Abstract
Purpose
To assess complications, local tumor recurrences, overall survival (OS), and estimates of cost-effectiveness for multisite cryoablation (MCA) of oligometastatic renal cell carcinoma (RCC).
Materials and Methods
A total of 60 computed tomography- and/or ultrasound-guided percutaneous MCA procedures were performed on 72 tumors in 27 patients (three women and 24 men). Average patient age was 63 years. Tumor location was grouped according to common metastatic sites. Established surgical selection criteria graded patient status. Median OS was determined by Kaplan–Meier method and defined life-years gained (LYGs). Estimates of MCA costs per LYG were compared with established values for systemic therapies.
Results
Total number of tumors and cryoablation procedures for each anatomic site are as follows: nephrectomy bed, 11 and 11; adrenal gland, nine and eight; paraaortic, seven and six; lung, 14 and 13; bone, 13 and 13; superficial, 12 and nine; intraperitoneal, five and three; and liver, one and one. A mean of 2.2 procedures per patient were performed, with a median clinical follow-up of 16 months. Major complication and local recurrence rates were 2% (one of 60) and 3% (two of 72), respectively. No patients were graded as having good surgical risk, but median OS was 2.69 years, with an estimated 5-year survival rate of 27%. Cryoablation remained cost-effective with or without the presence of systemic therapies according to historical cost comparisons, with an adjunctive cost-effectiveness ratio of $28,312–$59,554 per LYG.
Conclusions
MCA was associated with very low morbidity and local tumor recurrence rates for all anatomic sites, with apparent increased OS. Even as an adjunct to systemic therapies, MCA appeared cost-effective for palliation of oligometastatic RCC.
Renal cell carcinoma (RCC) was diagnosed in an estimated 58,240 new patients in the United States in 2010 (1). Approximately 25%–30% of patients diagnosed with local RCC have overt metastases at presentation, and 33% of patients with RCC at diagnosis develop metastatic disease; this suggests that the development of metastatic RCC (mRCC) is a possibility in more than 50% of all patients with RCC, or approximately 30,000 per year in the United States (2). Treatment responses of mRCC to conventional strategies of chemotherapy, radiation therapy, and hormone therapy have produced a median overall survival (OS) of 7–11 months and a 5-year OS rate of 10% (2). The associated high costs of emerging chemotherapy regimens have therefore required cost-effectiveness evaluations to justify minor survival benefits (3–8). It is also uncertain which patients benefit enough from systemic treatments to then be considered for local treatments of limited metastatic, or oligometastatic, RCC.
Metastasectomy, or the resection of oligometastatic RCC, is a surgical option primarily for pulmonary involvement, providing 5-year survival rates as high as 50% (9,10). Pulmonary metastasectomy has been considered cost-effective for soft-tissue sarcoma when considering only costs and no quality-of-life adjustments (11). However, a recent report on 1,463 patients with newly diagnosed RCC noted that only 21% underwent pulmonary metastasectomy, despite 62% presenting with mRCC at initial diagnosis (12). Therefore, a large unmet need exists for expanding the survival benefits of metastasectomy to the broadest possible group of patients with mRCC.
Reductions in morbidity and treatment cost, while still improving survival rates, are important for the adoption of minimally invasive treatments (13). Computed tomography (CT)-guided percutaneous cryoablation has been shown to be a well tolerated and effective treatment for primary RCC (14,15); however, studies have yet to explore its effectiveness in treating mRCC. The visible treatment zone of cryoablation, lower pain, and minimal morbidity allowed us to apply our established cryoablation techniques (15–18) to many anatomic sites for local control of limited mRCC.
The purpose of this study was to assess the potential role of multisite cryoablation (MCA) of oligometastatic RCC by evaluating complications, local recurrences, survival, and projected procedure costs in relation to systemic treatments. Estimates of MCA cost-effectiveness were compared versus best supportive care (BSC) and emerging chemoimmunotherapy regimens (3–8) to place an economic perspective on our outcomes for this select group of patients.
Materials and Methods
Patients
Consecutive patients with mRCC scheduled to undergo cryoablation read and signed an authorization form issued under the Health and Insurance Portability and Accountability Act of 1996. All patients also signed a separate consent form detailing the procedure, as well as an investigational review board approved consent form for prospective collection of procedure, imaging, and clinical parameters. Included in the study were 27 consecutive patients with mRCC (24 male, three female) with an average age of 63 years (range, 19–86 y). The eight procedural locations included lung (n = 13), liver (n = 1), and six soft-tissue sites: nephrectomy bed (n = 11), adrenal gland (n = 8), paraaortic (n = 6), superficial (n = 9), intraperitoneal location (n = 3), and bone (n = 13; Table 1).
