Abstract
Focal adhesion kinase (FAK) is a critical regulator of signal transduction in multiple cell types. Although this protein is activated upon T cell receptor (TCR) engagement, the cellular function that FAK plays in mature human T cells is unknown. By suppressing the function of FAK, we revealed that FAK inhibits TCR-mediated signaling by recruiting C-terminal Src kinase (Csk) to the membrane and/or receptor complex following TCR activation. Thus, in the absence of FAK, the inhibitory phosphorylation of Lck and/or Fyn is impaired. Together, these data highlight a novel role for FAK as a negative regulator TCR function in human T cells. These results also suggest that changes in FAK expression could modulate sensitivity to TCR stimulation and contribute to the progression of T cell malignancies and autoimmune diseases.
Introduction
Human T cells control the extent of the adaptive immune response following infection and in many pathological conditions (1-6). T cells are activated upon stimulation of the TCR by peptide-bound MHC complexes in combination with one or more co-stimulatory receptors (2). Constitutively active Lck is recruited to the TCR complex after antigen stimulation, where it phosphorylates ITAMs found in multiple TCR subunits (2, 7, 8). This event is critical for ZAP-70 activation (2). The adaptor proteins LAT and SLP-76 are then phosphorylated by ZAP-70. Together, LAT and SLP-76 recruit and control the activation of multiple effectors proteins like PLC-γ1 and PI3K, thereby triggering downstream signaling events like calcium influx and Akt activation (2, 9, 10). TCR activation culminates in transcriptional and morphological changes that regulate cytokine production, receptor expression, and the migratory properties of T cells (2).
The phosphorylation of Lck Y394 and Y505 controls Lck enzymatic activity to prevent inappropriate T cell responses. Lck Y505 phosphorylation stabilizes the protein in a closed, inactive conformation, which limits TCR function (11-15). This tyrosine residue is phosphorylated by C-terminal Src kinase (Csk) and de-phosphorylated by CD45 (11). The activity of Lck is also enhanced by the trans auto-phosphorylation of Y394, a residue found in the activation loop of the kinase domain (8, 11). Importantly, increases in Lck Y394 and decreases in Y505 phosphorylation are correlated with enhanced Lck activity (11). Thus, Lck activity is dictated by the balance of Lck Y394 and Y505 phosphorylation, and perturbations in the phosphorylation ratio of these two residues can increase or decrease TCR-induced signaling and T cell activation.
To phosphorylate Lck Y505, cytoplasmic Csk is recruited to the T cell membrane, a process that is vital for its function (16-19). The current model is that in unstimulated T cells Csk binds to phospho-Y317 on phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), also known as Csk-binding protein (Cbp) (18, 20-24). This interaction localizes Csk to the plasma membrane and enhances its catalytic function, which allows Csk to phosphorylate Lck Y505 (25). Upon TCR activation, PAG/Cbp is de-phosphorylated, after which Csk is transiently displaced from detergent-insoluble membrane lipid rafts (18, 23). This transient displacement allows CD45 to de-phosphorylate Lck Y505, resulting in the enhanced enzymatic function of Lck (11, 26). Within 5 min after TCR activation, Csk re-associates with lipid rafts, presumably because PAG Y317 is re-phosphorylated (18, 20). However, contrary to this model, Lck Y505 phosphorylation remains unchanged or increases after TCR stimulation (7, 8, 19, 27). Furthermore, the observation that PAG-deficient T cells do not have enhanced T cell activation suggests that alternative mechanisms exist to regulate Csk's recruitment to the membrane after TCR activation (21, 22, 28). Therefore the mechanisms that regulate Csk's recruitment to the membrane after TCR stimulation are not clear.
Actin cytoskeletal responses are essential for cytokine release and cellular spreading downstream of the TCR (29, 30). Focal adhesion kinase (FAK) is phosphorylated by Lck and/or Fyn upon TCR induction (31, 32). Previously, FAK was found to control cellular processes linked to actin polymerization. In line with this role, inhibiting FAK's expression or function in T cells, B cells, macrophages, and neutrophils impaired actin-dependent processes like adhesion or spreading (32-36). Thus, the observation that FAK regulates actin-dependent responses is likely to have important implications in TCR function. However, since FAK is expressed at low levels in human T cells compared to B cells (37), it may serve an alternative function downstream of the TCR.
In this study, we show that the transient knockdown of FAK results in enhanced or extended TCR-induced signal transduction, cytokine production, and CD69 expression in Jurkat E6.1 cells and CD4 human activated peripheral blood T cells (hAPBTs). Using total internal reflection fluorescence (TIRF) microscopy and immunoprecipitations, we found that Csk recruitment to the membrane and TCR complex following TCR induction requires FAK expression. After TCR activation, FAK-deficient cells also displayed decreased Lck Y505 phosphorylation and increased Lck Y394 and TCRζ chain phosphorylation. Together, these findings demonstrate that FAK negatively regulates TCR signaling by controlling Lck activation.
Materials and Methods
Human Samples
All human subject studies were in compliance with the Declaration of Helsinki. Healthy donors between the ages of 18-55 years old were recruited by the DeGowin Blood Center at the University of Iowa's Hospitals and Clinics. These donors provided written consent for their blood products to be used in research projects conducted at the University of Iowa in compliance with the University of Iowa's Institutional Review Board (IRB). Whole blood was passed through LRS cones (38), and these completely de-identified cones were then provided to investigators at the University of Iowa. Since the blood products from LRS cones were normally discarded, we did not require further IRB approval to use these blood samples.
Reagents and Antibodies
RPMI 1640, L-glutamine, penicillin-streptomycin, and PBS were acquired from Gibco. The FBS was obtained from Atlanta Biologicals and Denville. Human recombinant IL-2 used for ELISA was purchased from R&D Systems, while the human recombinant IL-2 used in growth media was obtained from AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: Human rIL-2 from Dr. Maurice Gately, Hoffman – La Roche Inc (39). The anti-phospho-LAT tyrosine 191, anti-actin and anti-FAK antibodies, PVDF membrane, and polybrene were obtained from Millipore. The anti-SLP-76 antibody was purchased from Antibody Solutions. The anti-Lck pY505, anti-SLP-76 pY128, anti-CD247 pY142, anti-Pyk2, anti-Csk, anti-Grb2, and anti-LAT antibodies were acquired from BD Biosciences. The anti-PLC-γ1 pY783, anti-PLC-γ1, anti-Lck, and anti-Src pY416 antibodies were purchased from Cell Signaling Technologies. The HRP-conjugated secondary antibodies and strepavidin-HRP were from Jackson Immunoresearch Laboratories. The IRdye 800CW- or IRdye 680-labeled secondary antibodies were obtained from Licor Biosciences. Protein A/G agarose beads were bought from Santa Cruz Technologies. Anti-human rIL-2, anti-human IFNγ, biotin-conjugated anti-human IL-2, and biotin-conjugated anti-human IFNγ antibodies were purchased from eBioscience. PE anti-human αβ T cell receptor, PE/Cy5 anti-CD69, Dylight 488 goat anti-mouse IgG1, DyLight 488 donkey anti-rabbit IgG, anti-CD3 (OKT3), and anti-CD4 (RPA-T4) antibodies were obtained from Biolegend. The anti-Akt pS473, Alexafluor 568 anti-rabbit, and Alexafluor 568 anti-mouse IgG1 antibodies and the magnetic Dynabeads were from Invitrogen. The goat anti-mouse IgG antibody was acquired from Rockland International. The FAK inhibitor, PF-573228, was purchased from Tocris Bioscience. Criterion Precast 4-15% polyacrylamide gels were purchased from Biorad. Oligonucleotides were generated at Integrated DNA Technologies. All chemicals used were research grade and purchased from a variety of sources.
Plasmids
The Luc miRNA cassette was a kind gift from Dr. Anton McCaffrey. Sequences for Fak-specific miRNAs were selected (Table SI) and used to replace the Luc targeting sequence as previously described (40). Two copies of the Luc and Fak 3183 miRNAs were PCR amplified and ligated into the pVetLeGFP vector developed by the University of Iowa's Gene Vector Transfer Core. The Luc miR- and Fak miR-GFP reporter lentiviruses were generated by the Gene Vector Transfer Core at the University of Iowa.
Transfection and stimulation of Jurkat E6.1 cells
Jurkat E6.1 T cells were grown to a density of 3-4×105 cells/ml in complete RPMI (RPMI 1640 supplemented with 10% FBS, 2mM L-glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin). Cells were transfected with Luc or Fak miRNA or the various FAK knockdown/re-expression vectors (5μg per 5×106 cells) using the Amaxa Cell Line Nucleofector Kit V (Lonza) and program X-001 and then incubated for 72 h. Prior to stimulation, cells were grown to a density of 2-5×105 cells/ml. Cells were stimulated with 10 μg/ml soluble anti-CD3 for various times as previously described (41-45).
Isolation and Growth of CD4 hAPBT
Peripheral blood mononuclear cells (PBMC) were obtained from LRS cones (38) as previously described (46). CD4 T cells were purified from non-adherent PBMC by negative selection using the Human CD4 T cell isolation kit (Stem Cell Technologies). Cells were routinely >95% pure. Human CD4 T cells then activated with anti-CD3 and anti-CD28 beads in the presence of 100 U/ml IL-2.
FAK knockdown and stimulation of CD4 hAPBT
Purified CD4 T cells were activated for three days with the antibody coated beads. The cells were then resuspended at 2×106 cells/ml in complete RPMI plus 100 U/mL recombinant IL-2 and 8 μg/ml polybrene. Luc or Fak miRNA-containing lentiviruses were then incubated with the cells (MOI 2) for 24 h. The virus was removed and the cells were incubated for 48-72 h. These cells were then stimulated by cross-linking with 2 μg/ml anti-CD3 and 2 μg/ml anti-CD4 as previously described (41, 42, 44). For imaging experiments, CD4 T cells were activated for 5-7 days with the Dynabeads as above, and transfections were carried out as described for the Jurkat cells.
Immunoblotting
Proteins in cellular lysates were separated by PAGE using 4-15% Criterion gels and transferred to PVDF membranes. The membranes were blocked at room temperature in TBST (10 mM TRIS pH 8.0, 150 mM NaCl, and 0.05% Tween 20) with 5% nonfat dry milk, 1% bovine serum albumin, or in SEA BLOCK Blocking Buffer (Thermo Scientific) diluted 1:1 in PBS. Immunoblotting was performed as described (42, 44, 45). Antibody binding was detected using ECL and a Fiji imager or using the Licor Odyssey Infrared Imager. Pan antibodies do not always recognize the phosphorylated forms of TCR signaling proteins, including those for LAT and SLP-76 (41). Therefore, stripped membranes were re-probed with a pan-actin antibody to ensure equivalent protein loading.
Analysis of Immunoblotting
The relative intensity of bands was determined using the gel-plotting macro of ImageJ or the analysis software in the Odyssey v3.0 program. Normalization of the phospho-protein intensity to the actin intensity was conducted as we have described previously (41-44). Upon normalization, the percent of maximal Luc phosphorylation was calculated:
% maximum Luc phosphorylation = (NI of timepoint ÷ NI of maximum Luc timepoint) × 100%. The average percentage from each least three independent experiments ± s.e.m. for every timepoint was then plotted using Microsoft Excel.
