Abstract
During development of the ventral spinal cord, the V2 interneurons emerge from p2 progenitors and diversify into two major subtypes, V2a and V2b, that play key roles in locomotor coordination. Dll4-mediated Notch activation in a subset of p2 precursors constitutes the crucial first step towards generating neuronal diversity in this domain. The mechanism behind the asymmetric Notch activation and downstream signaling events are, however, unknown at present. We show here that the Ascl1 and Neurog basic helix-loop-helix (bHLH) proneural factors are expressed in a mosaic pattern in p2 progenitors and that Foxn4 is required for setting and maintaining this expression mosaic. By binding directly to a conserved Dll4 enhancer, Foxn4 and Ascl1 activate Dll4 expression, whereas Neurog proteins prevent this effect, thereby resulting in asymmetric activation of Dll4 expression in V2 precursors expressing different combinations of proneural and Foxn4 transcription factors. Lineage tracing using the Cre-LoxP system reveals selective expression of Dll4 in V2a precursors, whereas Dll4 expression is initially excluded from V2b precursors. We provide evidence that BMP/TGFβ signaling is activated in V2b precursors and that Dll4-mediated Notch signaling is responsible for this activation. Using a gain-of-function approach and by inhibiting BMP/TGFβ signal transduction with pathway antagonists and RNAi knockdown, we further demonstrate that BMP/TGFβ signaling is both necessary and sufficient for V2b fate specification. Our data together thus suggest that the mosaic expression of Foxn4 and proneural factors may serve as the trigger to initiate asymmetric Dll4-Notch and subsequent BMP/TGFβ signaling events required for neuronal diversity in the V2 domain.
Keywords: V2 interneuron, Spinal cord, Foxn4, Ascl1, bHLH proneural factor, Dll4-Notch, BMP/TGFβ, Chick, Mouse
INTRODUCTION
Neuronal diversity during spinal cord (SC) development is initially generated by activities of two competing signaling pathways: sonic hedgehog (Shh) ventrally and bone morphogenetic proteins (BMPs)/Wnt dorsally (Martí et al., 1995; Ericson et al., 1997; Liem et al., 1997; Edlund and Jessell, 1999; Jessell, 2000; Muroyama et al., 2002). Additional pathways subsequently get involved (Sockanathan and Jessell, 1998; Novitch et al., 2003; Mizuguchi et al., 2006; Wildner et al., 2006; Del Barrio et al., 2007; Peng et al., 2007). Themed on the traditional paradigm, Hh signals are localized to the ventral SC and Gli repressor forms restrict activity in the dorsal SC (Jacob and Briscoe, 2003; Meyer and Roelink, 2003; Matise and Wang, 2011). Similarly, Wnt ligands are mostly restricted to dorsal regions, whereas inhibitors such as secreted Frizzled related proteins (sFRPs) are expressed in the ventral SC (Wodarz and Nusse, 1998; Kim et al., 2001; Kawano and Kypta, 2003). BMP/TGFβ signaling, however, does not phenocopy this model. For instance, though implicated in dorsal fate specification, expression of Tgfβ2 is observed in notochord and floor plate (García-Campmany and Martí, 2007). Additionally, expression of BMP/TGFβ signaling mediators Smad3, Smad4 and receptor-activated Smad1 and Smad5 is observed in almost all dorsoventral progenitor domains (Chesnutt et al., 2004; García-Campmany and Martí, 2007; Hazen et al., 2012). This suggests undeciphered instructive roles for this pathway during ventral neurogenesis.
The V2 interneurons (INs) emerging from the p2 progenitor domain diversify into two major subtypes: V2a INs expressing Chx10 (Vsx2 - Mouse Genome Informatics) and V2b INs expressing Gata2 and Gata3 (Ericson et al., 1997; Zhou et al., 2000). The winged helix/forkhead transcription factor (TF) Foxn4 is essential for Ascl1 and Dll4 expression in this domain (Li et al., 2005; Del Barrio et al., 2007). Notably, although Foxn4 is initially expressed in all p2 progenitors, Dll4 transcription is observed only in a subset of INs (Del Barrio et al., 2007; Peng et al., 2007). It has been speculated that Dll4+ precursors give rise to V2a INs, whereas the neighboring Dll4- precursors, which receive the Dll4 ligand and activate Notch pathway, differentiate into V2b INs (Peng et al., 2007). The restriction of Dll4 expression to a subset of precursors is the crucial step for generating asymmetry in immature postmitotic V2 precursors, which in turn is crucial for generating diversity. The mechanism behind this restriction, however, is presently unknown.
Notch ligands are regulated by proneural basic helix-loop-helix (bHLH) class of TFs (Bertrand et al., 2002; Castro et al., 2006; Henke et al., 2009). p2 progenitors express proneural TFs Ascl1, Neurog1 and Neurog2 as they initiate differentiation before onset of Dll4 expression. However, to date, no study has addressed the specific roles of these proneural genes in regulating Dll4 expression in V2 domain. Here, we provide evidence that Ascl1, Neurog1 and Neurog2 are expressed in a mosaic, balanced pattern in p2 progenitors and that Foxn4 is required for setting and maintaining this expression dynamic. The readout of this mosaic expression pattern results in asymmetric activation of Dll4 expression in V2 precursors expressing different combinations of proneural and Foxn4 TFs. One mechanism leading to this differential outcome involves direct binding of the proneural bHLH factors as well as Foxn4 to a conserved Dll4 enhancer. Asymmetric Dll4 activation and lateral inhibition may then generate two subsets of precursors with respect to Notch activation. We further show by lineage tracing that Dll4-Cre expression is initially excluded from Gata2-expressing V2b precursors. Finally, we show that Notch-mediated BMP/TGFβ signaling is required and sufficient for V2b fate specification. Thus, the intermingled expression of proneural TFs in p2 progenitors may serve as the trigger that initiates diversity in this ventral domain.
RESULTS
Mosaic expression pattern of proneural factors Ascl1, Neurog1 and Neurog2 in p2 progenitors dictates V2 subtype specification
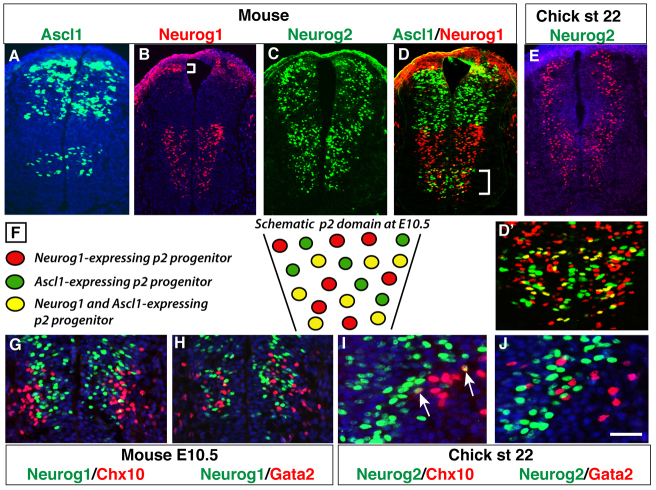
Although earlier studies have analyzed expression of proneural bHLH TFs Ascl1, Neurog1 and Neurog2 in the developing SC (Parras et al., 2002), no study has addressed the specific roles of these proneural factors in generating V2 subtype diversity. As a first step to characterize the function of these proneural factors in V2 fate specification, we carried out detailed immunostaining expression analysis of Ascl1, Neurog1 and Neurog2 in the ventral mouse and chick SCs. At embryonic day (E) 10.5, Ascl1 shows a distinct expression pattern in the ventral SC that previous studies have mapped to p2 IN progenitors (Fig. 1A). The broader Neurog1 and Neurog2 expression in the ventral neural tube also overlaps with the p2 domain (Fig. 1B,C). A similar expression pattern for Neurog proteins was seen in the chick neural tube (Fig. 1E). Interestingly, co-staining of Ascl1 and Neurog1 revealed a mosaic expression pattern with three types of p2 progenitors: progenitors expressing Ascl1 alone, those expressing Neurog1 alone, and those co-expressing both Ascl1 and Neurog1 (Fig. 1D′,F). Co-expression analysis revealed occasional overlap between Neurog1 and Neurog2 with Chx10 in V2a INs, whereas barely any overlap was observed between these two Neurogenins and Gata2 in V2b INs (Fig. 1G-J).
Fig. 1.
Mosaic expression pattern of proneural factors in p2 progenitors. (A-D) As detected by immunofluorescence, Ascl1, Neurog1 and Neurog2 are expressed in distinct patterns along the dorsoventral axis of the developing mouse spinal cord at E10.5. The bracket in B indicates the dorsal domain of Neurog1 expression. (E) Chicken Neurog2 displays an expression pattern similar to that of mouse Neurog2 at stage 22. (D′) Magnified view of the p2 region marked in D. (F) Schematic illustration of the salt and pepper expression pattern showing progenitors that express only Ascl1 or Neurog1 and those that express both Ascl1 and Neurog1. (G-J) Co-expression analysis of Chx10 or Gata2 with Neurog1 in mouse (G,H) and with Neurog2 in chick (I,J) spinal cords. No overlap is observed except for occasional Neurog2 and Chx10 co-expressing neurons (arrows in I). Scale bar: 60 μm for A-E; 30 μm for G,H; 10 μm for I,J.
