Abstract
BACKGROUND & AIMS
Central obesity could increase the risk for Barrett’s esophagus (BE) and esophageal adenocarcinoma by mechanical and/or metabolic mechanisms, such as hyperinsulinemia. We performed an epidemiologic study to determine whether prior type 2 diabetes mellitus (DM2) is associated with BE.
METHODS
We performed a population-based case-control study using the General Practice Research Database, a UK primary care database that contains information on more than 8 million subjects, to identify cases of BE (using previously validated codes; n = 14,245) and matched controls without BE (by age, sex, enrollment date, duration of follow-up evaluation, and practice region by incidence density sampling; n = 70,361). We assessed the association of a prior diagnosis of DM2 with BE using conditional univariate and multivariable regression analysis. Confounders assessed included smoking, obesity measured by body mass index (BMI), and gastroesophageal reflux disease.
RESULTS
BE cases were more likely than controls to have smoked (52.4% vs 49.9%), have a higher mean BMI (27.2 vs 26.9), and a higher prevalence of DM2 than controls (5.8% vs 5.3%). On multivariable analysis, DM2 was associated with a 49% increase in the risk of BE, independent of other known risk factors (odds ratio, 1.49; 95% confidence interval, 1.16–1.91). This association was stronger in women than men. Results remained stable with sensitivity analyses.
CONCLUSIONS
In a large population-based case-control study, DM2 was a risk factor for BE, independent of obesity (as measured by BMI) and other risk factors (smoking and gastroesophageal reflux disease). These data suggest that metabolic pathways related to DM2 should be explored in BE pathogenesis and esophageal carcinogenesis.
Keywords: Visceral Obesity, Insulin Resistance, Esophageal Adenocarcinoma, Epidemiology
Barrett’s esophagus (BE) is the strongest risk factor and most common precursor of esophageal adenocarcinoma (EAC), a malignancy with rapidly increasing incidence and persistently poor outcomes.1 The obesity epidemic has been suggested as one possible explanation for the increasing incidence of BE and EAC.2,3 However, epidemiologic studies assessing the association of obesity (when measured by body mass index [BMI], which measures overall adiposity) with BE often are contradictory,4–6 with some recent studies concluding that the increased risk of BE likely is mediated by central obesity.7–9
Increased visceral fat has been associated with BE and EAC independent of BMI.10,11 Both mechanical12 and nonmechanical endocrine pathways13 by which increased visceral fat may increase BE risk have been postulated. The metabolic pathways by which obesity might cause an increased risk of BE remain to be identified. Central obesity also is associated with type 2 diabetes mellitus (DM2), insulin resistance and hyperinsulinemia, and the metabolic syndrome, which have been associated with colon, pancreatic, breast, and prostate cancers.14–17 In these studies, pathways involving insulin and insulin growth factor (IGF) as mitogens, and/or aberrations in the IGF proproliferative anti-apoptotic pathways, have been implicated in pathogenesis.18 We previously showed that increased serum insulin, IGF1, and decreased insulin binding protein levels are associated with increased BE risk19 compared with population controls. DM2 also is characterized by hyperinsulinemia and insulin resistance.
In a study based on the national administrative database of the Veterans Administration in the United States, no association between DM2 and EAC was found; however, this study had several limitations.20 Another population-based study21 found an increased risk of EAC in subjects with DM2, but the increased risk was attenuated after adjusting for BMI. In addition, diagnosis of DM2, weight, and height were self-reported on surveys. There are currently no data regarding the association of DM2 with BE in the literature.
We proposed to evaluate this potential association by using the General Practice Research Database (GPRD), which is now part of the Clinical Practice Research Datalink. Studies that require validation of coded diagnostic outcomes, including DM2 and gastrointestinal conditions, have shown excellent agreement between the recorded diagnoses and the information on paper-based records when performed on GPRD data.22 Epidemiologic associations of different risk factors for malignancies also have been studied successfully using the GPRD.23 We hypothesized that DM2 is a risk factor for BE, independent of other known risk factors. To test this hypothesis, we performed a case-control study using the GPRD population-based database to assess the association of a prior diagnosis of DM2 with BE.
Methods
Approval of the University Hospitals Case Medical Center Institutional Review Board and the Independent Scientific Advisory Committee at the GPRD was obtained before the conduct of the study.
Study Population
Subjects were enrolled in the GPRD from May 1991 to April 2010.
Study Design
A case-control study was designed with selection of controls by incidence density sampling.24
Data Source
The GPRD was established in 1987 in the United Kingdom and has data on more than 8 million subjects. Recorded data on diagnoses and drug exposure are of high quality and have been validated in multiple studies,25,26 with many observational studies on BE and gastroesophageal re-flux disease (GERD) being completed and published using GPRD data.27–30
Cases
Cases were patients with a diagnosis of BE, diagnosed at least 1 year after the start of the up-to-standard follow-up period in GPRD (to exclude potentially prevalent BE cases) and identified by the appropriate diagnostic codes in the GPRD database (Medcode, 4614; read code, J101611 for BE; Medcode, 5596; read code, J102500 for Barrett’s ulcer of the esophagus). The first date of BE diagnosis was marked as the index date.