Table 1. Patient, Procedure, and Tumor Characteristics.
| Soft Tissue | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Characteristic | Nephrectomy Bed | Adrenal Gland | Paraaortic | Superficial | Intraperitoneal | Bone | Subtotal | Liver | Lung | Total |
| No. of patients | 7 | 5 | 5 | 5 | 3 | 7 | 32* | 1 | 6 | 37* |
| No. of procedures | 11 | 8 | 6 | 9 | 3 | 13 | 50† | 1 | 13 | 64† |
| No. of tumors | 11 | 9 | 7 | 12 | 5 | 13 | 57 | 1 | 14 | 72 |
| Mean tumor diameter (cm3) | 4.8 | 2.9 | 2.8 | 3.1 | 2.6 | 4.8 | 3.7 | 2 | 1.6 | 3.3 |
| Mean ablation diameter (cm3) | 6.4 | 4.5 | 4.6 | 5.0 | 4.7 | 6.8 | 5.5 | 4 | 3.3 | 5.1 |
| Mean no. of probes | 4.9 | 4.5 | 4.2 | 3 | 2.5 | 4.4 | 3.8 | 3 | 2.6 | 3.6 |
Note.—Lung tumor locations consisted of metastatic lesions in lung parenchyma and did not include mediastinal or hilar adenopathy. Retroperitoneal tumors included local recurrences following nephrectomy, metastatic adrenal masses, as well as any paraaortic/pericaval mass or adenopathy. Superficial tumor locations consisted of predominantly subcutaneous, muscular, and/or lymph node metastases within the extremities or torso wall. Intraperitoneal tumors were isolated within the abdominal cavity and not adherent to bowel. Tumors in bone locations were limited metastatic deposits in non–weight-bearing locations with the epicenter in osseous structures.
Ten patients had tumors in multiple areas, which overlapped in the final patient count. Actual patient count is 27.
Two patients had two procedures each in two areas, accounting for four extra procedures. Actual procedure count is 60.
Inclusion criteria for cryoablation consisted of a localized soft tissue mass smaller than 7 cm that was biopsyproven or deemed suspicious based on a CT image showing an enhancing, growing mass or positive findings on positron emission tomography. Patients should not have more than five cancerous foci in an organ site to avoid compromising safety in patients with advanced disease and to allow MCA to treat all metastases present at the time of the first procedure over the course of one or multiple procedures. These patients were generally referred by oncologists or surgeons for local control of oligometastatic RCC. Tumors in multiple locations were treated in single or multiple staged cryoablation procedures according to projected feasibility and/or safety. MCA was also used to treat additional foci developing over time in subsequent procedures. All cases were reviewed and performed by a single radiologist with 20 years of interventional and cross-sectional imaging experience (P.J.L.). Ablation was conducted within the context of providing thorough ablation for patients with oligometastatic RCC and a chance at providing active disease treatment comparable to surgical metastasectomy.
Patient charts were evaluated by a genitourinary oncologist (U.V.) with more than 10 years of experience. Patients were grouped into favorable (0 points), intermediate (1–2 points) and poor (3–5 points) risk categories by assigning points based on predetermined risk parameters (10). The five parameters for ascertaining this risk score were (i) a time from nephrectomy to recurrence of less than 12 months, (ii) serum hemoglobin level less than the age-specific lower limit of normal (< 13 g/dL in men and < 11.5 g/dL in women), (iii) serum calcium level more than 10 mg/dL after correction for serum albumin, (iv) Karnofsky performance status less than 80%, and (v) serum lactate dehydrogenase level greater than 300 U/L. Patients who received BSC or any chemoimmunotherapy regimen before or after MCA were also noted (eg, interferon [IFN] monotherapy, bevacizumab with IFN, sorafenib, or sunitinib).
Cryoablation Procedure
The primary technique goal for all cryoablation procedures was to achieve sufficient probe distribution (eg, approximately one cryoprobe for each centimeter of tumor diameter) to reach cytotoxic temperatures less than −20°C covering all tumor margins in an outpatient setting. Probe type (ie, 1.7-mm or 2.4-mm outer diameter) and number were recorded for each ablation site. Cryoablation planning techniques/procedural details and associated hydrodissection protection measures for renal, pulmonary, soft tissue, and breast tumors have been previously described (15–18).
Imaging and Follow-up
Real-time ultrasonography (LOGIQ 700; GE Medical Systems, Milwaukee, Wisconsin) was solely used to place and monitor cryoprobes during procedures in superficial locations, which included subcutaneous lesions residing in the head and neck or abdominal wall/flank. CT was used as the primary imaging modality for planning, procedure guidance, and treatment follow-up in the remaining seven procedural sites (Table 1). Magnetic resonance (MR) imaging was used as needed for improved tissue/tumor discrimination, in patients with iodine allergies, or when favored for radiation reduction (eg, in younger patients). Tumors and ablation zones were measured in three dimensions and noted on axial images in their greatest transverse and anteroposterior extent, with craniocaudal measurements obtained from sagittal and/or coronal reconstructions. In follow-up, enhanced CT or MR images were obtained at 1, 3, 6, 12, 18, and 24 months and yearly thereafter as available.
Complications
All treatment-related complications were categorized in accordance with the standardized Common Terminology Criteria for Adverse Events, version 3.0, of the National Cancer Institute, similar to published cryoablation series (15–18). Complications were not linked to cost estimates.