Correlation Analyses
Human CD4+ T cells were isolated by negative selection and activated using anti-CD3 and anti-CD28 coated beads in the presence of recombinant IL-2. On the indicated days, equal numbers of these cells were lysed using hot 2× sample buffer and were boiled and sonicated as described above. The expression of FAK, Csk, phospho-Lck Y505, and/or Pyk2 was detected by immunoblotting and normalized to actin expression in each lane. These normalized values obtained from each independent experiment were used to produce the scatter plots shown in Figure 10E and 10G. To generate the graphs shown in Figures 10F and 10H, equal numbers of freshly isolated CD4+ T cells (US CD4 T cell), unstimulated Jurkat E6.1 cells, unstimulated HuT78 T cells, and CD4+ T cells that were activated for 6 days using the anti-CD3 and anti-CD28 coated beads plus recombinant IL-2 (activated CD4 T cell) were lysed with hot 2× sample buffer. Each of these samples was loaded in duplicate onto a polyacrylamide gel, and immunoblotting was used to detect differences in FAK, Csk, and phospho-Lck Y505 expression in each sample. The densitometric intensity for FAK, Csk, and phospho-Lck Y505 in each lane was then normalized to actin expression. To generate the plots for the analysis of CD4 T cells and human T cell lines, the average expression of the duplicate samples was first calculated. Next, the average of these duplicate values from each of the four independent samples was determined and graphed. The Pearson Correlation Co-efficient and linear regression analysis were used to evaluate the relationship between FAK or Csk expression and Lck Y505 phosphorylation.
Immunoprecipitations
Control or FAK-deficient cells were stimulated with 2 μg/ml of soluble anti-CD3 for various times. These cells were lysed with IP lysis buffer, and Csk was then immunoprecipitated using 2 μg of anti-Csk antibody and protein A/G beads as described (41, 45). To precipitate the TCR complex from CD4 hAPBTs, the cells were treated with soluble anti-CD3 and anti-CD4 with or without anti-mouse IgG, and the samples were lysed as above. The protein A/G beads were then added to the supernatants and rotated overnight. Bound proteins were eluted using 25 μl of hot 2× buffer, and samples were boiled at 95°C for 4 min.
Cytokine Production
Control and FAK-deficient cells were washed and resuspended at 6×105 cells/ml in complete RPMI and stimulated with various doses of plate-bound OKT3 for 24 h. The production of IL-2 or IFN-γ was determined by standard TMB sandwich ELISA. The absorbance at 490 nm was measured using the Epoch plate reader (Biotek). Data were then normalized to account for the variations between human donors and/or independent experiments. The relative amount of cytokine that was produced in each sample was determined using this formula: % of maximum cytokine value in Fak miRNA-treated sample = (concentration of sample ÷ maximum concentration in Fak miRNA-treated sample) × 100%. These mean ± s.e.m. from three to four independent experiments was then calculated.
TCR Downregulation
Cells were resuspended at 1×107 cells/mL in RPMI 1640 and incubated for 10 min at 37°C. The cells were then stimulated with 1μg/ml soluble OKT3 for various times, transferred to ice, and fixed using 1% ice-cold methanol-free formaldehyde for 5 min. Cells were then stained with PE anti-human αβ on ice for 30 min. Cells were washed with FACS buffer (PBS, 5% FBS, and 0.05% sodium azide), and samples were collected on an Accuri flow cytometer. The mean fluorescence intensity was determined using Cflow Plus analysis program. Percent TCR surface expression was determined using the following equation: % Surface Expression = (MFI at timepoint) ÷ (MFI at 0 min) × 100%.
CD69 Expression
Control and FAK-deficient Jurkat cells suspended at 1×107 cells/mL in RPMI 1640 and stimulated with various doses of anti-CD3 for 4 h at 37°C. Cells were washed with FACS buffer and stained on ice with PE/Cy5 anti-human CD69 for 30 min. Samples were collected using an Accuri flow cytometer. The MFI of each sample was determined using the Accuri Cflow Plus software. The data were normalized such that CD69 expression at 0 min was equal to 1, and the average normalized value from three experiments ± s.e.m. was plotted using Microsoft Excel.
Cellular Imaging
Control or Fak-deficient T cells were stimulated on glass chamber slides coated with anti-CD3 (47). Cells were fixed with 3.2% paraformaldehyde and permeabilized with 0.25% Triton X-100. After blocking, primary antibodies against ZAP-70 pY319 or total Csk were then diluted in SEA BLOCK buffer prepared in PBS and incubated overnight at 4°C. Secondary antibodies were also incubated overnight at 4°C. The images were obtained using the Leica AM TIRF MC imaging system found in the University of Iowa's Central Microscopy Core. Images from 6-12 randomly selected fields per treatment were obtained using the 100× oil objective and a TIRF alignment of 150 nm at room temperature. The GFP-TIRF or mCherry-TIRF laser intensity and exposure times were set using the control cells stimulated for 3 min for the Jurkat cells or 7.5 min for CD4 hAPBTs, and these settings were kept constant for the remaining samples. Alternatively, the cells were stained using FITC-conjugated phalloidin overnight at 4°C and imaged using the 100× oil objective and the GFP-epifluorescence filter. All images were acquired using the Leica AF software and processed using the Fiji software package.
Imaging Analysis
All image analyses were performed using the Coloc 2 plug-in for the Fiji software package. To determine percent co-localization, twenty-five randomly-selected cells from each of the four independent Jurkat or three independent CD4 hAPBT experiments were analyzed. The Manders correlation coefficients were then determined and plotted using GraphPad Prism 5. Any cell that had a Costes P-value of less than 0.9 was excluded from further analysis to reduce the likelihood of false-positive co-localization. The mean channel intensity from these cells was also recorded to determine the amount of Csk and ZAP-70 phospho-tyrosine 319 staining. Stimulated cells that were stained with the secondary antibodies alone served as the negative control for these experiments.
Statistical Analysis
Statistical analysis was performed in Microsoft Excel using a two-tailed t-test assuming equal variance.
Results
MicroRNAs repress FAK expression in human T cells
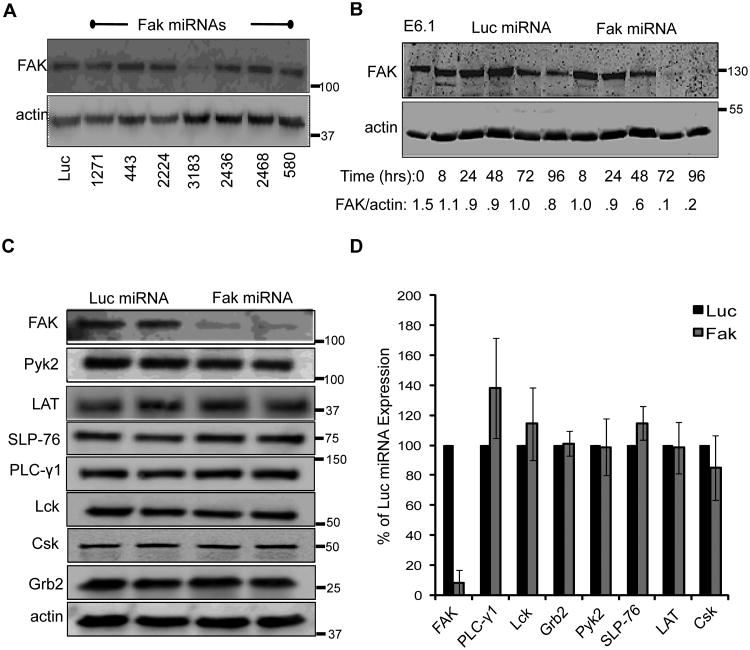
To elucidate if FAK is required for TCR function in mature human T cells, we transiently suppressed FAK expression using miRNAs. Seven RNA interference sequences were selected and used to replace the endogenous targeting sequences of human miR30 (40). We then tested the silencing efficacy of these sequences by transiently transfecting the Jurkat E6.1 human T cell line. By 72 h after transfection, only the sequence corresponding to nucleotides 3183-3202 inhibited FAK protein expression by greater than 90% compared to the control sequence against firefly luciferase (Luc miRNA) (Figure 1A). As shown in Figure 1B, FAK knockdown was detectable at 48 h and maximal suppression occurred at 72-96 h after transfection.
Figure 1.
MicroRNAs repress FAK expression in human T cells. A, Jurkat cells were transfected with either a control (Luc) miRNA or various miRNAs specific for FAK. Whole cell lysates were taken 72 h later and the expression of FAK and actin was analyzed by immunoblotting. B, Jurkat T cells were transfected with Luciferase- or FAK-specific miRNAs. Whole cell lysates were prepared at the indicated times and FAK and actin expression were analyzed by immunoblotting. The densitometric ratio of FAK expression compared to actin expression is shown below the graph. C. Whole cell lysates from Jurkat E6.1 T cells transfected with microRNAs against Luciferase or FAK were analyzed by immunoblot for FAK, Pyk2, LAT, SLP-76, PLC-γ1, Lck, Csk, and Grb2 and actin expression 72 h after transfection. (D) The normalized expression of FAK, Pyk2, LAT, SLP-76, PLC PLC-γ1, Lck, Csk, or Grb2 expression is shown as the mean of two to three experiments ± s.d.
Proline-rich tyrosine kinase 2 (Pyk2) is also expressed in human T cells and is activated by TCR stimulation (44, 48). Pyk2 shares 45% amino acid identity to FAK and is overexpressed in FAK−/− cells, where it compensates for multiple cellular functions (49-51). We found that transient knock down of FAK in Jurkat cells did not affect Pyk2 expression nor were Lck, Csk, SLP-76, LAT, Grb2, and PLC-γ1 protein levels altered (Figure 1C-1D). Thus, the Fak 3183 miRNA selectively and potently inhibits FAK expression in Jurkat cells.
FAK negatively regulates TCR-induced signaling in Jurkat cells and CD4 hAPBTs
Since FAK appears to integrate receptor-mediated signals that control actin reorganization in immune cells (33-36), we examined actin polymerization upon TCR induction. Jurkat cells expressing the Luc or FAK-specific miRNAs were stimulated on glass chamber slides treated with anti-TCR and stained to detect actin filaments that form upon T cell spreading (47). As shown in Figure S1A-S1B, the intensity of phalloidin staining and the degree of cellular spreading as measured by changes in cell area were comparable in control and FAK-deficient Jurkat cells. Therefore, it does not appear that TCR-induced actin remodeling that drives cellular spreading requires FAK.
The actin cytoskeleton also regulates TCR expression on resting and activated T cells (30). As such, we next examined TCR levels by flow cytometry. As shown in Figure S1C, the steady-state expression of the TCR was comparable in both cell lines. Additionally, no differences in the loss of TCR surface expression were observed following receptor activation in the control or FAK-deficient cells (Figure S1D). Thus, basal TCR expression and ligand-induced TCR downregulation are normal in the absence of FAK.
