Abstract
Inflammatory bowel disease (IBD), consisting of both Crohn’s disease (CD) and ulcerative colitis (UC), are chronic inflammatory conditions of the intestinal tract. As there is no cure for either CD or UC, these patients face numerous treatment decisions regarding their disease. The aims of this review are to evaluate literature regarding quantitative studies of patient preferences in therapy for IBD with a focus on the emerging technique of stated preference and its application in IBD. Numerous simple survey-based studies have been performed evaluating IBD patients’ preferences for medication frequency, mode of delivery, potential adverse events etc., as well as variations in these preferences. These studies are limited, however, as they are purely descriptive in nature with limited quantitative information on the relative value of treatment alternatives. Time trade-off and standard gamble studies have also been utilized to quantify patient utility for various treatment options or outcomes. However, these types of studies suffer from inaccurate assumptions regarding patient choice behavior. Stated preference is an emerging robust methodology increasingly utilized in health care that can determine the relative utility for a therapy option as well as its specific attributes (such as efficacy or adverse side effects). Stated preference techniques have begun to be applied in IBD and offer an innovative way of examining the numerous therapy options these patients and their providers face.
1. Inflammatory Bowel Disease: Overview and Treatment Options
Inflammatory bowel disease (IBD), consisting of two types of disease, Crohn’s disease (CD) and ulcerative colitis (UC), are chronic relapsing/remitting inflammatory conditions of the intestinal tract that have no cure. The etiology of IBD is unclear, but is believed to be multifactorial including a dysregulated immune system. IBD affects over 1.3 million Americans and the prevalence and incidence of IBD is increasing.1 The peak incidence of IBD occurs in the 2nd and 3rd decade of life, and this predominance in a younger population can result in a large economic burden from both chronic treatment as well as lost productivity. In CD, disease can occur anywhere from the mouth to the anus, is progressive over time, and is associated with several complications including abscesses, fistulae, and stricture formation from active disease. In contrast, UC is limited to the colon and therefore surgical removal of the colon, specifically a total proctocolectomy, provides a potential surgical “cure.” The two most common procedures performed are a total proctocolectomy with end ileostomy (an external ostomy bag) and restorative ileal pouch anal anastomosis (IPAA). However, the surgery itself has its own risks of morbidity and mortality; and quality of life after surgery can be compromised.
1.1 Treatment Options in IBD
When evaluating therapy options in IBD, it is important to consider that clinical drug efficacy trials in both UC and CD have traditionally had two different endpoints--clinical response and clinical remission. The former is improvement of clinical symptoms over a baseline score while the latter is an objective improvement to a pre-defined definition of remission based upon a disease severity indice.
In UC, treatment often begins in a “step-up” fashion with mesalamine (5-ASA) therapy, a relatively safe and effective therapy for mild-to-moderate UC. However, 5-ASA fails to induce a clinical remission in 50% or more of UC patients.2–8 For patients in whom 5-ASA therapy is inadequate to control their disease, the next drug utilized are often corticosteroids. Unfortunately, over 50% of patients either will suffer disease recurrence upon discontinuation of corticosteroids, or be unable to taper off corticosteroids at all due to recurrent disease at lower doses of the drug.9 Given that both short- and long-term corticosteroid use is associated with a significant number of potential adverse side effects, alternatives to corticosteroid therapy have been developed. These include potent classes of immunosuppressant medications called immunomodulators (including the thioprine analogs azathioprine and 6-MP) and anti-TNF therapies. Cyclosporine and tacrolimus have also been used as a bridge to thiopurines for refractory UC, particularly in patients who have failed to respond to intravenous corticosteroids.10,11
Because UC is limited to the colon, surgery offers a possible cure for the disease. The two most common operations performed for UC are total proctocolectomy with end ileostomy and restorative ileal pouch anal anastomsosis (IPAA). While UC patients having had surgery feel, after a period of adjustment, that they are better off than before surgery12, most patients and physicians who contemplate surgery consider it an option of “last resort.” At a national level, surgical rates have not changed significantly over the past decade.13 It is estimated, however, that 25–30% of UC patients eventually will require colectomy for medication-refractory disease.14,15
While many of the same therapies used in UC are utilized in CD, there are significant differences. Even if a patient is clinically asymptomatic, tissue damage can continues to progress and result in numerous complications.16,17 Up to 80% of CD patients will require intestinal surgery, with 30% requiring a second operation.1 50% of patients have perianal involvement (including perianal fistula and abscesses) which risk repeated surgeries and fecal incontinence.1 Furthermore, because disease can recur post-operatively, the goal of treatment is to avoid bowel resection surgery. Therefore there is no “surgical cure” for CD. Finally, CD itself has an increased overall-mortality compared to the general population, in part due to CD-related complications including malnutrition, post-operative complications, and intestinal cancer.18,19 Unfortunately, morbidity and mortality rates have not changed over the last 5 decades, despite improvements in medical therapies, as these medications often are not started early enough in disease progression (before irreversible damage occurs); or are not maintained in a regular fashion, allowing for progression of tissue damage during gaps in therapy.
As a result of these prognostic differences in CD, therapies and therapy algorithsm are also different. Mesalamine therapy has not been shown to be effective in CD.20,21 In patients with perianal disease or certain disease complications may be treated with antibiotic therapy for short amounts of time. Patients with mild disease can be started on corticosteroids or budesonide, a steroid medication with limited systemic absorption. However, given the potential for disease progression, even if asymptomatic patients, treatment algorithms have evolved over the past decade to emphasize a more aggressive approach to CD care. Studies have shown that a “top down” approach, with initiation of a combination of immunosuppressant medications from disease onset (typically a thiopurine analog plus an anti-TNF therapy), is associated with improved disease remission and decreased CD-related complications.22,23 While cyclosporine has not been found to be effective in CD, the thiopurine analogs, anti-TNF medications, and methotrexate have been shown to be effective in treating CD.22,24–31 An additional medication natalizumab, a humanized monoclonal antibody against the cell adhesion molecule α4-integrin, has also been shown to increase rates of remission and prevent disease relapse in refractory CD.32
Over the past decade, a greater understanding of the progression of IBD has also led to the increasing belief that a more rigorous endpoint, that of mucosal healing (e.g. endoscopic remission) should be the goal of IBD care.33 This has endorsed shifts in treatment algorithms to a more early-aggressive approach, especially in CD; and also has implications for how drug efficacy is evaluated in both diseases. However, patients’ willingness to accept the risks of additional therapy (see below) in the absence of symptoms can be at odds with such recommendations. Furthermore, certain populations of patients, such as pediatric patients, may be unwilling to accept long-term or lifelong risks of mono- or combination-immunosuppressant therapy, especially if they have never experienced any medical therapy for their disease.
1.2 Risks of Medical and Surgical Therapy in IBD
Medical therapy in IBD carries several potential risks. Corticosteroids are associated with numerous well-characterized side effects including near inevitable occurrence of bone disease and cataracts with long-term use. Furthermore, corticosteroid therapy has been associated with an increased mortality risk.34,35 Given their effects on the immune system, corticosteroids and the immunosuppressant medications have been associated with serious opportunistic infections. Corticosteroids have been associated with a 2–3 times increased odds of serious infections.36,37 Rates of infection for those on thiopurine analogs and anti-TNF therapy are as high as 5% per year, including a fatal central nervous system infection called progressive multifocal leucoencephalopathy (PML) with natalizumab.37–41 The thiopurine analogs and anti-TNF therapies also may increase the risk of certain cancers including an increased risk of cervical dysplasia and non-melanoma skin cancer.42–44 The risk of lymphoma associated with thiopurine analogs and anti-TNF use that may be as much as four times that of the general population; with combination therapy, this risk increases to 6–10 times that of the general population.45–48 A particularly aggressive and nearly universally fatal form of lymphoma, called hepatosplenic T-cell lymphoma (HSTCL), is associated with immunosuppression particularly in young adults.49–51 By virtue of retaining their colon, IBD patients also have a lifetime risk of colon cancer that is as high as three times that of the general population 52
As with all medications, there is the risk of medication failure for therapy in IBD. Best clinical trial efficacy rates indicate that at least one-third of patients will fail induction or maintenance of remission with these medications at one year.21,22,26,28,29,31,53–56 During this time, patients are exposed to the risks of medical therapy as well as the risk of continued active disease. The consequences of incompletely treated disease as a result of medication failure are poorly understood. However, indirect evidence suggests that patients are at increased risk of morbidity and mortality including hospitalization, complications of active disease (such as abscess or stricture formation) or emergent surgery which carries a substantially higher morbidity and mortality rate than elective or planned surgery.1,34,35,57–60.