Controls
Controls were selected from those study subjects who remained at risk (without a diagnosis of BE) at the index date (of the corresponding case). Each case was matched with up to 5 controls on 5 conditions: year of birth (±1 y), enrollment date in GPRD (±6 mo), sex, duration of GPRD follow-up period (±6 mo) before the case index date and practice region.
Exposure
The primary exposure of interest was an initial diagnosis of DM2 before the index date. DM2 was defined as a diagnosis of DM2 at baseline (by standard diagnosis codes for DM2 in the GPRD) and a medication code indicating either an oral hypoglycemic or insulin prescription being filled at least once before the index date (date of diagnosis of BE). This was performed to increase the specificity of the DM2 diagnosis.
Potential confounding variables that were considered included smoking, obesity categories, and a baseline diagnosis of GERD. History of smoking was abstracted as to whether the patient ever smoked (ever-smoker consisting of current and ex-smokers) or never smoked. Obesity was defined using BMI. We analyzed the variation in the weight of cases and controls throughout the entire follow-up period in the GPRD. We observed a mean change of only 0.539 9 kg/m2 during a median follow-up duration of 4.61 years (interquartile range, 2.38–7.24 y) in cases and controls. To enable maximal sample size and power we elected to use weight over the entire follow-up period available in the GPRD for cases and controls. The most recent weight and height values available were used to construct the BMI variable. When multiple weight values were available, the average of weights was used. Subjects were classified as morbidly obese (BMI, >40), obese (BMI, 30–39.9), overweight (BMI, 25–29.9), and normal weight (BMI, <25). GERD was defined using standard codes for reflux esophagitis and gastroesophageal reflux from the GPRD database (Medcodes 2535, 15054, 7104, 16605, 15579, 16450; read codes, J101100, J101111, J101112, J101113, J101114). We also included subjects who were on medications used for the treatment of GERD, including proton pump inhibitors and histamine-receptor antagonists. This information was obtained using the British National Formulary (BNF) mapping codes for proton pump inhibitors (BNF 010305*) and for histamine receptor antagonists (BNF 010301*).
Statistical Analysis
Sample size assessment
Because this was a matched case-control study, the power calculation was performed as per Dupont.31 Given the number of cases and controls analyzed in this study, we estimated 91% power to detect odds ratios (ORs) of 1.075, and 96% power to detect ORs of 1.08.
Analytical strategy
The analytical data set was created in SAS (versions 9.2 and 9.3; SAS, Cary, North Carolina) and the statistical analyses were conducted in Stata SE software (version 11.2; Stata, College Station, Texas). Conditional logistic regression was used to calculate the unadjusted and adjusted odds ratios along with the 95% confidence intervals as estimates of relative risk of BE associated with prior history of DM2. Besides the individual confounders including weight categories as per the World Health Organization classification, smoking history, and a baseline diagnosis of GERD (as defined earlier), we also included interaction terms of DM2 with weight categories and sex and GERD with smoking, separately. Because inclusion of interaction terms renders the traditional interpretation of the ORs in the conditional logistic regression models redundant, a more appropriate measure to assess the effect of an independent covariate on outcome probability is marginal effect. Marginal effect of a covariate refers to the expected odds of realizing the outcome of interest (BE in this case), given different realizations of other independent covariates.32,33
Results
A total of 84,606 subjects were included in the study (14,245 cases and 70,361 controls). Table 1 provides the descriptive statistics along with the unadjusted ORs for baseline variables. Given matching, age and sex were similar in cases and controls. The distribution of cases and control patients by practice region, one of the matching criteria, also was similar (Supplementary Table 1). The mean BMI of cases was slightly higher than that of controls; as indicated by the unadjusted ORs in Table 1, cases were more likely to be overweight or obese than controls. Cases were also more likely to have baseline GERD and be ever-smokers than controls. A higher proportion of cases had DM2 than controls (5.83% vs 5.33%); this difference was statistically significant (P > |z| = .016; OR, 1.10; 95% CI, 1.02–1.19). By using a more liberal definition of DM2: the presence of a diagnosis code of DM2, without the requirement for medication use (this will expand the cohort of subjects with DM2, including those who are controlled by diet), the prevalence in cases continued to be higher than in controls (7.48% vs 7.07%) (P > |z| = .073; OR, 1.07; 95% CI, 0.99–1.14). Information on race or ethnicity is not available in the GPRD database.
Table 1.