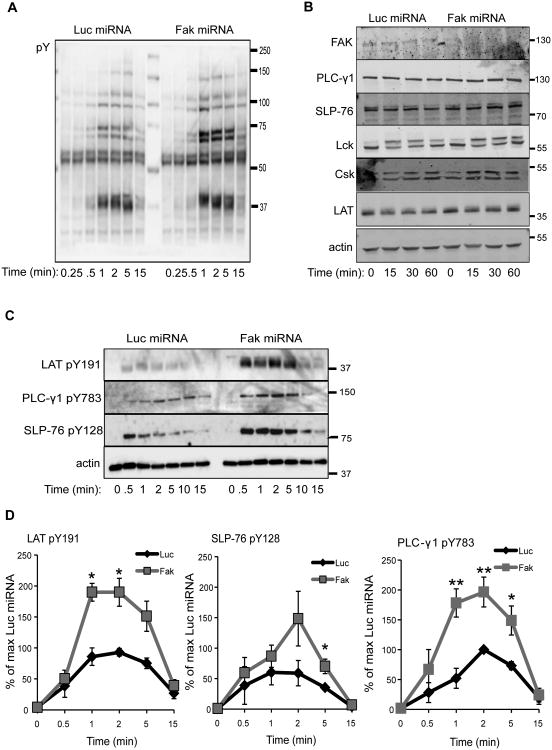
The actin cytoskeleton network also regulates signaling downstream of the TCR (29, 30). Therefore, we next investigated if TCR-induced signaling was altered in the absence of FAK. To that end, control and FAK-deficient Jurkat cells were stimulated for various times with a soluble, agonistic anti-TCR antibody. We then examined broad changes in early TCR-induced signaling using an anti-phosphotyrosine immunoblot. Surprisingly, TCR-inducible signaling appeared to be enhanced in the FAK-deficient Jurkat cells as measured by an anti-phosphotyrosine immunoblot (Figure 2A). This global increase in tyrosine phosphorylation was not due to altered protein expression of Lck, LAT, SLP-76, PLC-γ1, or Csk after TCR induction (Figure 2B). To confirm these findings, we employed a quantitative method to examine changes in TCR-induced signaling using phospho-specific antibodies and immunoblotting (43). Following activation with the stimulatory anti-TCR antibody, the site-specific phosphorylation of LAT Y191, PLC-γ1 Y783, and SLP-76 Y128 was examined. As shown in Figure 2C-2D, the phosphorylation kinetics of LAT, SLP-76, and PLC-γ1 were comparable between Luc and Fak miRNA-treated Jurkat cells, with maximum phosphorylation occurring at 1-2 min after stimulation and returning to baseline by 15 min. The magnitude of the phosphorylation of these proteins was strikingly different, however. When FAK expression was silenced in Jurkat cells, the site-specific phosphorylation of LAT, PLC-γ1, and SLP-76 was enhanced by 2-to-3-fold at 1-5 min after TCR stimulation (Figure 2C-2D). These data demonstrate that FAK suppresses the level of early TCR-mediated signaling in Jurkat cells.
Figure 2.
FAK controls the magnitude of early TCR-mediated signaling in Jurkat T cells. Control and FAK-deficient Jurkat cells were stimulated with soluble anti-TCR for various times. A, Total changes in TCR-induced phosphorylation were analyzed by immunoblotting with anti-phospho-tyrosine (Y). B, The expression of FAK, PLC-γ1 SLP-76, Lck, Csk, LAT, or actin was analyzed by immunoblotting. C, The site-specific phosphorylation of LAT Y191, SLP-76 Y128, and PLC-γ1 Y783 in TCR-stimulated control or FAK-deficient Jurkat T cells was analyzed by immunoblotting. D, The percent phosphorylation for LAT Y191, PLC-γ1 Y783, and SLP-76 Y128 compared to the Luciferase microRNA-transfected cells was calculated as described in Materials and Methods. The mean of three to four experiments ± s.e.m. is shown. * p ≤ 0.05; ** p ≤ 0.01
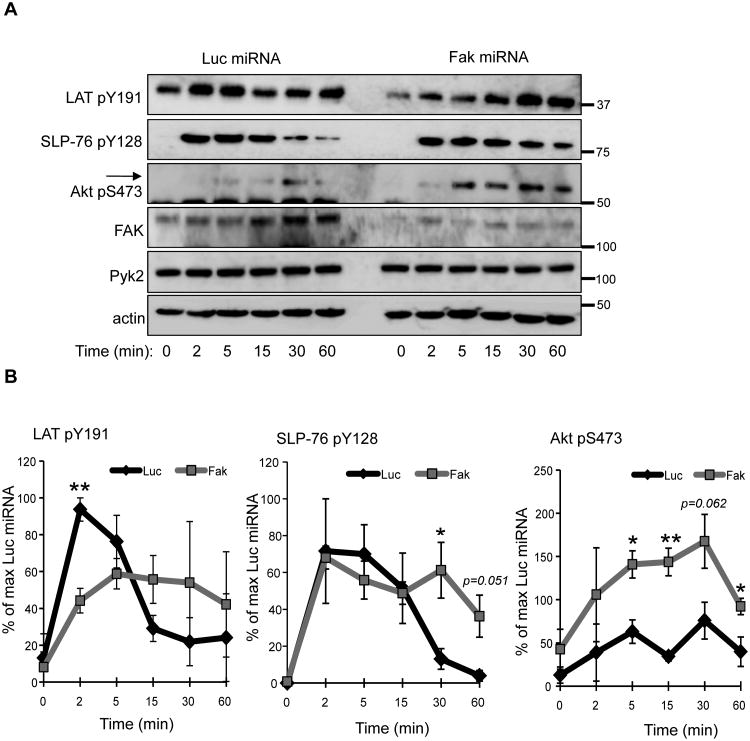
In Jurkat cells, many proximal signaling molecules including PLC-γ1 and Pyk2 have exaggerated TCR-induced activation compared to hAPBTs (41). Because of these differences, we also examined whether FAK deficiency altered TCR-induced signaling in primary human T cells. Transducing CD4 hAPBTs with GFP-expressing lentiviruses containing the Luc- or FAK-specific miRNAs led to greater than 75% silencing of FAK expression while having no effect on Pyk2 protein levels (Figure 3A), demonstrating that this suppression vector is also highly specific in primary human CD4 T cells. FAK-deficient CD4 hAPBTs initially displayed lower LAT Y191 phosphorylation after TCR activation. However, the phosphorylation of LAT appeared to last longer in FAK-deficient cells, although this change was not significant (Figure 3B). The TCR-inducible phosphorylation of SLP-76 was also significantly extended in the absence of FAK (Figure 3B). We next determined if Akt phosphorylation was altered, since this kinase regulates many T cell functions including cytokine production (52). Interestingly, the phosphorylation of Akt S473 was significantly enhanced in FAK-deficient cells compared to the control cells (Figure 3B). Together, these data reveal that TCR-induced signaling is enhanced and/or prolonged in the absence FAK, suggesting that FAK feedback inhibits TCR-mediated signaling in human T cells.
Figure 3.
The kinetics or extent of TCR signaling is altered in CD4 hAPBTs that lack FAK. A, CD4 hAPBTs were transduced with a lentivirus containing miRNAs against Luciferase (Luc) or FAK. The cells were then stimulated by soluble anti-CD3 and anti-CD4 cross-linking for various times. The site-specific phosphorylation of LAT Y191, SLP-76 Y128, and AKT S473 was analyzed by immunoblotting. The expression of FAK, Pyk2, and actin was also examined. B, Percent phosphorylation for LAT Y191, SLP-76 Y128, and Akt S473 relative to the Luciferase microRNA-transduced cells is depicted. Data are shown as mean of three experiments ± s.e.m. *p ≤ 0.05; **p ≤ 0.01.
FAK controls the threshold of TCR activation in human T cells
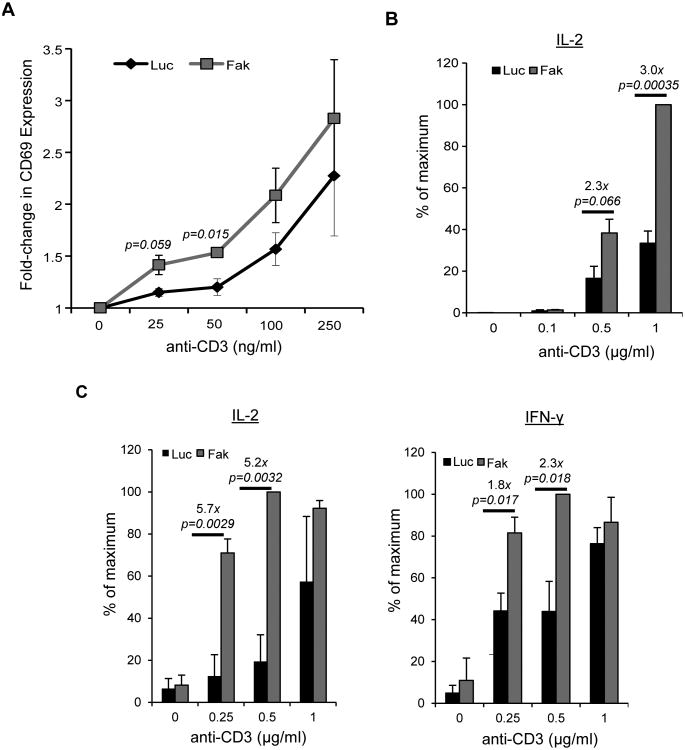
We next addressed if the changes in TCR-induced signaling described above altered downstream functions in human T cells. To that end, we measured differences in CD69 expression and cytokine production. As shown in Figure 4A, Jurkat cells treated with the Luc miRNA showed a modest upregulation in the expression of CD69 when stimulated with 100 ng/ml of soluble anti-TCR. CD69 expression in these cells had increased CD69 by approximately 2-fold after stimulation with 250 ng/ml of anti-TCR. By comparison, CD69 upregulation in FAK-deficient cells was similar to the control cells following stimulation with 2-4 fold lower doses (25-50 ng/ml) of anti-TCR. The control and Fak miRNA-treated cells showed similar CD69 responses at 250 ng/ml of anti-TCR (Figure 4A). We also found FAK-deficient Jurkat cells produced 2-to-3-fold more IL-2 production after stimulation with 0.5-1 μg/ml of anti-TCR (Figure 4B). FAK-deficient CD4 hAPBTs secreted approximately 5-fold more IL-2 and approximately 2-fold more IFN-γ following stimulation with 0.25-to-0.5 μg/ml of anti-TCR. No significant differences were observed at the 1μg/ml dose (Figure 4C). Thus, TCR induction occurs at lower doses of stimulation in the absence of FAK, suggesting that this protein fine-tunes the responsiveness of T cells to TCR activation.
Figure 4.
TCR-induced effector functions are enhanced in FAK-deficient T cells. A, CD69 expression in control or FAK-depleted Jurkat cells stimulated for 4 h with the indicated doses of soluble OKT3. The graph shows the mean fold change over the unstimulated samples ± s.e.m. from three independent experiments. B, Control or FAK-deficient Jurkat cells were stimulated for 24 h with various doses of plate-bound anti-TCR, and IL-2 production was measured by ELISA. The mean normalized value ± s.e.m. from three independent experiments is shown. C, CD4 hAPBTs were transduced with Luciferase (Luc) or Fak-specific miRNAs. Cells were then stimulated with various concentrations of plate-bound anti-TCR for 24 h. The production of IL-2 and IFNγ was measured by ELISA. The mean normalized value ± s.e.m of at least three independent replicates is shown.
