Abstract
Background
Nemaline myopathy—the most common non-dystrophic congenital myopathy—is caused by mutations in thin filament genes, of which the nebulin gene is the most frequently affected one. The nebulin gene codes for the giant sarcomeric protein nebulin, which plays a crucial role in skeletal muscle contractile performance. Muscle weakness is a hallmark feature of nemaline myopathy patients with nebulin mutations, and is caused by changes in contractile protein function, including a lower calcium-sensitivity of force generation. To date no therapy exists to treat muscle weakness in nemaline myopathy. Here, we studied the ability of the novel fast skeletal muscle troponin activator, CK-2066260, to augment force generation at submaximal calcium levels in muscle cells from nemaline myopathy patients with nebulin mutations.
Methods
Contractile protein function was determined in permeabilised muscle cells isolated from frozen patient biopsies. The effect of 5 µM CK-2066260 on force production was assessed.
Results
Nebulin protein concentrations were severely reduced in muscle cells from these patients compared to controls, while myofibrillar ultrastructure was largely preserved. Both maximal active tension and the calcium-sensitivity of force generation were lower in patients compared to controls. Importantly, CK-2066260 greatly increased the calcium-sensitivity of force generation—without affecting the cooperativity of activation—in patients to levels that exceed those observed in untreated control muscle.
Conclusions
Fast skeletal troponin activation is a therapeutic mechanism to augment contractile protein function in nemaline myopathy patients with nebulin mutations and with other neuromuscular diseases.
INTRODUCTION
Nemaline myopathy (NM) is the most common non-dystrophic congenital myopathy (incidence ~1:50 000).1 Hallmark features of NM are muscle weakness and the presence of nemaline bodies in skeletal muscle fibres.2 To date, seven genes have been implicated in NM. Strikingly, six of these genes code for proteins of the skeletal muscle thin filament: α-tropomyosin-3 and β-tropomyosin (TPM3 and TPM2), nebulin (NEB), actin α 1 (ACTA1), troponin T type 1 (TNNT1), and cofilin-2 (CFL2). The seventh implicated gene, KBTBD13, was recently discovered and the function of its protein product is unknown.3
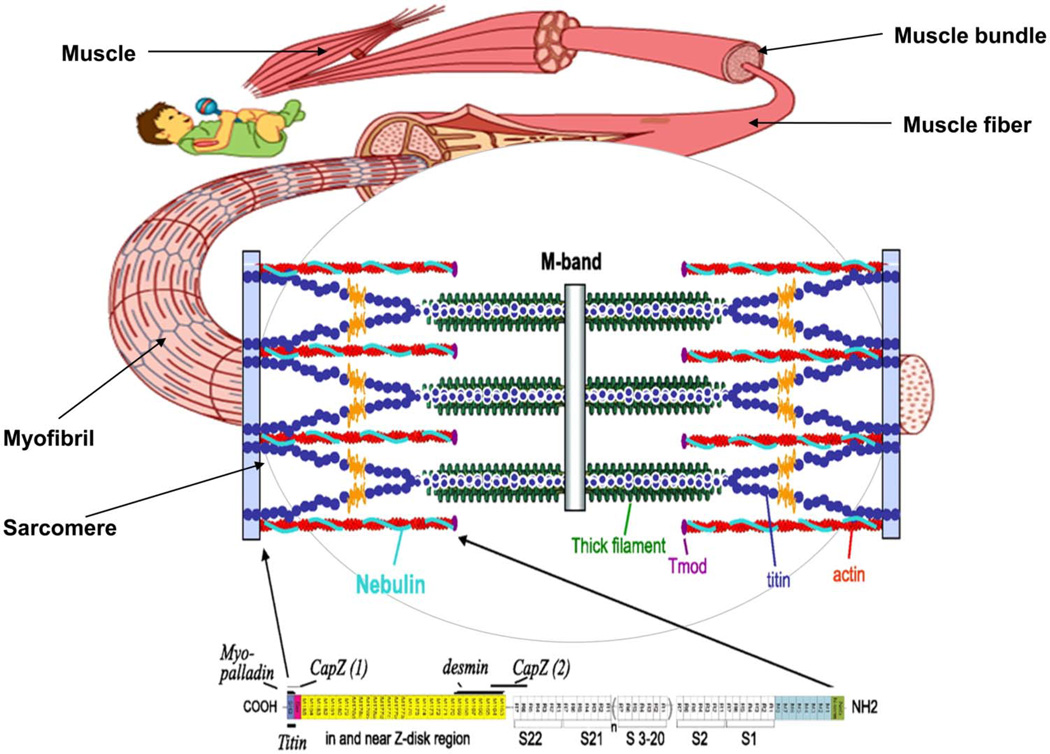
Mutations in the NEB gene are the most common cause of NM, likely accounting for >50% of NM cases.4 Nebulin is a giant sarcomeric protein (~800 kDa); its C-terminus is anchored in the Z-disk and its N-terminus is located close to the thin filament pointed end. Thus, a single nebulin molecule spans nearly the entire length of the thin filament (figure 1). Previous studies of a nebulin knockout mouse model showed that nebulin plays important roles in sarcomeric structure and contractile performance. Nebulin stabilises the thin filament and specifies its length,5–9 and evidence suggests that it also modulates both the kinetics of actomyosin cross bridge formation10,11 and the calcium-sensitivity of thin filament activation.11 Recent work from our group revealed that skeletal muscle fibres of NM patients with NEB mutations (NEB-NM) develop muscle weakness due to loss of these functions of nebulin; their myofibres contain thin filaments of shorter and non-uniform length, they show altered actomyosin cross bridge kinetics,12,13 and they have a lower calcium-sensitivity of force generation.13,14
Figure 1.
Schematic of nebulin’s location in the skeletal muscle sarcomere. A single nebulin molecule spans the thin filament with its C-terminus anchored in the Z-disk and its N-terminus located close to the thin filament pointed end. The highly modular central region (M9–M162) is divided into seven modular repeats that are arranged into 22 super-repeats.
To date, no therapy exists that enhances force generation in NEB-NM. Strategies to restore thin filament length or the kinetics of actomyosin interaction currently do not exist for skeletal muscle, and, in part due to the extremely large size of the nebulin gene and protein, effective genetic strategies to combat these effects are likely far off in the future. However, the lower calcium-sensitivity of force generation in NEB-NM might offer a more immediate therapeutic target, as recently a fast skeletal muscle troponin activator has been developed that amplifies the response of the thin filament to calcium in fast skeletal muscle fibres.15 The fast skeletal muscle troponin activator tirasemtiv (formerly CK-2017357) was shown to increase greatly the calcium-sensitivity of force generation in healthy rat skeletal muscle fibres,15 and is currently in phase II clinical studies for amyotrophic lateral sclerosis.16
The goal of our study was to test the ability of the novel fast skeletal troponin activator CK-2066260—a close structural analogue of tirasemtiv—to improve muscle cell strength in patients with NEB-NM. Replication of tirasemtiv pharmacology with a different molecule of the same mechanistic class (CK-2066260) strengthens the argument that the described effects are not specific to a single small molecule but instead reflect an effect related to the mechanism of action. Our findings indicate that CK-2066260 greatly increases the calcium-sensitivity of force generation in NEB-NM patients to levels that exceed those observed in untreated muscle cells from healthy controls. Thus, fast skeletal troponin activation is a therapeutic mechanism to augment muscle strength in NM patients with nebulin mutations and with other neuromuscular diseases.
METHODS
Muscle biopsies from NEB-NM patients
Quadriceps muscle specimens, remaining from diagnostic procedures or obtained during clinically indicated surgical procedures, were collected from four NM patients with confirmed NEB gene mutations, from four paediatric control subjects who were biopsied for other diagnostic purposes (with normal skeletal muscle histology), and from three adult control subjects with no medical history. The four biopsies from the NEB-NM patients and from the paediatric controls were collected following informed consent supervised by the Boston Children’s Hospital Institutional Review Board. The three adult control muscle biopsies were obtained under supervision of the Human Research Ethics Committee, Children’s Hospital at Westmead (CHW/10/45). All biopsies were stored frozen and unfixed at −80°C until use. See tables 1 and 2 for details on the clinical and genetic data of the subjects.
Table 1.