In UC, the option of surgery also carries risks, related to both the surgery itself and the post-operative quality of life. While the majority of patients will successfully undergo IPAA surgery, a minority will instead have a permanent externally draining ileostomy. Those who do have IPAA surgery still have six bowel movements a day on average. This means that some patients will have more bowel movements than prior to their colectomy. Additionally, patients with IPAA surgery are at risk for having fecal incontinence, impeding quality of life. Mortality rates with elective colectomy range are no higher than 1% at 35 months; and as low as 0.5% at 33 months.12,61,62 However, with emergent colectomy, mortality is much higher.57,60,63,64 As with all surgery, there is a risk of post-operative infection following colectomy. Reported rates range of septic complications, pelvic abscesses and wound dehiscence range from 1%–10%, depending on the institution.12,61,65
1.3 Decision Making in IBD
As illustrated above, patients with IBD face numerous decisions regarding therapy for their disease. For patients with mild UC, there are decisions about mesalamine therapy, specifically, route and frequency of administration. For the majority of UC patients, mesalamine therapy will be insufficient, and these patients face decisions regarding chronic repeated corticosteroid use, long-term immunosuppressant therapy, or colectomy surgery. Likewise, in CD, patients face decisions regarding chronic repeated corticosteroid use or initiation early in the disease with long-term immunosuppressant therapy, either as a single drug or as a combination of drugs.
Without the ability to predict which patients will respond to which therapies, or how aggressive disease will be, IBD patients and their physicians often find themselves in a variety of challenging clinical decision-making situations with no clear correct solutions. Because all potential therapies (medical and surgical) have potential risks and benefits, and thus implications for patient quantity and quality of life, patient collaboration in this decision-making is therefore essential. The last few decades have seen an increased importance placed on patient preferences in healthcare.66,67 Patient preferences arguably play a critical role in health care outcomes: patients’ preferences for therapies influence adherence, compliance and satisfaction with therapies, which in turn influences overall care. In turn, patient preferences can inform physicians in their daily interaction with patients; regulators in setting thresholds for therapy efficacy and risk; and national organizations in setting treatment guidelines. Thus, rigorous methodologies capable of accurately quantifying patient preferences in large patient populations are needed.
2. Preferences, Health-State Utility, and Patient-Reported Outcomes
The increased focus on patient-centered health care and medical research in recent years has heightened awareness of the importance of patients’ perspectives in assessing health-care interventions.68–71 Patients’ perspectives include information on outcomes such as pain that are not clinically observable. These outcomes are quantified using patient-reported outcome (PRO) instruments. For cost-utility studies, it is necessary to identify a common scale for combining and comparing dissimilar oucomes. Methods such as standard gamble (SG) and time-tradeoff (TTO) are widely used to obtain patient-derived health-state utilities for both clinically observable and PRO outcomes. Patients’ perspectives also are essential for obtaining values or weights indicating patients’ trade-off preferences for health outcomes, health-care processes, and convenience features of treatments. Stated-preference methods including contingent valuation and conjoint analysis or discrete-choice experiments (DCEs) have been increasingly used to quantify these subjective preference weights.
Thus PRO instruments, health-state utility methods, and stated-preference surveys were developed to answer different questions about patient concerns. Unfortunately, there is considerable misunderstanding about similarities and differences among methods and how these different patient-centered measurement techniques contribute to outcomes research. For example, PROs quantify health outcomes, but do not measure patient preferences.72 Health-state utility measurement techniques are used to calculate standardized quality-adjusted life years (QALYs) but do not account for the wide range of factors that contribute to patient satisfaction.73–81
The focus of this review is to evaluate the literature regarding quantitative studies of patient preferences in therapy for IBD. While most published studies have utilized conventional health-state utility measurement techniques, we evaluate limitations of this literature and explore the growing field of stated preference and its application in IBD.
3. Literature Review
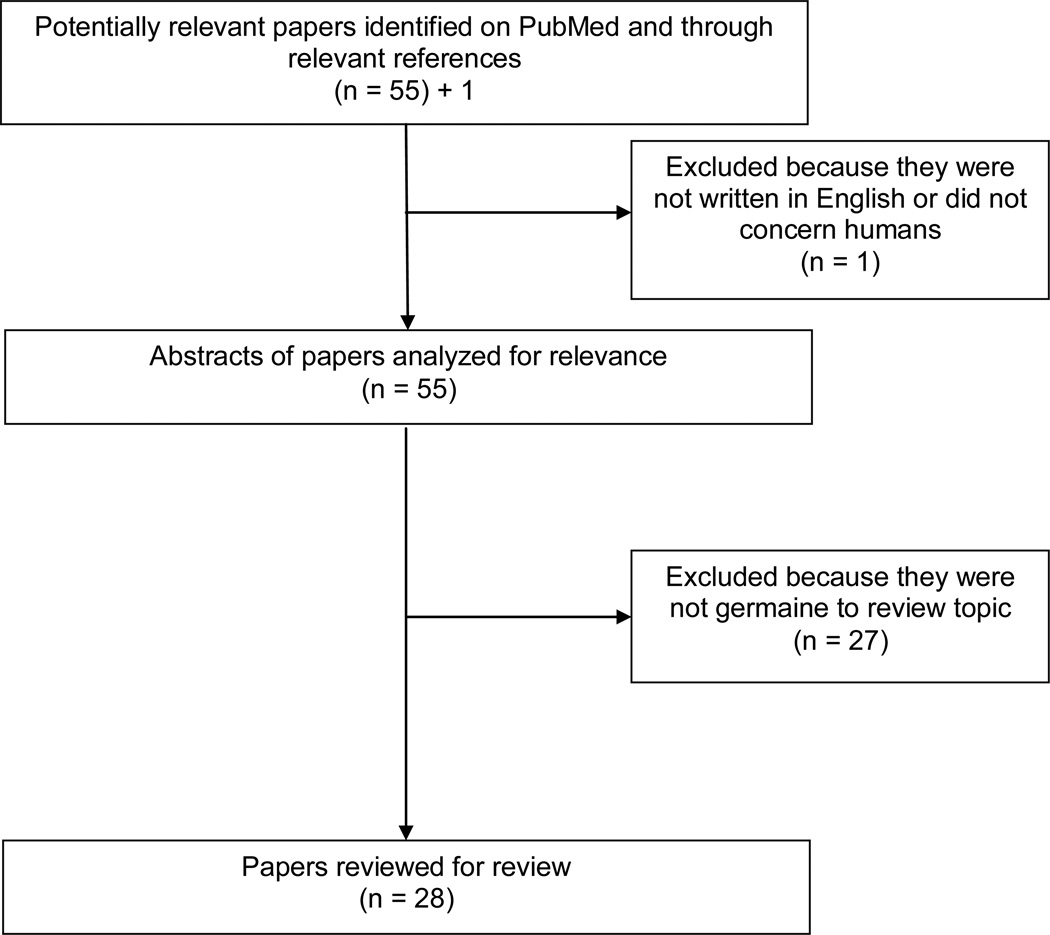
To identify published studies on patient preferences in IBD, a systematic PubMed search was conducted on December 20, 2012. The search used the following keywords and MESH headings: survey preferences or time trade off or standard gamble or conjoint analysis or discrete choice combined with inflammatory bowel disease or ulcerative colitis or Crohn’s disease. This approach identified 54 papers published between 1987 and 2012 (Figure 1). Two additional citations were identified through review of references or personal communication. Application of the limitations “English language” and “human studies” yielded 55 papers for analysis. Each study was systematically reviewed to assess for appropriateness for the current review. Studies were excluded because they did not evaluate patient preferences (9); were review or commentary articles (2); were not specific to IBD (5); did not evaluate preferences between treatment options and/or measured utilities for correlation with quality-of-life measurement tools only (6); was primarily a methodology analysis (1) or were cost-effectiveness analyses (4). Twenty-eight articles were evaluated for this review.
Figure 1.
Identification of Studies
4. Survey studies of IBD preferences
The most basic form of assessing patient preferences are simple questionnaire-based surveys. Given the variety of issues facing IBD patients and their providers, a number of studies have utilized survey methods to obtain information on a range of issues. Our search identified 15 survey studies on topics including information needs and delivery for IBD patients; perceptions regarding colorectal cancer screening and recommendations in IBD; preferences for shared decision-making; preferences for potential treatments (administration, efficacy, safety, dosing); adolescent and pediatric IBD preferences; and preferences regarding genetic testing in IBD (Table I).