The localization of Csk is altered in FAK-deficient cells
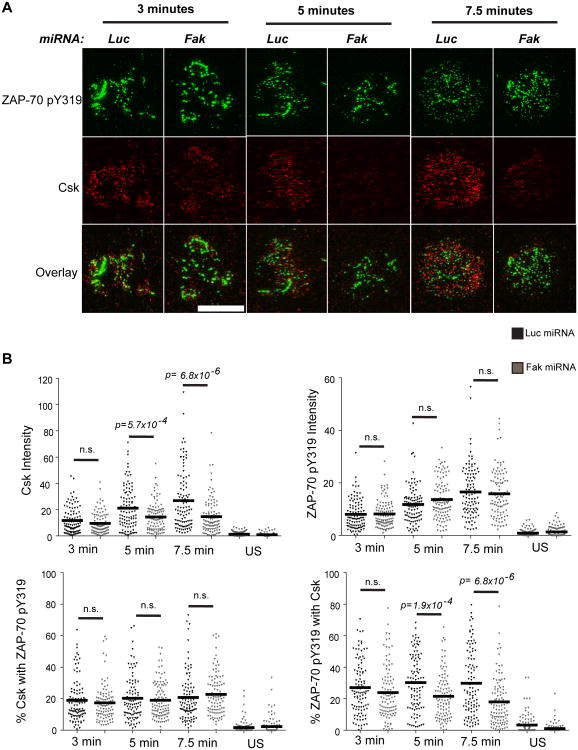
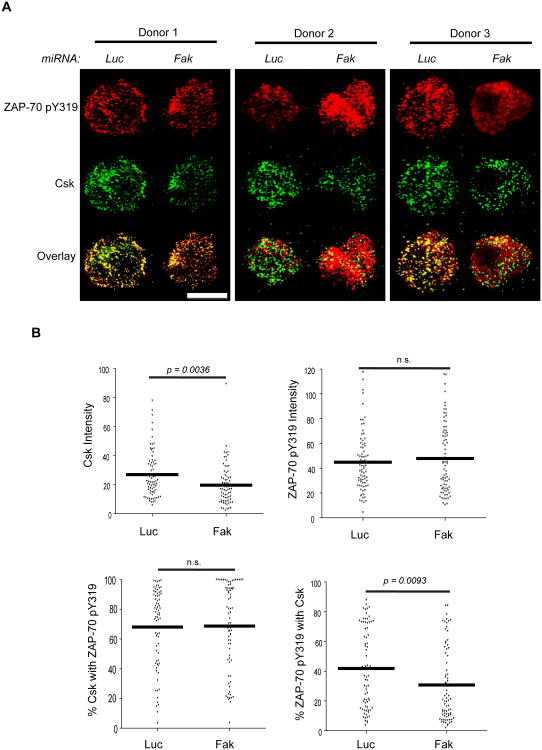
In other cell types, FAK associates with Csk (53-55). TCR-induced signaling is enhanced in Csk-deficient human T cells, and these cells are also more responsive to TCR induction (12, 13). Since this phenotype is strikingly similar to that we observed in FAK-deficient human T cells, we investigated if FAK controlled Csk function in human T cells. Csk is active when it is recruited to the plasma membrane (16-18). To assess if Csk was differentially recruited to the T cell membrane after TCR stimulation, we used TIRF microscopy. This type of microscopy will only excite proteins labeled with fluorochromes when they are within 100-200 nm from the cell-chamber slide interface and is therefore a useful way to track the recruitment of cytoplasmic proteins to the membrane (56). As seen in Figure 5, Csk was detectable in the membranes of TCR-stimulated control Jurkat cells at 3 min after receptor activation and its membrane expression increased over time. These results are consistent with previous membrane fractionation studies (17, 18). Csk was also found in the membrane of the control CD4 hAPBTs after TCR stimulation (Figure 6). Strikingly, there was a significant decrease in the intensity of Csk staining after TCR stimulation in FAK-deficient Jurkat cells and CD4 hAPBTs (Figure 5-6). We also analyzed phospho-ZAP-70 Y319 staining as an internal staining control, and we observed no detectable differences in the amount of phospho-ZAP-70 Y319 that was present in the membrane fractions (Figure 5-6). The latter observation is likely due to the fact that ZAP-70 turnover at the TCR is very rapid at these times (57). We did not select for FAK-deficient cells in these assays; therefore, those cells that had high Csk intensity were likely still FAK-sufficient. These results show that Csk membrane recruitment is defective in FAK-deficient T cells.
Figure 5.
Recruitment of Csk to membrane and TCR complex is impaired in FAK-deficient Jurkat T cells. A, Cells were stimulated on anti-TCR coated glass chamber slides for various times and stained with antibodies to detect ZAP-70 phospho-tyrosine 319 and total Csk. Cells were also stained with secondary antibodies alone (US) as a negative control. The images are representative of four independent experiments. The white scale bar is equal to 5 μm. B, For every experiment, twenty-five cells from each timepoint were analyzed using the Fiji analysis software. The intensity of Csk and ZAP-70 phospho-tyrosine 319 staining from 100 cells analyzed from four independent experiments was plotted. The horizontal line depicts the mean value (top graphs). The Manders correlation co-efficients were used to calculate Csk and phospho-ZAP-70 Y319 co-localization. The Manders co-efficients from 100 total Jurkat cells analyzed from four independent experiments were plotted. The horizontal line represents the mean value (bottom graphs). The p-values reflect the statistical differences between the control and FAK-deficient cells at the indicated times. n.s. means p > 0.05.
Figure 6.
Csk membrane and TCR localization is altered in FAK-deficient CD4 hAPBTs. A, CD4 hAPBTs were transfected with the Luc or FAK-specific microRNAs and incubated for 72 h. The cells were stimulated for 7.5 min with anti-TCR coated onto glass chamber slides. These cells were then stained with anti-phospho-ZAP70 Y319 and anti-Csk followed by the appropriate secondary antibodies. Representative cells from three separate human donors are shown. The white scale bar is equal to 5 μm. B, The intensity of Csk and ZAP-70 pY319 staining and co-localization between these proteins was analyzed as in Figure 9. The values from 75 cells taken from three independent experiments are shown and the mean values are represented by the horizontal lines. The p-values reflect the statistical differences between the control and FAK-deficient cells at the indicated times. n.s. means p > 0.05.
Csk interacts with the TCR complex (58, 59). Therefore, we next determined if the Csk and TCR association was mediated by FAK. Using phospho-ZAP-70 Y319 as a surrogate marker for the TCR (57), we found that a small fraction (15-20%) of membrane-associated Csk colocalized with phosphorylated ZAP-70 at 3-7.5 min after TCR stimulation. Similarly, 25-30% of phosphorylated ZAP-70 co-localized with Csk in Jurkat cells (Figure 5B: bottom graphs). When FAK expression was suppressed, the amount of phosphorylated ZAP-70 that colocalized with Csk was significantly decreased at 5-7.5 min after stimulation (Figure 5). Similar results were also obtained using CD4 hAPBTs, although there was substantially more colocalization between Csk and phosphorylated ZAP-70 in these cells (Figure 6). Jurkat cells are of thymocyte origin and do not express CD4 (41), which may explain these differences. These data indicate that Csk recruitment to the TCR complex is also impaired in the absence of FAK.
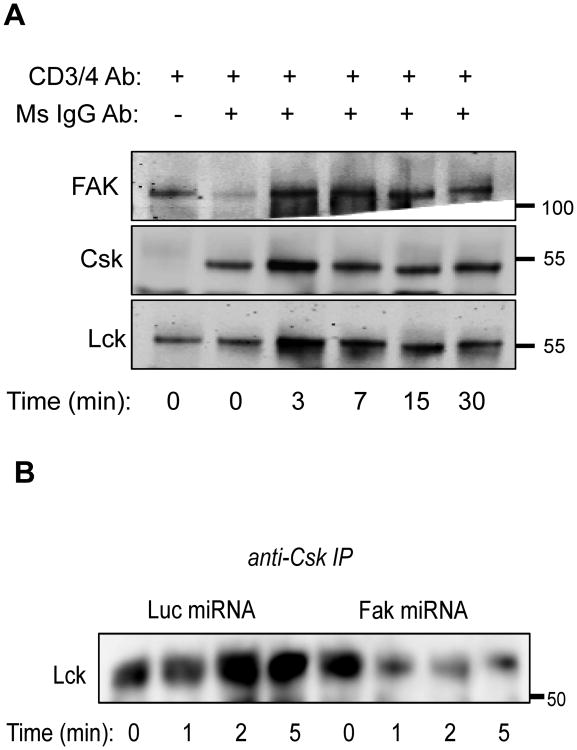
The results described above suggest that FAK associates with Csk at the TCR. To test this possibility, we stimulated CD4 hAPBTs for various times and pulled down the TCR/CD4 complex using the stimulatory anti-CD3 and anti-CD4 antibodies in the absence or presence of an anti-mouse crosslinking antibody. We found that FAK and Csk co-immunoprecipitated with the TCR-CD4 complex (Figure 7A). Consistent with published data (11, 53, 60, 61), Lck was also associated with the TCR-CD4 complex (Figure 7A). Interestingly, FAK and Lck were found to co-immunoprecipitate with the TCR-CD4 complex in the absence of crosslinking antibody, suggesting that these proteins constitutively associate with CD3 and/or CD4 in CD4 hAPBTs. At the 0 min timepoint, the addition of the crosslinking antibody caused Csk to associate with the TCR-CD4 complex. This increase in Csk's association with the TCR-CD4 complex could be the result of post-lysis stimulation. These data may also indicate that, upon crosslinking CD3 with CD4, the association between Csk and CD3 is stabilized (59), allowing us to detect the interaction by immunoprecipitation. We also attempted to verify these interactions using TIRF microscopy; however, FAK-specific antibodies detected many non-specific bands and could therefore not to be used for imaging studies (Figure S2 and unpublished observations). These data show that FAK is recruited to the TCR-CD4 complex, where it associates with Lck and its inhibitor Csk. Thus, FAK could regulate the function of these kinases.
Figure 7.
FAK forms a complex with Csk and Lck in human T cells. A, Activated CD4 T cells were stimulated by anti-CD3 and anti-CD4 cross-linking for various times. The TCR/CD4 complex was then immunoprecipitated, and the expression of FAK, Csk, and Lck was analyzed by immunoblotting. Data is representative of three independent experiments. B, Control or Fak-deficient Jurkat T cells were stimulated for various times with anti-CD3. Csk was immunoprecipitated and its association with Lck was assessed by immunoblotting. Data is representative of three experiments.
FAK, Csk, and Lck formed a stable complex in human T cells, and FAK-deficient T cells displayed hyper-active TCR function similar to Csk-deficient T cells. Therefore, FAK could serve as a scaffolding protein to recruit Csk to its substrate, Lck. To test this possibility, we stimulated control and FAK-deficient Jurkat cells with soluble anti-TCR for various times and performed Csk immunoprecipitations. We found that a cellular fraction of Lck was bound with Csk in TCR-stimulated cells. Strikingly, the TCR-inducible association between Lck and Csk was greatly reduced in the absence of FAK (Figure 7B). Thus, FAK facilitates complex formation between Csk and Lck after TCR induction.