To examine the biological relevance of the mosaic expression pattern of the proneural factors, we extended our expression analysis to Foxn4-/- spinal cords, where more V2a cells are generated at the expense of V2b neurons (Li et al., 2005; Panayi et al., 2010). Notably, at all stages of neurogenesis in wild-type embryos (E10.5-E12.5), there is complete overlap in expression of Ascl1 and Foxn4 in p2 progenitors (Fig. 2A-C). As reported previously, Ascl1 and Dll4 expression is completely downregulated in Foxn4-/- mutants, specifically in the p2 domain (Fig. 2D,D′,G,G′), consistent with the requirement of Ascl1 in specifying the V2b fate (Li et al., 2005). Expression of Neurog1 and Neurog2, by contrast, is maintained in the null mutant (Fig. 2E-F′). These results together thus suggest that Ascl1 expression is required for Dll4 expression, and that Neurog1 and Neurog2 in the absence of Ascl1 and Foxn4 expression are insufficient to induce Dll4 expression (Fig. 2H,H′).
Fig. 2.
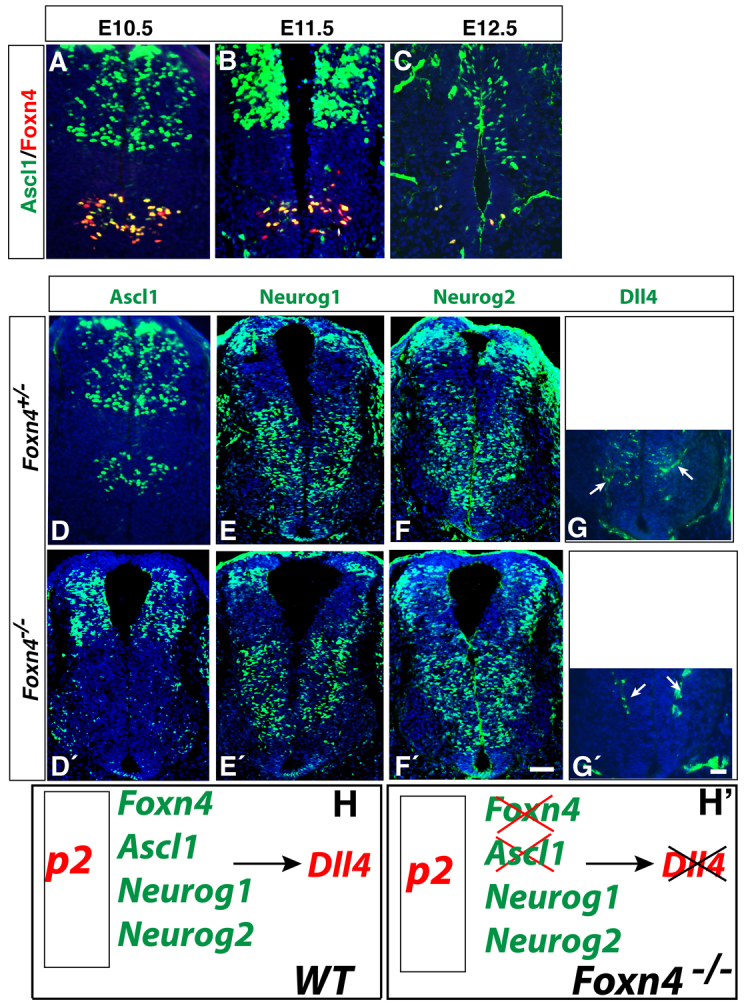
Foxn4-mediated equilibrium between Ascl1 and Neurog expression is essential for generating V2 interneuron diversity. (A-C) There is almost complete overlap between Ascl1 and Foxn4 expression in the p2 domain at E10.5-E12.5. (D-H′) In spinal cords of E11.5 Foxn4 mutant embryos, there is complete loss of Ascl1 (D,D′) and Dll4 expression (G,G′) in the p2 domain, whereas normal expression of Neurog1 and Neurog2 is observed (E-F′). Arrows in G,G′ indicate Dll4 expression in blood vessels. Schematic representation in H,H′ shows that Neurog expression is insufficient to induce Dll4 expression in the absence of Ascl1 and Foxn4. Scale bars: in F′, 11.8 μm for A-C; in F′, 20.7 μm for D-F′; in G′, 10 μm for G,G′.
Ascl1 and Neurog factors bind to a Dll4 enhancer but only Ascl1 can activate it
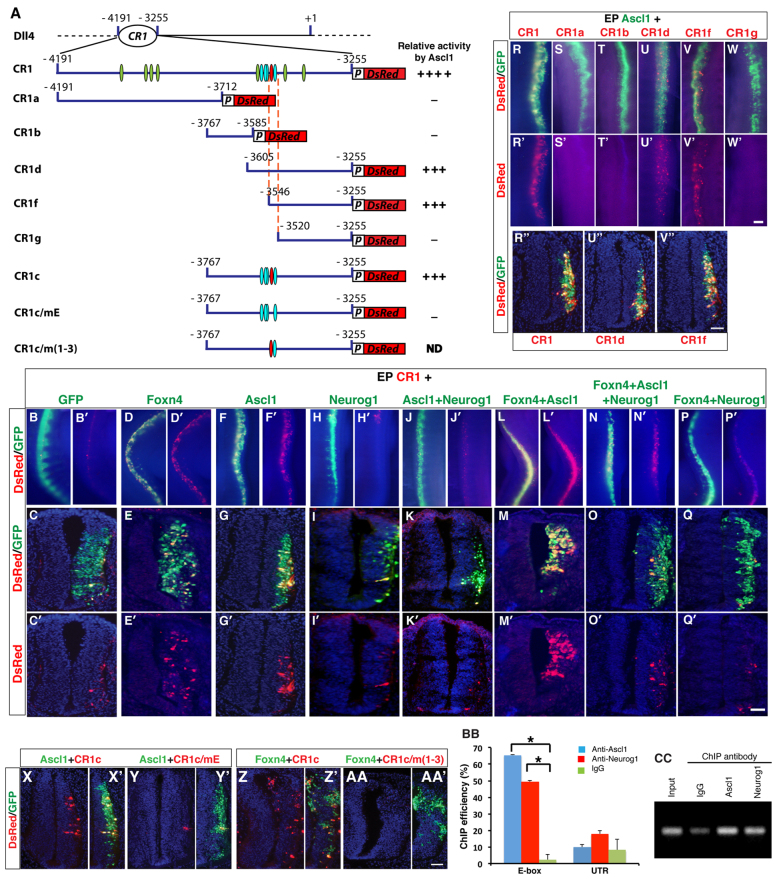
Dll4-Notch signaling has been demonstrated to determine the V2b versus V2a interneuron fate in the V2 domain (Del Barrio et al., 2007; Peng et al., 2007). It is therefore possible that proneural factors and Foxn4 may control the V2 fates by directly regulating Dll4 expression. To determine this, we tested whether Foxn4, Ascl1 and Neurog1 were able to activate gene expression through an evolutionarily conserved Dll4 enhancer CR1 found to be active in the retina (Luo et al., 2012) (Fig. 3A). In the chick spinal cord, CR1 was able to drive DsRed reporter expression in a pattern that mimics that of the mouse Dll4 along the dorsoventral axis, predominantly in the V2 domain (supplementary material Fig. S1). Co-transfection of the CR1-DsRed reporter construct with a Foxn4-GFP expression plasmid led to robust DsRed expression in the electroporated side of the spinal cord (Fig. 3D-E′), whereas co-transfection with a control GFP expression vector induced a low level of DsRed expression, presumably resulting from endogenously expressed Foxn4 and other factors (Fig. 3B-C′).
Fig. 3.
Direct activation of Dll4 expression by Foxn4 and Ascl1, but not Neurog1, through a phylogenetically conserved enhancer. (A) Schematics of truncated CR1 DsRed reporter constructs. Corresponding relative activities by Ascl1 are indicated to the right (ND, not determined). The green and red ovals in the CR1 fragment indicate the E-box motif CANNTG, with the red being the critical one. The four cyan ovals show the clustered ACGC Foxn4 binding motifs. In the CR1c/mE construct, the E-box is mutagenized to ATGATG, and in CR1c/m(1-3), the first three Foxn4 binding motifs are mutated to AAAA. The vertical dashed lines outline the critical 26-bp region containing the critical E-box and a ACGC motif. (B-Q′) DsRed expression in spinal cords co-electroporated with the CR1 reporter construct and indicated expression plasmids. Reporter activity was visualized in whole-mount spinal cords (B,B′,D,D′,F,F′,H,H′,J,J′,L,L′,N,N′,P,P′) or their cross sections (C,C′,E,E′,G,G′,I,I′,K,K′,M,M′,O,O′,Q,Q′). GFP alone showed only minimal endogenous enhancer activation (B-C′), whereas both Foxn4 and Ascl1 could strongly induce DsRed expression (D-G′). With Neurog1 barely any induction of DsRed expression was observed (H-I′). This repression was maintained even when Neurog1 was co-electroporated with Ascl1 (J-K′). Foxn4 and Ascl1 together, by contrast, caused synergistic increase of enhancer activation (L-M′), and electroporation of all three factors together could rescue Neurog1 inhibition to some extent (N-O′). Notably, Neurog1 and Foxn4 co-electroporation also resulted in significant reduction of ectopic enhancer activation observed with Foxn4 alone (P-Q′). (R-W′) Ascl1 regulation of truncated CR1 DsRed reporter constructs. As visualized in whole-mount embryos (R,R′,U,U′,V,V′) and transverse sections (R′,U′,V′), CR1d and CR1f exhibited high levels of DsRed expression, albeit less than full-length CR1. By contrast, CR1a, CR1b and CR1g had little or no activity (S-T′,W,W′). (X-AA′) E-box mutation nearly abolished DeRed reporter activation from CR1c by Ascl1 (X-Y′) while mutating Foxn4 binding motifs abrogated activation by Foxn4 (Z-AA′). (BB,CC) ChIP assay showing enrichment of the critical E-box region by anti-Ascl1 and anti-Neurog1 antibodies on chromatin of neural tubes pooled from E10.5, E11.5 and E12.5 wild-type embryos (BB,CC). Control DNA region from Dll4 3′ UTR was not significantly enriched (BB). Statistical significance was determined by Student’s t-test: *P<0.05. Scale bars: in Q′, 70 μm for B,B′,D,D′,F,F′,H,H′,J,J′,L,L′,N,N′,P,P′; in Q′, 30 μm for C,C′,E,E′,G,G′,I,I′,K,K′,M,M′,O,O′,Q,Q′; in W′, 54 μm for R-W′; in V′, 30 μm for R′,U′,V′; in AA′, 20 μm for X-AA′.