Baseline Characteristics of BE Cases and Controls
| Variable | Cases (n = 14,245) | Controls (n = 70,361) | Unadjusted OR (95% CI) |
|---|---|---|---|
| Mean age at index date, y (SD) | 64.0 (13.7) | 64.0 (13.6) | NAa |
| Male sex, n (%) | 9070 (63.7) | 44,723 (63.6) | NAa |
| Years before index date, mean (SD) | 12.1 (4.1) | 11.7 (4.4) | NAa |
| Years after index date, mean (SD) | 4.8 (3.4) | 4.5 (3.5) | NAa |
| BMI study period,b mean (SD) | 27.2 (4.6) | 26.9 (4.7) | 1.01 (1.01–1.02) |
| BMI categories, n (%) | |||
| Normal (BMI, <25) | 4214 (33.1) | 21,677 (36.5) | 1.00 |
| Overweight (BMI, 25–29.9) | 5519 (43.3) | 24,868 (41.9) | 1.15 (1.01–1.20) |
| Obese (BMI, 30–39.9) | 2825 (22.2) | 11,929 (20.1) | 1.22 (1.16–1.29) |
| Morbidly obese (BMI, >40) | 175 (1.4) | 872 (1.5) | 1.03 (0.87–1.22) |
| Diagnosis of GERD (%) | 12,009 (84.3) | 22,701 (32.3) | 12.73 (12.07–13.42) |
| Diagnosis of DM2 (%) | 830 (5.8) | 3752 (5.3) | 1.10 (1.02–1.19) |
| Ever-smokedc (%) (current + ex) | 7252 (52.4) | 32,820 (49.9) | 1.11 (1.07–1.15) |
SD, standard deviation.
NOTE. Data on 13 practice region distribution of cases and controls, which was also used in matching, are shown in Supplementary Table 1.
Data were not available (NA) because cases and control were matched on this covariate.
BMI was available in 12,733 (89.4%) cases and 59,346 (84.3%) controls.
Data on smoking were available in 13,845 (98.2%) cases and 65,715 (93.4%) controls.
As shown in Table 2, when adjusting for confounding variables, along with interaction terms, DM2 increased the risk of BE by almost 50% (OR, 1.49; 95% CI, 1.158–1.909). However, in the presence of the interaction terms in the logistic model, this OR may not reflect the true effect of DM2. For that, we turn to marginal effects as shown in Figure 1 and Table 3. Please refer to Supplementary Table 2 for additional details and discussion. This risk was somewhat attenuated (OR, 1.27; 95% CI, 1.04, 1.55) when the more liberal definition of DM2 stated earlier was used, but DM2 remained a significant predictor of BE risk independent of other risk factors (Supplementary Table 3). The association appeared to be stronger in females, compared with males (Table 4).
Table 2.
Multivariable Model of Association of DM2 With BE
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| DM2 | 1.49 | 1.16–1.91 | .002 |
| Normal BMI (BMI, <25) | Control | ||
| Overweight (BMI, 25–29.9) | 1.04 | 0.99–1.01 | .156 |
| Obese (BMI, 30–39.9) | 1.06 | 0.99–1.14 | .060 |
| Morbidly obese (BMI, >40) | 0.98 | 0.80–1.21 | .878 |
| No DM2 and normal BMI | Control | ||
| DM2 and overweight | 0.82 | 0.64–1.07 | .139 |
| DM2 and obese | 0.73 | 0.56–0.95 | .019 |
| DM2 and morbidly obese | 0.53 | 0.31–0.92 | .023 |
| DM2 and male | 0.75 | 0.61–0.92 | .005 |
| Baseline GERD | 12.94 | 11.93–14.04 | <.0001 |
| Ever-smoker | 1.10 | 1.00–1.22 | .043 |
| Baseline GERD and ever-smoker | 0.82 | 0.73–0.91 | <.001 |
NOTE. There were 64,651 participants total: 12,511 cases and 52,140 controls.
Figure 1.
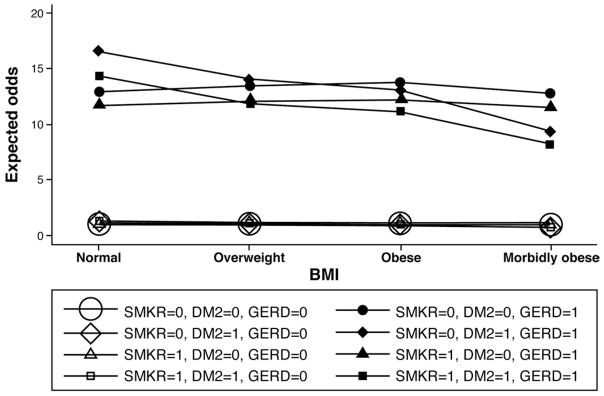
Marginal effects analysis for multivariable model of association of BE with DM2. The plot depicts the expected odds (risks) of developing BE for patients with or without the following baseline conditions for different BMI categories: GERD, DM2, and whether smoked. The 4 lines at the bottom of the graph, indicating small positive risk of BE for all weight categories, correspond to the groups of patients without GERD but with or without smoking and DM2 in the baseline. The 4 lines at the top of the graph pertain to the groups of patients with GERD in the baseline. Clearly, having GERD in the baseline is associated significantly with the development of BE, even after accounting for DM2 and smoking status in the baseline. The graph also suggests that for subjects with DM2 in the baseline, increasing BMI appears to be protective because the expected odds decline monotonically from normal BMI patients to morbidly obese patients.