Table I.
Survey Studies of IBD Patient Preferences
| Study | Study Population and Sample Size | Study Aim |
|---|---|---|
| Wong S et al.82 | 241 UC and CD patients | Evaluate the information needs and preferred mode of information delivery in patients with IBD |
| Conrad S et al.83 | 1056 UC and CD patients | Evaluate information needs, preferred sources of information and preferred role in decision-making |
| Bernstein KI et al.84 | 74 UC and CD patients (newly diagnosed) | Evaluate information needs and preferred method of information delivery for newly diagnosed IBD patients |
| Siegel CA et al.85 | 199 UC patients | Evaluate UC patients’ perception of colon cancer risk and assess preferences for colectomy surgery in the setting of dysplasia |
| Baars JE et al.86 | 617 CD patients 450 UC patients |
Evaluate IBD patients preferences regarding shared decision-making |
| Allen PB et al.87 | 125 UC and CD patients | Evaluate preferences for infliximab versus adalimumab |
| Lewis JR et al.88 | 30 UC and CD patients | Evaluate IBD patient preferences for genetic testing in IBD |
| Gray JR et al.89 | 100 UC patients | Evaluate patients preferences regarding therapies including efficacy, dosing frequency, cost, etc. |
| Knopf JM et al.90 | 22 IBD patients and their parents | To determine differences and similarities between pediatric patients and their parents with regards to decision-making |
| Kennedy ED et al.91 | 127 CD patients | Evaluate patient preferences for postoperative maintenance therapies |
| Konda V et al.92 | 82 CD patients 25 UC patients 7 other (presumably indeterminant) |
Evaluate patient interest in genetic testing and willingness to accept uncertainty of these tests |
| Rutter MD et al.93 | 281 patients | Evaluate patient preferences and experience with colonoscopy surveillance in UC |
| Cheung WY et al.94 | 69 general practitioners caring for IBD patients | Evaluate physician preferences for open access outpatient follow-up for their IBD patients |
| Green TJ et al.95 | 76 CD 49 UC |
Evaluate the dietary practices and effectiveness of these diets in pediatric IBD patients |
| Probert CS et al.96 | 70 UC and CD patients | Evaluate patient preferences for receiving information regarding their disease |
All of these studies provide some insights regarding attitudes of both patients and physicians toward IBD care. However, these studies generally are descriptive in nature (typically presenting percentages of respondents as results), with limited quantitative information on the perceived value of treatment alternatives. More specifically, while a survey may tell us the average score a given outcome has on a Likert or visual-analog scale, such metrics do not provide valid estimates of intensity of preferences.72,97 The resulting data thus do not provide information on what patients would be willing to give up to obtain outcome A, or what specifically makes outcome A preferable to outcome B. For example, in the study by Allen et al, the authors evaluated patients’ stated preferences for infliximab (an intravenous medication) versus adalimumab (a self-injectable medication), and described a trend in respondents towards a preference for infliximab.87 However, the authors could not evaluate what features (frequency of infusions, mode of administration, etc) of the drugs evaluated could have explained this finding, the intensity of preference for these features or the drugs themselves, or what would cause patients to change their mind regarding their preferences.
Eliciting patient trade-off preferences in a realistic, although hypothetical, clinical context is more likely to be informative about how patients would evaluate actual therapeutic decisions. For example, researchers in one study asked patients if they would be willing to accept a medication with a 98% remission rate but risks similar to that of infliximab (0.4% mortality risk).98 Over 60% of patients said the benefits of this drug did not justify its risks. However, it is unclear what implicit alternative treatment patients assumed in answering the question. If patients had been told that they could take this drug or face colectomy surgery because of complications resulting from disease progression, it is possible that a greater number of patients would consider the hypothetical medication to be acceptable.
5. Time trade-off and standard-gamble studies in IBD
Health-state utility is a well-known cardinal index of the quality of a given health state. Utilities can be measured at population or individual levels, and vary as people’s health changes. Changes in health states can be expressed as incremental utility elicited by either time-tradeoff or standard-gamble question formats. Utilities can be converted to QALYs that are used in cost-utility analysis. QALYs weight durations in each health state by the average utility of that state and facilitate health-outcome comparisons across groups of people, health outcomes and durations.
In time-trade-off (TTO) studies, respondents evaluate specific treatment-outcome scenarios and are asked how much of a reduction in expected life years they would accept for living in perfect health instead of living the rest of their expected lifetime in the compromised health state. Health-state utility is measured as the ratio of equivalent years in perfect health to years in compromised health.
Standard-gamble studies determines the chance of the worst imaginable health state (usually assumed to be death) that a patient would be willing to accept in return for the best possible health (usually defined as perfect health). The patient typically imagines a treatment that would completely cure their symptoms but involves a risk of immediate death. Setting the utility of death at zero and the utility of best possible health at 1, and assuming that expected utility is the sum of outcomes weighted by probabilities, one minus the indicated risk of death indicates the health-state utility.
A significant number of the TTO and SG studies evaluating health-state utilities for various IBD therapies have been small studies, often with sample sizes of less than 50 patients (Table II). The majority have focused on patients with UC, and specifically preferences for continued medical management of UC versus colectomy surgery. This decision branch lends itself rather elegantly to analysis, as it involves two very different decisions and their corresponding varying health-state utilities. One study directly evaluated utilities for colectomy surgery versus continued medical therapy in UC. Arseneau and colleagues evaluated 48 UC patients’ utilities in steroid-refractory UC for colectomy surgery, therapy with infliximab and/or therapy with cyclosporine for use in Markov modeling for these various treatment decisions.99 However, one prominent finding in their results was the highly variable measure of optimal treatment choice (e.g. that which maximized their QALYs) among patients, with total colectomy being the optimal treatment choice for 37% of their patient sample. When utilizing the average utility for analysis, medical therapy was superior to colectomy surgery. However, in their probabilistic sensitivity analysis utilizing multiple simulations of treatment decisions per patient, only one-third of patients had highly robust and reproducible optimal treatment decisions. The authors concluded that assessing individual patient utilities was important given the significant number of UC patients for which there was no clear superior treatment.
Table II.
Time trade-off (TTO) and standard gamble (SG) studies in IBD
| Study | Study Type | Study Population and Sample Size | Study Aim |
|---|---|---|---|
| McLeod RS et al.100 | TTO | 20 pre- & post-colectomy UC patients 93 post-colectomy UC patients |
Evaluate perceived quality of life by UC patients pre-colectomy and post-colectomy surgery |
| Kennedy ED et al.101 | SG | 65 CD patients | Determine if CD patients value absolute reduction in post-operative disease recurrence attributable to 5-ASA therapy* |
| Arseneau KO et al.102 | SG | 32 CD patients 15 non-CD patients |
Determine utilities for CD patients for varying perianal fistula treatments† |
| Arseneau KO et al.99 | TTO | 48 UC patients | Determine utilities for steroid-refractory UC for colectomy versus varying medical therapy† |
| Byrne CM et al.103 | TTO/SG | 41 CD patients 92 colorectal surgeons 74 gastroenterologists |
Compare preferences for surgical intervention in CD between patients, gastroenterologists and surgeons |
| Waljee AK et al.104 | TTO | 150 non-UC patients 150 UC patients 150 UC post-colectomy patients |
Evaluate differences in utility of active UC versus post-colectomy state |
| Waljee AK et al.105 | SG/TTO | 150 UC patients 150 post-colectomy UC patients |
Evaluate discount rate used by UC patients when evaluating colectomy |
| Brown LK et al.106 | TTO | 17 physicians 150 post-colectomy UC patients 69 moderate UC patients |
Compare physician and patient preferences for active UC versus colectomy |
further discussion in letter to editor107
utilities determined for Markov modeling
Three studies examined the perceived and actual utility of pre- and post-colectomy outcomes in UC. McLeod et al. examined 20 UC patients’ perceptions of their post-operative quality of life prior to surgery and compared this to their realized 1-year post-operative quality of life utilities; and further examined 93 UC patients over one year after colectomy surgeries.100 They found that mean realized post-operative utilities were significantly higher than perceived utilities and did not vary by colectomy surgery type.100 Walijee et al also evaluated differences in utility of UC patients with active disease versus those having a colectomy. They surveyed patients without UC, patients with UC without colectomy and post-colectomy UC patients with standardized scenarios for moderately active UC and post-colectomy states.104 Those without UC viewed both the UC and post-colectomy scenarios equally poorly. UC patients, however, reported similar utilities for both active UC and the post-colectomy scenarios. This was in contrast to those patients who had a colectomy: these patients perceived active UC as significantly worse than the post-colectomy state.104 Finally, a recent study compared physicians and UC patients’ preferences for active UC versus colectomy.106 They evaluated 17 physicians, 150 UC patients having had a colectomy and 69 patients with moderately active UC with standardized scenarios for active disease and post-surgery. Both the physicians and those patients having a colectomy viewed UC more poorly than the post-colectomy state; however, patients who had not had a colectomy but had moderately active UC indicated that both their active disease and the post-colectomy state had equal utilities.106
Taken together, this evidence would seem to suggest that patients with UC who have not undergone colectomy surgery associate this treatment and the post-surgery state with significantly diminished quality of life versus those who have actually had the surgery. A study evaluated this apparent preference by UC patients for more long-term health risks (e.g. immunosuppression) versus short-term health risks (such as colectomy surgery) by focusing on the standard 5% discounted rate for future health traditionally used in TTO/SG studies.105 The authors determined that this standard discount rate was a substantial underestimation of measured UC patients’ discount rate, suggesting that UC patients either have a strong aversion to immediate risks in favor of future risks or place a lower value on future health in favor of more immediate health.105 However, in all comparisons of pre- and post-colectomy health states, it is important to appreciate that the comparison is not equivalent: those having undergone colectomy surgery more likely did so due to medication-refractory disease. Their disease history (including duration of disease and medication use history) would be expected to impact their risk preferences.