Clinical and pathological data of nemaline myopathy patients and characteristics of control subjects
| Patient ID |
Biopsy ID |
Gender | Biopsy location |
Age at biopsy |
Fibre typing | Rod characteristics | Clinical form |
Age of onset |
Maximal motor ability |
Clinical status |
|---|---|---|---|---|---|---|---|---|---|---|
| Nebulin based NM patients | ||||||||||
| 26–2 | T33 | Female | Quadriceps | 13 months | Extreme type 1 predominance | Numerous large rods in type 1 fibres | Intermediate | Birth | Sat, but never walked | Non-ambulant, requires ventilation and G-tube feedings at 13 years old |
| 258–2 | T1069 | Male | Quadriceps | 4 months | 60–70% type 1 fibres, most smaller than type 2s | Subsarcolemmal rods in all fibre types | Typical | Birth | Ambulatory since 18 months | Ambulatory at 5 years old, requires G-tube and BIPAP at night |
| 974–1 | T1033 | Male | Unspecified | 2 months | Hypotrophic type 1, no fibre type predominance | Rods predominantly in hypotrophic type 1 fibres | Intermediate | Birth | Rolling over | Unable to sit at 2 years old, requires G-tube, breathes independently |
| 988–1 | T887 | Male | Quadriceps | 2.5 months | Normal fibre type proportions with significant fibre size variation | Subsarcolemmal rods in all fibre types | Indeterminate | Birth | n/a | Severely hypotonic, requires G-tube and nocturnal ventilation at 5 months |
| Infant control subjects | ||||||||||
| 212–1 | T141 | Male | Quadriceps | 3 years | Normal | n/a | n/a | n/a | Normal | Unaffected |
| 213–1 | T142 | Female | Quadriceps | 2 years | Normal | n/a | n/a | n/a | Normal | Unaffected |
| 218–1 | T147 | Female | Quadriceps | 4 years | Normal | n/a | n/a | n/a | Normal | Unaffected |
| 219–1 | T148 | Female | Quadriceps | 5 years | Normal | n/a | n/a | n/a | Normal | Unaffected |
| Adult control subjects | ||||||||||
| n/a | C1 | Male | Quadriceps | 20 | Normal | n/a | n/a | n/a | Normal | Unaffected |
| n/a | C2 | Male | Quadriceps | 25 | Normal | n/a | n/a | n/a | Normal | Unaffected |
| n/a | C3 | Male | Quadriceps | 30 | Normal | n/a | n/a | n/a | Normal | Unaffected |
BIPAP, bi-level positive airways pressure; NM, nemaline myopathy
Table 2.
Genetic data of nemaline myopathy patients
| Patient ID | Biopsy ID | NEB mutations | Nebulin defects |
|---|---|---|---|
| 26–2 | T33 | c.[7431+1916_7536+372del]+[7431+1916_7536+372del] | p.[Arg2478_Asp2512del]+[Arg2478_Asp2512del] |
| 258–2 | T1069 | c.[3567+3_3567+7delAAGT]+[18124C>T] | Exon 33 splice defect+p.Gly6041Stop |
| 974–1 | T1033 | c.[7431+1916_7536+372del]+[24842_24841delAG] | p.[Arg2478_Asp2512del]+[Arg8280SerfsStop2] |
| 988–1 | T887 | c.[1152+1G>A]+[17013+1G>T] | Exon 13 splice defect+exon 107 splice defect |
Pt 26–2 is homozygous for del exon 55.
Pt 258–2 is compound heterozygous for exon 33 splice site mutation and exon 114 nonsense mutation.
Pt 974–1 is compound heterozygous for del exon 55 and 2 bp deletion in exon 177.
Pt 988–1 is compound heterozygous for two splice site mutations in intron 13 and 107.
Reference sequences are NM_001164507 and NP_004534.2.
Nebulin protein values
To assess nebulin protein concentrations, muscle samples were homogenised and analysed on 1% agarose electrophoresis gels, as previously described.17 To prevent protein degradation, all buffers contained protease inhibitors (phenylmethylsulfonyl fluoride (PMSF), 0.5 mM; leupeptin, 0.04 mM; E64, 0.01 mM). Gels were scanned and analysed with One-D scan EX (Scanalytics Inc, Rockville, Maryland, USA) software. The integrated optical density of nebulin and myosin heavy chain (MHC) was determined. For western blot analysis, one- or two-colour infrared western blots were scanned (Odyssey Infrared Imaging System, Li-Cor Biosciences, Nebraska, USA) and the images analysed with One-D scan EX.
Pathological evaluation
For electron microscopy, samples were fixed and processed per standard histological techniques for either routine histochemical staining or ultrastructural examination at the time of biopsy, and all slides and ultrastructural images were reviewed by a neuropathologist (MWL).
Muscle mechanics
From the frozen samples, muscle fibre bundles were isolated as described previously.14 In brief, from the biopsies smaller sections (2×2 mm) were isolated in liquid nitrogen. Subsequently, they were placed for 24 h at −20°C in 4 ml 50% glycerol/relaxing solution containing high concentrations of protease inhibitors (PMSF 0.5 mM, leupeptin 0.04 mM, E64 0.01 mM). Subsequently, the sections were placed on a ‘roller band’ for 24 h at 4°C, followed by submersing them in skinning solution for 24 h at 4°C. Finally, the skinning solution was substituted with a glycerol/relaxing solution with lower concentrations of protease inhibitors and stored at −20° C until further use. On the day of an experiment, small strips (cross sectional area (CSA) ~0.07 mm2) were dissected from the glycerinated sections and washed thoroughly with relaxing solution. The strips were mounted using aluminium T clips between a length motor (ASI 403A, Aurora Scientific Inc, Ontario, Canada) and a force transducer element (ASI 315C-I, Aurora Scientific Inc) in a skinned fibre apparatus (ASI 802D, Aurora Scientific Inc) that was mounted on an inverted microscope (Zeiss Axio Observer A1). Sarcomere length was set using a high speed VSL camera and ASI 900B software (Aurora Scientific Inc). Mechanical experiments were performed at a sarcomere length of ~2.1 µm, a length selected to minimise force differences due to shorter thin filaments in fibres from NEB-NM patients,14 and at 2.6 µm to study the effect of sarcomere length on activation. Fibre width and diameter were measured at three points along the fibre and the CSA was determined assuming an elliptical cross section. Fibre dimensions were determined using an inverted microscope and a custom made prism that was mounted in the bath. The dimensions of the muscle strips were measured using a 40× objective and a high speed VSL camera with calibrated ASI 900B software (Aurora Scientific Inc).
Three different types of bathing solutions were used during the experimental protocols: a relaxing solution (40 mM BES; 10 mM; 6.86 mM MgCl2; 5.96 mM Na-ATP; 3.28 mM K-propionate; 33 mM creatine phosphate; 10 mM EGTA DTT; 0.5 mM PMSF; 0.2 mM Leupeptin; 0.05 mM E64; 200 U/ml creatine phosphokinase), a pre-activating solution with low EGTA concentration (40 mM BES; 1 mM EGTA; 6.66 mM MgCl2; 5.98mMNa-ATP; 30.44 mM K-propionate; 33 mM creatine phosphate; 1 mM DTT (dithiothreitol); 0.5 mM PMSF; 0.2 mM leupeptin; 0.05 mM E64; 200 U/ml creatine phosphokinase), and an activating solution (40 mM BES; 10 mM CaCO3-EGTA; 6.64 mM MgCl2; 6.23mM Na-ATP; 2.1 mM K-propionate; 15 mM creatine phosphate; 1 mM DTT; 0.5 mM PMSF; 0.2 mM leupeptin; 0.05 mM E64; 200 U/ml creatine phosphokinase). The temperature of the bathing solutions was kept constant at 15°C using a temperature controller (ASI 825A, Aurora Scientific Inc).