Table 3.
Marginal Effects of Individual Variables (DM2, BMI, GERD, and Sex) on BE Risk
| Row | DM2a | BMI | GERD | Male | Smoker | Marginal effect | 95% lower CI limit | 95% upper CI limit |
|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 3 | 0 | 0 | 0 | 1.06 | 1.00 | 1.13 |
| 2 | 0 | 3 | 0 | 0 | 1 | 1.17 | 1.04 | 1.31 |
| 3 | 0 | 3 | 0 | 1 | 0 | 1.06 | 1.00 | 1.13 |
| 4 | 0 | 3 | 0 | 1 | 1 | 1.17 | 1.04 | 1.31 |
| 5 | 0 | 3 | 1 | 0 | 0 | 13.77 | 12.38 | 15.16 |
| 6 | 0 | 3 | 1 | 0 | 1 | 12.44 | 11.20 | 13.68 |
| 7 | 0 | 3 | 1 | 1 | 0 | 13.77 | 12.38 | 15.16 |
| 8 | 0 | 3 | 1 | 1 | 1 | 12.44 | 11.20 | 13.68 |
| 9 | 0 | 4 | 0 | 0 | 0 | 0.98 | 0.78 | 1.19 |
| 10 | 0 | 4 | 0 | 0 | 1 | 1.09 | 0.84 | 1.33 |
| 11 | 0 | 4 | 0 | 1 | 0 | 0.98 | 0.78 | 1.19 |
| 12 | 0 | 4 | 0 | 1 | 1 | 1.09 | 0.84 | 1.33 |
| 13 | 0 | 4 | 1 | 0 | 0 | 12.74 | 9.92 | 15.56 |
| 14 | 0 | 4 | 1 | 0 | 1 | 11.51 | 8.96 | 14.06 |
| 15 | 0 | 4 | 1 | 1 | 0 | 12.74 | 9.92 | 15.56 |
| 16 | 0 | 4 | 1 | 1 | 1 | 11.51 | 8.96 | 14.06 |
| 17 | 1 | 3 | 0 | 0 | 0 | 1.15 | 0.92 | 1.39 |
| 18 | 1 | 3 | 0 | 0 | 1 | 1.27 | 0.99 | 1.56 |
| 19 | 1 | 3 | 0 | 1 | 0 | 0.86 | 0.72 | 1.01 |
| 20 | 1 | 3 | 0 | 1 | 1 | 0.95 | 0.77 | 1.14 |
| 21 | 1 | 3 | 1 | 0 | 0 | 14.94 | 11.75 | 18.13 |
| 22 | 1 | 3 | 1 | 0 | 1 | 13.50 | 10.63 | 16.37 |
| 23 | 1 | 3 | 1 | 1 | 0 | 11.18 | 9.08 | 13.29 |
| 24 | 1 | 3 | 1 | 1 | 1 | 10.10 | 8.22 | 11.99 |
| 25 | 1 | 4 | 0 | 0 | 0 | 0.78 | 0.42 | 1.14 |
| 26 | 1 | 4 | 0 | 0 | 1 | 0.86 | 0.46 | 1.26 |
| 27 | 1 | 4 | 0 | 1 | 0 | 0.58 | 0.31 | 0.86 |
| 28 | 1 | 4 | 0 | 1 | 1 | 0.64 | 0.34 | 0.95 |
| 29 | 1 | 4 | 1 | 0 | 0 | 10.10 | 5.42 | 14.79 |
| 30 | 1 | 4 | 1 | 0 | 1 | 9.13 | 4.90 | 13.36 |
| 31 | 1 | 4 | 1 | 1 | 0 | 7.56 | 3.97 | 11.15 |
| 32 | 1 | 4 | 1 | 1 | 1 | 6.83 | 3.59 | 10.07 |
CI, confidence interval.
NOTE. To simplify results, we omitted the marginal effects for BMI categories 1 and 2, however, we provide the complete list of marginal effects in Supplementary Table 2. For rows 1 through 16, the marginal effect for male and female were the same, which was an artifact of the estimated model in which DM2 and male sex were interacted, which assumes a value of 0 when a patient is nondiabetic irrespective of sex.
Values were assigned as follows for DM2: 1, type 2 diabetes mellitus was present; 0, otherwise; weight: 1, normal; 2, overweight; 3, obese; 4, morbidly obese; sex: 1, male; 0, female.
Table 4.