Recurrences
The ideal goal of cryoablation is to achieve complete ablation of a tumor focus with minimal damage to surrounding soft tissues. Local recurrences were noted as nodular enhancing rim of the ablation zone and/or contiguous mass effect.
Survival
Median OS for patients undergoing MCA was determined by using the Kaplan–Meier estimator in the Lifetest procedure in SAS software (version 9.2; SAS Institute, Cary, North Carolina). OS was measured from the time of the first procedure until death or until the most recent follow-up for vital status determination. Because of modest sample sizes (or numbers of events), OS statistics (eg, median, 1-y rate) were estimated more conservatively by using linear interpolation between successive event times on the Kaplan–Meier curve (19). All point estimates of OS statistics were accompanied by a 95% CI.
Cost
A total cost of $12,833 per cryoablation procedure represents a high-end estimate from average professional fees ($2,500), disposable equipment fees ($5,333 for four cryoprobes), and hospital fees ($5,000). Mean cost of more frequent follow-up imaging examinations of $42,000 encompassed six follow-up CT imaging sessions at $7,000 each (eg, at 1, 3, 6, 12, 18, and 24 mo and yearly thereafter). Each CT session reflected our institution's 2010 Medicare technical component guidelines of $2,171, $2,396, and $1,390 for chest, abdomen, and pelvic CT, respectively, and professional fees of approximately $350 per scan. No significant cost difference was assumed for MR imaging based on our 2010 Medicare guideline of $2,171 for each MR examination per anatomic site. A mean number of procedures was calculated and used to determine the cost per patient. The overlapping schedule in follow-up imaging after a second ablation did not justify counting follow-up imaging charges more than once.
Results
Patients, Procedure, and Follow-up
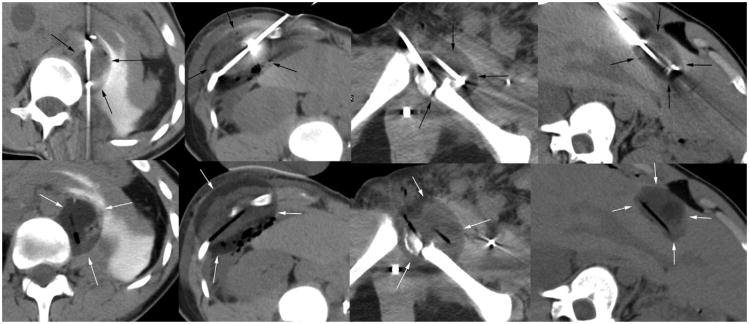
A total of 27 patients underwent 60 procedures for 72 tumors (Table 1). The mean number of procedures per patient was 2.2. The cryoablation zone was well defined by CT as a hypodense ice ball in soft tissues, with an average ablation diameter of 5.1 cm, generated by a mean probe number of 3.6 per patient for a mean tumor diameter of 3.3 cm. Figures 1–3 show four different treatment sites in a 19-year-old patient with mRCC. Grouping of our patients according to established risk criteria (10) yielded 0% categorized as having favorable risk, 67% categorized as having intermediate risk (18 of 27), and 33% categorized as having poor risk (nine of 27). Of the study patients, 22% (n = 6) and 67% (n = 18) received chemoimmunotherapy before or after MCA, respectively, with a total of 70% of patients (n = 19) receiving a systemic regimen at some point. Table 2 shows the details of chemoimmunotherapy before and after MCA.
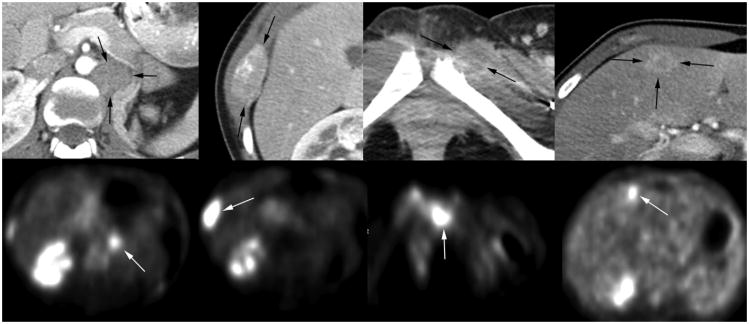
Figure 1.
Images from a 19-year-old woman with tubulocystic RCC in whom a systemic course of immunotherapy had failed. She presented (from left to right) with initial metastases (arrows) involving the paraaortic region of the nephrectomy bed and right lower lateral rib, and then developing in the left symphysis pubis and segment 4B of the liver. Top row shows preoperative, early enhanced CT images at the dominant cross-sectional appearance of each metastasis as a result of the hypervascular nature of her tumor foci. Bottom row depicts corresponding positron emission tomography images showing high metabolic activity (white arrows).
Figure 3.
Early enhanced axial CT images from comparable anatomic levels for the mRCC foci in Figures 1 and 2 (arrows, respectively) approximately 12 months after MCA at each site. Note the near-complete resorption and only thin remaining scar at each site, suggesting thorough treatment response and healing.