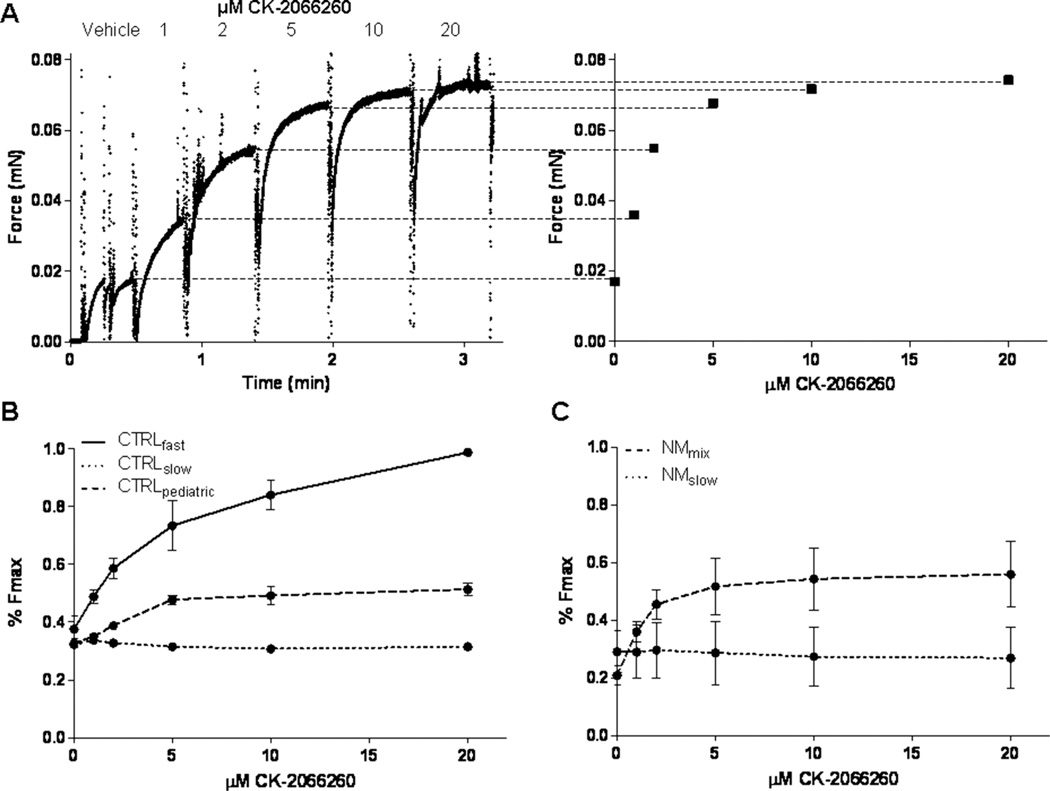
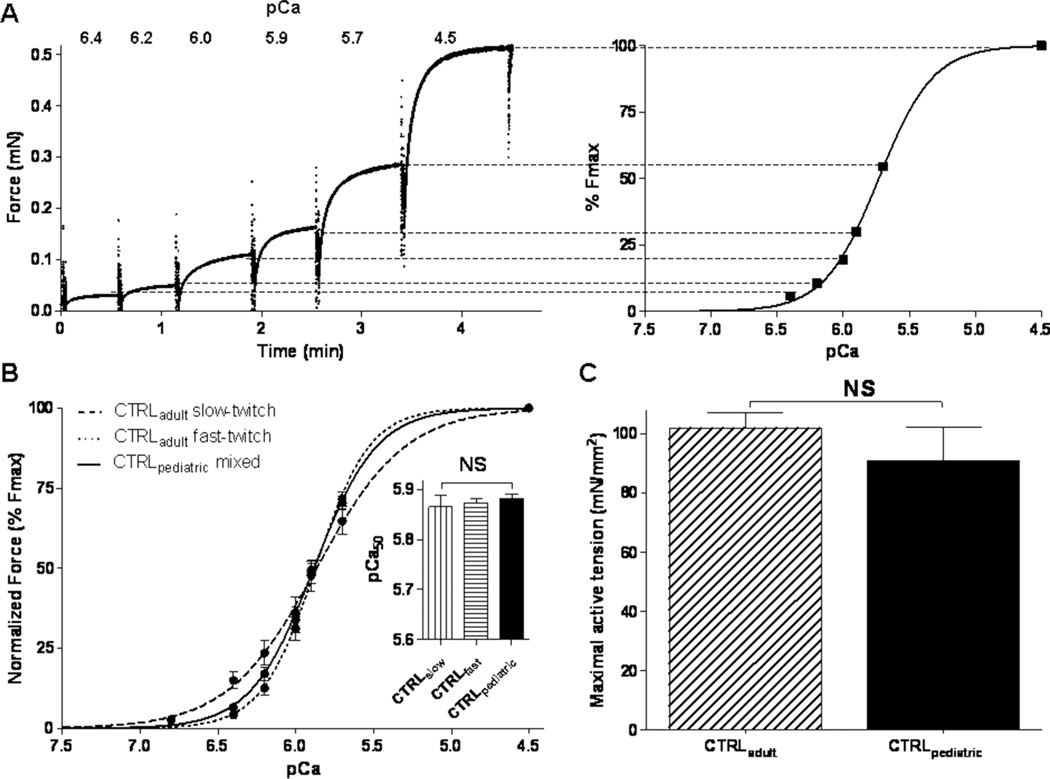
To determine a dose–response curve for the fast skeletal troponin activator CK-2066260 in muscle fibres from controls and from NEB-NM patients, tissue was exposed to pCa solutions of 6.0—the pCa that yielded about 35% of maximal active tension—with increasing concentrations (1, 2, 5, 10 and 20 µM) of CK-2066260 dissolved in 1% dimethylsulfoxide (DMSO) as vehicle. Note that 1% DMSO did not affect muscle fibre contractility (data not shown). Figures 5B,C show that CK-2066260 enhanced submaximal force generation with a maximal effect at 5 µM, which is in accordance with previously reported dose–response curves of its analogue CK-2017357.15 Accordingly, experiments were conducted with either CK-2066260 (5 µM) or vehicle alone (1% DMSO). To determine the force–pCa relation (pCa=−log of molar free Ca2+ concentration), skinned muscle fibre bundles were sequentially bathed in solutions with pCa values ranging from 4.5 to 9.0 and the steady state force was measured (figure 3A). Measured force values were normalised to the maximal force obtained at pCa 4.5. The obtained force–pCa data were fitted to the Hill equation, providing the pCa50 and the Hill coefficient, nH, an index of myofilament cooperativity.
Figure 5.
Dose–response relationship of CK-2066260 in skinned muscle preparations. (A) Example of a force trace from a skinned muscle bundle that is sequentially exposed to incremental CK-2066260 concentrations. (B) Dose–response relation of CK-2066260 in fast twitch and slow twitch CTRLadult single fibres and CTRLpaediatric bundles with a mixed myosin heavy chain (MHC) composition. (C) Dose–response relationship of CK-2066260 in predominantly slow twitch (<12% fast twitch) and NEB-NM (nebulin nemaline myopathy) bundles with a mixed MHC composition. Vehicle (1% dimethylsulfoxide) was present in all experiments. CTRLadult, adult controls; CTRLpaediatric, paediatric controls; NM, nemaline myopathy.
Figure 3.
Maximal active tension and calcium-sensitivity of force generation in skinned muscle fibres from adult and paediatric controls. (A) Example of a force trace from a skinned muscle preparation that is sequentially exposed to incremental Ca2+ concentrations (here expressed as pCa: −log of molar free Ca2+ concentration). (B) No significant differences in calcium-sensitivity of force generation (reflected by the pCa50 value) were found between skeletal muscle bundles from CTRLpaediatric and both slow twitch and fast twitch single fibres from CTRLadult. (C) No significant difference in maximal active tension was found between skeletal muscle fibres from adult and paediatric controls. CTRLadult, adult controls; CTRLpaediatric, paediatric controls.
MHC composition of bundles used for contractility experiments
For determination of the MHC isoform composition of the muscle fibre preparations we used specialised SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis).14 In brief, muscles fibres were denatured by boiling for 2 min in SDS sample buffer. The stacking gel contained a 4% acrylamide concentration (pH 6.7), and the separating gel contained 7% acrylamide (pH 8.7) with 30% glycerol (v/v). The gels were run for 24 h at 15°C and a constant voltage of 275 V. Finally, the gels were silver stained, scanned, and analysed with One-D scan EX (Scanalytics Inc, Rockville, Maryland, USA) software.
Statistical analyses
Data are presented as mean±SEM. For statistical analyses, t tests and two way analysis of variance (ANOVA) were used as appropriate. A value of p<0.05 was considered to be statistically significant.