There is no option for a surgically curative procedure in CD. However, up to 80% of CD patients will require intestinal surgery during their disease history, with a significant portion requiring a second operation.1 A study evaluated CD patients’ utilities for various surgical interventions versus medical therapy for their CD, and compared these utilities to those of surgeons and gastroenterologists using scenarios illustrating different surgical outcomes.103 They found that gastroenterologists were more strongly averse to surgery than either surgeons or CD patients; and in half of the scenarios provided, gastroenterologists’ and CD patients’ utilities diverged in a significant fashion, most notably regarding an open or laparoscopic ileocolic bowel resection, one of the most common procedures performed in CD.103 Furthermore, gastroenterologists and colorectal surgeons’ utilities were similar in less than half of the scenarios provided.103
Given the lack of a surgical cure in CD, disease therapy relies much more heavily on medical management with a goal of preventing first surgeries and/or decreasing the risk of recurrence of disease necessitating subsequent surgeries. In a Markov model examining 1-year cost and utility for infliximab (an infusion medication) compared to the oral medications 6-MP and metronidazole for perianal fistula therapy in CD, utilities were evaluated from CD and control patients and found no differences in measured utilities for various therapy options.102 Another study aimed to examine the utilities patients had regarding mesalamine therapy postoperatively to prevent disease recurrence.101 However, a subsequent letter to the journal editors remarked that the authors of this study were not evaluating utilities per se, but rather actions, an important distinction within the utility theory framework that can lead to miscalculations of actual utilities expected based upon these measured actions.107
5.1 Advantages and Disadvantages of Time Trade-off and Standard Gamble
Using traditional methods such as TTG and SG to obtain utility values for QALY estimation is widely accepted for health-technology assessment because these methods allow for a simple method of integrating mortality, morbidity and preferences for therapies into a single estimation representing the equivalent years of perfect health. This allows for relatively simple comparisons with other QALY-based measurements (including some quality of life questionnaires) and utilization of these QALYs for cost-utility analyses.
However, it is exactly this simplicity that can become problematic due to inaccurate assumptions regarding patient preferences. TTO and SG studies suffer from a number of fundamental limitations that have been recognized for several decades.77–81 The clinically-artificial method of eliciting patient utilities in TTO/SG studies employs cardinal-utility, a ratio-scale metric rejected by nineteenth-century utility theorists in favor of ordinal-utility measures. Ordinal utility is the basis of virtually all subsequent applied-economics research.108
Numerous validity tests of QALY studies also have rejected the assumptions of independence, procedural and description invariance, linearity over time and comparability across groups of patients.109,110 Furthermore, in the interest of simplicity, conventional TTO and SG applications assume that health history or current health state do not affect relative preferences. To the extent that disease attributes (including duration of disease, history of complications and current disease symptoms) would be expected to impact patient preferences, these conventional methodologies may lead to biased comparisons between and within groups of patients. Moreover, conventional health-state utility measurement techniques are unable to capture the impact of acute conditions, treatment risks, or process-related factors such as the method of administration or treatment duration. Such factors can play a significant role in understanding patients’ preferences for IBD treatments.
Additionally, TTO and SG studies have been shown to have significant failings in populations with low numeracy. A full discussion regarding numerical issues in patient-preference studies is outside the scope of this review; however treatment decisions and patient preferences do require patients to integrate and weigh pros and cons of medical decisions in a numerical fashion. Several factors have been found to be important in how patients perceive risks. This includes framing, which refers to how risk is presented;111–113 how numbers are presented (such as frequencies, which have been shown to be easiest to understand, versus proportions or probabilities which are more artificial constructs and require additional conditional math);114–118 and base-rate neglect (susceptibility to numerators with relative neglect of the corresponding denominator).114–117,119 These issues are attenuated in low-numeracy populations, a significant issue in the United States.111,112,114–117,120–124 And patients with low-numeracy skills have proven difficulties appropriately completing SG and TTO tasks appropriately.122,125,126
6. Discrete-Choice Experiments in IBD
DCEs, also known as choice-format conjoint analysis, employ a multi-attribute preference-elicitation technique that quantifies the strength of preferences for features of products, services or health-care interventions. Interventions, such as medical or surgical treatments in IBD, derive value from their specific attributes, features or outcomes, including treatment efficacies, tolerability, convenience, and potential serious adverse risks. In turn, each of these attributes has varying levels, such as efficacy rates or adverse risk rates. DCEs systematically elicit tradeoffs among constructed outcome combinations to generate choice data that quantifies implicit decision weights indicating relative utility for both treatment attributes as well as the treatment option as a whole. Because DCEs measure the rate at which patients accept tradeoffs among different treatment attributes, it is also possible to calculate a maximum acceptable risk or maximum probability of an adverse event that participants are willing to tolerate in exchange for a given treatment benefit. DCE increasingly has been applied in the field of healthcare for eliciting patient preferences for a range of medical and surgical therapies across many disease states.127–133
Given the numerous treatment options, both medical and surgical, in UC and CD, there are a number of opportunities to evaluate patient and provider preferences (Table III). The majority of published studies have evaluated preferences in CD, and all have utilized larger sample sizes of study participants than prior methodologies. In a DCE examining preferences for therapies in CD, the three most important attributes of medical therapy were achievement of a lasting remission, frequency of medication administration and how quickly the patient achieved a response to therapy.134 One of the first examined CD patients’ preferences for lethal adverse events in exchange for medication efficacy.135 One of the risks in this study was for the rare but lethal neurological side effect PML associated with natalizumab. At the time that the study was conducted, natalizumab had been removed from the market by the FDA due to concerns regarding cases of reported PML in the approved indication for multiple sclerosis. The authors found that CD patients were willing to accept high levels of PML well above the clinical exposure in exchange for clinically realistic improvements in treatment outcomes.135 Following this study, natalizumab was approved for CD patients, subject to certain restrictions.
Table III.