Table 2. Patients Receiving Systemic Regimens before or after MCA.
| Timing | IFN Monotherapy | Bevacizumab/IFN | Sorafenib | Sunitinib | Other | Total |
|---|---|---|---|---|---|---|
| Before MCA | 0 | 0 | 3 (11) | 2 (7) | 4 (15) | 6 (22)* |
| After MCA | 1 (4) | 1 (4) | 5 (19) | 5 (19) | 11 (41) | 18 (67)* |
| Total | 1 (4) | 1 (4) | 8 (30) | 7 (4) | 15 (56) | 19 (70)† |
Note.—Values in parentheses are percentages. IFN = interferon, MCA = multisite cryoablation.
Multiple patients received more than one systemic regimen, which results in overlapping data. The actual numbers of patients receiving chemoimmunotherapy before or after MCA were six and 18, respectively.
Some patients received chemoimmunotherapy before and after MCA, resulting in overlapping data. Actual number of patients receiving systemic regimens at some point was 19.
Complications
A total of 17 complications (28%) were grade 1/2 in severity and resolved on their own without any significant consequences (Table 3). Examples of such mild to moderate complications include hematuria and pleural effusions not requiring significant (if any) treatment. One procedure (1.7%) on a metastasis in the L5 vertebral body resulted in a grade 3 “foot drop” when the ablation zone abutted the S1 nerve root, resulting in decreased motor strength requiring an ankle brace.
Table 3. Rates of Complications per Procedure.
| Location | No. of Procedures | Grade | Grade > 3 Complications | |||
|---|---|---|---|---|---|---|
|
| ||||||
| 1/2 | 3 | 4 | 5 | |||
| Soft tissue | ||||||
| Nephrectomy bed | 11 | 4 | — | — | — | — |
| Adrenal gland | 8 | 2 | — | — | — | — |
| Paraaortic | 6 | — | — | — | — | — |
| Superficial | 9 | 2 | — | — | — | — |
| Intraperitoneal | 3 | 1 | — | — | — | — |
| Bone | 13 | 2 | 1 | — | — | — |
| Subtotal | 50 | 11 | 1 | — | — | — |
| Liver | 1 | — | — | — | — | — |
| Lung | 13 | 6 | — | — | — | — |
| Total | 64* | 17 (28.3) | 1 (1.7) | 0 | 0 | 1 (1.7) |
Note.—Values in parentheses are percentages.
Two patients had two procedures each in two areas, accounting for four extra procedures. The actual procedure count is 60; therefore, percentages are calculated divided by 60.
Recurrences
The median follow-up time for all patients was 16 months from the date of their last available CT or MR imaging study. Overall, ablation of 72 tumor sites resulted in one procedural recurrence (1%) and one satellite recurrence (1%; Table 4).
Table 4. Total Procedural and Satellite Recurrences by Anatomic Location of Tumor.
| Location | No. of Tumors | Total Local Recurrences |
|---|---|---|
| Soft Tissue | ||
| Nephrectomy bed | 11 | — |
| Adrenal gland | 9 | — |
| Paraaortic | 7 | — |
| Superficial | 12 | — |
| Intraperitoneal | 5 | — |
| Bone | 13 | 1 |
| Subtotal | 57 | 1 |
| Liver | 1 | 1 |
| Lung | 14 | — |
| Total | 72 | 2 (3) |
Note.—Values in parentheses are percentages.
Survival
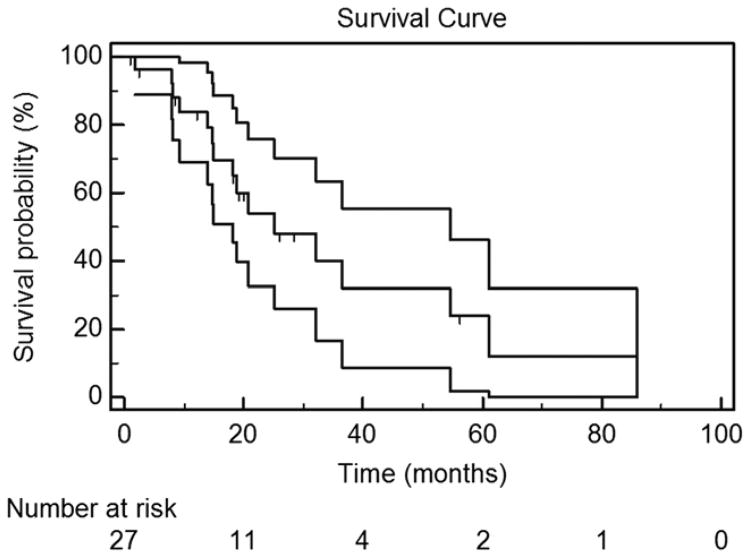
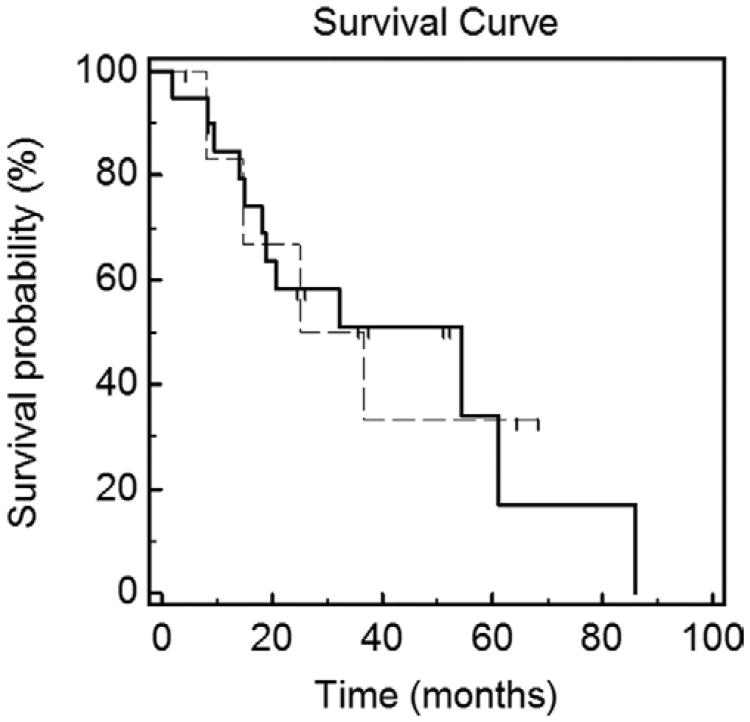