RESULTS
Muscle fibres of NEB-NM patients have lower nebulin protein concentrations, while myofibrillar ultrastructure is preserved
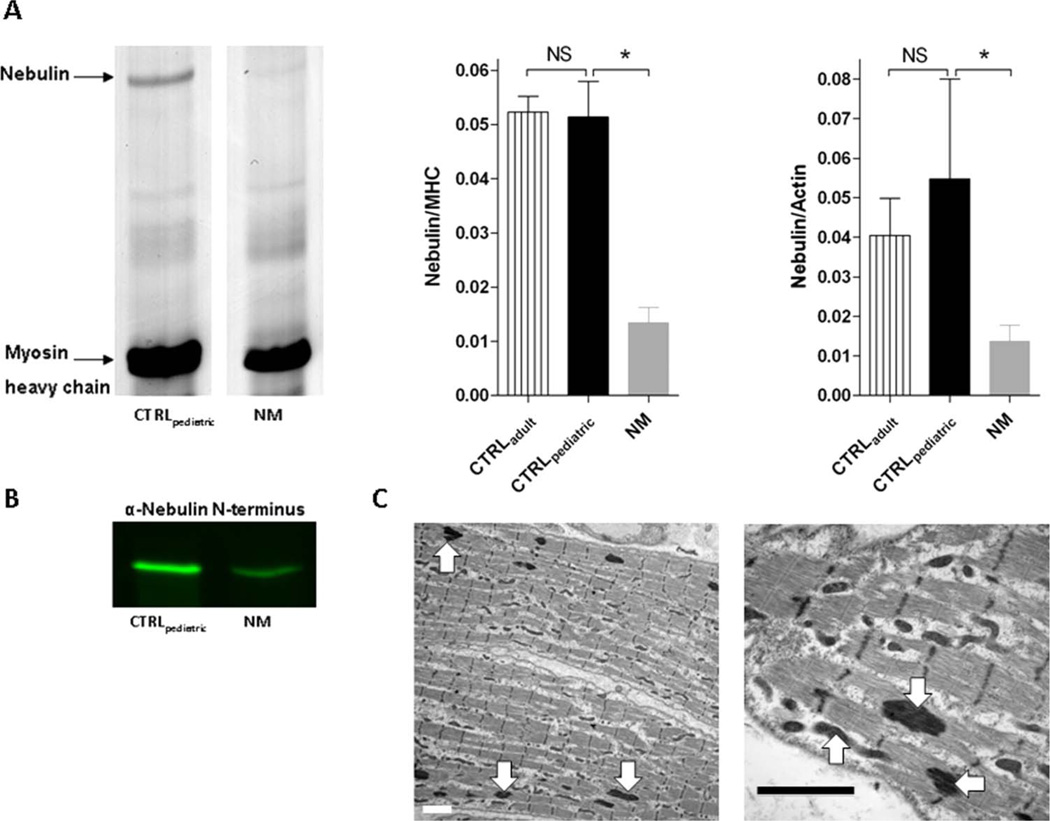
To study the effect of mutations in NEB on its protein product, nebulin protein concentrations were determined in quadriceps muscle of paediatric NEB-NM patients and these were compared to those in quadriceps muscle from controls. For all muscle samples, 1% agarose gels showed a single band at the appropriate molecular weight (~800 kDa) of nebulin (figure 2A), which was confirmed to be nebulin by western blot studies using an antibody against nebulin’s N-terminus (figure 2B). Quantitative densitometry revealed that both the nebulin/MHC ratio and the nebulin/actin ratio in muscle from paediatric controls (CTRLpaediatric; 0.051±0.007 for nebulin/MHC and 0.055±0.025 for nebulin/actin, respectively) was not significantly different from the ratio found in adult controls (CTRLadult; 0.052±0.003 for nebulin/MHC and 0.040±0.009 for nebulin/actin, respectively). As expected from previous studies,12–14 muscle from NEB-NM patients exhibited a significantly lower nebulin/MHC ratio (0.013±0.003) and a lower nebulin/actin ratio (0.014±0.004) compared to muscle from CTRLpaediatric and CTRLadult (figure 2A), indicating that the molecular basis for contractile deficits in muscle fibres from NEB-NM patients is related to a relative deficiency of nebulin protein.
Figure 2.
Nebulin protein values and myofibrillar structure in muscle fibres from nebulin nemaline myopathy (NEB-NM) patients. (A) Protein gel example of muscle sample from CTRLpaediatric and NEB-NM patients (left). Nebulin protein values normalised over myosin heavy chain values were not significantly different between CTRLpaediatric and CTRLadult, and were significantly lower in muscle from NEBNM patients (middle). Nebulin protein values normalised over actin protein values were not significantly different between CTRLpaediatric and CTRLadult, and were significantly lower in muscle from NEB-NM patients (right). (B) Western blot studies using an antibody against nebulin’s N-terminus revealed one single band at the appropriate molecular weight (~800 kDa) of nebulin; also note the lower intensity of the nebulin band in the NEB-NM patient, suggesting nebulin deficiency. (C) Electron microscopy of NEB-NM patient revealed the presence of electron dense nemaline rods in muscle fibres (arrows), surrounded by areas of appropriate myofibrillar organisation. Bar=2 µm. CTRLpaediatric, paediatric controls; NM, nemaline myopathy.
Electron micrographs from NEB-NM patient muscle biopsies were used to evaluate myofibrillar structure. The organisation of the contractile apparatus was normal in patients with NEB-NM (figure 2C), with the exception of areas containing nemaline rods. Thus, despite the lower nebulin protein concentrations found in muscle from NEB-NM patients, their myofibrillar ultrastructure was largely preserved.
The contractile performance of skinned muscle fibres from paediatric controls resembles that of adult controls
Skeletal muscle fibres from NEB-NM patients exhibit shorter and non-uniform thin filament lengths, and therefore produce maximal active tension at a sarcomere length of ~2.1 µm.14 To minimise potential force differences due to shorter thin filaments in NEB-NM, most of our studies were carried out at a sarcomere length of 2.1 µm in both control and NEB-NM fibres.
Previous work compared contractility of skeletal muscle fibres from paediatric NEB-NM patients to that of fibres from adult controls. In addition to adult controls, in the present study we also included a paediatric control group to evaluate the potential confounding effect of age differences on maximal active tension and calcium-sensitivity of force generation.
Figure 3A shows a typical force response of a muscle fibre to various calcium concentrations. From the force–pCa curves, the pCa50—the calcium concentration that produces 50% of maximal force—was determined. The pCa50 of quadriceps fibre bundles from paediatric controls was not significantly different from that of slow twitch or fast twitch single fibres from adult controls (5.87±0.02 for CTRLslow (n=6), 5.87±0.01 for CTRLfast (n=23), and 5.88±0.01 for CTRLpaediatric (n=15), respectively, figure 3B). As the force–pCa curves indicate, the cooperativity of activation (nH) in slow twitch (1.52±0.16, n=6) was lower than fast twitch (2.50±0.16, n=23) CTRLadult fibres. Accordingly, the cooperativity of activation in CTRLpaediatric bundles (nH: 2.14±0.09, n=15) was intermediate, but not significantly different from that of CTRLslow and CTRLfast, reflecting the mix of slow and fast twitch fibres in the CTRLpaediatric bundles.
Maximal active tension was 92.5±9.6 mN/mm2 in slow twitch CTRLadult fibres (n=6), 107.1±6.4 mN/mm2 in fast twitch CTRLadult fibres (n=23), and 91.0±11.0 mN/mm2 in CTRLpaediatric bundles (n=15) that contained a mix of slow and fast twitch muscle fibres (figure 3C). Thus, maximal active tension of muscle fibres from paediatric controls was not significantly different from that of adult controls.
These data indicate that the maximal active tension and the calcium-sensitivity of force generation of skinned quadriceps fibres from paediatric controls are comparable to that of adult controls. As it is most ideal to compare data from paediatric NEB-NM patients to data obtained from paediatric controls, we limit our further comparisons to these two groups.