The TCR-inducible phosphorylation and function of Lck is altered in FAK-deficient T cells
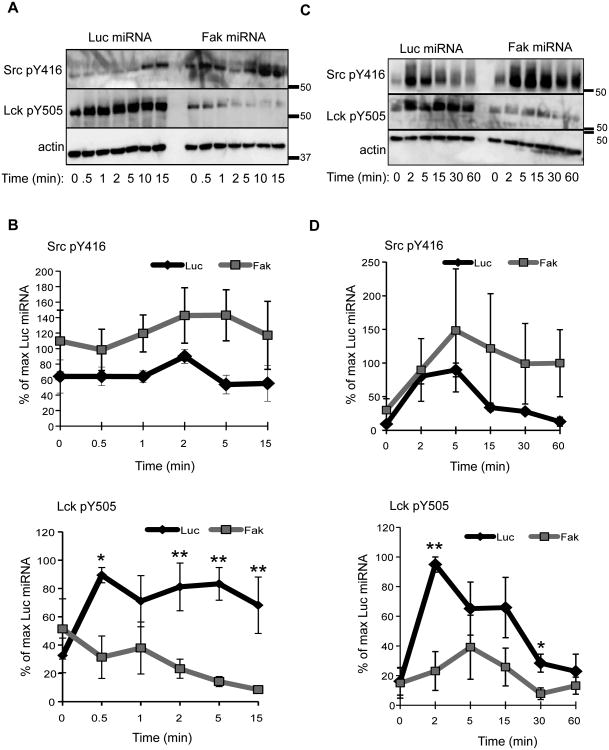
The recruitment of Csk to the membrane, the TCR, and/or Lck was defective in FAK-deficient T cells (Figures 5-6). In Csk-deficient T cells, the phosphorylation of Lck Y394 and Y505 is enhanced and reduced, respectively, and these alterations result in enhanced Lck activity (12, 13). To examine if the defects in Csk localization were correlated with differences in Lck phosphorylation, we stimulated control or FAK-deficient Jurkat cells and CD4 hAPBTs and examined the phosphorylation of Lck Y394 and Y505. We used an anti-Src pY416 antibody to detect changes in Lck Y394 phosphorylation, which recognizes all Src kinases when they are phosphorylated on their activating sites. Consistent with previous reports (7, 8, 12), both Lck Y394 and Y505 were phosphorylated in unstimulated Jurkat cells and CD4 hAPBTs, and TCR stimulation enhanced the phosphorylation of these sites in the control cells. Strikingly, the levels of Lck Y505 induced by TCR activation were significantly reduced in the FAK-deficient Jurkat cells and CD4 hAPBTs. The phosphorylation of Lck Y394, the auto-phosphorylation site, was also increased by approximately 2-fold in the FAK-deficient cells (Figure 8). This result suggests that Lck's enzymatic activity is enhanced in FAK-deficient cells. Thus, the defects in Csk recruitment observed in FAK-deficient cells are correlated with decreased Lck Y505 and increased Y394 phosphorylation.
Figure 8.
The phosphorylation of Lck is altered in FAK-deficient human T cells. A, Luciferase (Luc) or Fak miRNA-containing Jurkat T cells were stimulated as in Figure 2. The site-specific phosphorylation of Src Y416 (equivalent to Lck Y394) and Lck Y505 was then analyzed by immunoblotting. B, Quantification of three to four experiments examining the phosphorylation of Lck in control and FAK-deficient Jurkat cells. Data is shown as mean ± s.e.m. C, Control or Fak-deficient primary human CD4 T cells were stimulated as in Figure 3. D, Percent phosphorylation of Src Y416 and Lck Y505 in stimulated control or FAK-depleted primary human CD4 T cells was determined. Data is depicted as mean of three experiments ± s.e.m. * p ≤ 0.05; ** p ≤ 0.01.
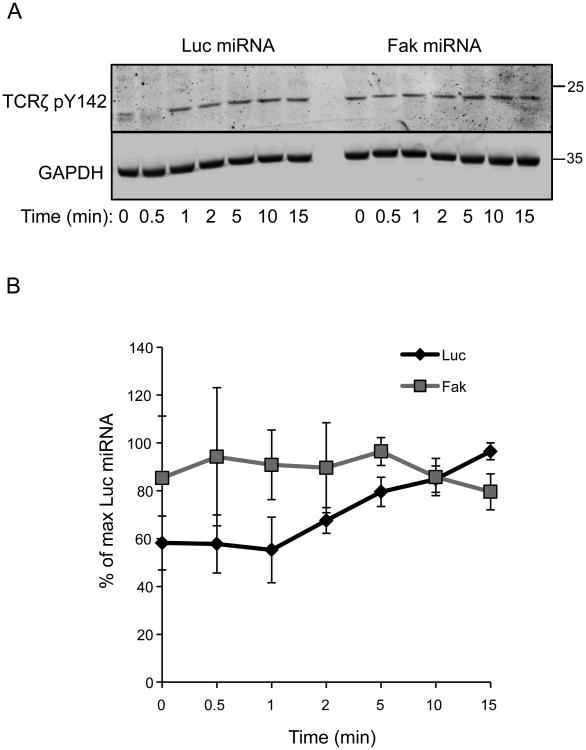
In Csk-deficient Jurkat cells, TCRζ chain phosphorylation occurs with faster kinetics and is modestly elevated at early times after TCR stimulation (62). Therefore, we also examined if TCRζ Y142 phosphorylation was altered in FAK-deficient Jurkat cells. In the Jurkat cells expressing the Luc-specific miRNA, the site-specific phosphorylation of TCRζ Y142 was detected in unstimulated cells and increased by approximately 40% after 15 min of stimulation (Figure 9A-9B). By comparison, the phosphorylation of TCRζ Y142 was enhanced by approximately 1.5-fold in the FAK-deficient T cells at 0-5 min after TCR stimulation. Thus, FAK-deficient T cells have higher levels of TCRζ phosphorylation in resting conditions and early after TCR stimulation. TCRζ Y142 phosphorylation was comparable in the control and FAK-deficient T cells greater than 5 min after receptor activation. Since ITAM phosphorylation appears to be the limiting factor that mediates ZAP-70 recruitment to the TCR (63), these data may also explain why no significant differences in phospho-ZAP-70's membrane recruitment were observed by TIRF microscopy (Figures 5-6). Collectively, these results suggest that Lck's function is enhanced in FAK-deficient T cells and that FAK-deficient T cells are more poised for TCR activation, resulting in increased TCR-dependent signaling and function.
Figure 9. TCRζ chain phosphorylation is altered in FAK-deficient T cells.
A, Control or FAK-deficient Jurkat T cells were stimulated as in Figure 2. The phosphorylation of TCRζ Y142 was then assessed by immunoblotting. B, Quantification of four independent experiments examining TCRζ Y142 phosphorylation in the control and FAK-deficient Jurkat cells. The graph shows the mean ± s.e.m.
FAK expression correlates with Lck Y505 phosphorylation in human T cells
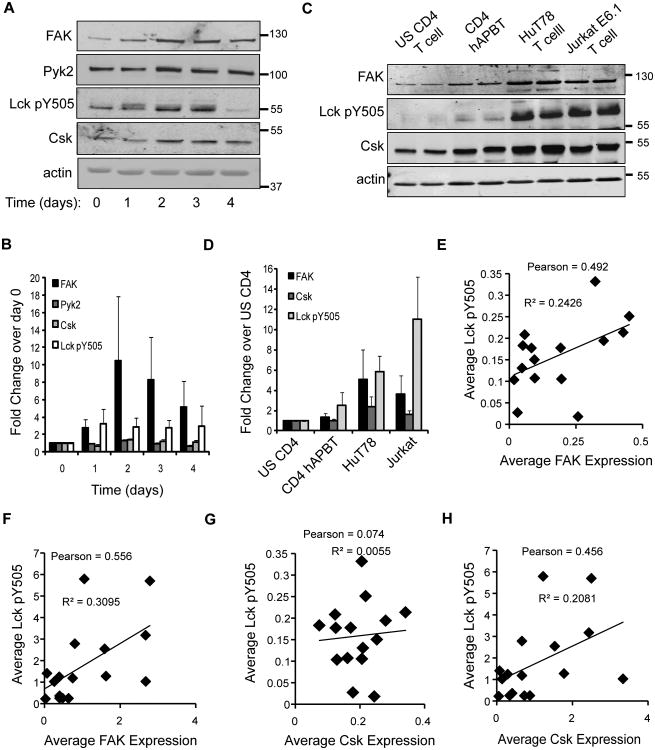
FAK expression has been reported to increase upon T cell activation (64). We found that FAK protein levels were upregulated by 3-to-10-fold in primary CD4 T cells that were stimulated for 1-4 days with anti-CD3 and anti-CD28, while Pyk2 levels remained unchanged (Figure 10A-10B). The human T cell lines Jurkat E6.1 and HuT78 also expressed slightly more FAK protein relative to primary CD4 T cells (Figure 10C-10D), indicating that FAK-dependent functions may be differentially regulated in these cell lines compared to primary human CD4 T cells.
Figure 10.
FAK expression correlates with the phosphorylation of Lck tyrosine 505. A, Primary human CD4 T cells were activated in culture with magnetic beads coated with anti-CD3 and anti-CD28 in the presence of 100 U/ml recombinant IL-2 for the indicated times. The expression of FAK, Pyk2, Csk, Lck Y505, or actin was assessed by immunoblotting. B, The expression of the proteins was normalized to actin expression. The fold change in protein expression relative to day 0 stimulation was then calculated. The data is shown as mean of three experiments ± s.e.m. C, Equal numbers of freshly isolated CD4 T cells (US CD4 T cell), CD4 T cells activated for 6 days with anti-CD3 and anti-CD28-coated beads (CD4 hAPBT), unstimulated HuT78 T cells, or unstimulated Jurkat E6.1 T cells were lysed. Immunoblotting was then used to examine FAK, phospho-Lck Y505, Lck, Csk, or actin expression. D, Relative expression of the various proteins was calculated as in (B). Fold change in protein expression relative to unstimulated (US) CD4 T cell sample was calculated, and the mean of four experiments ± s.e.m. is shown. E-H Linear regression and Pearson Correlation analyses were used to examine the relationship between Lck Y505 phosphorylation and average FAK expression or average Csk expression in CD4 T cells (E and G) or in CD4 T cells and human T cell lines (F and H).
Like FAK expression, Lck Y505 phosphorylation was also increased in activated CD4 hAPBTs (Figure 10A-10B). Basal Lck Y505 phosphorylation was also augmented in HuT78 T cells and Jurkat cells compared to primary human T cells (Figure 10C-10D). Thus, it appeared that Lck Y505 phosphorylation correlated with FAK expression. To investigate this possibility, linear regression and Pearson correlation analyses were performed. As shown in Figure 10E, there was a strong linear relationship between Lck Y505 phosphorylation and FAK expression (p=0.093), and the Pearson correlation between these proteins trended toward significance (p=0.062). Similarly, a significant linear relationship (*p=0.025) and Pearson correlation (*p=0.025) was observed when we compared Lck Y505 phosphorylation and FAK expression in human non-stimulated and activated CD4 T cells, unstimulated Jurkat cells, and unstimulated HuT78 T cells (Figure 10F). Thus, changes in FAK expression appear to serve as a rheostat to tune both basal and TCR-inducible Lck Y505 phosphorylation in primary human CD4 T cells and transformed human T cell lines.