Figure 4 displays estimated OS. Of the 27 patients, 16 have died as of the time of manuscript submission. The median OS, or total life-years gained (LYGs), for patients undergoing MCA was 32.3 months, or 2.69 years. The 5-year OS rate was estimated to be 27%. Figure 5 displays the estimated OS for patients who received only BSC throughout their treatment (median survival, 30.9 mo) versus patients who received systemic therapy (median survival, 54.6 mo).
Figure 4.
Kaplan–Meier OS estimate in the 27 study-eligible patients. The top and bottom lines represent the 95% CI of each successive estimate of the survival rate. The median OS was 32.3 months (95% CI, 14.0–80.8 mo). The 2-year OS rate was 57% (95% CI, 37%–76%). The 3-year OS rate was 45% (95% CI, 24%–66%). The 5-year OS rate was 27% (95% CI, 4%–50%).
Figure 5.
Kaplan–Meier OS estimate for patients who received chemotherapeutic targeted therapy regimens before and after MCA (dashed line) versus patients who received only BSC throughout treatment (solid line). The median OS was 54.6 months for patients who were administered systemic therapy before/after MCA, and the median OS was 30.9 months for patients who received only BSC.
Cost
In all cases, “upper-bound” cost estimates produced total cost of each cryoablation procedure and frequent imaging follow-up of $54,833 ($12,833 per procedure plus $42,000 total for imaging follow-up). Multiple metastatic lesions were treated in an average of 2.2 procedures per patient, making the estimated upper-bound cost per patient of $70,233 (ie, $12,833 * 2.2 + $42,000). Table 5 demonstrates our modified cost-effectiveness evaluations for MCA based on comparisons with established values from the British National Health Service for five mRCC therapies: BSC, IFN monotherapy, bevacizumab with IFN, sorafenib, and sunitinib (3–8). Therefore, MCA was considered in its adjunctive role, whereby the adjunctive cost-effectiveness ratio (ACER) for MCA was still cost-effective when costs were added to the established chemoimmunotherapy regimens, with the average being $41,567 per LYG.
Table 5. Cost-effectiveness Estimates for Five Established Therapies for Widespread mRCC (3) in Conjunction with High-End Estimates of Cost for MCA.
| Outcome | BSC | IFN Monotherapy | Bevacizumab with IFN | Sorafenib | Sunitinib | MCA* |
|---|---|---|---|---|---|---|
| LYGs | 1.30 | 1.63 | 1.96 | 1.60 | 2.16 | 2.69 |
| QALYs | 0.91 | 1.19 | 1.45 | 1.15 | 1.62 | NA |
| QALY/LYG (%) | 0.70 | 0.73 | 0.74 | 0.72 | 0.75 | |
| Total cost ($)† | 5,927 | 14,091 | 89,968 | 46,421 | 66,170 | 70,233 |
| Cost per LYG ($) | 4,559 | 8,645 | 45,902 | 29,013 | 30,634 | 26,108 |
| ACER for adjunctive MCA‡ | ||||||
| Cost per LYG ($) | 28,312 | 31,347 | 59,554 | 43,366 | 50,707 | 44,657§ |
ACER = adjunctive cost-effectiveness ratio, BSC = best supportive care, IFN = interferon, LYG = life-year gained, MCA = multisite cryoablation, mRCC = metastatic renal cell carcinoma, QALY = quality-adjusted life-year.
Assumes 2.2 cryoablation procedures per patient and more image intensive follow-up.
Conversion factor of 1.67 from pounds to dollars was used to allow easier comparison, and conforms to the difference between established definitions of cost efficacy of $100,000 (29).