Discrete-Choice Experiment studies in IBD
| Study | Study Population and Sample Size | Study Aim |
|---|---|---|
| Hodgkins P et al.138 | 400 UC patients | To determine preferences for 5-ASA therapy based on self-reported adherence |
| Johnson FR et al.137 | 315 gastroenterologists 580 CD patients |
To measure and compare acceptability of medication risks and benefits between adult CD patients and gastroenterologists |
| Lichtenstein GR et al.134 | 252 CD patients | To determine treatment preferences for and relative importance of treatment attributes for CD |
| Johnson FR et al.136 | 345 CD patients > 18 years old 150 parents of CD patients < 18 years old |
To measure and compare risk preferences for medical therapy between parents of juvenile patients and adult patients |
| Johnson FR et al.135 | 580 CD patients | To measure CD patients’ willingness to accept serious adverse events in exchange for medication efficacy |
Two further studies in CD have evaluated differences in preferences for medication risks and benefits between populations of patients and/or providers. A study by Johnson and colleagues compared risk preferences between juvenile and adult CD patients, an important issue for providers caring for these patients as they transition between the pediatric and adult health care systems.136 They found that parents of juvenile CD patients and adult CD patients had similar risk tolerances for medication efficacies in CD therapies.136 An additional study compared CD patients and gastroenterologists with respect to their willingness to accept therapy risks and benefits.137 Utilizing DCE methodology, the authors were able to evaluate preferences across varying age groups of CD patients, various risks of medical therapies and various efficacies of the therapies. The authors found that gastroenterologists’ risk tolerances differed by the age of their patients, with less risk tolerance for the older IBD patient compared to the middle-aged or younger IBD patient.137 Furthermore, significant differences existed in disease severity tolerance and various risk tolerance tolerances for resultant drug efficacy: for example, middle-aged patients were significantly more tolerant of the risk of lymphoma than physicians if a medication was completely efficacious and resulted in a disease remission (rather than a clinical improvement but not complete remission).137
A multicenter multi-country DCE study evaluated UC patient preferences for various aspects of 5-ASA therapy and further evaluated differences in these preferences for self-proclaimed “good” versus “poor” medication adherers.138 The study found that preferences varied by adherence-reporting as well as country for important attributes in 5-ASA therapy. Patients who self-reported good adherence preferred 5-ASA therapies that improved stool frequency and promoted mucosal healing. North Americans (US and Canada) preferred reduced pill quantity or frequency of taking medications. Furthermore, clinical characteristics of the participants (for example, disease activity within the past year) affected importance of preferences regarding therapy efficacy.138
6.1 Advantages and Disadvantages of Discrete-Choice Experiments
One of the most powerful advantages of DCE is that it does not require the restrictive assumptions of conventional QALY metrics. By offering realistic benefit-risk tradeoff scenarios within a non-expected utility framework, DCEs more accurately quantify preference data, from which utility is derived. Houtven et al showed how to derive maximum acceptable risk from generalized utility theory with an example using a DCE study in IBD.110
DCE also allows for subgroup analysis by health history, current health state or other covariates of interest which may affect risk preferences. For example, Johnson et al. found that duration of disease, proximity of last disease flare and disease history all affected risk preferences for treatment SAEs in exchange for treatment efficacy.135 As it is reasonable to expect risk preferences to be influenced by patient history, DCE offers a rigorous framework with which to assess this impact.
As patient preferences play a key role in patient satisfaction, which in turn influences adherence and ultimately clinical outcomes, DCE-measured preference data are more patient-centered than QALYs. Furthermore, by measuring preferences for attributes of a medical therapy as well as the therapy overall, DCE also provides information on the total value of an intervention or on the marginal effect of modifying a single factor on the value. By collecting data within carefully devised experimental designs, DCEs can introduce variability and reduce or even completely eliminate collinearity, making possible precise estimates of attribute contributions to therapy utilities. This also allows for preference measurement on future interventions including those that may not be currently available. Finally, within the given choice format, DCE can assess numeracy, present risks in multiple formats, and perform inherent tests of validity and consistenty including logic testing, test-retest and transitivity.
While DCEs stated-preference measures can quantify preferences for both health outcomes and process-related factors, the resulting relative importance weights are not generalizable across therapeutic areas. An important limitation of DCEs include their hypothetical nature: simulated decision-making using hypothetical therapeutic options does not have the same medical, emotional and financial consequences as actual therapeutic decisions. In addition, outcomes in real clinical settings are inherently probabilistic in nature. Eliciting patients’ willingness to accept benefit-risk tradeoffs is cognitively challenging because of the low numeracy level of many respondents. Resulting preference data thus may be subject to considerable measurement error. Finally, DCE studies can be difficult to implement, both in terms of design methodologies as well as analysis,139,140 which can affect comprehension and acceptability among physicians and regulators.
7. Discussion
Understanding patients’ preferences for therapies that affect their care is important in every aspect of health care. There is an increased importance placed on patient autonomy within medical decision making, both in health care policy, as well as by patients and physicians. Furthermore, health-care expenditures are often shouldered wholly or in part by patients themselves, which often results in rationing of care based upon preferences for perceived risks and benefits.116 This is a particular problem for illnesses such as IBD that disproportionately affect younger patients who may not have access to resources through employment and health insurance.
Additionally, understanding IBD patient preferences takes on particular importance because the most appropriate treatment option in IBD is not always obvious. Therefore, patient preferences for treatment efficacy, potential adverse effects or surgery play an important part of clinical decision making. Understanding those preferences can have far-reaching consequences. For example, surgery in UC is thought of as an option of last resort or a product of medication failure. However, if it is found that UC patients view colectomy surgery as equivalent to long term use of immunosuppressants with their associated risks; or superior to incompletely effective medical therapy, then the conversations physicians have with UC patients as well as society-based practice algorithms may change. Likewise, as seen in CD, patients’ preferences for risks of therapies in exchange for durable treatment efficacy can influence acceptability of these drugs including drug approval. Therefore, accurate assessment of patient preferences is important not only in understanding patients’ goals in therapy but also in potentially setting benchmarks for therapy guidelines and therapies themselves.
It is clear that IBD patients do have preferences for medical and surgical therapies for their disease. The next challenge, therefore, is accurately describing and quantifying these preferences. Traditional methods of eliciting patient preferences such as SG and TTO have constituted the bulk of studies in IBD. And these studies have offered important insights, such as the discovery that post-surgical UC patients value their surgery after the fact more than they did prior to going to surgery. This has important implications in counseling UC patients who may be facing surgery but wary of its impact on quality of life.
However, clinical decision-making in IBD is more complicated than a single decisionnode. And failure to account for all the attributes that constitute a particular therapeutic option, or the alternative options, can lead to inaccurate assumptions regarding patient preferences for these decisions. For example, if a patient with active disease is facing surgery or life-long immunosuppressant therapy with its potential risks, it is possible that this patient may not discount the surgical alternative as greatly as previously judged using traditional methods of preference elicitation.
A more serious limitation of SG and TTO is the growing recognition that the fundamental axiom of these studies, namely the expected utility theory, may actually be violated in normal human preference behavior. This results in bias in preference assessment when utilizing these techniques. This violation of the expected utility theory has been recognized in economics for some time; but is increasingly appreciated when assessing preferences for health care. As a result, more rigorous methodologies, such as DCE, are being applied to assess preferences for health care, both goods and services. The realistic presentation of clinical scenarios with the ability to quantify maximum acceptable risks for treatment attributes and therapies allows DCE to uniquely handle the myriad of choices and risks facing IBD patients and evaluate preferences across a number of clinical outcomes and risk probabilities. As patient preference studies continue, it is easy to envision DCE studies evaluating a number of critical issues in IBD: how much risk of serious adverse events are UC patients willing to accept from immunosuppressants before they prefer surgical options for their disease? How much additional medication risk (from mono- or combination-therapy) are patients with CD willing to accept to avoid a relapse of their disease? What specific attributes of a medication therapy (injection versus infusion; frequency of dosing, etc) make one medication preferable to another for a given efficacy? And how do these preferences change with age, gender, disease duration or other clinical features of IBD phenotype? One can easily see how the answers to such questions could facilitate not only interactions between patients and physicians in the office, but also how regulators and society as a whole view therapeutic strategies in IBD.
8. Conclusion
IBD patients face an increasing number of choices regarding care of their chronic illness. These choices range from mode and frequency of drug administration to preferences regarding types of serious potential risks to decisions regarding forgoing medical therapy in favor of surgical interventions. As a result of these increased choices, IBD patient preferences carry a growing importance in the decisions regarding their care, both at a personal and public policy level. Therefore, rigorous methodologies to evaluate patient preferences and willingness to accept risks are needed. SG and TTO have traditionally examined preferences in IBD care, but have inherent flaws that may bias their results. DCE offers the ability to provide systematic, quantifiable measurements of patient preferences, which in turn can inform physicians, surgeons, and public health policy regarding the care of this challenging disease.
Key Points for Decision Makers.
Accurately quantifying patient preferences for potential risks and benefits of therapy options is crucial in numerous clinical situations in IBD where no clear correct decision exists, and therefore patient preferences play a critical role in patient-centered decision-making.
Traditional estimates of health-state utilities have several important limitations that can render inaccurate estimations of patient preferences for therapies in IBD. Stated-preference methods, including discrete-choice experiments, employ a non-expected utility framework to more accurately assess patient preferences for overall therapies and therapy-specific attributes (including efficacy and averse effects), and represent a powerful tool to quantify preference data.
Acknowledgments
Funding for this study came in part from NIH K08 DK084347-01.
Meenakshi Bewtra conducted the literature search and summarized the findings. Meenakshi Bewtra and F. Reed Johnson interpreted the findings and developed the manuscript.
Meenakshi Bewtra has received a research grant from Centocor.