We also examined if Csk expression and Lck Y505 phosphorylation were correlated. Unlike FAK and Lck pY505 expression, Csk expression did not change after TCR stimulation (Figure 10A-10B), and the expression of the Lck pY505 and Csk was not correlated as determined by linear regression analysis (p=0.93) or Pearson correlation (p=0.79; Figure 10G). Jurkat cells and HuT78 T cells expressed more Csk protein at basal levels than primary CD4 T cells (Figure 9A-9B). Therefore, the linear relationship between Csk and Lck Y505 phosphorylation was stronger in these cells (p=0.076). However, the Pearson correlation was not significant (Figure 10H; p = 0.076). Therefore, unlike FAK, Csk expression is not increased after human CD4 T cells are activated, and differences in Csk expression do not positively correlate with enhanced Lck Y505 phosphorylation in human T cells.
Discussion
In this study, we evaluated the role that FAK plays in TCR-induced effector function in human T cells. Surprisingly, we found that TCR-mediated signaling is enhanced or extended and that TCR-induced effector functions are augmented in Jurkat cells and CD4 hAPBTs that lack FAK. It appears that FAK controls the function of the Lck by recruiting Csk to the membrane and/or receptor complex in TCR-stimulated cells (Figure S3). These studies reveal that FAK regulates Csk activity and Lck/Fyn function downstream of the TCR and demonstrate for the first time that FAK is a negative regulator of TCR function.
While this manuscript was under review, Wiemer and co-workers published a report examining how FAK controlled CD4 T cell activation. To do so, FAK's function was impaired using PF562271, an inhibitor that suppresses the catalytic function of both FAK and its related kinase, Pyk2. In contrast to the results we obtained using FAK-deficient human CD4 T cells, treatment with PF562271 appeared to suppress proximal TCR-mediated signaling and proliferation (32). Wiemer and colleagues used doses of PF562271 that were substantially higher than those required to suppress FAK's enzymatic activity in vitro and in cell-based assays (65). This is a major limitation of their study, as higher doses of PF562271 have been reported to suppress the in vitro catalytic functions of Pyk2, cyclin-dependent kinases, Fyn, and Lck (65). To confirm the results of their inhibitor experiments, this group also conditionally deleted FAK in murine CD4 T cells, which resulted in an approximate 65% knockdown of FAK protein expression. In contrast to the results obtained with PF562271, anti-CD3 and anti-CD28 induced proliferation was normal-to-elevated in these FAK-deficient murine CD4 T cells (32). Therefore, it is likely that the differences seen in this published study and ours are due to off-target effects of PF562271.
The activation of Lck and Fyn is tightly regulated to prevent inappropriate T cell activation. This regulation is achieved by altering Lck's localization or phosphorylation (11, 66). Lck Y505 plays a seminal role in inhibiting Lck activity: the in vitro kinase activity of Lck is reduced when Lck Y505 is phosphorylated, and T cells that express a Lck Y505F mutant are hyper-activated (67-69). Defective TCR-inducible Lck Y505 phosphorylation has been observed in hyper-responsive CD4 T cells isolated from patients with acute coronary syndromes (70). Thus, deregulated Lck Y505 phosphorylation can promote T cell hyper-activation. In this study, we showed that the TCR-inducible phosphorylation of Lck Y505 was significantly decreased in the absence of FAK. This defect was also accompanied by an increase in Lck Y394 auto-phosphorylation (8) and enhanced TCR-induced function. Our data support the idea that Lck Y505 phosphorylation is vitally important to inhibit TCR-induced responses and demonstrate that FAK is a critical regulator of Lck Y505 phosphorylation downstream of the TCR. This inhibitory function may be achieved by suppressing Lck's catalytic function and/or limiting its accessibility to its protein substrates, including the TCRζ chain (14, 15).
Several reports have demonstrated that Csk regulates TCR function in both the basal and activated states. Indeed, knocking down Csk or inhibiting its function increases TCR-dependent signaling and effector responses (12, 13, 18), demonstrating that this kinase is indispensable for proper mature T cell function. Csk been reported to bind to the plasma membrane, where it is exerts its inhibitory effects on TCR function (12, 16-18). We have confirmed by high resolution TIRF imaging that Csk is brought to the membrane following TCR activation. Our data suggest that FAK regulates the membrane localization and function of Csk in activated T cells. We found that the translocation of Csk to the membrane following TCR activation was dependent upon FAK. This reduction in Csk protein found in the membrane was correlated with increased Lck Y394 and TCRζ chain phosphorylation, decreased Lck Y505 phosphorylation, and increased sensitivity to TCR induction. However, we did not see an increase in the magnitude of ZAP-70 Y319 or Erk1/Erk2 phosphorylation (data not shown). These observations are strikingly similar to those made using Csk-deficient Jurkat cells or human CD4 T cells (13). Thus, FAK and Csk appear to cooperatively inhibit TCR function following its activation.
We also demonstrated that FAK associated with Csk at the activated TCR. Using TIRF microscopy, we also found that a small fraction of the membrane-associated Csk co-localized with phosphorylated ZAP-70. Thus, Csk may be actively sequestered from the activated TCR complex, as was recently reported in murine CD8 T cells (58). The Csk and phosphorylated ZAP-70 interaction was significantly reduced in FAK-deficient Jurkat cells and CD4 hAPBTs. Therefore, it appears that FAK regulates Csk recruitment to or retention at the TCR, where Csk could inhibit the pool of active Lck that is found at the stimulated receptor (14, 60, 61). Further work is needed to address how FAK regulates Csk's function. We hypothesize that FAK Y397 serves to localize Csk to the plasma membrane after TCR induction. Interestingly, this site has the consensus binding motif for the SH2 domain of Csk (71). The SH2 domain of Csk has been reported to bind to FAK (53, 55); therefore, Csk may directly interact with FAK Y397. Furthermore, FAK may be recruited the plasma membrane and/or TCR by directly binding Lck/Fyn or the TCR co-receptor CD4 (72, 73). These hypotheses will be addressed in future experiments.
The current model states that Csk's function is regulated by the plasma-membrane anchored protein, PAG. According to this model, Csk is associated with PAG via its SH2 domain, which binds to phospho-Y317 on PAG (23, 24). This interaction allows Csk to phosphorylate Lck Y505 to suppress tonic TCR signals to prevent aberrant T cell development or activation (19, 25, 62, 74). After TCR stimulation, the current paradigm suggests that PAG is de-phosphorylated, which subsequently releases Csk from the plasma membrane (18, 20, 23). After Csk moves into the cytoplasm, this model then states that CD45 must de-phosphorylate Lck Y505 to induce T cell activation (11, 26, 75). Csk is then recruited back into the plasma membrane once activated Fyn phosphorylates PAG Y317. Indeed, PAG overexpression inhibits TCR-dependent signaling and function (20, 23), supporting the idea that PAG is an important regulator of Csk function in T cells.
Although this model persists as the mode by which Csk feedback inhibits TCR signaling, several more recent publications have challenged this paradigm. First, we and others have demonstrated that Lck Y505 phosphorylation does not decrease after TCR activation. Instead, Lck Y505 phosphorylation remains unchanged or modestly increases upon TCR stimulation (7, 8, 19, 27). Second, despite the fact that Lck Y505 is hyper-phosphorylated in CD45-deficient T cells, Lck's catalytic function is actually increased in the absence of CD45, likely because CD45 also de-phosphorylates Lck Y394 (67, 75-77). Finally, Lck is catalytically active when it is phosphorylated on both Y394 and Y505 (8). Thus, Lck Y505 de-phosphorylation does not appear to be required to induce Lck's catalytic function; however, it may serve to localize Lck to the TCR, where it can induce T cell activation (14).
Several reports have also called into question the relevance of the PAG-Csk axis in regulating TCR signaling events. Csk's SH2 domain associates with PAG and Csk's enzymatic function is enhanced when bound to PAG (20, 23); however, the loss of PAG does not appear to limit Csk function. Consistent with this idea, genetic deletion of PAG in murine T cells did not dramatically alter Csk's localization to lipid rafts or inhibit T cell development or activation (21, 22). Similarly, knocking down PAG in human T cells modestly increased early TCR-mediated signaling events, but actually suppressed downstream effector functions (28, 78). These results are in sharp contrast to Csk-deficient T cells that have developmental defects and enhanced TCR-induced activation (19, 62, 74). Additionally, after it is transiently displaced, Csk appears to be recruited back into the plasma membrane before PAG is re-phosphorylated (18, 20). These data strongly suggest that PAG is not the primary regulator of Csk function in activated T cells. It is possible that other membrane adaptors like LIME or Dok1 may recruit Csk to the membrane in the absence of PAG (19, 79). Alternatively, PAG may regulate Csk's function in unstimulated T cells to suppress tonic TCR signaling, while other mechanisms may control Csk's function following TCR activation. Moreover, PAG may only regulate Csk's function at specific cellular locations distinct from sites of TCR activation. Our data strongly suggest that FAK is a protein that recruits Csk to the TCR complex after antigen stimulation in mature CD4 T cells and that this process suppresses TCR-induced function.
Immune cell activation via antigen receptors is intricately linked to protective immunity against pathogens and in the development of allergic disease and autoimmunity (80-83). Like the TCR, signal transduction downstream of the BCR and Fc-epsilon receptor (FcεR) is initiated by members of the Src family kinases and suppressed by Csk (11, 84, 85). Our data support a model wherein changes in FAK expression regulate the activity of Lck by recruiting its inhibitor Csk to the TCR complex. Interestingly, we found that FAK associated with both Lck and the TCR/CD4 complex, an interaction that was previously reported for the related proteins Lyn and IgM-BCR (86). Thus, FAK could similarly inhibit BCR function by recruiting Csk to the receptor complex to block Lyn activation. We also showed that changes in FAK expression regulated the antigen sensitivity of the TCR. Similar to our observations, the magnitude of FcεR-mediated signaling was higher in cells that expressed low levels of FAK (87). Thus, the expression of FAK may also fine-tune FcεR-mediated signaling. Together, these data suggest that FAK may be a universal negative regulator of antigen receptor signaling, a finding that would have broad implications in the treatment of a variety of human disorders.
Collectively, our data demonstrate that FAK is a negative regulator of TCR function in human T cells. The fact that FAK expression appears to fine-tune TCR activation suggests that FAK signaling could be regulated to manipulate T cell responses in different disease states. FAK function could be dampened to enhance T cell responsiveness to low affinity tumor or pathogen-derived antigens. Moreover, conditions that arise due to hyper-reactive T cell responses may benefit from enhancing the function of FAK to downmodulate TCR activation. The balance between Th1 and Th2-driven immune responses could possibly be skewed by altering FAK signaling, since strength of TCR signal controls the polarization of these cells (88). Ultimately, FAK signaling could serve as an important node to amplify or dampen normal and aberrant T cell function in numerous human diseases.
Supplementary Material
Acknowledgments
We would like to thank Dr. Anton McCaffrey for helpful discussions and reagents.
Footnotes
This work was supported by an American Heart Association Predoctoral Fellowship to NMC (11PRE7390070). NMC and SFC were supported by the NIH Predoctoral Training Grant in Immunology (T32AI007485). This work was supported by a Scientist Development Grant 0830244N from the American Heart Association and R01 CA136729 from the National Institutes of Health to JCDH.