ACER for the adjunctive role for MCA when paired with systemic regimens assumes costs are additive and divided by a total LYG of 2.69 for MCA.
Average cost per LYG.
Discussion
This study suggests feasibility, safety, and potential cost-effectiveness of MCA as an adjunct to the palliative care of patients with oligometastatic RCC. We first summarize our findings and then cover their individual implications. Local tumor recurrence and procedure morbidity for this study were minimal and did not appear dependent on tumor location. Our projected 5-year survival rate of 27% in patients treated with MCA approaches the encouraging rates noted for pulmonary metastasectomy of mRCC (9–11). Overall, 30% of our patients with oligometastatic RCC did not receive any chemotherapy before or after MCA and appeared to benefit from an additional 1.39 years versus BSC (3). Our estimates of MCA cost-effectiveness suggest the worthiness of more detailed cost considerations (13,20–22) for ablation in an adjunctive role.
A unique aspect of cryoablation is its flexibility for pulmonary and soft-tissue locations, which are common in mRCC (12). Although resection may be favored in patients with multiple pulmonary metastases, 92% of patients with mRCC present with only one or two metastases, of which 45%–64% reside within the lung (12). Therefore, nearly half of mRCC locations are scattered in soft-tissue locations, such as lymph nodes, the nephrectomy bed, adrenal glands, pancreas, liver, and brain. Radiofrequency (RF) ablation has also been described for mRCC metastases of the adrenal gland, paraaortic nodes, and multiple pulmonary masses (23–27). Yet, cryoablation has benefits of low procedural pain (14) and excellent visualization of the low-density ice margins for thorough control and treatment planning (14–18). Cryoablation has also been noted to have excellent resorption of nonfibrous areas, suggestive of good healing of the necrotic tissue (15,17), whereas no significant resorption was noted at 1-year follow-up for RF ablation of adrenal mRCC metastases (22). The improved survival seen for our MCA group may have also been achievable with the use of RF or microwave ablation for the pulmonary ablations of oligometastatic RCC, similar to local surgical benefits. The largest reported single-center (25) and multicenter (26) experiences with lung RF ablation have showed encouraging survival for primary lung cancer and colorectal metastases; however, limitations continue to persist as a result of high impedance of the lung, which alters the electrical resistance of the tissue, making the ablation zone difficult to predict. Of the two series that reported pulmonary RF ablation for mRCC (27,28), local recurrences ranged from 9% to 36% and median survival estimates were between 21 and 60 months. Similar to the present study, these studies emphasize the heterogeneity of patients with oligometastatic RCC and their exposure to chemoimmunotherapy and/or metastasectomy. We therefore sought to place our survival rates in better context with outcomes from any established systemic series (3–8).
Relative improvements in OS are routinely measured in LYGs but should also assess relative morbidities. Our LYG estimates for MCA may therefore be better considered as an adjunct to systemic treatments, or ACER, for appropriate cost-effectiveness considerations. Also, in patients who were not in a favorable risk category, our median OS of 2.69 years was achieved in 67% of patients considered to be at intermediate risk and 33% of those considered to be at poor risk, most of whom would not have been surgical candidates (10). As a predominantly outpatient procedure with low procedural discomfort (14) and only minimal or transient reduction in functional status, MCA will also likely display favorable conversion of LYGs to future quality-adjusted life-year assessments.
We explored inflated cost estimates for MCA to gain insight whether the palliative use of MCA had reasonable potential for future more detailed cost-effectiveness or already exacerbated terminal health care costs. Our cost estimates also contain billing charges, rather than estimates of direct and/or indirect costs (11). These cost overestimates served as a potential economic counterbalance to any survival benefit noted for MCA, especially because ablation may be perceived as only adding costs to a palliative care setting.
Incremental cost effectiveness ratios have been used to evaluate treatment costs of mRCC in relation to mean LYGs (11); however, incremental assessments could not be accurately calculated because some of our patients received systemic regimens before or after MCA. As incremental cost effectiveness ratios below $100,000 per LYG were considered cost-effective (29), we used this parameter to assess the cost-effectiveness of MCA in an adjunctive role by adding the cost of MCA to each therapy under comparison, then dividing this total cost by the overall LYGs for MCA observed in the study. We termed this approach the ACER to more accurately estimate scenarios encountered by our patients receiving palliative MCA.
Our cost-effectiveness estimates were done to place the adjunctive role of MCA to consider the impact of its added cost for palliation (29). We acknowledge that thorough cost-effectiveness estimates should include utility estimates (eg, quality-adjusted life-years) as well as sensitivity analyses for probability and cost assumptions within the framework of a Markov or Monte Carlo decision model (20–22). Such in-depth analyses are beyond the scope of this study, which focused on the feasibility, safety, and OS assessments of MCA for palliation in relation to potential cost implications.