Multivariable Models of Association of DM2 With BE Stratified by Sex
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Males | |||
| DM2 | 1.13 | 0.865–1.477 | .371 |
| Normal BMI (BMI, <25) | Control | ||
| Overweight (BMI, 25–29.9) | 0.96 | 0.896–1.025 | .212 |
| Obese (BMI, 30–39.9) | 0.87 | 0.803–0.952 | .002 |
| Morbidly obese (BMI, >40) | 0.60 | 0.407–0.873 | .008 |
| No DM2 and normal BMI | Control | ||
| DM2 and overweight | 0.77 | 0.563–1.060 | .110 |
| DM2 and obese | 0.80 | 0.574–1.121 | .197 |
| DM2 and morbidly obese | 1.07 | 0.474–2.412 | .872 |
| Baseline GERD | 14.45 | 12.938–16.144 | <.001 |
| Ever-smoker | 1.21 | 1.068–1.360 | .003 |
| Baseline GERD*ever-smoker | 0.74 | 0.646–0.853 | <.001 |
| Females | |||
| DM2 | 1.41 | 0.982–2.017 | .063 |
| Normal BMI (BMI, <25) | Control | ||
| Overweight (BMI, 25–29.9) | 1.17 | 1.071–1.273 | <.001 |
| Obese (BMI, 30–39.9) | 1.40 | 1.265–1.543 | <.001 |
| Morbidly obese (BMI, >40) | 1.34 | 1.046–1.722 | .021 |
| No DM2 and normal BMI | Control | ||
| DM2 and overweight | 0.98 | 0.623–1.548 | .938 |
| DM2 and obese | 0.66 | 0.428–1.013 | .057 |
| DM2 and morbidly obese | 0.38 | 0.182–0.799 | .011 |
| Baseline GERD | 11.25 | 9.972–12.684 | <.001 |
| Ever-smoker | 1.00 | 0.846–1.177 | .980 |
| Baseline GERD*ever-smoker | 0.92 | 0.760–1.102 | .353 |
CI, confidence interval.
NOTE. Female sex: n = 24,302; 4587 cases and 19,715 controls; male sex: n = 40,349; 7924 cases and 32,425 controls.
Given the lack of validation of BE codes in the GPRD, the association of BE with DM2 was assessed by using 2 additional definitions of BE: (1) the presence of 2 or more BE diagnostic codes and an endoscopy code after the index date, and (2) the presence of 2 or more BE diagnostic codes after the index date. When BE was defined as the presence of 2 or more BE codes and at least one endoscopy code, DM2 was associated with a 4-fold increased risk of BE (OR, 4.24; 95% CI, 1.39–12.91; P = .011) (Supplementary Table 4 and Supplementary Figure 1). When BE was defined by the presence of at least 2 diagnostic codes, the association of DM2 with BE was attenuated (OR, 1.35; 95% CI, 0.87–2.09; P = .177) (Supplementary Table 5, Supplementary Figure 2).
Discussion
In this large population-based case-control study, we have identified DM2 as a risk factor for BE independent of known risk factors such as GERD, obesity as measured by BMI, and smoking, with the suggestion of a stronger influence of DM2 on BE risk in females than in males. This follows other studies published by us10,19 and others reporting on the association of BE risk with central obesity and its known sequelae.7 It appears that this association is mediated by visceral and not subcutaneous abdominal fat.34 One of the postulated mechanisms for this association is via the IGF1 pathway, which is known to be upregulated in individuals with increased visceral fat.35
Several investigators have assessed the potential nonme-chanical pathways by which abdominal visceral fat can increase esophageal injury, BE, and EAC risk. Recent studies have focused on the association of BE with systemic sequelae of increased visceral fat, including the metabolic syndrome,36,37 systemic inflammation,36 and insulin resistance.19 In a prior study, BE risk was associated with increased serum insulin, insulin-like growth factor levels, and insulin resistance. Results from the present study are a logical extension of these studies, in which we found an increased risk of BE in subjects with DM2, a more clinically relevant and easily measured disease state. The IGF pathway is known to promote tissue proliferation by stimulating the activation of the phosphoinositol-3-kinase/AKT/ mammalian target of Rapamycin pathway as well as other pathways that participate in tissue proliferation.38–40 This study also raises the possibility that DM2 and visceral obesity-induced hyperinsulinemia may counteract the protective effect of estrogen on leptin-associated esophageal metaplasia and neoplasia. Increased serum leptin levels have been shown to be associated with BE in men.41,42 Estrogen has been found to influence leptin-receptor expression and sensitivity of hypothalamus to leptin, driving subcutaneous body fat accrual over visceral fat.43 Visceral fat–induced hyperinsulinemia may counteract this effect.
Diagnostic criteria for BE in the United Kingdom include any esophageal columnar metaplasia without the requirement for intestinal metaplasia. It is difficult to estimate the proportion of subjects with BE who have documented intestinal metaplasia given the absence of histopathology data in the GPRD database. However some recent studies have supported the neoplastic potential of nonintestinal columnar metaplasia in the esophagus.44 Biomarkers predicting progression in intestinalized columnar metaplasia have been described in nonintestinalized esophageal columnar mucosa as well.45,46
Our study also reveals the complexity of determining how obesity may contribute to metaplastic and neoplastic diseases. The strength of the association between BE and DM2 was different between men and women. Gender differences also were noted in the study by Edelstein et al,8,47 which examined the association of obesity with BE. Curiously, our multivariate model (Table 2) also found that increasing BMI in subjects with DM2 was protective. We cannot offer a clear explanation or mechanism for this finding. Obesity is associated closely with GERD,48 and this finding may be related to a sex-specific inter-action between obesity and GERD. Alternatively, this may reflect greater health care seeking among women, leading to more endoscopic evaluation than men. However, it is interesting to note that increasing BMI has been associated with a protective effect against breast cancer in premenopausal women.49 Our findings support further investigations on the hormonal changes that mediate the association of central adiposity with BE and EAC.