Footnotes
F. Reed Johnson declares no conflicts of interest.
References
- 1.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 2.Harrell LE, Hanauer SB. Mesalamine derivatives in the treatment of Crohn's disease. Gastroenterol Clin North Am. 2004;33:303–317. ix–x. doi: 10.1016/j.gtc.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Hanauer S, Sninsky C, Robinson M, et al. An oral preparation of mesalamine as long-term maintenance therapy for ulcerative colitis. A randomized, placebo-controlled trial. The Mesalamine Study Group. Ann Intern Med. 1996;124:204–211. doi: 10.7326/0003-4819-124-2-199601150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Hanauer SB, Sandborn WJ, Kornbluth A, et al. Delayed-release oral mesalamine at 4.8 g/day (800 mg tablet) for the treatment of moderately active ulcerative colitis: the ASCEND II trial. Am J Gastroenterol. 2005;100:2478–2485. doi: 10.1111/j.1572-0241.2005.00248.x. [DOI] [PubMed] [Google Scholar]
- 5.Sninsky CA, Cort DH, Shanahan F, et al. Oral mesalamine (Asacol) for mildly to moderately active ulcerative colitis. A multicenter study. Ann Intern Med. 1991;115:350–355. doi: 10.7326/0003-4819-115-5-350. [DOI] [PubMed] [Google Scholar]
- 6.Hanauer S, Schwartz J, Robinson M, et al. Mesalamine capsules for treatment of active ulcerative colitis: results of a controlled trial. Pentasa Study Group. Am J Gastroenterol. 1993;88:1188–1197. [PubMed] [Google Scholar]
- 7.Levine DS, Riff DS, Pruitt R, et al. A randomized, double blind, dose-response comparison of balsalazide (6.75 g), balsalazide (2.25 g), and mesalamine (2.4 g) in the treatment of active, mild-to-moderate ulcerative colitis. Am J Gastroenterol. 2002;97:1398–1407. doi: 10.1111/j.1572-0241.2002.05781.x. [DOI] [PubMed] [Google Scholar]
- 8.Sandborn WJ, Regula J, Feagan BG, et al. Delayed-release oral mesalamine 4.8 g/day (800-mg tablet) is effective for patients with moderately active ulcerative colitis. Gastroenterology. 2009;137 doi: 10.1053/j.gastro.2009.08.069. 1934-43.e1-3. [DOI] [PubMed] [Google Scholar]
- 9.Faubion WA, Jr, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–260. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 10.Actis GC, Fadda M, David E, Sapino A. Colectomy rate in steroid-refractory colitis initially responsive to cyclosporin: a long-term retrospective cohort study. BMC Gastroenterol. 2007;7:13. doi: 10.1186/1471-230X-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Lama Y, Gisbert JP, Mate J. The role of tacrolimus in inflammatory bowel disease: a systematic review. Dig Dis Sci. 2006;51:1833–1840. doi: 10.1007/s10620-006-9209-y. [DOI] [PubMed] [Google Scholar]
- 12.Michelassi F, Lee J, Rubin M, et al. Long-term functional results after ileal pouch anal restorative proctocolectomy for ulcerative colitis: a prospective observational study. Ann Surg. 2003;238:433–441. doi: 10.1097/01.sla.0000086658.60555.ea. discussion 442-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bewtra M, Su C, Lewis JD. Trends in hospitalization rates for inflammatory bowel disease in the United States. Clin Gastroenterol Hepatol. 2007;5:597–601. doi: 10.1016/j.cgh.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Langholz E, Munkholm P, Davidsen M, Binder V. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology. 1994;107:3–11. doi: 10.1016/0016-5085(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 15.Blam ME, Stein RB, Lichtenstein GR. Integrating anti-tumor necrosis factor therapy in inflammatory bowel disease: current and future perspectives. Am J Gastroenterol. 2001;96:1977–1997. doi: 10.1111/j.1572-0241.2001.03931.x. [DOI] [PubMed] [Google Scholar]
- 16.Pariente B, Cosnes J, Danese S, et al. Development of the Crohn's disease digestive damage score, the Lemann score. Inflamm Bowel Dis. 2011;17:1415–1422. doi: 10.1002/ibd.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV., Jr Risk factors associated with progression to intestinal complications of Crohn's disease in a population-based cohort. Gastroenterology. 2010;139:1147–1155. doi: 10.1053/j.gastro.2010.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jess T, Winther KV, Munkholm P, Langholz E, Binder V. Mortality and causes of death in Crohn's disease: follow-up of a population-based cohort in Copenhagen County, Denmark. Gastroenterology. 2002;122:1808–1814. doi: 10.1053/gast.2002.33632. [DOI] [PubMed] [Google Scholar]
- 19.Bewtra M, Kaiser LM, TenHave T, Lewis JD. Crohn's disease and ulcerative colitis are associated with elevated standardized mortality ratios: a meta-analysis. Inflamm Bowel Dis. 2013;19:599–613. doi: 10.1097/MIB.0b013e31827f27ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanauer SB, Stromberg U. Oral Pentasa in the treatment of active Crohn's disease: A meta-analysis of double-blind, placebo-controlled trials. Clin Gastroenterol Hepatol. 2004;2:379–388. doi: 10.1016/s1542-3565(04)00122-3. [DOI] [PubMed] [Google Scholar]
- 21.Hanauer SB, Korelitz BI, Rutgeerts P, et al. Postoperative maintenance of Crohn's disease remission with 6-mercaptopurine, mesalamine, or placebo: a 2-year trial. Gastroenterology. 2004;127:723–729. doi: 10.1053/j.gastro.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 23.Burger D, Travis S. Conventional medical management of inflammatory bowel disease. Gastroenterology. 2011;140:1827.e2–1837.e2. doi: 10.1053/j.gastro.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 24.McDonald JW, Feagan BG, Jewell D, Brynskov J, Stange EF, Macdonald JK. Cyclosporine for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2005;(2) doi: 10.1002/14651858.CD000297.pub2. CD000297. [DOI] [PubMed] [Google Scholar]
- 25.Feagan BG, Fedorak RN, Irvine EJ, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease. North American Crohn's Study Group Investigators. N Engl J Med. 2000;342:1627–1632. doi: 10.1056/NEJM200006013422202. [DOI] [PubMed] [Google Scholar]
- 26.Prefontaine E, Macdonald JK, Sutherland LR. Azathioprine or 6-mercaptopurine for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2010;(6) doi: 10.1002/14651858.CD000545.pub3. CD000545. [DOI] [PubMed] [Google Scholar]
- 27.Prefontaine E, Sutherland LR, Macdonald JK, Cepoiu M. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2009;(1) doi: 10.1002/14651858.CD000067.pub2. CD000067. [DOI] [PubMed] [Google Scholar]
- 28.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 29.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 30.Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–838. doi: 10.7326/0003-4819-146-12-200706190-00159. [DOI] [PubMed] [Google Scholar]
- 31.Schreiber S, Rutgeerts P, Fedorak RN, et al. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn's disease. Gastroenterology. 2005;129:807–818. doi: 10.1053/j.gastro.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 32.Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn's disease. N Engl J Med. 2005;353:1912–1925. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- 33.Osterman MT. Mucosal healing in inflammatory bowel disease. J Clin Gastroenterol. 2013;47:212–221. doi: 10.1097/MCG.0b013e3182732ff5. [DOI] [PubMed] [Google Scholar]
- 34.Lichtenstein GR, Cohen R, Feagan BG, et al. Safety of Infliximab and Other Crohn's Disease Therapies: TreatTM Registry Data with 24,575 Patient-Years of Follow-up. American Journal of Gastroenterology. 2008;103 [Google Scholar]
- 35.Lewis JD, Gelfand JM, Troxel AB, et al. Immunosuppressant medications and mortality in inflammatory bowel disease. Am J Gastroenterol. 2008;103:1428–1435. doi: 10.1111/j.1572-0241.2008.01836.x. quiz 1436. [DOI] [PubMed] [Google Scholar]
- 36.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn's disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621–630. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Toruner M, Loftus EV, Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–936. doi: 10.1053/j.gastro.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Siegel CA, Sands BE. Review article: practical management of inflammatory bowel disease patients taking immunomodulators. Aliment Pharmacol Ther. 2005;22:1–16. doi: 10.1111/j.1365-2036.2005.02520.x. [DOI] [PubMed] [Google Scholar]