To test the roles of proneural factors, the enhancer construct was co-electroporated with Ascl1- and Neurog-GFP expression plasmids. Similar to Foxn4, Ascl1 efficiently induced DsRed expression (Fig. 3F-G′); however, maximal reporter expression level was achieved only by cotransfection with both Ascl1 and Foxn4 expression plasmids (Fig. 3L-M′), indicating a synergistic effect. Neurog1 and Neurog2, on the other hand, were unable to drive the reporter expression (compare Fig. 3B-C′ with 3H-I′; data not shown). Furthermore, co-transfection of Neurog1 with Ascl1, Foxn4, or both Ascl1 and Foxn4 resulted in greatly reduced reporter expression (Fig. 3J-K′,N-Q′), indicating that Dll4 expression is activated by Ascl1 and Foxn4 but repressed by Neurog proneural factors through the CR1 enhancer.
To narrow down the region in CR1 that can be activated by Ascl1, we tested a series of deletion reporter constructs by co-electroporation with the Ascl1-GFP expression plasmid (Fig. 3A). Prominent DsRed expression, albeit weaker than with CR1, was observed with the CR1d and CR1f reporter constructs (Fig. 3A,R-R′,U-V′). However, no reporter expression was seen with the CR1a, CR1b and CR1g constructs (Fig. 3A,S-T′,W,W′). These results thus define a 26-bp sequence between CR1f and CR1g that can be activated by Ascl1 (Fig. 3A). Interestingly, this region falls within the previously defined Foxn4 critical region and contains both an E-box and a Foxn4 binding motif that are highly conserved among many vertebrate species (supplementary material Fig. S2A,B) (Luo et al., 2012). Mutating the E-box and cluster of Foxn4 binding motifs nearly abolished DsRed reporter activation from the CR1 enhancer by Ascl1 and Foxn4, respectively (Fig. 3A,X-AA′).
We performed an electrophoretic mobility shift assay (EMSA) to test whether Ascl1 and Neurog proteins were able to directly bind to the minimal enhancer region using an oligo probe containing the critical E-box (Probe 2) and a control upstream probe that lacks an E-box (Probe 1) (supplementary material Fig. S2B). Although unable to form any complex with Probe 1, in the presence of the ubiquitous bHLH factor E12, in vitro translated Ascl1 formed a strong complex with Probe 2, which could be abrogated by excess wild-type but not mutant cold probes (supplementary material Fig. S2C, lanes 1-12, and S2D). Similarly, Neurog1 formed a specific complex with Probe 2 in the presence of E12 and the DNA-protein complex remained when both Ascl1 and Neurog1 were present (supplementary material Fig. S2C, lanes 13-18, and S2D). In addition, we carried out chromatin immunoprecipitation (ChIP) assays to show that both Ascl1 and Neurog1 were able to occupy in vivo the critical E-box region of the CR1 enhancer in cell culture and mouse embryonic neural tubes, whereas no enrichment was shown for control DNA in Dll4 3′ UTR (Fig. 3BB,CC; supplementary material Fig. S2E). Thus, Ascl1 and Neurog factors may bind to the same E-box in the Dll4 enhancer but have differential effects on Dll4 gene expression.
Dll4 is selectively expressed in V2a precursors to non-cell-autonomously specify the V2b fate
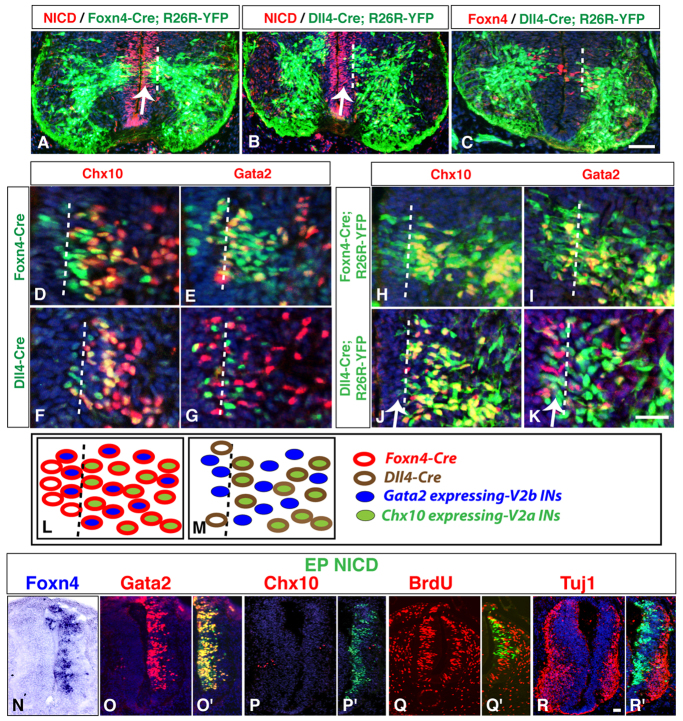
Dll4-Notch signaling has been shown to determine the V2b versus V2a fate choices adopted by p2 progenitors (Del Barrio et al., 2007; Peng et al., 2007), suggesting that Dll4 expression may exhibit cell-type specificity. To test this possibility, we produced a bacterial artificial chromosome (BAC) Dll4-Cre transgenic line (unpublished) to map the progeny of Dll4-expressing precursors by crossing with the R26R-YFP reporter line (Srinivas et al., 2001). For comparison we included Foxn4-Cre; R26R-YFP embryos in the analysis (Li et al., 2010). Whereas YFP-expressing progenies of both Cre lines arise from the p2 domain that expresses the Notch intracellular domain (NICD) (Fig. 4A,B), Dll4 expression is initiated later than that of Foxn4 (Fig. 4A-C). Analysis of Cre expression in E11.5 Foxn4-Cre embryos showed extensive overlap with that of the V2a IN marker Chx10 and V2b-specific Gata2 (Fig. 4D,E). Cre expression in Dll4-Cre mice, however, showed a different pattern. Whereas there was extensive overlap between Cre and Chx10 in V2a neurons, there was no colocalization between Cre and Gata2 in V2b nascent neurons (Fig. 4F,G). Similar analysis using YFP staining revealed near complete overlap of YFP with Chx10 and Gata2 in V2 INs in Foxn4-Cre; R26R-YFP embryos (Fig. 4H,I). By contrast, in Dll4-Cre; R26R-YFP embryos, although good co-expression of YFP was observed in Chx10-expressing neurons (Fig. 4J), a transient zone with no overlap of Gata2 and YFP expression in early precursors was clearly observed (Fig. 4K, arrow). Schematic comparison of Cre expression patterns in Dll4-Cre and Foxn4-Cre embryos indicates that whereas Dll4-expressing precursors become V2a neurons, the transient Dll4-negative precursors adopt the V2b fate (Fig. 4L,M).
Fig. 4.
Lineage tracing of Dll4-expressing precursors in the V2 domain. (A-C) In E11.5 Foxn4-Cre; R26R-YFP spinal cords, there was an extensive overlap of YFP with NICD in the progenitor domain (indicated by the arrow in A), whereas in Dll4-Cre; R26R-YFP spinal cords, there was hardly any overlap (indicated by the arrow in B). In addition, in Dll4-Cre; R26R-YFP spinal cords, Foxn4 immunostaining showed that YFP expression was initiated later than Foxn4 (C). (D,E) In E11.5 Foxn4-Cre spinal cords, there was an extensive overlap between Cre and Chx10 or Gata2 in V2 neurons. (F,G) In E11.5 Dll4-Cre spinal cords, Cre was co-expressed with Chx10, but excluded from Gata2 expressing cells. (H-K) In E11.5 Foxn4-Cre; R26R-YFP spinal cords, both Chx10- and Gata2-immunoreactive subtypes were immunoreactive for YFP. Similar tracing analysis with Dll4-Cre; R26R-YFP mice revealed extensive overlap between YFP and Chx10 expression (J) but showed a transient exclusion of YFP from Gata2-immunoreactive V2b precursors at early stages of differentiation (K, highlighted by the dashed line). (L,M) Schematic illustrating the Cre expression patterns in E11.5 Foxn4-Cre and Dll4-Cre spinal cords. Dotted vertical lines in A-M mark the transition zone between proliferating progenitors and differentiating neurons. (N-R′) Overexpression of activated Notch receptor (NICD) in the chick spinal cord caused widespread induction of Foxn4 and Gata2 expression (N-O′) but downregulation of Chx10 expression (P,P′). NICD-expressing cells hardly colocalized with those labeled by bromodeoxyuridine (BrdU) and failed to express Tuj1 (Q-R′). Scale bars: in C, 20 μm for A-C; in K, 12 μm for D-K; in R, 20 μm for N-R′.
Because misexpressed Dll4 promoted V2b differentiation (Peng et al., 2007), our data imply that Foxn4- and Ascl1-activated Dll4 non-cell-autonomously activates Notch signaling to specify the V2b fate. Consistent with this and a previous report (Peng et al., 2007), overexpression of the constitutively active NICD robustly induced Foxn4 and Gata2 expression, but markedly reduced Chx10+ V2a cells in the chick SC (Fig. 4N-P′). The induced Gata2+ cells appeared to be V2b precursors, as they were mostly located in the ventricular zone, postmitotic, yet negative for the mature neuron marker Tuj1 (Tubb3 - Mouse Genome Informatics) (Fig. 4O,O′,Q-R′). Given that Foxn4 and Ascl1 can directly activate the Dll4 enhancer and that Ascl1 expression can be induced by Dll4 (Fig. 3) (Peng et al., 2007; Luo et al., 2012), our data suggest that Foxn4, Ascl1 and Dll4-Notch signaling may form a feedback regulatory loop to specify the V2b cell fate in the V2 domain.