Although this was a large population-based study, there were potential limitations. The validity of diagnostic codes for BE in the GPRD database has not been studied systematically. However, prior studies on BE subjects in the GPRD database have found that this information was available in almost 60% of subjects when a detailed review of the medical chart was performed.27–29 Furthermore, the association of BE with DM2 remained largely robust when different definitions of BE were used (Supplementary Tables 4 and 5). Protopathic or detection bias may exist. It is possible that having a chronic disease such as DM2 is more likely to result in contact with providers, leading to higher rates of endoscopy and hence more detection of BE. We assessed the rate of endoscopic use in subjects with a comparable chronic disease such as coronary artery disease. We found that 16% of subjects with a DM2 diagnosis had endoscopy, whereas 22.5% of subjects with coronary artery disease had endoscopy. This suggests that subjects with DM2 were not more likely to have endoscopy than those with other comparable chronic diseases. Nonsteroidal anti-inflammatory drug use recently was shown to be protective against the development of BE.50 Information available in the GPRD database includes prescriptions only and hence would not be able to capture over-the-counter nonsteroidal anti-inflammatory drug use, which is known to be fairly prevalent. Hence, we chose not include this variable in the final model. Finally, a case-control study can establish association but not causation. In this study we assessed the association of the presence of a DM2 diagnosis before the diagnosis of BE was established. This makes the alternative hypothesis that BE leads to the development of DM2 much less likely.
In summary, in this large population-based case-control study, DM2 was found to be a risk factor for BE, independent of smoking, obesity (measured by BMI), and a diagnosis of gastroesophageal reflux. The effect of DM2 was more pronounced in women compared with men. This may reflect the influence of the insulin-insulin growth factor pathway, which has been shown to be upregulated in BE and EAC in prior studies. Patients with DM2 may be at a higher risk of developing BE and, potentially, esophageal adenocarcinoma. Prospective studies are needed to confirm this association.
Supplementary Material
Acknowledgments
Funding
This research was supported by an investigator-initiated grant from Takeda Pharmaceuticals, Inc. Prasad Iyer and Amitabh Chak are members of the National Cancer Institute–supported Barrett’s Esophagus Translational Research Network (U54 CA163060); and Prasad Iyer also is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (RC4DK090413). This study is based in part on data from the General Practice Research Database obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. However, the interpretation and conclusions contained in this study are those of the author/s alone.
Abbreviations used in this paper
- BE
Barrett’s esophagus
- BMI
body mass index
- BNF
British National Formulary
- DM2
type 2 diabetes mellitus
- EAC
esophageal adenocarcinoma
- GERD
gastroesophageal reflux disease
- GPRD
general practice research database
- IGF
insulin-insulin growth factor
- OR
odds ratio
Footnotes
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at http://dx.doi.org/10.1016/j.cgh.2013.03.024.
Conflicts of interest
These authors disclose the following: Prasad Iyer and Amitabh Chak have received research funding from Takeda Pharmaceuticals. The remaining authors disclose no conflicts.
References
- 1.Sharma P. Clinical practice. Barrett’s esophagus. N Engl J Med. 2009;361:2548–2556. doi: 10.1056/NEJMcp0902173. [DOI] [PubMed] [Google Scholar]
- 2.Whiteman DC, Sadeghi S, Pandeya N, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57:173–180. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 3.Chow WH, Blot WJ, Vaughan TL, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90:150–155. doi: 10.1093/jnci/90.2.150. [DOI] [PubMed] [Google Scholar]
- 4.Cook MB, Greenwood DC, Hardie LJ, et al. A systematic review and meta-analysis of the risk of increasing adiposity on Barrett’s esophagus. Am J Gastroenterol. 2008;103:292–300. doi: 10.1111/j.1572-0241.2007.01621.x. [DOI] [PubMed] [Google Scholar]
- 5.Kamat P, Wen S, Morris J, et al. Exploring the association between elevated body mass index and Barrett’s esophagus: a systematic review and meta-analysis. Ann Thorac Surg. 2009;87:655–662. doi: 10.1016/j.athoracsur.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Abrams JA, Sharaiha RZ, Gonsalves L, et al. Dating the rise of esophageal adenocarcinoma: analysis of Connecticut tumor registry data, 1940–2007. Cancer Epidemiol Biomarkers Prev. 2011;20:183–186. doi: 10.1158/1055-9965.EPI-10-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corley DA, Kubo A, Levin TR, et al. Abdominal obesity and body mass index as risk factors for Barrett’s esophagus. Gastroenterology. 2007;133:34–41. doi: 10.1053/j.gastro.2007.04.046. quiz 311. [DOI] [PubMed] [Google Scholar]
