. 2004;287(4):H1544–53. doi: 10.1152/ajpheart.00792.2003. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein R, Meyer T. Role of everolimus in pancreatic neuroendocrine tumors. Expert Rev Anticancer Ther. 2011;11(11):1653–65. doi: 10.1586/era.11.145. [DOI] [PubMed] [Google Scholar]
- 30.Snigur GL, Pisarev VB, Spasov AA, Samokhina MP, Bulanov AE. Mechanisms of toxic effect of streptozotocin on beta-cells in the islets of Langerhans. Bull Exp Biol Med. 2009;148(6):937–9. doi: 10.1007/s10517-010-0856-9. [DOI] [PubMed] [Google Scholar]