- 39.Siegel CA, Hur C, Korzenik JR, Gazelle GS, Sands BE. Risks and benefits of infliximab for the treatment of Crohn's disease. Clin Gastroenterol Hepatol. 2006;4:1017–1024. doi: 10.1016/j.cgh.2006.05.020. quiz 976. [DOI] [PubMed] [Google Scholar]
- 40.Colombel JF, Loftus EV, Jr, Tremaine WJ, et al. The safety profile of infliximab in patients with Crohn's disease: the Mayo clinic experience in 500 patients. Gastroenterology. 2004;126:19–31. doi: 10.1053/j.gastro.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 41.Carson KR, Focosi D, Major EO, et al. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a Review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol. 2009;10:816–824. doi: 10.1016/S1470-2045(09)70161-5. [DOI] [PubMed] [Google Scholar]
- 42.Marehbian J, Arrighi HM, Hass S, Tian H, Sandborn WJ. Adverse events associated with common therapy regimens for moderate-to-severe Crohn's disease. Am J Gastroenterol. 2009;104:2524–2533. doi: 10.1038/ajg.2009.322. [DOI] [PubMed] [Google Scholar]
- 43.Kane S, Khatibi B, Reddy D. Higher incidence of abnormal Pap smears in women with inflammatory bowel disease. Am J Gastroenterol. 2008;103:631–636. doi: 10.1111/j.1572-0241.2007.01582.x. [DOI] [PubMed] [Google Scholar]
- 44.Hutfless S, Fireman B, Kane S, Herrinton LJ. Screening differences and risk of cervical cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2008;28:598–605. doi: 10.1111/j.1365-2036.2008.03766.x. [DOI] [PubMed] [Google Scholar]
- 45.Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121–1125. doi: 10.1136/gut.2004.049460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn's disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009;7:874–881. doi: 10.1016/j.cgh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herrinton LJ, Liu L, Weng X, Lewis JD, Hutfless S, Allison JE. Role of thiopurine and anti-TNF therapy in lymphoma in inflammatory bowel disease. Am J Gastroenterol. 2011;106:2146–2153. doi: 10.1038/ajg.2011.283. [DOI] [PubMed] [Google Scholar]
- 48.Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617–1625. doi: 10.1016/S0140-6736(09)61302-7. [DOI] [PubMed] [Google Scholar]
- 49.Khan WA, Yu L, Eisenbrey AB, et al. Hepatosplenic gamma/delta T-cell lymphoma in immunocompromised patients. Report of two cases and review of literature. Am J Clin Pathol. 2001;116:41–50. doi: 10.1309/TC9U-FAV7-0QBW-6DFC. [DOI] [PubMed] [Google Scholar]
- 50.Mackey AC, Green L, Leptak C, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease: update. J Pediatr Gastroenterol Nutr. 2009;48:386–388. doi: 10.1097/mpg.0b013e3181957a11. [DOI] [PubMed] [Google Scholar]
- 51.Moran G, Dillon J, Green J. Crohn's disease, hepatosplenic T-cell lymphoma and no biological therapy: are we barking up the wrong tree? Inflamm Bowel Dis. 2009;15:1281–1282. doi: 10.1002/ibd.20802. [DOI] [PubMed] [Google Scholar]
- 52.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn's disease. Gastroenterology. 2004;126:402–413. doi: 10.1053/j.gastro.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 54.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 55.Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab pegol for Crohn's disease. N Engl J Med. 2007;357:239–250. doi: 10.1056/NEJMoa062897. [DOI] [PubMed] [Google Scholar]
- 56.Su C, Lichtenstein GR. Treatment of inflammatory bowel disease with azathioprine and 6-mercaptopurine. Gastroenterol Clin North Am. 2004;33:209–234. viii. doi: 10.1016/j.gtc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Kaplan GG, McCarthy EP, Ayanian JZ, Korzenik J, Hodin R, Sands BE. Impact of hospital volume on postoperative morbidity and mortality following a colectomy for ulcerative colitis. Gastroenterology. 2008;134:680–687. doi: 10.1053/j.gastro.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Roberts SE, Williams JG, Yeates D, Goldacre MJ. Mortality in patients with and without colectomy admitted to hospital for ulcerative colitis and Crohn's disease: record linkage studies. BMJ. 2007;335:1033. doi: 10.1136/bmj.39345.714039.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alves A, Panis Y, Mathieu P, et al. Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg. 2005;140:278–283. doi: 10.1001/archsurg.140.3.278. discussion 284. [DOI] [PubMed] [Google Scholar]
- 60.Hyman NH, Cataldo P, Osler T. Urgent subtotal colectomy for severe inflammatory bowel disease. Dis Colon Rectum. 2005;48:70–73. doi: 10.1007/s10350-004-0750-5. [DOI] [PubMed] [Google Scholar]
- 61.Fazio VW, Ziv Y, Church JM, et al. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg. 1995;222:120–127. doi: 10.1097/00000658-199508000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delaney CP, Fazio VW, Remzi FH, et al. Prospective, age-related analysis of surgical results, functional outcome, and quality of life after ileal pouch-anal anastomosis. Ann Surg. 2003;238:221–228. doi: 10.1097/01.sla.0000080825.95166.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alves A, Panis Y, Bouhnik Y, Maylin V, Lavergne-Slove A, Valleur P. Subtotal colectomy for severe acute colitis: a 20-year experience of a tertiary care center with an aggressive and early surgical policy. J Am Coll Surg. 2003;197:379–385. doi: 10.1016/S1072-7515(03)00434-4. [DOI] [PubMed] [Google Scholar]
- 64.Pal S, Sahni P, Pande GK, Acharya SK, Chattopadhyay TK. Outcome following emergency surgery for refractory severe ulcerative colitis in a tertiary care centre in India. BMC Gastroenterol. 2005;5:39. doi: 10.1186/1471-230X-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bach SP, Mortensen NJ. Ileal pouch surgery for ulcerative colitis. World J Gastroenterol. 2007;13:3288–3300. doi: 10.3748/wjg.v13.i24.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sacristan JA. Patient-centered medicine and patient-oriented research: improving health outcomes for individual patients. BMC Med Inform Decis Mak. 2013;13:6. doi: 10.1186/1472-6947-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Truog RD. Patients and doctors--evolution of a relationship. N Engl J Med. 2012;366:581–585. doi: 10.1056/NEJMp1110848. [DOI] [PubMed] [Google Scholar]
- 68.Laine C, Davidoff F. Patient-centered medicine. A professional evolution. JAMA. 1996;275:152–156. [PubMed] [Google Scholar]
- 69.Mead N, Bower P. Patient-centredness: a conceptual framework and review of the empirical literature. Soc Sci Med. 2000;51:1087–1110. doi: 10.1016/s0277-9536(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 70.Rademakers J, Delnoij D, Nijman J, de Boer D. Educational inequalities in patient-centred care: patients' preferences and experiences. BMC Health Serv Res. 2012;12 doi: 10.1186/1472-6963-12-261. 261-6963-12-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bensing J. Bridging the gap. The separate worlds of evidence-based medicine and patient-centered medicine. Patient Educ Couns. 2000;39:17–25. doi: 10.1016/s0738-3991(99)00087-7. [DOI] [PubMed] [Google Scholar]
- 72.Mohamed AF, Hauber AB, Johnson FR, Coon CD. Patient preferences and linear scoring rules for patient-reported outcomes. Patient. 2010;3:217–227. doi: 10.2165/11537880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 73.Bala MV, Zarkin GA. Are QALYs an appropriate measure for valuing morbidity in acute diseases? Health Econ. 2000;9:177–180. doi: 10.1002/(sici)1099-1050(200003)9:2<177::aid-hec497>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 74.Giesler RB, Ashton CM, Brody B, et al. Assessing the performance of utility techniques in the absence of a gold standard. Med Care. 1999;37:580–588. doi: 10.1097/00005650-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 75.O'Leary JF, Fairclough DL, Jankowski MK, Weeks JC. Comparison of time-tradeoff utilities and rating scale values of cancer patients and their relatives: evidence for a possible plateau relationship. Med Decis Making. 1995;15:132–137. doi: 10.1177/0272989X9501500205. [DOI] [PubMed] [Google Scholar]