- 8.Edelstein ZR, Farrow DC, Bronner MP, et al. Central adiposity and risk of Barrett’s esophagus. Gastroenterology. 2007;133:403–411. doi: 10.1053/j.gastro.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Das A, Thomas S, Zablotska LB, et al. Association of esophageal adenocarcinoma with other subsequent primary cancers. J Clin Gastroenterol. 2006;40:405–411. doi: 10.1097/00004836-200605000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Nelsen EM, Kirihara Y, Takahashi N, et al. Distribution of body fat and its influence on esophageal inflammation and dysplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2012;10:728–734. doi: 10.1016/j.cgh.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Serag HB, Graham DY, Satia JA, et al. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. Am J Gastroenterol. 2005;100:1243–1250. doi: 10.1111/j.1572-0241.2005.41703.x. [DOI] [PubMed] [Google Scholar]
- 12.Pandolfino JE, El-Serag HB, Zhang Q, et al. Obesity: a challenge to esophagogastric junction integrity. Gastroenterology. 2006;130:639–649. doi: 10.1053/j.gastro.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Tilg H, Moschen AR. Visceral adipose tissue attacks beyond the liver: esophagogastric junction as a new target. Gastroenterology. 2010;139:1823–1826. doi: 10.1053/j.gastro.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 14.Jinjuvadia R, Lohia P, Jinjuvadia C, et al. The association between metabolic syndrome and colorectal neoplasm: systemic review and meta-analysis. J Clin Gastroenterol. 2013;47:33–44. doi: 10.1097/MCG.0b013e3182688c15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansen D, Stocks T, Jonsson H, et al. Metabolic factors and the risk of pancreatic cancer: a prospective analysis of almost 580,000 men and women in the Metabolic Syndrome and Cancer Project. Cancer Epidemiol Biomarkers Prev. 2010;19:2307–2317. doi: 10.1158/1055-9965.EPI-10-0234. [DOI] [PubMed] [Google Scholar]
- 16.Rosato V, Bosetti C, Talamini R, et al. Metabolic syndrome and the risk of breast cancer. Recenti Prog Med. 2011;102:476–478. doi: 10.1701/998.10859. [DOI] [PubMed] [Google Scholar]
- 17.Pelucchi C, Serraino D, Negri E, et al. The metabolic syndrome and risk of prostate cancer in Italy. Ann Epidemiol. 2011;21:835–841. doi: 10.1016/j.annepidem.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 19.Greer KB, Thompson CL, Brenner L, et al. Association of insulin and insulin-like growth factors with Barrett’s oesophagus. Gut. 2012;61:665–672. doi: 10.1136/gutjnl-2011-300641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubenstein JH, Davis J, Marrero JA, et al. Relationship between diabetes mellitus and adenocarcinoma of the oesophagus and gastric cardia. Aliment Pharmacol Ther. 2005;22:267–271. doi: 10.1111/j.1365-2036.2005.02544.x. [DOI] [PubMed] [Google Scholar]
- 21.Neale RE, Doecke JD, Pandeya N, et al. Does type 2 diabetes influence the risk of oesophageal adenocarcinoma? Br J Cancer. 2009;100:795–798. doi: 10.1038/sj.bjc.6604908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ. 1991;302:766–768. doi: 10.1136/bmj.302.6779.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang YX, Hennessy S, Lewis JD. Type 2 diabetes mellitus and the risk of colorectal cancer. Clin Gastroenterol Hepatol. 2005;3:587–594. doi: 10.1016/s1542-3565(05)00152-7. [DOI] [PubMed] [Google Scholar]
- 24.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, et al. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 25.Jick SS, Kaye JA, Vasilakis-Scaramozza C, et al. Validity of the general practice research database. Pharmacotherapy. 2003;23:686–689. doi: 10.1592/phco.23.5.686.32205. [DOI] [PubMed] [Google Scholar]
- 26.Lawrenson R, Williams T, Farmer R. Clinical information for research; the use of general practice databases. J Public Health Med. 1999;21:299–304. doi: 10.1093/pubmed/21.3.299. [DOI] [PubMed] [Google Scholar]
- 27.Solaymani-Dodaran M, Logan RF, West J, et al. Mortality associated with Barrett’s esophagus and gastroesophageal reflux disease diagnoses-a population-based cohort study. Am J Gastroenterol. 2005;100:2616–2621. doi: 10.1111/j.1572-0241.2005.00340.x. [DOI] [PubMed] [Google Scholar]
- 28.Solaymani-Dodaran M, Logan RF, West J, et al. Risk of extra-oesophageal malignancies and colorectal cancer in Barrett’s oesophagus and gastro-oesophageal reflux. Scand J Gastroenterol. 2004;39:680–685. doi: 10.1080/00365520410004802. [DOI] [PubMed] [Google Scholar]