BMP signaling promotes V2b interneuron generation
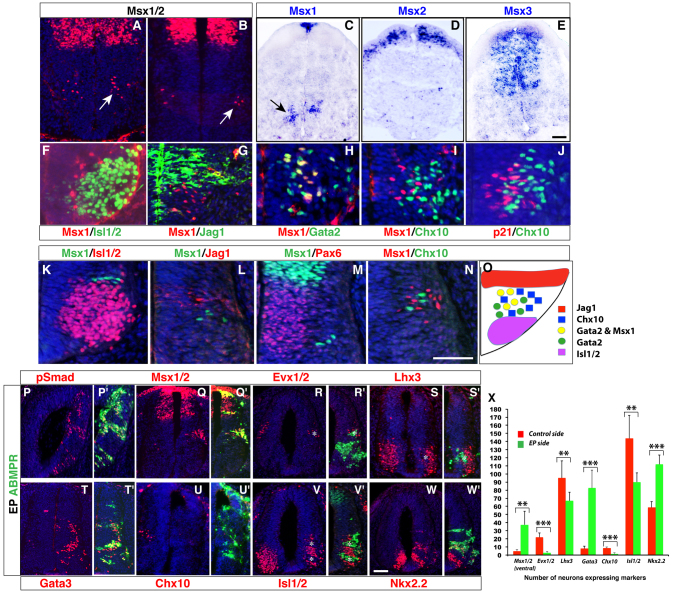
During our analysis of p2 progenitor markers, we observed Msx expression in this domain using an antibody that detects all three Msx proteins (Msx1, 2 and 3). The ventral Msx expression is first seen in mice at E10.5 (Fig. 5A), while in the chick spinal cord it is observed between stages 17-18 when differentiation is initiated (Fig. 5B). To characterize expression patterns of individual murine Msx genes, we performed in situ hybridization, which showed that only Msx1 is expressed in the ventral spinal cord (Fig. 5C-E). By double-immunolabeling, we determined that Msx1 is expressed specifically in the V2 domain, as it is excluded from the Isl1/2+ motoneurons located ventrally, the Jag+ V1 INs located dorsally, and from the Pax6+ progenitors (Fig. 5F,G,K-M). In the V2 domain, Msx1 is co-expressed with Gata2 mostly in the more medially located V2b precursors but excluded from Chx10+ V2a cells (Fig. 5H,I,N). This expression pattern is similar to p21 (Fig. 5J), which marks nascent INs as they undergo a transition from late G1 to early G0 phase (Misra et al., 2008).
Fig. 5.
BMP/TGFβ signaling promotes V2b cell fates at the expense of V2a interneurons in the ventral spinal cord. (A-N) Msx expression analysis in the ventral spinal cord. Cells immunoreactive for Msx1/2 were observed in the ventral spinal cord of E10.5 mouse embryos (arrow in A) and stage 18 chick embryos (arrow in B). In situ hybridization on E11.5 mouse spinal cords revealed Msx1 expression in dorsal as well as ventral domains (C, arrow points to ventral Msx1 expression), Msx2 expression in a dorsal region (D), and Msx3 expression in a broad dorsal domain (E). In E10.5 mouse (F-J) and stage 20 chick (K-N) ventral spinal cords, no Msx1 expression was observed in ventrally located motoneurons that express Isl1/2 (F,K), dorsal cells that express Jagged1 (G,L), or in Pax6-positive progenitors (M). Msx1 overlaps with Gata2 in proximal Gata2-expressing cells, but is shut off in distal Gata2-expressing cells (H). It does not colocalize with Chx10 in V2a neurons (I,N). p21 expression, like Msx1, also precedes Chx10 expression in this domain (J). Transverse sections through the spinal cord are shown in A-N. (O) Schematic of expression patterns of Msx1 and other markers in the ventral spinal cord. (P-W′) Overexpression of ABMPR increased the number of pSmad-, Msx1/2-, Gata3- and Nkx2.2-immunoreactive cells on the electroporated side (P-Q′,T,T′,W,W′), but inhibited the number of Evx1/2-, Lhx3-, Chx10- and Isl1/2-immunoreactive cells (R-S′,U-V′). The asterisks in (R-S′,V,V′) indicate the exclusion of Evx1/2-, Lhx3- and Isl1/2-positive cells from the regions with overexpressed ABMPR (green). (X) Quantification of the effect of ABMPR overexpression on ventral cell fates. Each histogram represents the mean±s.d. for three embryos. Statistical significance was determined by Student’s t-test: **P<0.005, ***P<0.0001. Scale bars: in E, 10 μm for A,B; in E, 15 μm for C-E; in N, 37 μm for F,G; in N, 18.5 μm for H-J; in N, 20 μm for K-N; in W, 13 μm for P-W′.
As Msx genes are known BMP signaling targets (Liu et al., 2004), our observations raised the possibility that BMP signaling may have a role in V2b IN specification. To test this possibility, we transfected a construct encoding the activated BMP receptor 1b (ABMPR) into chick SCs. Exogenous activation of BMP receptors was confirmed by ectopic induction of phosphorylated (p) Smad (pSmad1,5,8) and Msx (Fig. 5P-Q′,X). We found that misexpressed ABMPR increased the number of Gata3+ cells by approximately tenfold while reducing the number of Chx10+ cells by >80% (Fig. 5T-U′,X). Interestingly, ectopic induction of Gata3+ cells was largely limited to the ventral spinal cord (supplementary material Fig. S3A-B). ABMPR overexpression also significantly promoted Nkx2.2+ cells but decreased Lhx3+ cells, Evx1/2+ V0 cells and Isl1/2+ motoneurons (Fig. 5R-S′,V-W′,X). Together, these results suggest that activation of BMP signaling is sufficient to induce V2b and inhibit V2a IN differentiation.
Inhibition of BMP/TGFβ signaling causes loss of V2b interneurons
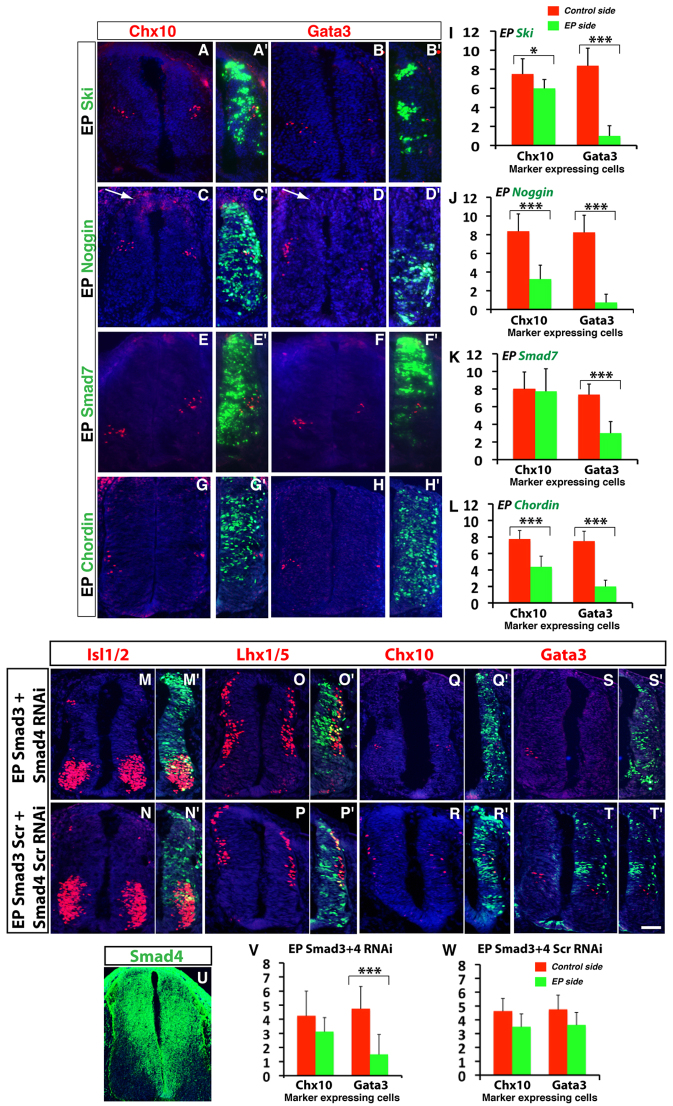
To determine whether BMP/TGFβ signaling is necessary for V2b neuron generation, we overexpressed BMP (noggin, Smad7 and chordin) and TGFβ (Ski and Smad7) inhibitors in chick SCs. Electroporation of all of these inhibitors caused great to near complete depletion of Gata3+ neurons (Fig. 6B,B′,D,D′,F,F′,H-L), indicating a requirement of BMP/TGFβ signaling in specifying V2b cells. For Chx10+ V2a cells, overexpressed Ski, noggin and chordin caused a reduction of 20%, 61% and 43%, respectively, but Smad7 had no effect (Fig. 6A,A′,C,C′,E,E′,G,G′,I-L). As V2a INs could be inhibited both by activating and inhibiting the BMP/TGFβ signaling pathway, these effects are possibly secondary to ectopic induction of V2b cells.
Fig. 6.