The calcium-sensitivity of force generation is lower in skinned muscle fibres from NEB-NM patients
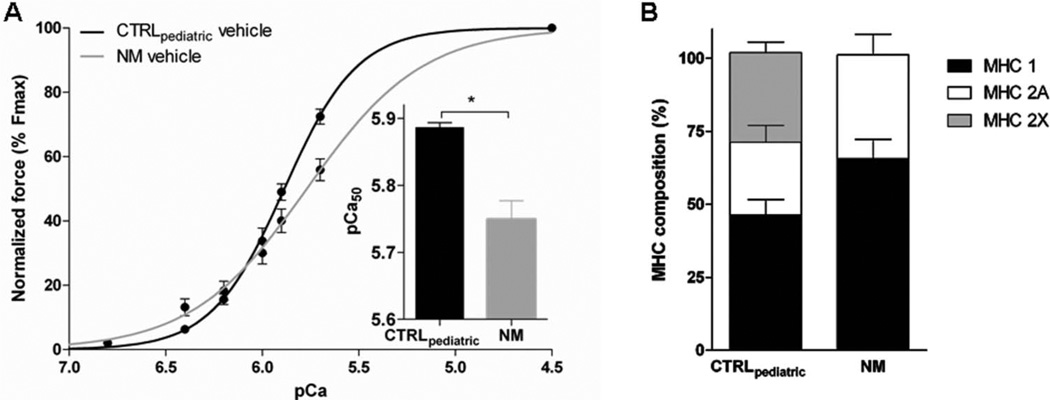
The calcium-sensitivity of force generation, as reflected by the pCa50, was lower in quadriceps fibres from NEB-NM patients (5.77±0.02, n=20) compared to CTRLpaediatric (5.88±0.01, n=15). As shown in figure 4A, the slope of the force–pCa relation was less steep in NEB-NM patients compared to CTRLpaediatric (nH: 1.43±0.12 in NEB-NM (n=20) vs 2.14 ±0.09 in CTRLpaediatric (n=15)), indicating that the cooperativity of activation was lower in nebulin deficient muscle. Maximal active tension was also lower in muscle fibres from NEB-NM patients compared to CTRLpaediatric fibres (15.4±3.5 mN/mm2, n=20 vs 91.0±11.0 mN/mm2, n=15, respectively). Thus, the calcium-sensitivity of force generation and maximal active tension were lower in muscle fibres from NEB-NM patients compared to those from control subjects.
Figure 4.
The calcium-sensitivity of force generation in skinned muscle fibres from nebulin nemaline myopathy (NEB-NM) patients and from paediatric controls (CTRLpaediatric). (A) Calcium-sensitivity of force generation was lower in NEB-NM bundles compared to CTRLpaediatric bundles (p<0.05). (B) Myosin heavy chain (MHC) composition analyses revealed a trend to more MHC 1 in NEB-NM bundles compared to CTRLpaediatric bundles (p=0.06). In addition, bundles from NEB-NM patients lack the MHC 2× isoform.
Since the contractile performance of muscle bundles may be affected by its fibre type composition, we used SDS-PAGE to determine the MHC isoforms of the bundles used for contractile measurements. CTRLpaediatric bundles were composed of 46.4 ±5.2% MHC 1, 24.8±5.8% MHC 2A, and 30.7±3.6% MHC 2× (figure 4B). Bundles from NEB-NM patients had 63.7±6.7% MHC 1 and 35.6±7.1% MHC 2A. MHC 2× was not observed in bundles from NEB-NM patients. The difference in MHC 1 between CTRLpaediatric and NEB-NM patients had a p value of 0.06 suggesting that there is a trend towards a higher percentage of MHC 1. This skewing to more oxidative fibre types is not surprising, as an increased number of oxidative fibres is often noted in muscle biopsies from patients with NM.18
The effect of the fast skeletal muscle troponin activator CK-2066260 on the calcium-sensitivity of force generation
Dose– response relationship
Since troponin activators increase the calcium-sensitivity of force generation, we set out to determine whether the troponin activator CK-2066260 might improve the contractile properties of nebulin deficient muscle. To determine the concentration of CK-2066260 at which it exerts its maximal effect, we first established dose–response curves for the different muscle fibre preparations relating the concentration of drugs to the percentage of maximal force (figure 5). For this purpose, muscle fibres were activated at pCa 6.0—this pCa was chosen because it yielded ~35% of maximal force—in the presence of incremental CK-2066260 concentrations. For a typical response of a fibre to incremental concentrations of CK-2066260, see figure 2A.
Since the bundles of both the NEB-NM patients and CTRLpaediatric specimens contained a mix of both slow twitch and fast twitch fibres, and because we were also interested in the compound’s effect on pure slow twitch and fast twitch fibres, we also tested individual slow and fast twitch fibres from CTRLadult. Slow twitch single fibres from CTRLadult (n=2) and NEB-NM patient bundles composed of predominantly (>90%) slow twitch fibres (n=2) did not respond to CK-2066260 at any of the concentrations tested (figure 5B,C). In fast twitch single fibres from adult CTRLadult (n=2), mixed CTRLpaediatric bundles (n=2), and mixed NEB-NM patient bundles (n=3), force enhancement was near maximal at 5 µM CK-2066260 (figure 2B,C). Therefore, a concentration of 5 µM CK-2066260 was used to investigate the effect of the compound on contractile performance of skinned muscle fibres.
Calcium-sensitivity of force generation
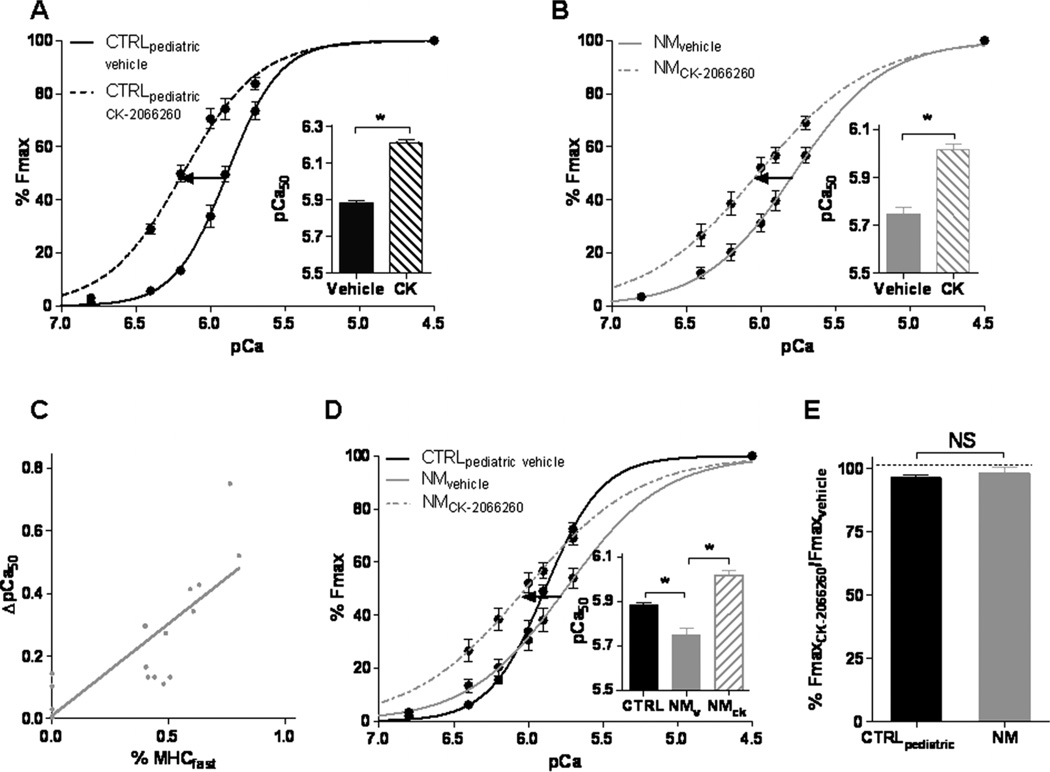
The presence of 5 µM CK-2066260 induced a leftward shift in the force–pCa relation in CTRLpaediatric, as reflected by the increase of the pCa50 value from 5.88±0.01 (n=6) in vehicle only to 6.18±0.01 (n=6) in CK-2066260 (figure 6A). The cooperativity of activation was lower in the presence of 5 µM CK-2066260 (nH 1.70±0.11 vs 2.47±0.13 in vehicle (n=6)). Importantly, in NEB-NM patient bundles a notable leftward shift in the force–pCa relation was observed in the presence of CK-2066260. The pCa50 increased from 5.78±0.04 to 6.02±0.02 (n=19), without affecting nH (1.15±0.11 vs 1.33±0.14 (n=19) in vehicle) (figure 6B). The magnitude of increase in pCa50 induced by CK-2066260 was not significantly different in CTRLpaediatric fibres (0.26±0.04 pCa units) compared to nebulin deficient NM fibres (0.24±0.05 pCa units). However, when taking into account that the fibre bundles from NEB-NM patients contained a higher percentage of non-responsive fibres expressing MHC 1 (figure 4B), the effect size of the compound in fibres expressing MHC 2 might be larger in NEB-NM patient fibres. Indeed, a significant correlation was observed between the percentage of fast twitch fibres in the fibre bundles from NEB-NM patients and the magnitude of increase in pCa50 upon 5 µM CK-2066260 administration (figure 6C, Pearson r=0.79).