Abbreviations used in the manuscript are: Csk, C-terminal Src kinase; FAK, focal adhesion kinase; hAPBTs, human activated peripheral blood T cells; miRNA, microRNA; PAG, phosphoprotein associated with glycosphingolipid-associated microdomains; Pyk2, proline-rich tyrosine kinase 2; TIRF, total internal reflection fluorescence
References
- 1.Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol. 2010;10:838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annual review of immunology. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jutel M, Akdis CA. T-cell subset regulation in atopy. Curr Allergy Asthma Rep. 2011;11:139–145. doi: 10.1007/s11882-011-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jager A, Kuchroo VK. Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand J Immunol. 2010;72:173–184. doi: 10.1111/j.1365-3083.2010.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atalar K, Afzali B, Lord G, Lombardi G. Relative roles of Th1 and Th17 effector cells in allograft rejection. Curr Opin Organ Transplant. 2009;14:23–29. doi: 10.1097/MOT.0b013e32831b70c2. [DOI] [PubMed] [Google Scholar]
- 6.Lahoute C, Herbin O, Mallat Z, Tedgui A. Adaptive immunity in atherosclerosis: mechanisms and future therapeutic targets. Nat Rev Cardiol. 2011;8:348–358. doi: 10.1038/nrcardio.2011.62. [DOI] [PubMed] [Google Scholar]
- 7.Dong S, Corre B, Nika K, Pellegrini S, Michel F. T cell receptor signal initiation induced by low-grade stimulation requires the cooperation of LAT in human T cells. PloS one. 2010;5:e15114. doi: 10.1371/journal.pone.0015114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nika K, Soldani C, Salek M, Paster W, Gray A, Etzensperger R, Fugger L, Polzella P, Cerundolo V, Dushek O, Hofer T, Viola A, Acuto O. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity. 2010;32:766–777. doi: 10.1016/j.immuni.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz-Orcutt N, Houtman JC. PI3 kinase function is vital for the function but not formation of LAT-mediated signaling complexes. Molecular immunology. 2009;46:2274–2283. doi: 10.1016/j.molimm.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Shim EK, Jung SH, Lee JR. Role of two adaptor molecules SLP-76 and LAT in the PI3K signaling pathway in activated T cells. Journal of immunology. 2011;186:2926–2935. doi: 10.4049/jimmunol.1001785. [DOI] [PubMed] [Google Scholar]
- 11.Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunological reviews. 2009;228:9–22. doi: 10.1111/j.1600-065X.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- 12.Schoenborn JR, Tan YX, Zhang C, Shokat KM, Weiss A. Feedback circuits monitor and adjust basal Lck-dependent events in T cell receptor signaling. Sci Signal. 2011;4:ra59. doi: 10.1126/scisignal.2001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vang T, Abrahamsen H, Myklebust S, Enserink J, Prydz H, Mustelin T, Amarzguioui M, Tasken K. Knockdown of C-terminal Src kinase by siRNA-mediated RNA interference augments T cell receptor signaling in mature T cells. European journal of immunology. 2004;34:2191–2199. doi: 10.1002/eji.200425036. [DOI] [PubMed] [Google Scholar]
- 14.Rossy J, Owen DM, Williamson DJ, Yang Z, Gaus K. Conformational states of the kinase Lck regulate clustering in early T cell signaling. Nature immunology. 2013;14:82–89. doi: 10.1038/ni.2488. [DOI] [PubMed] [Google Scholar]
- 15.Stirnweiss A, Hartig R, Gieseler S, Lindquist JA, Reichardt P, Philipsen L, Simeoni L, Poltorak M, Merten C, Zuschratter W, Prokazov Y, Paster W, Stockinger H, Harder T, Gunzer M, Schraven B. T cell activation results in conformational changes in the Src family kinase Lck to induce its activation. Science signaling. 2013;6:ra13. doi: 10.1126/scisignal.2003607. [DOI] [PubMed] [Google Scholar]
- 16.Cloutier JF, Chow LM, Veillette A. Requirement of the SH3 and SH2 domains for the inhibitory function of tyrosine protein kinase p50csk in T lymphocytes. Molecular and cellular biology. 1995;15:5937–5944. doi: 10.1128/mcb.15.11.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otahal P, Pata S, Angelisova P, Horejsi V, Brdicka T. The effects of membrane compartmentalization of csk on TCR signaling. Biochimica et biophysica acta. 2011;1813:367–376. doi: 10.1016/j.bbamcr.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Torgersen KM, Vang T, Abrahamsen H, Yaqub S, Horejsi V, Schraven B, Rolstad B, Mustelin T, Tasken K. Release from tonic inhibition of T cell activation through transient displacement of C-terminal Src kinase (Csk) from lipid rafts. The Journal of biological chemistry. 2001;276:29313–29318. doi: 10.1074/jbc.C100014200. [DOI] [PubMed] [Google Scholar]
- 19.Schoenborn JR, Tan YX, Zhang C, Shokat KM, Weiss A. Feedback circuits monitor and adjust basal Lck-dependent events in T cell receptor signaling. Science signaling. 2011;4:ra59. doi: 10.1126/scisignal.2001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson D, Bakinowski M, Thomas ML, Horejsi V, Veillette A. Phosphorylation-dependent regulation of T-cell activation by PAG/Cbp, a lipid raft-associated transmembrane adaptor. Molecular and cellular biology. 2003;23:2017–2028. doi: 10.1128/MCB.23.6.2017-2028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobenecker MW, Schmedt C, Okada M, Tarakhovsky A. The ubiquitously expressed Csk adaptor protein Cbp is dispensable for embryogenesis and T-cell development and function. Molecular and cellular biology. 2005;25:10533–10542. doi: 10.1128/MCB.25.23.10533-10542.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu S, Huo J, Tan JE, Lam KP. Cbp deficiency alters Csk localization in lipid rafts but does not affect T-cell development. Molecular and cellular biology. 2005;25:8486–8495. doi: 10.1128/MCB.25.19.8486-8495.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brdicka T, Pavlistova D, Leo A, Bruyns E, Korinek V, Angelisova P, Scherer J, Shevchenko A, Hilgert I, Cerny J, Drbal K, Kuramitsu Y, Kornacker B, Horejsi V, Schraven B. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. The Journal of experimental medicine. 2000;191:1591–1604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, Nagai K, Tarakhovsky A, Okada M. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000;404:999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- 25.Takeuchi S, Takayama Y, Ogawa A, Tamura K, Okada M. Transmembrane phosphoprotein Cbp positively regulates the activity of the carboxyl-terminal Src kinase, Csk. The Journal of biological chemistry. 2000;275:29183–29186. doi: 10.1074/jbc.C000326200. [DOI] [PubMed] [Google Scholar]
- 26.Cahir McFarland ED, Hurley TR, Pingel JT, Sefton BM, Shaw A, Thomas ML. Correlation between Src family member regulation by the protein-tyrosine-phosphatase CD45 and transmembrane signaling through the T-cell receptor. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:1402–1406. doi: 10.1073/pnas.90.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burkhardt AL, Stealey B, Rowley RB, Mahajan S, Prendergast M, Fargnoli J, Bolen JB. Temporal regulation of non-transmembrane protein tyrosine kinase enzyme activity following T cell antigen receptor engagement. The Journal of biological chemistry. 1994;269:23642–23647. [PubMed] [Google Scholar]
- 28.Smida M, Cammann C, Gurbiel S, Kerstin N, Lingel H, Lindquist S, Simeoni L, Brunner-Weinzierl MC, Suchanek M, Schraven B, Lindquist JA. PAG/Cbp suppression reveals a contribution of CTLA-4 to setting the activation threshold in T cells. Cell communication and signaling : CCS. 2013;11:28. doi: 10.1186/1478-811X-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez TS, Billadeau DD. T cell activation and the cytoskeleton: you can't have one without the other. Advances in immunology. 2008;97:1–64. doi: 10.1016/S0065-2776(08)00001-1. [DOI] [PubMed] [Google Scholar]
- 30.Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–259. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 31.Fukai I, Hussey RE, Sunder-Plassmann R, Reinherz EL. A critical role for p59(fyn) in CD2-based signal transduction. European journal of immunology. 2000;30:3507–3515. doi: 10.1002/1521-4141(2000012)30:12<3507::AID-IMMU3507>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 32.Wiemer AJ, Wernimont SA, Cung TD, Bennin DA, Beggs HE, Huttenlocher A. The focal adhesion kinase inhibitor PF-562,271 impairs primary CD4+ T cell activation. Biochemical pharmacology. 2013 doi: 10.1016/j.bcp.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parsons SA, Sharma R, Roccamatisi DL, Zhang H, Petri B, Kubes P, Colarusso P, Patel KD. Endothelial paxillin and focal adhesion kinase (FAK) play a critical role in neutrophil transmigration. European journal of immunology. 2012;42:436–446. doi: 10.1002/eji.201041303. [DOI] [PubMed] [Google Scholar]
- 34.Kasorn A, Alcaide P, Jia Y, Subramanian KK, Sarraj B, Li Y, Loison F, Hattori H, Silberstein LE, Luscinskas WF, Luo HR. Focal adhesion kinase regulates pathogen-killing capability and life span of neutrophils via mediating both adhesion-dependent and -independent cellular signals. Journal of immunology. 2009;183:1032–1043. doi: 10.4049/jimmunol.0802984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owen KA, Pixley FJ, Thomas KS, Vicente-Manzanares M, Ray BJ, Horwitz AF, Parsons JT, Beggs HE, Stanley ER, Bouton AH. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. The Journal of cell biology. 2007;179:1275–1287. doi: 10.1083/jcb.200708093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tse KW, Dang-Lawson M, Lee RL, Vong D, Bulic A, Buckbinder L, Gold MR. B cell receptor-induced phosphorylation of Pyk2 and focal adhesion kinase involves integrins and the Rap GTPases and is required for B cell spreading. The Journal of biological chemistry. 2009;284:22865–22877. doi: 10.1074/jbc.M109.013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitney GS, Chan PY, Blake J, Cosand WL, Neubauer MG, Aruffo A, Kanner SB. Human T and B lymphocytes express a structurally conserved focal adhesion kinase, pp125FAK. DNA and cell biology. 1993;12:823–830. doi: 10.1089/dna.1993.12.823. [DOI] [PubMed] [Google Scholar]
- 38.Dietz AB, Bulur PA, Emery RL, Winters JL, Epps DE, Zubair AC, Vuk-Pavlovic S. A novel source of viable peripheral blood mononuclear cells from leukoreduction system chambers. Transfusion. 2006;46:2083–2089. doi: 10.1111/j.1537-2995.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- 39.Lahm HW, Stein S. Characterization of recombinant human interleukin-2 with micromethods. J Chromatogr. 1985;326:357–361. doi: 10.1016/s0021-9673(01)87461-6. [DOI] [PubMed] [Google Scholar]
- 40.Keck K, Volper EM, Spengler RM, Long DD, Chan CY, Ding Y, McCaffrey AP. Rational design leads to more potent RNA interference against hepatitis B virus: factors effecting silencing efficiency. Mol Ther. 2009;17:538–547. doi: 10.1038/mt.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartelt RR, Cruz-Orcutt N, Collins M, Houtman JC. Comparison of T cell receptor-induced proximal signaling and downstream functions in immortalized and primary T cells. PloS one. 2009;4:e5430. doi: 10.1371/journal.pone.0005430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins M, Bartelt RR, Houtman JC. T cell receptor activation leads to two distinct phases of Pyk2 activation and actin cytoskeletal rearrangement in human T cells. Molecular immunology. 2010;47:1665–1674. doi: 10.1016/j.molimm.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Houtman JC, Houghtling RA, Barda-Saad M, Toda Y, Samelson LE. Early phosphorylation kinetics of proteins involved in proximal TCR-mediated signaling pathways. Journal of immunology. 2005;175:2449–2458. doi: 10.4049/jimmunol.175.4.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins M, Tremblay M, Chapman N, Curtiss M, Rothman PB, Houtman JC. The T cell receptor-mediated phosphorylation of Pyk2 tyrosines 402 and 580 occurs via a distinct mechanism than other receptor systems. Journal of leukocyte biology. 2010;87:691–701. doi: 10.1189/jlb.0409227. [DOI] [PubMed] [Google Scholar]