Inhibition of BMP/TGFβ signaling blocks V2b interneuron specification. (A-H′) BMP/TGFβ antagonists inhibit V2b differentiation. Electroporation of Ski, noggin and chordin all resulted in diminished Chx10- or Gata3-immunoreactive cells (A-H′). However, in all instances, Gata3 inhibition was much more pronounced. Smad7 overexpression also caused a decrease of Gata3-immunoreactive cells but was not significantly inhibitory for Chx10 expression (E-F′). Arrows in C,D indicate altered roof plates associated with noggin overexpression. (I-L) Quantification of overexpression analysis. Each histogram represents the mean ± s.d. for three embryos. Statistical significance was determined by Student’s t-test: *P<0.05, ***P<0.0001. (M-W) Simultaneous knockdown of Smad3 and Smad4 leads to loss of V2b interneurons. Co-electroporation of Smad3 and Smad4 RNAi plasmids caused a general reduction in differentiation in the dorsal spinal cord, as observed with Isl1/2 and Lhx1/5 immunostaining (M,M′,O,O′). It greatly reduced Gata3-immunoreactive neurons but caused no significant change in Chx10-immunoreactive cells (Q,Q′,S,S′). Co-electroporation of the scrambled controls resulted in no significant alteration of these marker-immunoreactive cells (N,N′,P,P′,R,R′,T,T′). (U) At E11.5, Smad4 appears to be expressed in all progenitors of the spinal cord. (V,W) Quantification of the RNAi knockdown experiments. Each histogram represents the mean ± s.d. for three embryos. Statistical significance was determined by Student’s t-test: ***P<0.0001. Scale bar: 9 μm for A-H′; 25 μm for M-T′; 20 μm for U.
To investigate directly the role of BMP/TGFβ pathway in V2b fate specification, we examined Smad3 knockout mice and conditional Smad4 knockout animals (Datto et al., 1999; Chu et al., 2004). Neither model showed any overt V2 phenotype at early stages examined (data not shown), suggesting redundancy between signaling components. We therefore analyzed the effect of knocking down both Smad3 and Smad4 simultaneously by RNAi constructs. Knocking down both Smads caused some general inhibition of differentiation, especially in the dorsal SC, as observed with Isl1/2 and Lhx1/5 staining (Fig. 6M,M′,O,O′). Notably for Isl1/2 the inhibition was restricted to the dorsal dI3 population and the ventral motoneurons were not inhibited (Fig. 7M). With respect to V2 fates, simultaneous Smad inhibition caused a 68% reduction of Gata3-expressing V2b INs but no significant change in Chx10+ V2a INs (Fig. 6Q,Q′,S,S′,V). Co-electroporation of scrambled Smad3 and Smad4 RNAi constructs did not cause significant alteration in either Gata3+ or Chx10+ INs (Fig. 6R,R′,T,T′,W). Consistent with a role of these Smads in V2 fate specification, Smad4 expression was observed in progenitors throughout the spinal cord (Fig. 6U). Smad3 has already been shown to be expressed in the V2 domain (Martí et al., 1995). Together, these data demonstrate a requirement of BMP/TGFβ signal transduction in V2b IN specification.
Fig. 7.
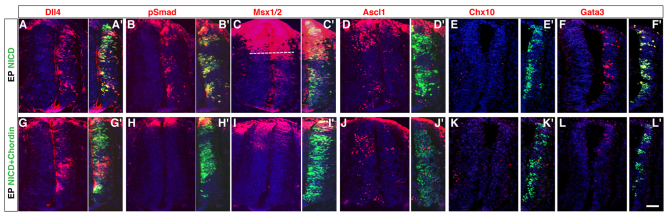
Notch functions upstream of BMP signaling. (A-F′) Overexpression of NICD induced widespread expression of Dll4, pSmad, Msx1/2 and Gata3 but caused marked downregulation of Ascl1 and Chx10 expression on the electroporated side. (G-L′) Co-electroporation of chordin with NICD failed to induce ectopic pSmad and Msx1/2 expression, diminished Gata3 induction, but still led to induction of Dll4 expression and inhibition of Ascl1 and Chx10 expression. Transverse sections through chick spinal cords at stages 22-24 are shown in all panels. Right side inserts show GFP expression to indicate the extent of transfection. The dashed line in C indicates the ventral limit of endogenous Msx expression. Scale bar: 10 μm.
Dll4-initiated Notch signaling acts upstream of BMP/TGFβ signaling in V2b precursors
Given that both Dll4-Notch and BMP/TGFβ signaling are involved in V2b fate specification, it is important to decipher the hierarchy and interactions of these two signal transduction pathways. Overexpression of NICD caused widespread ectopic expression of pSmad and BMP target Msx1/2 in the chick SC, including the V2 domain (Fig. 7B-C′). Consistent with activation of Notch signaling by NICD, robust Dll4 and Gata3 expression was induced but Ascl1 and Chx10 expression was strongly suppressed (Fig. 7A,A′,D-F′). By contrast, activation of the BMP pathway by ABMPR could not induce Dll4 expression (data not shown). Moreover, ABMPR-activated Gata3 induction was not suppressed by the dominant-negative Maml1, a Notch signaling inhibitor (supplementary material Fig. S3) (Peng et al., 2007). To further delineate the effects of the two pathways, we co-transfected BMP inhibitor chordin with NICD. In the co-electroporated embryos, Dll4 induction and Ascl1 and Chx10 inhibition were still observed, but pSmad and Msx1/2 upregulation was completely blocked and Gata3 induction greatly diminished (Fig. 7G-L′). These results suggest that the activation of BMP signaling in V2b precursors depends on and occurs downstream of Dll4-Notch signaling during V2 IN development.
DISCUSSION
Asymmetric Dll4 activation by proneural and Foxn4 TFs as a basis of V2 subtype diversity
Although widely expressed, proneural TFs Ascl1, Neurog1 and Neurog2 have predominantly cross-repressive patterns with minimal overlap (Gowan et al., 2001; Parras et al., 2002; Helms and Johnson, 2003; Nakada et al., 2004; Helms et al., 2005; Ge et al., 2006; Battiste et al., 2007; Sugimori et al., 2007; Osório et al., 2010; Quiñones et al., 2010). This repression is validated in the developing SC also, where Ascl1, Neurog1 and Atoh1 are expressed in discrete cross-repressive domains (Gowan et al., 2001; Parras et al., 2002; Nakada et al., 2004; Helms et al., 2005; Kele et al., 2006). Functionally, proneural factors have a crucial role in neuronal differentiation, a function largely dependent on activating Notch ligands. Of these, Dll1 expression is regulated by an enhancer having two distinct subdomains: one that requires Ascl1 binding for activity and the other that binds Neurog2 but is only partly required for SC expression (Bertrand et al., 2002). Dll3 enhancer, by contrast, has one critical E-box subdomain that can bind Ascl1, Neurog1 and Ascl1/Neurog2 heterodimers. Dll3 expression is mostly dependent on Ascl1, but not Neurog1 (Henke et al., 2009).
Differential regulation of Delta ligands is dictated largely by the expression pattern of proneural factors. V2 domain is unique, as it has progenitors expressing Dll4, Ascl1 and Neurog in exclusive as well as overlapping patterns. It thereby provides a context to analyze whether these factors have distinct roles based on exclusive expression patterns, or based on intrinsic properties. In this study, we show that although both Ascl1 and Neurog factors can bind to a conserved Dll4 enhancer, only Ascl1 can ectopically activate it. A balanced mosaic expression of the proneural factors is therefore crucial for initiating asymmetric expression of Dll4 in p2 progenitors. Although our studies focused on one conserved enhancer, it is conceivable that other Dll4 enhancers might be regulated in a different manner. Overall, mutually exclusive expression patterns might facilitate the regular function of proneural factors by heterodimerization with ubiquitously expressed bHLH E-proteins, whereas overlapping expression might be a mechanism adopted to create a mixture of functional and nonfunctional heterodimers with E-proteins and each other, thereby creating a basis for asymmetric activation of Notch ligand expression.
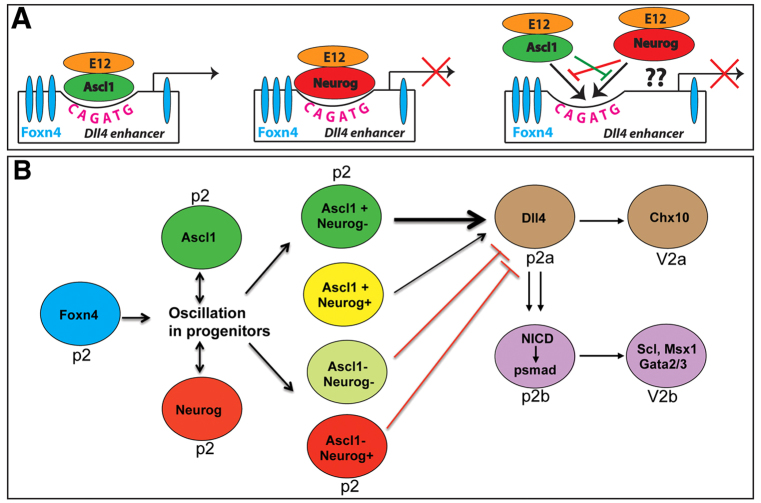
The generation of neural diversity by bHLH TFs is dependent on inputs from other regionally expressed factors (Powell and Jarman, 2008). One such example is the synergistic activation of Dll1 by simultaneous binding to its enhancer of Ascl1 and regionally expressed Brn1 (Pou3f3 - Mouse Genome Informatics) and Brn2 (Pou3f2 - Mouse Genome Informatics) (Castro et al., 2006). In the V2 domain Foxn4 is required for Ascl1 expression and V2b IN specification (Li et al., 2005; Del Barrio et al., 2007). Interestingly, the CR1 Dll4 enhancer contains a functional E-box and Foxn4 binding motifs located in close proximity (Fig. 8). Foxn4 may therefore operate at two levels for Dll4 regulation, one by directly binding and activating the enhancer and the other by regulating expression of Ascl1, which in turn can also bind to and activate the Dll4 enhancer. The juxtaposed location of the highly conserved binding motifs suggests a mechanism that requires proximity of these two TF binding sites for optimal Dll4 enhancer activity, perhaps dependent on direct interaction between Foxn4 and Ascl1. This may explain the synergistic effect of Foxn4 and Ascl1 on the Dll4 enhancer and the observed Foxn4 and Ascl1 co-expression in p2 progenitors at all stages of neurogenesis.