Figure 6.
Effect of CK-2066260 on calcium-sensitivity of force generation in skinned muscle bundles at a sarcomere length of 2.1 µm. (A) CK-2066260 (5 µM) increased the calcium-sensitivity of force generation (reflected by the pCa50 value) in muscle fibres from CTRLpaediatric (p<0.05). (B) 5 µM CK-2066260 increased calcium-sensitivity of force generation in muscle fibres from nebulin nemaline myopathy (NEB-NM) patients (p<0.05). (C) The CK-2066260 induced shift on the pCa50 value was correlated to the NM bundle’s MHCfast content (Pearson r=0.79). (D) In the presence of 5 µM CK-2066260, the pCa50 of NEB-NM patients exceeded that of normal CTRLpaediatric values. (E) No significant effect of CK-2066260 on maximal active tension in muscle fibres from CTRLpaediatric and NEB-NM patients was observed. CTRLpaediatric, paediatric controls; MHC, myosin heavy chain; NM, nemaline myopathy.
Comparing skeletal muscle fibres of NEB-NM patients treated with 5 µM CK-2066260 to untreated CTRLpaediatric fibres reveals that the fast troponin activator enhances calcium-sensitivity of force generation in NEB-NM patients above normal control values (figure 6D). We also investigated whether the maximal active tension was affected by CK-2066260. FmaxCK-2066260 expressed as percentage of Fmaxvehicle was 96.5±1.2% (n=10) in CTRLpaediatric and 98.3±2.2% (n=18) in NEB-NM patients (figure 6E), indicating that CK-2066260 did not affect maximal active tension. Thus, the effect of CK-2066260 is that it completely restores the impaired calcium-sensitivity of force generation in skeletal muscle fibres from NEB-NM patients.
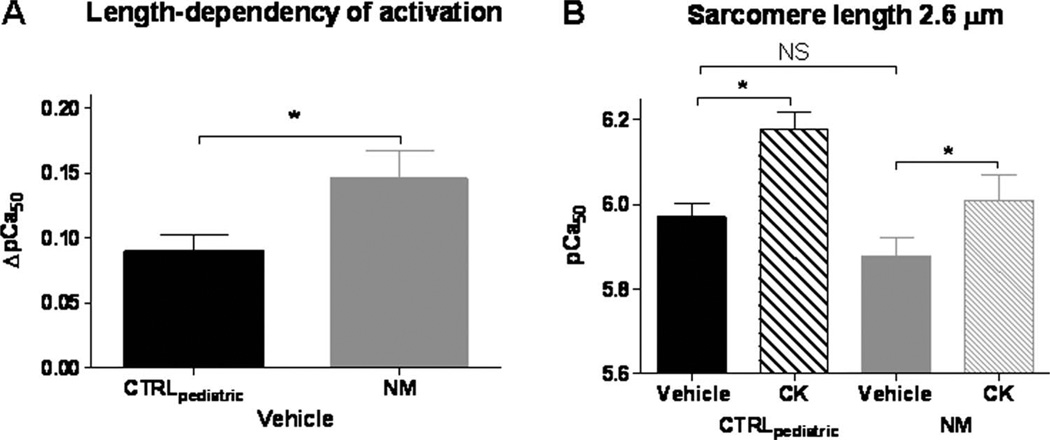
The experiments described above were performed at a sarcomere length of 2.1 µm. Because skeletal muscle operates at a range of sarcomere lengths, we also studied a longer sarcomere length (2.6 µm). The calcium-sensitivity of force generation increases when sarcomere length increases (figure 7A), a well known phenomenon called length dependency of activation. Interestingly, this length dependency of activation (expressed as ΔpCa50: the increase in pCa50 at a sarcomere length of 2.6 µm compared to that at 2.1 µm) is higher in NEB-NM patients (ΔpCa50: 0.15±0.02, n=11) compared to CTRLpaediatric (ΔpCa50: 0.090±0.01, n=10) (figure 7A). Due to this more prominent length dependency of activation in fibres from NEB-NM patients, the calcium-sensitivity of force generation in these patients (5.88±0.04) was not significantly different from CTRLpaediatric (5.97±0.03) (see figure 7B) at a sarcomere length of 2.6 µm. As shown in figure 7B, also at a sarcomere length of 2.6 µm, CK-2066260 induced a pronounced increase in the calcium-sensitivity of force generation in skinned fibre bundles from both CTRLpaediatric (pCa50 increased from 5.97±0.03 in vehicle to 6.18±0.04 in CK-2066260) and NEB-NM patients (pCa50 increased from 5.88±0.04 to 6.01±0.06).
Figure 7.
The calcium-sensitivity of force generation and the effect of CK-2066260 at a sarcomere length of 2.6 µm. (A) Length dependency of activation (expressed as ΔpCa50: the increase in pCa50 at a sarcomere length of 2.6 µm compared to that at 2.1 µm) was more prominent in nebulin nemaline myopathy (NEB-NM) patients compared to CTRLpaediatric (p<0.05). (B) At a sarcomere length of 2.6 µm, 5 µM CK-2066260 increased the pCa50 significantly in both muscle fibres from CTRLpaediatric and NEB-NM patients (p<0.05). At this longer sarcomere length, no significant difference in pCa50 between CTRLpaediatric fibres and NEB-NM fibres was observed. CTRLpaediatric, paediatric controls; NM, nemaline myopathy.
For an overview of the pCa50 data and for a detailed description of the number of preparations used for each parameters see table 1 of the supplemental data.
DISCUSSION
As lower calcium-sensitivity of force generation contributes to weakness of NEB-NM muscle, we studied the ability of the novel fast skeletal muscle troponin activator, CK-2066260, to augment force generation at submaximal levels of activation in skeletal muscle fibres from NEB-NM patients. CK-2066260 greatly increases the calcium-sensitivity of force generation in fast twitch fibres at both sarcomere lengths that were studied to levels that are similar (sarcomere length 2.6 µm) or exceed (sarcomere length 2.1 µm) those observed in control subjects. Thus, fast skeletal troponin activation might be a therapeutic mechanism to augment muscle strength in NEB-NM patients.
Force generation is lower in muscle fibres from NEB-NM patients compared to those from controls
The severe reductions in force levels generated by fibres of NM patients are clearly due to contractile deficits caused by defects in sarcomeric proteins,18 rather than lower neural activation, ineffective excitation contraction coupling, or other non-contractile defects. Mechanisms that have been shown to contribute to muscle weakness in NEB-NM include myofibrillar disarray,14,19 shorter thin filaments,14 altered cross bridge cycling kinetics,12,13 and a reduced calcium-sensitivity of force generation.13
Previous studies indicated that the calcium-sensitivity of force generation is lower in muscle fibres from paediatric NEB-NM patients compared to fibres from controls.13 Because control subjects in prior studies were adults, rather than age matched paediatric control subjects (due to the lack of availability of the latter), it could not be ruled out that the age differences between groups confounded the results. Here, we overcame this limitation by including a control group of paediatric patients, with an average age that was close to that of the paediatric NEB-NM patients (table 1), and who were biopsied during investigation of diseases that turned out to be nonneuromuscular in basis and who had histologically normal muscle. Importantly, the contractile performance of muscle fibres from these paediatric controls closely resembles that of adult controls (figure 3B,C), thereby validating our previous work in which paediatric NEB-NM patients were compared to adult controls. The NEB-NM patients that we investigated in the present study have NEB mutations that are distinct from those in our previous work,13 but their fibres exhibited similar reductions in calcium-sensitivity of force generation (figure 4A), confirming the findings of our previous studies,13 and suggesting that lower calcium-sensitivity of force generation is a general characteristic in NEB-NM patients.