- 45.Cruz-Orcutt N, Houtman JC. PI3 kinase function is vital for the function but not formation of LAT-mediated signaling complexes. Molecular immunology. 2009;46:2274–2283. doi: 10.1016/j.molimm.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Chapman NM, Yoder AN, Houtman JC. Non-catalytic functions of Pyk2 and Fyn regulate late stage adhesion in human T cells. PloS one. 2012;7:e53011. doi: 10.1371/journal.pone.0053011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bunnell SC, Kapoor V, Trible RP, Zhang W, Samelson LE. Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity. 2001;14:315–329. doi: 10.1016/s1074-7613(01)00112-1. [DOI] [PubMed] [Google Scholar]
- 48.Ostergaard HL, Lysechko TL. Focal adhesion kinase-related protein tyrosine kinase Pyk2 in T-cell activation and function. Immunologic research. 2005;31:267–282. doi: 10.1385/IR:31:3:267. [DOI] [PubMed] [Google Scholar]
- 49.Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK- cell migration. The EMBO journal. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weis SM, Lim ST, Lutu-Fuga KM, Barnes LA, Chen XL, Gothert JR, Shen TL, Guan JL, Schlaepfer DD, Cheresh DA. Compensatory role for Pyk2 during angiogenesis in adult mice lacking endothelial cell FAK. The Journal of cell biology. 2008;181:43–50. doi: 10.1083/jcb.200710038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Owen JD, Ruest PJ, Fry DW, Hanks SK. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both auto- and activation loop phosphorylation sites and inhibits adhesion-dependent tyrosine phosphorylation of Pyk2. Molecular and cellular biology. 1999;19:4806–4818. doi: 10.1128/mcb.19.7.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kane LP, Weiss A. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunological reviews. 2003;192:7–20. doi: 10.1034/j.1600-065x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 53.Bergman M, Joukov V, Virtanen I, Alitalo K. Overexpressed Csk tyrosine kinase is localized in focal adhesions, causes reorganization of alpha v beta 5 integrin, and interferes with HeLa cell spreading. Molecular and cellular biology. 1995;15:711–722. doi: 10.1128/mcb.15.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tremblay L, Hauck W, Aprikian AG, Begin LR, Chapdelaine A, Chevalier S. Focal adhesion kinase (pp125FAK) expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. International journal of cancer Journal international du cancer. 1996;68:164–171. doi: 10.1002/(sici)1097-0215(19961009)68:2<169::aid-ijc4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 55.Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 56.Millis BA. Evanescent-wave field imaging: an introduction to total internal reflection fluorescence microscopy. Methods in molecular biology. 2012;823:295–309. doi: 10.1007/978-1-60327-216-2_19. [DOI] [PubMed] [Google Scholar]
- 57.Bunnell SC, Hong DI, Kardon JR, Yamazaki T, McGlade CJ, Barr VA, Samelson LE. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. The Journal of cell biology. 2002;158:1263–1275. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borger JG, Filby A, Zamoyska R. Differential Polarization of C-Terminal Src Kinase between Naive and Antigen-Experienced CD8+ T Cells. Journal of immunology. 2013;190:3089–3099. doi: 10.4049/jimmunol.1202408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Wet B, Zech T, Salek M, Acuto O, Harder T. Proteomic characterization of plasma membrane-proximal T cell activation responses. The Journal of biological chemistry. 2011;286:4072–4080. doi: 10.1074/jbc.M110.165415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hashimoto-Tane A, Yokosuka T, Ishihara C, Sakuma M, Kobayashi W, Saito T. T-cell receptor microclusters critical for T-cell activation are formed independently of lipid raft clustering. Molecular and cellular biology. 2010;30:3421–3429. doi: 10.1128/MCB.00160-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 62.Vang T, Abrahamsen H, Myklebust S, Enserink J, Prydz H, Mustelin T, Amarzguioui M, Tasken K. Knockdown of C-terminal Src kinase by siRNA-mediated RNA interference augments T cell receptor signaling in mature T cells. European journal of immunology. 2004;34:2191–2199. doi: 10.1002/eji.200425036. [DOI] [PubMed] [Google Scholar]
- 63.Altan-Bonnet G, Germain RN. Modeling T cell antigen discrimination based on feedback control of digital ERK responses. PLoS biology. 2005;3:e356. doi: 10.1371/journal.pbio.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsuchida M, Manthei ER, Alam T, Knechtle SJ, Hamawy MM. T cell activation up-regulates the expression of the focal adhesion kinase Pyk2: opposing roles for the activation of protein kinase C and the increase in intracellular Ca2+ Journal of immunology. 1999;163:6640–6650. [PubMed] [Google Scholar]
- 65.Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, Richter D, Emerson E, Lin J, Kath J, Coleman K, Yao L, Martinez-Alsina L, Lorenzen M, Berliner M, Luzzio M, Patel N, Schmitt E, LaGreca S, Jani J, Wessel M, Marr E, Griffor M, Vajdos F. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer research. 2008;68:1935–1944. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- 66.Filipp D, Ballek O, Manning J. Lck, Membrane Microdomains, and TCR Triggering Machinery: Defining the New Rules of Engagement. Frontiers in immunology. 2012;3:155. doi: 10.3389/fimmu.2012.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D'Oro U, Sakaguchi K, Appella E, Ashwell JD. Mutational analysis of Lck in CD45-negative T cells: dominant role of tyrosine 394 phosphorylation in kinase activity. Molecular and cellular biology. 1996;16:4996–5003. doi: 10.1128/mcb.16.9.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abraham N, Miceli MC, Parnes JR, Veillette A. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Nature. 1991;350:62–66. doi: 10.1038/350062a0. [DOI] [PubMed] [Google Scholar]
- 69.Abraham KM, Levin SD, Marth JD, Forbush KA, Perlmutter RM. Delayed thymocyte development induced by augmented expression of p56lck. The Journal of experimental medicine. 1991;173:1421–1432. doi: 10.1084/jem.173.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pryshchep S, Goronzy JJ, Parashar S, Weyand CM. Insufficient deactivation of the protein tyrosine kinase lck amplifies T-cell responsiveness in acute coronary syndrome. Circulation research. 2010;106:769–778. doi: 10.1161/CIRCRESAHA.109.206052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Songyang Z, Shoelson SE, McGlade J, Olivier P, Pawson T, Bustelo XR, Barbacid M, Sabe H, Hanafusa H, Yi T, et al. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol Cell Biol. 1994;14:2777–2785. doi: 10.1128/mcb.14.4.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berg NN, Ostergaard HL. T cell receptor engagement induces tyrosine phosphorylation of FAK and Pyk2 and their association with Lck. Journal of immunology. 1997;159:1753–1757. [PubMed] [Google Scholar]
- 73.Garron ML, Arthos J, Guichou JF, McNally J, Cicala C, Arold ST. Structural basis for the interaction between focal adhesion kinase and CD4. Journal of molecular biology. 2008;375:1320–1328. doi: 10.1016/j.jmb.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 74.Schmedt C, Saijo K, Niidome T, Kuhn R, Aizawa S, Tarakhovsky A. Csk controls antigen receptor-mediated development and selection of T-lineage cells. Nature. 1998;394:901–904. doi: 10.1038/29802. [DOI] [PubMed] [Google Scholar]
- 75.McNeill L, Salmond RJ, Cooper JC, Carret CK, Cassady-Cain RL, Roche-Molina M, Tandon P, Holmes N, Alexander DR. The differential regulation of Lck kinase phosphorylation sites by CD45 is critical for T cell receptor signaling responses. Immunity. 2007;27:425–437. doi: 10.1016/j.immuni.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 76.Burns CM, Sakaguchi K, Appella E, Ashwell JD. CD45 regulation of tyrosine phosphorylation and enzyme activity of src family kinases. The Journal of biological chemistry. 1994;269:13594–13600. [PubMed] [Google Scholar]
- 77.D'Oro U, Ashwell JD. Cutting edge: the CD45 tyrosine phosphatase is an inhibitor of Lck activity in thymocytes. Journal of immunology. 1999;162:1879–1883. [PubMed] [Google Scholar]
- 78.Smida M, Posevitz-Fejfar A, Horejsi V, Schraven B, Lindquist JA. A novel negative regulatory function of the phosphoprotein associated with glycosphingolipid-enriched microdomains: blocking Ras activation. Blood. 2007;110:596–615. doi: 10.1182/blood-2006-07-038752. [DOI] [PubMed] [Google Scholar]
- 79.Brdickova N, Brdicka T, Angelisova P, Horvath O, Spicka J, Hilgert I, Paces J, Simeoni L, Kliche S, Merten C, Schraven B, Horejsi V. LIME: a new membrane Raft-associated adaptor protein involved in CD4 and CD8 coreceptor signaling. The Journal of experimental medicine. 2003;198:1453–1462. doi: 10.1084/jem.20031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Datta S, Milner JD. Altered T-cell receptor signaling in the pathogenesis of allergic disease. The Journal of allergy and clinical immunology. 2011;127:351–354. doi: 10.1016/j.jaci.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nature medicine. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sakaguchi S, Benham H, Cope AP, Thomas R. T-cell receptor signaling and the pathogenesis of autoimmune arthritis: insights from mouse and man. Immunology and cell biology. 2012;90:277–287. doi: 10.1038/icb.2012.4. [DOI] [PubMed] [Google Scholar]
- 83.Zouali M, Sarmay G. B lymphocyte signaling pathways in systemic autoimmunity: implications for pathogenesis and treatment. Arthritis and rheumatism. 2004;50:2730–2741. doi: 10.1002/art.20487. [DOI] [PubMed] [Google Scholar]
- 84.Kurosaki T, Hikida M. Tyrosine kinases and their substrates in B lymphocytes. Immunological reviews. 2009;228:132–148. doi: 10.1111/j.1600-065X.2008.00748.x. [DOI] [PubMed] [Google Scholar]
- 85.Abramson J, Pecht I. Regulation of the mast cell response to the type 1 Fc epsilon receptor. Immunological reviews. 2007;217:231–254. doi: 10.1111/j.1600-065X.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 86.Mlinaric-Rascan I, Yamamoto T. B cell receptor signaling involves physical and functional association of FAK with Lyn and IgM. FEBS letters. 2001;498:26–31. doi: 10.1016/s0014-5793(01)02474-7. [DOI] [PubMed] [Google Scholar]
- 87.Hamawy MM, Swieter M, Mergenhagen SE, Siraganian RP. Reconstitution of high affinity IgE receptor-mediated secretion by transfecting protein tyrosine kinase pp125FAK. The Journal of biological chemistr. 1997;272:30498–30503. doi: 10.1074/jbc.272.48.30498. [DOI] [PubMed] [Google Scholar]
- 88.Nakayama T, Yamashita M. The TCR-mediated signaling pathways that control the direction of helper T cell differentiation. Seminars in immunology. 2010;22:303–309. doi: 10.1016/j.smim.2010.04.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.