Fig. 8.
Model for asymmetric activation of Dll4 expression by Foxn4 and proneural factors and subsequent activation of Notch and BMP/TGFβ signaling in p2 progenitors. (A) Oscillating expression of neurogenic factors in p2 domain combined with the synergistic interaction of Ascl1 and the opposing activity of Neurog factors on the Dll4 enhancer creates four kinds of progenitors in this domain. There is strong Dll4 expression in p2 progenitors expressing Ascl1 alone and weaker expression in those expressing both Ascl1 and Neurog. However, p2 progenitors expressing Neurog factors alone or none of these transcription factors will lack Dll4 expression. The curved CAGATC site indicates the critical E-box and cyan ovals indicate Foxn4 binding motifs in the Dll4 enhancer. (B) The mosaic expression pattern of proneural factors due to their oscillations coupled with lateral inhibition/cis-inhibition leads to the generation of p2a progenitors with high levels of Dll4 ligand and neighboring p2b progenitors with high levels of activated Notch. The activated Notch in turn cell-autonomously activates BMP/TGFβ signaling essential for V2b fate specification.
Because of the apparently complete colocalization between Foxn4 and Ascl1 in p2 progenitors, our data suggest the presence of four types of progenitors in the p2 domain: (1) those expressing Foxn4 and Ascl1; (2) those expressing Foxn4, Ascl1 and Neurog (1 or/and 2); (3) those expressing Neurog; and (4) those that express none of these TFs (Fig. 8A). We propose that this mosaic expression pattern of Foxn4 and proneural factors initiates asymmetric expression of Dll4 in p2 progenitors, which eventually leads to the specification of different V2 IN subtypes (Fig. 8). As expression of proneural genes has been shown to oscillate in neural progenitors (Kageyama et al., 2008; Shimojo et al., 2008; Kageyama et al., 2009), the mosaic expression pattern observed here may result in part from this oscillatory expression and may represent only a snapshot of the dynamic expression levels and asymmetry of Foxn4 and proneural factors in p2 neural progenitors. We found that although both Ascl1 and Neurog1 were able to bind the conserved E-box in the Dll4 enhancer, only Ascl1 could activate the enhancer, whereas Neurog proteins inhibited the enhancer activation by Ascl1 and Foxn4 (Fig. 3; supplementary material Fig. S2), presumably by competition for common cofactors, competition for binding sites, or by disruption of pre-formed complexes. Thus, p2 progenitors that express both Foxn4 and Ascl1 are expected to express Dll4 strongly, whereas those expressing all three TFs will have a weak Dll4 expression, and those expressing Neurog alone or none of these TFs will lack Dll4 expression (Fig. 8A). The uneven expression of TFs coupled with lateral inhibition/cis-inhibition subsequently generates p2a progenitors with high levels of Dll4 ligand and neighboring p2b progenitors with high levels of activated Notch. The activated Notch in turn cell-autonomously induces the BMP/TGFβ signal transduction pathway to specify the V2b fate (Fig. 8B).
Requirement of BMP/TGFβ signaling in V2b interneuron specification
Neurogenesis along the dorsoventral axis of the neural tube is initiated by extracellular inductive signals. BMP signals initiate patterning of the dorsal neural tube (Liem et al., 1997). Conversely, their downregulation is essential for specification of ventral fates (McMahon et al., 1998; Liem et al., 2000; Patten and Placzek, 2002; Timmer et al., 2002). Notably, Smad3 expression pattern supports this notion, as Smad3 is excluded specifically from motoneuron progenitors (García-Campmany and Martí, 2007). However, none of the previous studies looked specifically at the effect of BMP/TGFβ signaling on the V2 fate specification. We show in this study that overexpression of activated BMP receptor 1b could efficiently induce Gata2/3-expressing neurons. We further demonstrated the necessity of BMP/TGFβ signaling in V2b IN generation by overexpression of pathway inhibitors as well as simultaneous knockdown of Smad3 and Smad4 expression. Our study thus for the first time reveals an important instructive role for BMP/TGFβ signaling in ventral V2b IN fate specification.
BMP/TGFβ signaling acts downstream of Notch signaling in V2b interneuron generation
Our results show that Dll4 ligand expression represents the earliest step for initiating V2 subtype diversification. BMP/TGFβ signaling acts at a later step, initiated downstream in a subpopulation of p2 progenitors that are receiving Notch signaling. This conclusion is based on two observations. First, activation of Notch signaling induced pSmad expression that could be inhibited by chordin; however, chordin was unable to inhibit Notch-activated Dll4 expression. Second, activation of BMP signaling by dominant active ABMPR failed to induce Dll4 expression (data not shown) and co-expression of dominant-negative Maml1 did not prevent Gata3 induction. Nevertheless, there appeared to be a slight reduction in Gata3 induction compared with ABMPR overexpression alone. This might reflect sequestration of the endogenous Notch signaling operating in this region by dominant-negative Maml1. It is interesting to note that perturbations to Notch signaling lead to interconversion of V2 subtype fates (Li et al., 2005; Del Barrio et al., 2007; Peng et al., 2007). Inhibition of BMP/TGFβ signaling, by contrast, just inhibited V2b fates but no fate conversions were observed. This is true both when pathway inhibitors as well as RNAi knockdown was utilized. This indicates that BMP/TGFβ activation is a late event that comes into play after binary V2 fate decisions have been made. So although required for downstream activation of V2b specific markers, BMP/TGFβ signaling may not be involved in the binary fate decision per se.
MATERIALS AND METHODS
Animals
All experiments with mice were performed in accordance with animal protocols approved by Rutgers University. The C57BL/6J mice were purchased from the Jackson Laboratory and CD1 mice from the Charles River Laboratories. The Foxn4 knockout (Li et al., 2004), Foxn4-Cre (Li et al., 2010), Smad3 knockout (Datto et al., 1999), Smad4loxp/loxp (Chu et al., 2004) and R26R-YFP (Srinivas et al., 2001) mice were generated previously and maintained by breeding with C57/BL6J mice. The stage of mouse embryos was determined by taking the morning when the copulation plug was seen as E0.5. All genotypes described were confirmed by polymerase chain reaction (PCR).
In ovo electroporation and expression constructs
Electroporation was performed on stage 11-12 chick embryos using a BTX square wave electroporator as described (Li et al., 2005). Transfected embryos were incubated for 48 hours and processed for immunohistochemistry (Li et al., 2005). For RNAi knockdown, embryos were collected 30 hours post-transfection. Following full-length cDNAs were subcloned into the pCIG vector (Megason and McMahon, 2002): Ascl1 (Parras et al., 2002), chordin (Sasal et al., 1995), Ski (Colmenares and Stavnezer, 1989), noggin (Smith and Harland, 1992), Smad7 (Nakao et al., 1997) and Neurog2 (Gowan et al., 2001). The mouse NICD region spanning amino acids 1753-2185 (Gaiano et al., 2000) was also subcloned into pCIG. Bmp4 (Sela-Donenfeld and Kalcheim, 2002) was subcloned into the pcDNA3 vector, and Myc-Neurog1 (Ma et al., 1999) and dominant active BMPR-1b (ABMPR) (Timmer et al., 2002) into the pMWiii vector. The expression plasmid for dominant negative Maml1 was described previously (Peng et al., 2007). Transfection with the Bmp4 or ABMPR expression plasmids was visualized by co-electroporating the pCIG vector.
shRNA plasmids
Previously generated Smad3 shRNA and its control were used for Smad3 knockdown (García-Campmany and Martí, 2007). Smad4 shRNA plasmid was generated by inserting the already characterized region of Smad4 effective for gene silencing (Jazag et al., 2005) into the pBS/U6 (pU6) RNAi vector (Sui et al., 2002): 5′-GATCCGGCAGCCATAGTGAAGGACTGTTCAAGAGACAGTCCTTC ACTATGGCTGCCTTTTTTTG-3′ and 5′-AATTCAAAAAAAGGCAGCCATAGTGAAGGACTGTCTCTTGAACAGTCCTTCACTATGGCTGCCG-3′. Scrambled control sequences generated by Genescript software were: 5′-GATCCGGAGTAAGCTACCGGTAAGGCTTCAAGAGAGCCTTACCGGTAGCTTACTCCTTTTTTTG-3′ and 5′-AATTCAAAAAAAGGAGTAAGCTACCGGTAAGGCTCTCTTGAAGCCTTACCGGTAGCT-TACTCCG-3′.
Immunostaining, RNA in situ hybridization and BrdU labeling
In-ovo BrdU labeling, immunostaining and in situ hybridization were performed as described previously (Li et al., 2004; Mo et al., 2004; Misra et al., 2008). The following primary antibodies were used: rabbit anti-Foxn4 (Li et al., 2004); mouse anti-Ascl1, -Lhx3, -Isl1/2, -Evx1/2, -Nkx2.2, -Lhx1/5, and -Msx1/2 (Developmental Studies Hybridoma Bank); mouse anti-Neurog1 and rabbit anti-Neurog2 (Lo et al., 2002); mouse anti-Tuj1 and -Cre (Covance); rat anti-BrdU (Sigma); chick anti-GFP and rabbit anti-Jag1 (Abcam); goat anti-Neurog1, -Neurog2 and -DsRed, mouse anti-Gata3 and -Smad4, and rabbit anti-Gata2 (Santa Cruz); rabbit anti-Pax6 (Millipore); sheep anti-Chx10 (Exalpha); guinea pig anti-Chx10 and -Gata2 (Peng et al., 2007); rabbit anti-NICD and -pSmad1,5,8 (Cell Signaling); goat anti-Dll4 (R&D); rabbit anti-Ascl1 (Gowan et al., 2001); and guinea pig anti-Lbx1 (Müller et al., 2002). Images were captured with a Nikon Eclipse 80i microscope. Probes used for RNA in situ hybridization were: chicken Foxn4 (Li et al., 2005) and mouse Msx1, Msx2 and Msx3 (Wang et al., 1996).