- 29.Solaymani-Dodaran M, Logan RF, West J, et al. Risk of oesophageal cancer in Barrett’s oesophagus and gastro-oesophageal reflux. Gut. 2004;53:1070–1074. doi: 10.1136/gut.2003.028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Serag H, Hill C, Jones R. Systematic review: the epidemiology of gastro-oesophageal reflux disease in primary care, using the UK general practice research database. Aliment Pharmacol Ther. 2009;29:470–480. doi: 10.1111/j.1365-2036.2008.03901.x. [DOI] [PubMed] [Google Scholar]
- 31.Dupont WD. Power calculations for matched case-control studies. Biometrics. 1988;44:1157–1168. [PubMed] [Google Scholar]
- 32.Ai C, Norton EC. Interaction terms in logit and probit models. Econ Lett. 2003;80:123–129. [Google Scholar]
- 33.Buis ML. Stata tip 87: interpretation of interactions in nonlinear models. Stata J. 2010:305–308. [Google Scholar]
- 34.El-Serag HB, Kvapil P, Hacken-Bitar J, et al. Abdominal obesity and the risk of Barrett’s esophagus. Am J Gastroenterol. 2005;100:2151–2156. doi: 10.1111/j.1572-0241.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 35.Nam SY, Kim BC, Han KS, et al. Abdominal visceral adipose tissue predicts risk of colorectal adenoma in both sexes. Clin Gastroenterol Hepatol. 2010;8:443–450. e441–442. doi: 10.1016/j.cgh.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Ryan AM, Healy LA, Power DG, et al. Barrett esophagus: prevalence of central adiposity, metabolic syndrome, and a proinflammatory state. Ann Surg Jun. 2008;247:909–915. doi: 10.1097/SLA.0b013e3181612cac. [DOI] [PubMed] [Google Scholar]
- 37.Leggett CL, Nelsen E, Tian J, et al. Metabolic syndrome as a risk factor for Barrett’s esophagus: a population based case control study. Mayo Clin Proc. 2013;88:157–165. doi: 10.1016/j.mayocp.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen SC, Chou CK, Wong FH, et al. Overexpression of epidermal growth factor and insulin-like growth factor-I receptors and auto-crine stimulation in human esophageal carcinoma cells. Cancer Res. 1991;51:1898–1903. [PubMed] [Google Scholar]
- 39.Liu YC, Leu CM, Wong FH, et al. Autocrine stimulation by insulin-like growth factor I is involved in the growth, tumorigenicity and chemoresistance of human esophageal carcinoma cells. J Biomed Sci. 2002;9:665–674. doi: 10.1159/000067282. [DOI] [PubMed] [Google Scholar]
- 40.Takaoka M, Harada H, Andl CD, et al. Epidermal growth factor receptor regulates aberrant expression of insulin-like growth factor-binding protein 3. Cancer Res. 2004;64:7711–7723. doi: 10.1158/0008-5472.CAN-04-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kendall BJ, Macdonald GA, Hayward NK, et al. Leptin and the risk of Barrett’s oesophagus. Gut. 2008;57:448–454. doi: 10.1136/gut.2007.131243. [DOI] [PubMed] [Google Scholar]
- 42.Thompson OM, Beresford SA, Kirk EA, et al. Serum leptin and adiponectin levels and risk of Barrett’s esophagus and intestinal metaplasia of the gastroesophageal junction. Obesity (Silver Spring) 2010;18:2204–2211. doi: 10.1038/oby.2009.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao Q, Horvath TL. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am J Physiol Endocrinol Metab. 2008;294:E817–E826. doi: 10.1152/ajpendo.00733.2007. [DOI] [PubMed] [Google Scholar]
- 44.Kelty CJ, Gough MD, Van Wyk Q, et al. Barrett’s oesophagus: intestinal metaplasia is not essential for cancer risk. Scand J Gastroenterol. 2007;42:1271–1274. doi: 10.1080/00365520701420735. [DOI] [PubMed] [Google Scholar]
- 45.Hahn HP, Blount PL, Ayub K, et al. Intestinal differentiation in metaplastic, nongoblet columnar epithelium in the esophagus. Am J Surg Pathol. 2009;33:1006–1015. doi: 10.1097/PAS.0b013e31819f57e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu W, Hahn H, Odze RD, et al. Metaplastic esophageal columnar epithelium without goblet cells shows DNA content abnormalities similar to goblet cell-containing epithelium. Am J Gastroenterol. 2009;104:816–824. doi: 10.1038/ajg.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edelstein ZR, Bronner MP, Rosen SN, et al. Risk factors for Barrett’s esophagus among patients with gastroesophageal reflux disease: a community clinic-based case-control study. Am J Gastroenterol. 2009;104:834–842. doi: 10.1038/ajg.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Serag H. Role of obesity in GORD-related disorders. Gut. 2008;57:281–284. doi: 10.1136/gut.2007.127878. [DOI] [PubMed] [Google Scholar]
- 49.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 50.Omer ZB, Ananthakrishnan AN, Nattinger KJ, et al. Aspirin protects against Barrett’s esophagus in a multivariate logistic regression analysis. Clin Gastroenterol Hepatol. 2012;10:722–727. doi: 10.1016/j.cgh.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