The mechanisms underlying the lower calcium-sensitivity of force generation in NEB-NM patients are unclear, but it seems likely that they are a direct consequence of the severely low nebulin protein concentrations that we observed here (figure 2A) and that were also found in our previous work.12,13 This concept is supported by studies of a nebulin deficient mouse model, which revealed that nebulin augments the calcium-sensitivity of force generation,11 presumably through its interaction with other regulatory proteins such as tropomyosin and troponin or as a consequence of nebulin’s effect on cross bridge cycling kinetics.20–22 Accordingly, a recent study of muscle fibres from an NEB-NM patient with only slightly reduced nebulin protein concentrations showed no change in calcium-sensitivity of force generation.23
A novel aspect of the present study was our examination of the length dependency of activation through determination of the calcium-sensitivity of force generation at sarcomere lengths of both 2.1 µm and 2.6 µm. Interestingly, at a sarcomere length of 2.6 µm the calcium-sensitivity of force generation in muscle fibres from NEB-NM patients was not significantly lower than in those from CTRLpaediatric. Hence, the reduced calcium-sensitivity of force generation contributes to weakness in NEB-NM patients mainly when muscles operate at short lengths. The absence of a difference in calcium-sensitivity of force generation at the longer sarcomere length is explained by the exaggerated length dependency of activation in nebulin deficient fibres from NM patients (as shown in figure 7A). These findings are consistent with previous studies on nebulin deficient mouse muscle, which also revealed a lower calcium-sensitivity of force generation compared to that of wild type muscle fibres at a sarcomere length of 2.1 µm,11 but no difference at a longer sarcomere length.8,10 Both these findings and our results imply that nebulin plays a role in the length dependency of activation.20 This length dependency of activation is most prominent in (nebulin-free) cardiac muscle, where it underlies the Frank–Starling law of the heart, but is much less pronounced in skeletal muscle.24 The presence of nebulin provides an explanation for why activation in skeletal muscle possesses less length dependency: nebulin increases calcium-sensitivity of force generation at a short sarcomere length. Thus, nebulin deficient muscle from NM patients lacks this feature provided by nebulin, leading to reduced calcium-sensitivity of force generation at short sarcomere lengths.
The novel fast skeletal muscle specific troponin activator CK-2066260: clinical implications for NEB-NM patients
To date, no effective therapy is available for NEB-NM patients. In the present work, we studied the effect of targeting the lower calcium-sensitivity of force generation in NEB-NM muscle fibres by using an analogue of a recently developed compound that amplifies the response of the thin filament to calcium in fast skeletal muscle fibres.15 The compound that we used structurally and functionally resembles the fast skeletal muscle troponin activator, tirasemtiv (formerly CK-2017357).16 This troponin activator was shown to greatly enhance calcium-sensitivity of force generation in healthy rat and human skeletal muscle fibres15 and is currently being studied as therapy for amyotrophic lateral sclerosis.16 Tirasemtiv slows the dissociation rate of calcium from the troponin complex,15 thereby stabilising the open conformation of the troponin/tropomyosin complex to enhance cross-bridge formation at a given calcium concentration. The analogue CK-2066260 is a close structural analogue of tirasemtiv and has a similar mechanism of action. Like CK-2017357, CK-2066260 is selective for the fast skeletal troponin complex and has little effect on slow skeletal muscle (figure 5B,C). In the present study, we observed that CK-2066260 greatly increased the calcium-sensitivity of force generation—without affecting nH—in skeletal muscle fibres from NEB-NM patients to levels that exceed those observed in untreated control muscle (figure 6D).
Other troponin activators, such as levosimendan25 and EMD 57033,26,27 were shown in the past to also increase the calcium-sensitivity of force generation in human skeletal muscle fibres. However, a disadvantage of these agents is that, since they are cardiac/slow skeletal specific, they also affect cardiac function. Increasing the calcium-sensitivity of force generation in cardiac muscle might slow ventricular relaxation which compromises ventricular filling and might cause diastolic dysfunction. Thus, troponin activators that are specific for fast twitch muscle fibres—such as CK-2066260—are appealing compounds to ameliorate skeletal muscle weakness in skeletal muscle specific myopathies such as NM.
Considering that the normal activation level of muscle is submaximal— between 10% and 65% of its maximal force28—during normal activity, the potential of troponin activators for the treatment of muscle weakness in NM patients is high. Our findings reveal that CK-2066260 increases force generation of muscle fibres from NEB-NM patients by 20–140% (figure 6B) at the tested submaximal levels of activation. Thus, although the muscle fibres from patients have a diminished capacity to produce maximal force and CK-2066260 did not fully restore maximal muscle fibre force back to control values, the substantial increase in force at submaximal muscle activation produced by CK-2066260 may still improve quality of life in these patients. The benefit from troponin activators involves both increased force development and increased efficiency by reducing the amount of cytosolic calcium that is required to generate a given level of force. Considering that the energy utilisation of the calcium pump SERCA (sarcoendoplasmic reticulum calcium transport ATPase) accounts for 30–40% of total ATP consumption during muscle contraction,29 the use of a troponin activator has the potential to reduce the amounts of calcium that cycle each contraction, thereby reducing muscle fatigue, which is especially important for the respiratory muscles in patients with NM. Note that respiratory failure, due to diaphragm weakness, is the main cause of death in neonates and children with NM.2 In support of this potential role for troponin activators in attenuating respiratory failure, a recent study with a cardiac/ slow skeletal specific agent confirmed an improved neuromechanical efficiency and reduced development of fatigue of the human diaphragm during loading tasks in vivo.30
It should be noted that the potential therapeutic value of troponin activators such as CK-2066260 is not restricted to NM patients which suffer from a lower calcium-sensitivity of force generation; Russell and coworkers15 reported force augmentation in the face of limited neural input. In particular, patients with disorders involving defective excitation–contraction coupling such as minicore myopathy, central core myopathy or myotubular myopathy, might benefit by maximising the effect of suboptimal calcium influx. However NEB-NM patients might have a larger window for improvement because they suffer from a lower calcium-sensitivity of force generation. Thus, troponin activation is a potential therapeutic mechanism to improve force in NM patients with nebulin mutations as well as potentially with other neuromuscular diseases.
Supplementary Material
Acknowledgements
Special thanks to Ruud Zaremba for the analyses of myosin heavy chain isoforms and to Elizabeth DeChene for assistance acquiring clinical data and specimens.
Funding This work was supported by the ERA-NET E-Rare (7th Framework Programme) grant NEMMYOP and SarcoSi to CACO, by a grant from ‘De Drie Lichten’ to JMdW, a VIDI grant (016.126.319) from the Dutch Organization for Scientific Research to CACO, National Institutes of Health of the USA grants R01 AR05387 to HG, R01 AR044345 to AHB, K08 AR059750 and L40 AR057721 to MWL, and generous support from A Foundation Building Strength for Nemaline Myopathy, and from the Lee and Penny Anderson Family Foundation.
Footnotes
Contributors All authors contributed to the conception and design, analysis and interpretation of data, drafting the article or revising it critically for important intellectual content, and the approval of the version to be published.
Competing interests FIM and JRJ are employees of Cytokinetics, Inc. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Ethics approval Boston Children’s Hospital IRB and the HREC, Children’s Hospital at Westmead (CHW/10/45).