Analysis of the CR1 Dll4 enhancer activity
Dll4 enhancer alignment and reporter constructs were described previously (Luo et al., 2012). In addition, the critical E-box in the CR1c construct was mutated to ATGATG from CAGATG using the mutagenesis service of Genewiz. The enhancer reporter plasmids were co-transfected with various TF expression plasmids into the spinal cord of developing chick embryos as described above. Whole-mount images were taken with a fluorescence microscope, or the tissue was fixed and processed for cryosection and staining.
Electrophoretic mobility shift assay
EMSA was carried out as previously described (Liu et al., 2000). In vitro translated Ascl1, E12 and Neurog1 protein products were generated by a TNT T7 or Sp6 coupled reticulocyte lysate system (Promega) using Ascl1, E12 and Neurog1 expression plasmids (Henke et al., 2009). Competition was performed by adding excess amount of wild-type or mutant cold oligonucleotides to the reaction mixtures. The E-box was mutated from CAGATG to ATGATG in the mutant Probe 2 oligonucleotide (supplementary material Fig. S2B).
Chromatin immunoprecipitation assay
ChIP assays were performed on chromatin prepared from neural tube tissue pooled from E10.5, E11.5 and E12.5 wild-type mouse embryos using the Magna ChIP HiSens kit from Millipore. Isolated chromatin was sheared using a Diagenode Bioruptor for 30 minutes at 50% power with 30 seconds on:off cycles. Sixty micrograms of chromatin was incubated with 5 μg antibodies overnight. Antibodies used were rabbit anti-Ascl1 and goat anti-Neurog1 purchased from Bioss and Santa Cruz, respectively. ChIP enrichment was quantified by quantitative reverse transcription PCR (qRT-PCR) with SYBR Green mix from ABI. Percentage ChIP efficiency was calculated as described by Henke et al. (Henke et al., 2009). The following primers were used: for the critical E-box, 5′-CCACGGCTGCCAGGCTCTGCCAG-3′ and 5′-GGAGATTTGCAAACTGTTGCTGCCAC-3′, and for 3′ UTR, 5′-CCTCCCTCACACCCATTTCT-3′ and 5′-TGTAGAAAGGCCAGTGCTTCTG-3′. ChIP assays were also performed on chromatin DNA prepared from 293T cells co-transfected with the CR1 reporter construct and Ascl1, Neurog1 and E12 expression plasmids according to the instruction of the Simple Enzymatic Chromatin IP kit (Cell Signaling).
Supplementary Material
Acknowledgments
We thank Dr Min Zou for thoughtful comments on the manuscript and are grateful to Drs Xiao-Fan Wang, Jane Johnson, Lee Niswander, Chaya Kalcheim, Edward De Robertis, Thomas Lufkin, Richard Harland, Kamal Sharma, Elisa Marti, and Masahiro Kawabata for generously sharing plasmids, antibodies or knockout mice. This paper is dedicated to the memory of Dr Aaron Shatkin.
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
K.M., H.L., S.L., M.M. and M.X. designed and performed the experiments, and analyzed the data. K.M. and M.X. wrote the manuscript. All authors participated in the editing process.
Funding
This work was supported in part by the New Jersey Commission on Spinal Cord Research [09-3087-SCR-E-0 to M.X. and 11-2955-SCR-E-0 to K.M.] and the National Institutes of Health [EY020849 and EY012020 to M.X.]. The Matise lab is supported by grants from the NIH/NICHD and National Science Foundation (NSF). Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.092536/-/DC1
References
- Battiste J., Helms A. W., Kim E. J., Savage T. K., Lagace D. C., Mandyam C. D., Eisch A. J., Miyoshi G., Johnson J. E. (2007). Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development 134, 285–293 [DOI] [PubMed] [Google Scholar]
- Bertrand N., Castro D. S., Guillemot F. (2002). Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 3, 517–530 [DOI] [PubMed] [Google Scholar]
- Castro D. S., Skowronska-Krawczyk D., Armant O., Donaldson I. J., Parras C., Hunt C., Critchley J. A., Nguyen L., Gossler A., Göttgens B., et al. (2006). Proneural bHLH and Brn proteins coregulate a neurogenic program through cooperative binding to a conserved DNA motif. Dev. Cell 11, 831–844 [DOI] [PubMed] [Google Scholar]
- Chesnutt C., Burrus L. W., Brown A. M., Niswander L. (2004). Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev. Biol. 274, 334–347 [DOI] [PubMed] [Google Scholar]
- Chu G. C., Dunn N. R., Anderson D. C., Oxburgh L., Robertson E. J. (2004). Differential requirements for Smad4 in TGFbeta-dependent patterning of the early mouse embryo. Development 131, 3501–3512 [DOI] [PubMed] [Google Scholar]
- Colmenares C., Stavnezer E. (1989). The ski oncogene induces muscle differentiation in quail embryo cells. Cell 59, 293–303 [DOI] [PubMed] [Google Scholar]
- Datto M. B., Frederick J. P., Pan L., Borton A. J., Zhuang Y., Wang X. F. (1999). Targeted disruption of Smad3 reveals an essential role in transforming growth factor β-mediated signal transduction. Mol. Cell. Biol. 19, 2495–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Barrio M. G., Taveira-Marques R., Muroyama Y., Yuk D. I., Li S., Wines-Samuelson M., Shen J., Smith H. K., Xiang M., Rowitch D., et al. (2007). A regulatory network involving Foxn4, Mash1 and delta-like 4/Notch1 generates V2a and V2b spinal interneurons from a common progenitor pool. Development 134, 3427–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund T., Jessell T. M. (1999). Progression from extrinsic to intrinsic signaling in cell fate specification: a view from the nervous system. Cell 96, 211–224 [DOI] [PubMed] [Google Scholar]
- Ericson J., Rashbass P., Schedl A., Brenner-Morton S., Kawakami A., van Heyningen V., Jessell T. M., Briscoe J. (1997). Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell 90, 169–180 [DOI] [PubMed] [Google Scholar]
- Gaiano N., Nye J. S., Fishell G. (2000). Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron 26, 395–404 [DOI] [PubMed] [Google Scholar]
- García-Campmany L., Martí E. (2007). The TGFbeta intracellular effector Smad3 regulates neuronal differentiation and cell fate specification in the developing spinal cord. Development 134, 65–75 [DOI] [PubMed] [Google Scholar]
- Ge W., He F., Kim K. J., Blanchi B., Coskun V., Nguyen L., Wu X., Zhao J., Heng J. I., Martinowich K., et al. (2006). Coupling of cell migration with neurogenesis by proneural bHLH factors. Proc. Natl. Acad. Sci. USA 103, 1319–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowan K., Helms A. W., Hunsaker T. L., Collisson T., Ebert P. J., Odom R., Johnson J. E. (2001). Crossinhibitory activities of Ngn1 and Math1 allow specification of distinct dorsal interneurons. Neuron 31, 219–232 [DOI] [PubMed] [Google Scholar]
- Hazen V. M., Andrews M. G., Umans L., Crenshaw E. B., 3rd, Zwijsen A., Butler S. J. (2012). BMP receptor-activated Smads confer diverse functions during the development of the dorsal spinal cord. Dev. Biol. 367, 216–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms A. W., Johnson J. E. (2003). Specification of dorsal spinal cord interneurons. Curr. Opin. Neurobiol. 13, 42–49 [DOI] [PubMed] [Google Scholar]
- Helms A. W., Battiste J., Henke R. M., Nakada Y., Simplicio N., Guillemot F., Johnson J. E. (2005). Sequential roles for Mash1 and Ngn2 in the generation of dorsal spinal cord interneurons. Development 132, 2709–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke R. M., Meredith D. M., Borromeo M. D., Savage T. K., Johnson J. E. (2009). Ascl1 and Neurog2 form novel complexes and regulate Delta-like3 (Dll3) expression in the neural tube. Dev. Biol. 328, 529–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Briscoe J. (2003). Gli proteins and the control of spinal-cord patterning. EMBO Rep. 4, 761–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazag A., Kanai F., Ijichi H., Tateishi K., Ikenoue T., Tanaka Y., Ohta M., Imamura J., Guleng B., Asaoka Y., et al. (2005). Single small-interfering RNA expression vector for silencing multiple transforming growth factor-β pathway components. Nucleic Acids Res. 33, e131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M. (2000). Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat. Rev. Genet. 1, 20–29 [DOI] [PubMed] [Google Scholar]
- Kageyama R., Ohtsuka T., Shimojo H., Imayoshi I. (2008). Dynamic Notch signaling in neural progenitor cells and a revised view of lateral inhibition. Nat. Neurosci. 11, 1247–1251 [DOI] [PubMed] [Google Scholar]
- Kageyama R., Ohtsuka T., Shimojo H., Imayoshi I. (2009). Dynamic regulation of Notch signaling in neural progenitor cells. Curr. Opin. Cell Biol. 21, 733–740 [DOI] [PubMed] [Google Scholar]
- Kawano Y., Kypta R. (2003). Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 116, 2627–2634 [DOI] [PubMed] [Google Scholar]
- Kele J., Simplicio N., Ferri A. L., Mira H., Guillemot F., Arenas E., Ang S. L. (2006). Neurogenin 2 is required for the development of ventral midbrain dopaminergic neurons. Development 133, 495–505 [DOI] [PubMed] [Google Scholar]
- Kim A. S., Anderson S. A., Rubenstein J. L., Lowenstein D. H., Pleasure S. J. (2001). Pax-6 regulates expression of SFRP-2 and Wnt-7b in the developing CNS. J. Neurosci. 21, RC132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Mo Z., Yang X., Price S. M., Shen M. M., Xiang M. (2004). Foxn4 controls the genesis of amacrine and horizontal cells by retinal progenitors. Neuron 43, 795–807 [DOI] [PubMed] [Google Scholar]