Numerous weaknesses in this feasibility and efficacy-based study relate to the relatively small patient population. More importantly, our patients were selected for oligometastatic RCC, the treatment of which may yield more favorable survival outcomes than seen in traditional stage IV disease. An important caveat to the observed results must take into consideration the inherent selection bias in studies comparing oligometastatic disease versus widespread stage IV disease. However, with the medical literature lacking studies that specifically address the rate of oligometastatic disease within drug trials, appropriate comparisons could not be feasibly conceived. Conversely, none of our patients were eligible for surgery based on established surgical selection criteria (10), and most had undergone systemic treatments that had failed. Indeed, some degree of systemic response may be a good indicator of long-term oligometastatic RCC status, and facilitates local ablation outcomes or OS, even though 30% of our patients received only MCA. Our observed OS was therefore considered adjunctive to systemic regimens when used to calculate LYGs and estimated cost-effectiveness.
Future comprehensive “social” cost-effectiveness analysis would require enumeration of additional costs on the patient's end (eg, travel, foregone wages, costs incurred by family members). Inclusion of these costs would increase the total cost estimates, yet would likely not compensate for our already upper-bound cost estimates.
In summary, percutaneous cryoablation of oligometastatic RCC was performed with minimal morbidity, complications, and/or local recurrence rates and may be associated with greater OS than systemic treatments alone. Our cost assessments also reveal data suggesting that cryoablation can achieve favorable results in an adjunctive role while remaining cost-effective.
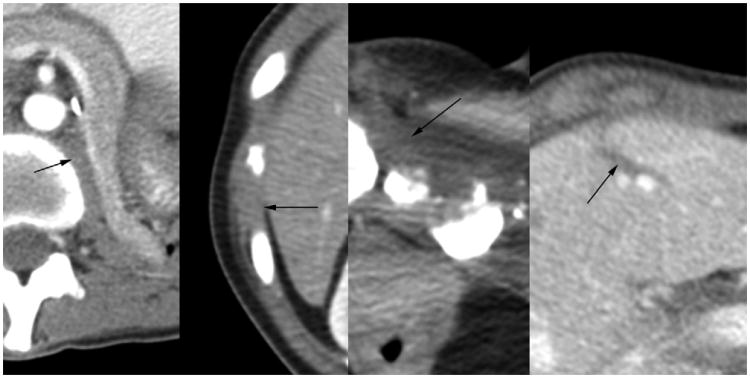
Figure 2.
Nonenhanced axial CT images matching the locations noted in Figure 1, showing cryoprobe in place (top row) and then removed (bottom row) to better define low-density ice (arrows) encompassing the prior tumor regions. All procedures were performed on an outpatient basis, with the patient discharged approximately 4 hours later.
Acknowledgments
This study was partially supported by National Institutes of Health Cancer Center Support Grant CA-22453. None of the authors have identified a conflict of interest.
Abbreviations
- ACER
adjunctive cost-effectiveness ratio
- BSC
best supportive care
- IFN
interferon
- LYG
life-year gained
- MCA
multisite cryoablation
- mRCC
metastatic renal cell carcinoma
- OS
overall survival
- RCC
renal cell carcinoma
- RF
radiofrequency
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ather M, Masood N, Siddiqui T. Current management of advanced and metastatic renal cell carcinoma. Urol J. 2010;7:1–9. [PubMed] [Google Scholar]
- 3.Thompson-Coon J, Hoyle M, Green C, Liu Z, Welch K, Moxham T, Stein K. Bevacizumab, sorafenib tosylate, sunitinib and temsirolimus for renal cell carcinoma: a systematic review and economic evaluation. Health Technol Assess. 2010;14:1–184. doi: 10.3310/hta14020. [DOI] [PubMed] [Google Scholar]
- 4.Pauker SG, Kassirer JP. Decision analysis. N Engl J Med. 1987;316:250–258. doi: 10.1056/NEJM198701293160505. [DOI] [PubMed] [Google Scholar]
- 5.Yang JC, Haworth L, Sherry RM, et al. A randomised trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. New Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 7.Rini BI, Halabi S, Rosenberg JE, et al. CALGB 90206: a phase III trial of bevacizumab plus interferon-alpha versus interferon-alpha monotherapy in metastatic renal cell carcinoma. 2008 ASCO Genitourinary Cancers Symposium Proceedings. Abstract 350. [Google Scholar]
- 8.Chowdhury S, Larkin JMG, Gore ME. Recent advances in the treatment of renal cell carcinoma and the role of targeted therapies. Eur J Cancer. 2008;44:2152–2161. doi: 10.1016/j.ejca.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann HS, Neef H, Krohe K, Andreev P, Silber RE. Prognostic factors and survival after pulmonary resection of metastatic renal cell carcinoma. Eur Urol. 2005;48:77. doi: 10.1016/j.eururo.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Eggener SE, Yossepowitch O, Kundu S, Motzer RJ, Russo P. Risk score and metastasectomy independently impact prognosis in patients with recurrent renal cell carcinoma. J Urol. 2008;180:873–878. doi: 10.1016/j.juro.