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Wallgren-Pettersson C, Laing NG. Report of the 70th ENMC International Workshop: Nemaline myopathy, 11–13 June 1999, Naarden, The Netherlands. Neuromuscul Disord. 2000;10:299–306. doi: 10.1016/s0960-8966(99)00129-7. [DOI] [PubMed] [Google Scholar]
- 2.North KN, Laing NG, Consortium I. Nemaline myopathy: current concepts. The ENMC International Consortium and Nemaline Myopathy. J Med Genet. 1997;34:705–713. doi: 10.1136/jmg.34.9.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sambuughin N, Yau KS, Olivé M, Duff RM, Bayarsaikhan M, Lu S, Gonzalez-Mera L, Sivadorai P, Nowak KJ, Ravenscroft G, Mastaglia FL, North KN, Ilkovski B, Kremer H, Lammens M, Van Engelen BGM, Fabian V, Lamont P, Davis MR, Laing NG, Goldfarb LG. Dominant mutations in KBTBD13, a member of the BTB/Kelch family, cause nemaline myopathy with cores. Am J Hum Genet. 2010;87:842–847. doi: 10.1016/j.ajhg.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelin K, Hilpelä P, Donner K, Sewry C, Akkari PA, Wilton SD, Wattanasirichaigoon D, Bang ML, Centner T, Hanefeld F, Odent S, Fardeau M, Urtizberea JA, Muntoni F, Dubowitz V, Beggs AH, Laing NG, Labeit S, De la Chapelle A, Wallgren-Pettersson C. Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc Natl Acad Sci. 1999;96:2305–2310. doi: 10.1073/pnas.96.5.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pappas CT, Krieg PA, Gregorio CC. Nebulin regulates actin filament lengths by a stabilization mechanism. J Cell Biol. 2010;189:859–870. doi: 10.1083/jcb.201001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castillo A, Nowak R, Littlefield KP, Fowler VM, Littlefield RS. A nebulin ruler does not dictate thin filament lengths. Biophys J. 2009;96:1856–1865. doi: 10.1016/j.bpj.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gokhin D, Bang M. Reduced thin filament length in nebulin-knockout skeletal muscle alters isometric contractile properties. Am J Resp Crit Care. 2009;296:1123–1132. doi: 10.1152/ajpcell.00503.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witt CC, Burkart C, Labeit D, McNabb M, Wu Y, Granzier H, Labeit S. Nebulin regulates thin filament length, contractility, and Z-disk structure in vivo. EMBO J. 2006;25:3843–3855. doi: 10.1038/sj.emboj.7601242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bang M-L, Li X, Littlefield R, Bremner S, Thor A, Knowlton KU, Lieber RL, Chen J. Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle. J Cell Biol. 2006;173:905–916. doi: 10.1083/jcb.200603119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bang M-L, Caremani M, Brunello E, Littlefield R, Lieber RL, Chen J, Lombardi V, Linari M. Nebulin plays a direct role in promoting strong actin-myosin interactions. FASEB J. 2009;23:4117–4125. doi: 10.1096/fj.09-137729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandra M, Mamidi R, Ford S, Hidalgo C, Witt CC, Ottenheijm CA, Labeit S, Granzier H. Nebulin alters cross-bridge cycling kinetics and increases thin filament activation: a novel mechanism for increasing tension and reducing tension cost. J Biol Chem. 2009;284:30889–30896. doi: 10.1074/jbc.M109.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawlor MW, Ottenheijm CA, Lehtokari V-L, Cho K, Pelin K, Wallgren-Pettersson C, Granzier H, Beggs AH. Novel mutations in NEB cause abnormal nebulin expression and markedly impaired muscle force generation in severe nemaline myopathy. Skelet Muscle. 2011;1:23. doi: 10.1186/2044-5040-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ottenheijm CAC, Hooijman P, DeChene ET, Stienen GJ, Beggs AH, Granzier H. Altered myofilament function depresses force generation in patients with nebulin-based nemaline myopathy (NEM2) J Struct Biol. 2010;170:334–343. doi: 10.1016/j.jsb.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ottenheijm CAC, Witt CC, Stienen GJ, Labeit S, Beggs AH, Granzier H. Thin filament length dysregulation contributes to muscle weakness in nemaline myopathy patients with nebulin deficiency. Hum Mol Genet. 2009;18:2359–2369. doi: 10.1093/hmg/ddp168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell AJ, Hartman JJ, Hinken AC, Muci AR, Kawas R, Driscoll L, Godinez G, Lee KH, Marquez D, Browne WF, Chen MM, Clarke D, Collibee SE, Garard M, Hansen R, Jia Z, Lu P-P, Rodriguez H, Saikali KG, Schaletzky J, Vijayakumar V, Albertus DL, Claflin DR, Morgans DJ, Morgan BP, Malik FI. Activation of fast skeletal muscle troponin as a potential therapeutic approach for treating neuromuscular diseases. Nat Med. 2012;18:452–455. doi: 10.1038/nm.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shefner J, Cedarbaum JM, Cudkowicz ME, Maragakis N, Lee J, Jones D, Lou WM, Mahoney K, Chen M, Saikali K, Mao J, Russell AJ, Hansen RL, Malik F, Wolff AA. Safety, tolerability and pharmacodynamics of a skeletal muscle activator in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2012;13:430–438. doi: 10.3109/17482968.2012.684214. [DOI] [PubMed] [Google Scholar]
- 17.Warren CM, Krzesinski PR, Greaser ML. Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis. 2003;24:1695–1702. doi: 10.1002/elps.200305392. [DOI] [PubMed] [Google Scholar]
- 18.Sanoudou D, Beggs AH. Clinical and genetic heterogeneity in nemaline myopathy—a disease of skeletal muscle thin filaments. Trends Mol Med. 2001;7:362–368. doi: 10.1016/s1471-4914(01)02089-5. [DOI] [PubMed] [Google Scholar]
- 19.Ryan MM, Ilkovski B, Strickland CD, Schnell C, Sanoudou D, Midgett C, Houston R, Muirhead D, Dennett X, Shield LK, De Girolami U, Iannaccone ST, Laing NG, North KN, Beggs AH. Clinical course correlates poorly with muscle pathology in nemaline myopathy. Neurology. 2003;60:665–673. doi: 10.1212/01.wnl.0000046585.81304.bc. [DOI] [PubMed] [Google Scholar]
- 20.Ottenheijm CAC, Granzier H. Lifting the nebula: novel insights into skeletal muscle contractility. Physiology. 2010;25:304–310. doi: 10.1152/physiol.00016.2010. [DOI] [PubMed] [Google Scholar]
- 21.Lukoyanova N, VanLoock MS, Orlova A, Galkin VE, Wang K, Egelman EH. Each actin subunit has three nebulin binding sites: implications for steric blocking. Curr Biol. 2002;12:383–388. doi: 10.1016/s0960-9822(02)00678-4. [DOI] [PubMed] [Google Scholar]
- 22.Ogut O, Hossain MM, Jin J-P. Interactions between nebulin-like motifs and thin filament regulatory proteins. J Biol Chem. 2003;278:3089–3097. doi: 10.1074/jbc.M205853200. [DOI] [PubMed] [Google Scholar]
- 23.Ochala J, Lehtokari V-L, Iwamoto H, Li M, Feng H-Z, Jin J-P, Yagi N, Wallgren-Pettersson C, Pénisson-Besnier I, Larsson L. Disrupted myosin cross-bridge cycling kinetics triggers muscle weakness in nebulin-related myopathy. FASEB J. 2011;25:1903–1913. doi: 10.1096/fj.10-176727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konhilas JP, Irving TC, De Tombe PP. Length-dependent activation in three striated muscle types of the rat. J Phys. 2002;544:225–236. doi: 10.1113/jphysiol.2002.024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Hees HWH, Dekhuijzen PNR, Heunks LMA. Levosimendan enhances force generation of diaphragm muscle from patients with chronic obstructive pulmonary disease. Am J Resp Crit Care. 2009;179:41–47. doi: 10.1164/rccm.200805-732OC. [DOI] [PubMed] [Google Scholar]
- 26.Ochala J, Li M, Ohlsson M, Oldfors A, Larsson L. Defective regulation of contractile function in muscle fibres carrying an E41K beta-tropomyosin mutation. J Phys. 2008;586:2993–3004. doi: 10.1113/jphysiol.2008.153650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochala J, Gokhin DS, Pénisson-Besnier I, Quijano-Roy S, Monnier N, Lunardi J, Romero NB, Fowler VM. Congenital myopathy-causing tropomyosin mutations induce thin filament dysfunction via distinct physiological mechanisms. Hum Mol Genet. 2012;21:4473–4485. doi: 10.1093/hmg/dds289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jasmin BJ, Gardiner PF. Patterns of EMG activity of rat plantaris muscle during swimming and other locomotor activities. J Appl Phys. 1987;63:713–718. doi: 10.1152/jappl.1987.63.2.713. [DOI] [PubMed] [Google Scholar]
- 29.Barclay CJ, Woledge RC, Curtin NA. Energy turnover for Ca2+ cycling in skeletal muscle. J Muscle Res Cell M. 2007;28:259–274. doi: 10.1007/s10974-007-9116-7. [DOI] [PubMed] [Google Scholar]
- 30.Doorduin J, Sinderby CA, Beck J, Stegeman DF, Van Hees HWH, Van der Hoeven JG, Heunks LMA. The calcium sensitizer levosimendan improves human diaphragm function. Am J Resp Crit Care. 2012;185:90–95. doi: 10.1164/rccm.201107-1268OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.