- Li S., Misra K., Matise M. P., Xiang M. (2005). Foxn4 acts synergistically with Mash1 to specify subtype identity of V2 interneurons in the spinal cord. Proc. Natl. Acad. Sci. USA 102, 10688–10693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Misra K., Xiang M. (2010). A Cre transgenic line for studying V2 neuronal lineages and functions in the spinal cord. Genesis 48, 667–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem K. F., Jr, Tremml G., Jessell T. M. (1997). A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell 91, 127–138 [DOI] [PubMed] [Google Scholar]
- Liem K. F., Jr, Jessell T. M., Briscoe J. (2000). Regulation of the neural patterning activity of sonic hedgehog by secreted BMP inhibitors expressed by notochord and somites. Development 127, 4855–4866 [DOI] [PubMed] [Google Scholar]
- Liu W., Khare S. L., Liang X., Peters M. A., Liu X., Cepko C. L., Xiang M. (2000). All Brn3 genes can promote retinal ganglion cell differentiation in the chick. Development 127, 3237–3247 [DOI] [PubMed] [Google Scholar]
- Liu Y., Helms A. W., Johnson J. E. (2004). Distinct activities of Msx1 and Msx3 in dorsal neural tube development. Development 131, 1017–1028 [DOI] [PubMed] [Google Scholar]
- Lo L., Dormand E., Greenwood A., Anderson D. J. (2002). Comparison of the generic neuronal differentiation and neuron subtype specification functions of mammalian achaete-scute and atonal homologs in cultured neural progenitor cells. Development 129, 1553–1567 [DOI] [PubMed] [Google Scholar]
- Luo H., Jin K., Xie Z., Qiu F., Li S., Zou M., Cai L., Hozumi K., Shima D. T., Xiang M. (2012). Forkhead box N4 (Foxn4) activates Dll4-Notch signaling to suppress photoreceptor cell fates of early retinal progenitors. Proc. Natl. Acad. Sci. USA 109, E553–E562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Fode C., Guillemot F., Anderson D. J. (1999). Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 13, 1717–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí E., Bumcrot D. A., Takada R., McMahon A. P. (1995). Requirement of 19K form of Sonic hedgehog for induction of distinct ventral cell types in CNS explants. Nature 375, 322–325 [DOI] [PubMed] [Google Scholar]
- Matise M. P., Wang H. (2011). Sonic hedgehog signaling in the developing CNS where it has been and where it is going. Curr. Top. Dev. Biol. 97, 75–117 [DOI] [PubMed] [Google Scholar]
- McMahon J. A., Takada S., Zimmerman L. B., Fan C. M., Harland R. M., McMahon A. P. (1998). Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 12, 1438–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megason S. G., McMahon A. P. (2002). A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development 129, 2087–2098 [DOI] [PubMed] [Google Scholar]
- Meyer N. P., Roelink H. (2003). The amino-terminal region of Gli3 antagonizes the Shh response and acts in dorsoventral fate specification in the developing spinal cord. Dev. Biol. 257, 343–355 [DOI] [PubMed] [Google Scholar]
- Misra K., Gui H., Matise M. P. (2008). Prox1 regulates a transitory state for interneuron neurogenesis in the spinal cord. Dev. Dyn. 237, 393–402 [DOI] [PubMed] [Google Scholar]
- Mizuguchi R., Kriks S., Cordes R., Gossler A., Ma Q., Goulding M. (2006). Ascl1 and Gsh1/2 control inhibitory and excitatory cell fate in spinal sensory interneurons. Nat. Neurosci. 9, 770–778 [DOI] [PubMed] [Google Scholar]
- Mo Z., Li S., Yang X., Xiang M. (2004). Role of the Barhl2 homeobox gene in the specification of glycinergic amacrine cells. Development 131, 1607–1618 [DOI] [PubMed] [Google Scholar]
- Müller T., Brohmann H., Pierani A., Heppenstall P. A., Lewin G. R., Jessell T. M., Birchmeier C. (2002). The homeodomain factor lbx1 distinguishes two major programs of neuronal differentiation in the dorsal spinal cord. Neuron 34, 551–562 [DOI] [PubMed] [Google Scholar]
- Muroyama Y., Fujihara M., Ikeya M., Kondoh H., Takada S. (2002). Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 16, 548–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada Y., Hunsaker T. L., Henke R. M., Johnson J. E. (2004). Distinct domains within Mash1 and Math1 are required for function in neuronal differentiation versus neuronal cell-type specification. Development 131, 1319–1330 [DOI] [PubMed] [Google Scholar]
- Nakao A., Afrakhte M., Morén A., Nakayama T., Christian J. L., Heuchel R., Itoh S., Kawabata M., Heldin N. E., Heldin C. H., et al. (1997). Identification of Smad7, a TGFbeta-inducible antagonist of TGF-β signalling. Nature 389, 631–635 [DOI] [PubMed] [Google Scholar]
- Novitch B. G., Wichterle H., Jessell T. M., Sockanathan S. (2003). A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron 40, 81–95 [DOI] [PubMed] [Google Scholar]
- Osório J., Mueller T., Rétaux S., Vernier P., Wullimann M. F. (2010). Phylotypic expression of the bHLH genes Neurogenin2, Neurod, and Mash1 in the mouse embryonic forebrain. J. Comp. Neurol. 518, 851–871 [DOI] [PubMed] [Google Scholar]
- Panayi H., Panayiotou E., Orford M., Genethliou N., Mean R., Lapathitis G., Li S., Xiang M., Kessaris N., Richardson W. D., et al. (2010). Sox1 is required for the specification of a novel p2-derived interneuron subtype in the mouse ventral spinal cord. J. Neurosci. 30, 12274–12280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parras C. M., Schuurmans C., Scardigli R., Kim J., Anderson D. J., Guillemot F. (2002). Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev. 16, 324–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten I., Placzek M. (2002). Opponent activities of Shh and BMP signaling during floor plate induction in vivo. Curr. Biol. 12, 47–52 [DOI] [PubMed] [Google Scholar]
- Peng C. Y., Yajima H., Burns C. E., Zon L. I., Sisodia S. S., Pfaff S. L., Sharma K. (2007). Notch and MAML signaling drives Scl-dependent interneuron diversity in the spinal cord. Neuron 53, 813–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell L. M., Jarman A. P. (2008). Context dependence of proneural bHLH proteins. Curr. Opin. Genet. Dev. 18, 411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones H. I., Savage T. K., Battiste J., Johnson J. E. (2010). Neurogenin 1 (Neurog1) expression in the ventral neural tube is mediated by a distinct enhancer and preferentially marks ventral interneuron lineages. Dev. Biol. 340, 283–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasal Y., Lu B., Steinbelsser H., De Robertis E. M. (1995). Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature 378, 419 [DOI] [PubMed] [Google Scholar]
- Sela-Donenfeld D., Kalcheim C. (2002). Localized BMP4-noggin interactions generate the dynamic patterning of noggin expression in somites. Dev. Biol. 246, 311–328 [DOI] [PubMed] [Google Scholar]
- Shimojo H., Ohtsuka T., Kageyama R. (2008). Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron 58, 52–64 [DOI] [PubMed] [Google Scholar]
- Smith W. C., Harland R. M. (1992). Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell 70, 829–840 [DOI] [PubMed] [Google Scholar]
- Sockanathan S., Jessell T. M. (1998). Motor neuron-derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell 94, 503–514 [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C. S., William C. M., Tanabe Y., Jessell T. M., Costantini F. (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimori M., Nagao M., Bertrand N., Parras C. M., Guillemot F., Nakafuku M. (2007). Combinatorial actions of patterning and HLH transcription factors in the spatiotemporal control of neurogenesis and gliogenesis in the developing spinal cord. Development 134, 1617–1629 [DOI] [PubMed] [Google Scholar]
- Sui G., Soohoo C., Affar B., Gay F., Shi Y., Forrester W. C., Shi Y. (2002). A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc. Natl. Acad. Sci. USA 99, 5515–5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer J. R., Wang C., Niswander L. (2002). BMP signaling patterns the dorsal and intermediate neural tube via regulation of homeobox and helix-loop-helix transcription factors. Development 129, 2459–2472 [DOI] [PubMed] [Google Scholar]
- Wang W., Chen X., Xu H., Lufkin T. (1996). Msx3: a novel murine homologue of the Drosophila msh homeobox gene restricted to the dorsal embryonic central nervous system. Mech. Dev. 58, 203–215 [DOI] [PubMed] [Google Scholar]
- Wildner H., Müller T., Cho S. H., Bröhl D., Cepko C. L., Guillemot F., Birchmeier C. (2006). dILA neurons in the dorsal spinal cord are the product of terminal and non-terminal asymmetric progenitor cell divisions, and require Mash1 for their development. Development 133, 2105–2113 [DOI] [PubMed] [Google Scholar]
- Wodarz A., Nusse R. (1998). Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14, 59–88 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Yamamoto M., Engel J. D. (2000). GATA2 is required for the generation of V2 interneurons. Development 127, 3829–3838 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.