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porter GA, Cantor SB, Walsh GL, et al. Cost-effectiveness of pulmonary resection and systemic chemotherapy in the management of metastatic soft tissue sarcoma: a combined analysis from the University of Texas M. D. Anderson and Memorial Sloan-Kettering Cancer Centers. J Thorac Cardiovasc Surg. 2004;127:1366–1372. doi: 10.1016/j.jtcvs.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Naito S, Yamamoto N, Takayama T, et al. Prognosis of Japanese metastatic renal cell carcinoma patients in the cytokine era: a cooperative group report of 1463 patients. Eur Urol. 2010;57:317–325. doi: 10.1016/j.eururo.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 13.Pandharipande PV, Gazelle GS. Comparative effectiveness research: What it means for radiology. Radiology. 2009;253:600–605. doi: 10.1148/radiol.2533091286. [DOI] [PubMed] [Google Scholar]
- 14.Allaf ME, Varkarakis IM, Bhayani SB, Inagaki T, Kavoussi LR, Solomon SB. Pain control requirements for percutaneous ablation of renal tumors: cryoablation versus radiofrequency ablation--initial observations. Radiology. 2005;237:366–370. doi: 10.1148/radiol.2371040829. [DOI] [PubMed] [Google Scholar]
- 15.Littrup PJ, Ahmed A, Aoun HD, et al. CT-guided percutaneous cryotherapy of renal masses. J Vasc Interv Radiol. 2007;18:383–392. doi: 10.1016/j.jvir.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Littrup PJ, Duan Y, et al. Thoracic masses treated with percutaneous cryotherapy: initial experience with more than 200 procedures. Radiology. 2005;235:289–298. doi: 10.1148/radiol.2351030747. [DOI] [PubMed] [Google Scholar]
- 17.Littrup PJ, Jallad B, Chandiwala-Mody P, D'Agostini M, Adam BA, Bouwman D. Cryotherapy for breast cancer: a feasibility study without excision. J Vasc Interv Radiol. 2009;20:1329–1341. doi: 10.1016/j.jvir.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Littrup PJ, Jallad B, Vorugu V, Littrup G, Currier B, George M, Herring D. Lethal isotherms of cryoablation in a phantom study: effects of heat load, probe size, and number. J Vasc Interv Radiol. 2009;20:1343–1351. doi: 10.1016/j.jvir.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee E, Wang JW. Statistical methods for survival data analysis. 3rd. Hoboken, NJ: Wiley; 2003. [Google Scholar]
- 20.Pandharipande PV, Gervais DA, Hartman RI, et al. Renal mass biopsy to guide treatment decisions for small incidental renal tumors: a cost-effectiveness analysis. Radiology. 2010;256:836–846. doi: 10.1148/radiol.10092013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandharipande PV, Gervais DA, Mueller PR, Hur C, Gazelle GS. Radiofrequency ablation versus nephron-sparing surgery for small unilateral renal cell carcinoma: cost-effectiveness analysis. Radiology. 2008;248:169–178. doi: 10.1148/radiol.2481071448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gazelle GS, McMahon PM, Beinfeld MT, Halpern EF, Weinstein MC. Metastatic colorectal carcinoma: cost-effectiveness of percutaneous radiofrequency ablation versus that of hepatic resection. Radiology. 2004;233:729–739. doi: 10.1148/radiol.2333032052. [DOI] [PubMed] [Google Scholar]
- 23.Mouracade P, Dettloff H, Schneider M, Debras B, Jung JL. Radio-frequency ablation of solitary adrenal gland metastasis from renal cell carcinoma. Urology. 2009;74:1341–1343. doi: 10.1016/j.urology.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 24.Arellano RS, Flanders VL, Lee SI, Mueller PR, Gervais DA. Imaging-guided percutaneous radiofrequency ablation of retroperitoneal metastatic disease in patients with gynecologic malignancies: clinical experience with eight patients. AJR Am J Roentgenol. 2010;194:1635–1638. doi: 10.2214/AJR.09.3561. [DOI] [PubMed] [Google Scholar]
- 25.Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243:268–275. doi: 10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]
- 26.Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study) Lancet Oncol. 2008;9:621–628. doi: 10.1016/S1470-2045(08)70155-4. [DOI] [PubMed] [Google Scholar]
- 27.Soga N, Yamakado K, Gohara H, et al. Percutaneous radiofrequency ablation for unresectable pulmonary metastases from renal cell carcinoma. BJU Int. 2009;104:790–794. doi: 10.1111/j.1464-410X.2009.08459.x. [DOI] [PubMed] [Google Scholar]
- 28.Shu Yan Huo A, Lawson Morris D, King J, Glenn D. Use of percutaneous radiofrequency ablation in pulmonary metastases from renal cell carcinoma. Ann Surg Oncol. 2009;16:3169–175. doi: 10.1245/s10434-009-0664-5. [DOI] [PubMed] [Google Scholar]
- 29.Folland S, Goodman AC, Stano M. Cost benefit analysis and other tools of economic evaluation. In: Folland S, Goodman AC, Stano M, editors. The Economics of Health and Health Care. 6th. Upper Saddle River, NJ: Prentice Hall; 2010. pp. 74–96. [Google Scholar]