- 76.Lin MR, Yu WY, Wang SC. Examination of assumptions in using time tradeoff and standard gamble utilities in individuals with spinal cord injury. Arch Phys Med Rehabil. 2012;93:245–252. doi: 10.1016/j.apmr.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 77.Bleichrodt H, Pinto J. The Validity of Qalys Under Non-expected Utility. The Economic Journal. 2005;115:533–550. [Google Scholar]
- 78.Brazier J, Rowen D, Yang Y, Tsuchiya A. Comparison of health state utility values derived using time trade-off, rank and discrete choice data anchored on the full health-dead scale . Eur J Health Econ. 2012;13:575–587. doi: 10.1007/s10198-011-0352-9. [DOI] [PubMed] [Google Scholar]
- 79.Hauber AB. Healthy-years equivalent: wounded but not yet dead. Expert. Rev Pharmacoecon Outcomes Res. 2009;9:265–269. doi: 10.1586/erp.09.22. [DOI] [PubMed] [Google Scholar]
- 80.Johnson FR. Editorial: Moving the QALY forward or just stuck in traffic? Value Health. 2009;12(Suppl 1):S38–S39. doi: 10.1111/j.1524-4733.2009.00521.x. [DOI] [PubMed] [Google Scholar]
- 81.Nord E, Daniels N, Kamlet M. QALYs: some challenges. Value Health. 2009;12(Suppl 1):S10–S15. doi: 10.1111/j.1524-4733.2009.00516.x. [DOI] [PubMed] [Google Scholar]
- 82.Wong S, Walker JR, Carr R, et al. The information needs and preferences of persons with longstanding inflammatory bowel disease. Can J Gastroenterol. 2012;26:525–531. doi: 10.1155/2012/735386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Conrad S, Huppe A, Raspe H. Preference of patients with inflammatory bowel disease regarding information and shared decision-making: results from a cross-sectional survey in Germany. Z Gastroenterol. 2012;50:364–372. doi: 10.1055/s-0031-1281949. [DOI] [PubMed] [Google Scholar]
- 84.Bernstein KI, Promislow S, Carr R, Rawsthorne P, Walker JR, Bernstein CN. Information needs and preferences of recently diagnosed patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:590–598. doi: 10.1002/ibd.21363. [DOI] [PubMed] [Google Scholar]
- 85.Siegel C, Schwartz L, Woloshin S, et al. When should ulcerative colitis patients undergo colectomy for dysplasia? Mismatch between patient preferences and physician recommendations. Inflammatory Bowel Disease. 2010;16:1658–1662. doi: 10.1002/ibd.21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baars J, Siegel C, van't Spijker A, Markus T, Kuipers E, van der Woude C. Inflammatory bowel disease-patients are insufficiently educated about the basic characteristics of their disease and the associated risk of colorectal cancer. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2010;42:777–784. doi: 10.1016/j.dld.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 87.Allen PB, Lindsay H, Tham TC. How do patients with inflammatory bowel disease want their biological therapy administered? BMC Gastroenterol. 2010;10 doi: 10.1186/1471-230X-10-1. 1-230X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewis JR, Konda V, Rubin DT. Genetic testing for inflammatory bowel disease: focus group analysis of patients and family members. Genet Test Mol Biomarkers. 2009;13:495–503. doi: 10.1089/gtmb.2008.0102. [DOI] [PubMed] [Google Scholar]
- 89.Gray JR, Leung E, Scales J. Treatment of ulcerative colitis from the patient's perspective: a survey of preferences and satisfaction with therapy. Aliment Pharmacol Ther. 2009;29:1114–1120. doi: 10.1111/j.1365-2036.2009.03972.x. [DOI] [PubMed] [Google Scholar]
- 90.Knopf JM, Hornung RW, Slap GB, DeVellis RF, Britto MT. Views of treatment decision making from adolescents with chronic illnesses and their parents: a pilot study. Health Expect. 2008;11:343–354. doi: 10.1111/j.1369-7625.2008.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kennedy ED, To T, Steinhart AH, Detsky A, Llewellyn-Thomas HA, McLeod RS. Do patients consider postoperative maintenance therapy for Crohn's disease worthwhile? Inflamm Bowel Dis. 2008;14:224–235. doi: 10.1002/ibd.20300. [DOI] [PubMed] [Google Scholar]
- 92.Konda V, Huo D, Hermes G, Liu M, Patel R, Rubin DT. Do patients with inflammatory bowel disease want genetic testing? Inflamm Bowel Dis. 2006;12:497–502. doi: 10.1097/00054725-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 93.Rutter MD, Saunders BP, Wilkinson KH, Schofield G, Forbes A. Intangible costs and benefits of ulcerative colitis surveillance: a patient survey. Dis Colon Rectum. 2006;49:1177–1183. doi: 10.1007/s10350-006-0546-x. [DOI] [PubMed] [Google Scholar]
- 94.Cheung WY, Dove J, Lervy B, Russell IT, Williams JG. Shared care in gastroenterology: GPs' views of open access to out-patient follow-up for patients with inflammatory bowel disease. Fam Pract. 2002;19:53–56. doi: 10.1093/fampra/19.1.53. [DOI] [PubMed] [Google Scholar]
- 95.Green TJ, Issenman RM, Jacobson K. Patients' diets and preferences in a pediatric population with inflammatory bowel disease. Can J Gastroenterol. 1998;12:544–549. doi: 10.1155/1998/928706. [DOI] [PubMed] [Google Scholar]
- 96.Probert CS, Mayberry JF. Inflammatory bowel disease: patients' expectations in the 1990s. J R Soc Med. 1991;84:131–132. doi: 10.1177/014107689108400305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Johnson FR, Hauber AB, Osoba D, Hsu MA, Coombs J, Copley-Merriman C. Are chemotherapy patients' HRQoL importance weights consistent with linear scoring rules? A stated-choice approach. Qual Life Res. 2006;15:285–298. doi: 10.1007/s11136-005-0581-4. [DOI] [PubMed] [Google Scholar]
- 98.Siegel CA, Levy LC, Mackenzie TA, Sands BE. Patient perceptions of the risks and benefits of infliximab for the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1–6. doi: 10.1002/ibd.20283. [DOI] [PubMed] [Google Scholar]
- 99.Arseneau KO, Sultan S, Provenzale DT, et al. Do patient preferences influence decisions on treatment for patients with steroid-refractory ulcerative colitis? Clin Gastroenterol Hepatol. 2006;4:1135–1142. doi: 10.1016/j.cgh.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 100.McLeod RS, Churchill DN, Lock AM, Vanderburgh S, Cohen Z. Quality of life of patients with ulcerative colitis preoperatively and postoperatively. Gastroenterology. 1991;101:1307–1313. doi: 10.1016/0016-5085(91)90081-u. [DOI] [PubMed] [Google Scholar]
- 101.Kennedy ED, Detsky AS, Llewellyn-Thomas HA, et al. Can the standard gamble be used to determine utilities for uncertain health states? An example using postoperative maintenance therapy in Crohn's disease. Med Decis Making. 2000;20:72–78. doi: 10.1177/0272989X0002000109. [DOI] [PubMed] [Google Scholar]
- 102.Arseneau KO, Cohn SM, Cominelli F, Connors AF., Jr Cost-utility of initial medical management for Crohn's disease perianal fistulae. Gastroenterology. 2001;120:1640–1656. doi: 10.1053/gast.2001.24884. [DOI] [PubMed] [Google Scholar]
- 103.Byrne CM, Solomon MJ, Young JM, Selby W, Harrison JD. Patient preferences between surgical and medical treatment in Crohn's disease. Dis Colon Rectum. 2007;50:586–597. doi: 10.1007/s10350-006-0847-0. [DOI] [PubMed] [Google Scholar]
- 104.Waljee AK, Higgins PD, Waljee JF, et al. Perceived and actual quality of life with ulcerative colitis: a comparison of medically and surgically treated patients. Am J Gastroenterol. 2011;106:794–799. doi: 10.1038/ajg.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Waljee AK, Morris AM, Waljee JF, Higgins PD. Individual health discount rate in patients with ulcerative colitis. Inflamm Bowel Dis. 2011;17:1328–1332. doi: 10.1002/ibd.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brown LK, Waljee AK, Higgins PD, Waljee JF, Morris AM. Proximity to disease and perception of utility: physicians' vs patients' assessment of treatment options for ulcerative colitis. Dis Colon Rectum. 2011;54:1529–1536. doi: 10.1097/DCR.0b013e31823436a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leshno M. On using the standard gamble to determine utilities for uncertain health states. Med Decis Making. 2001;21:82–83. doi: 10.1177/0272989X0102100115. [DOI] [PubMed] [Google Scholar]
- 108.Deaton A, Muellbauer J. Economics and consumer behavior. Cambridge, UK: Cambridge University Press; 1980. [Google Scholar]
- 109.Starmer C. Developments in non-expected utility theory: the hunt for a descriptive theory of choice under risk. Journal of Economic Literature. 2000;38:332–382. [Google Scholar]
- 110.Van Houtven G, Johnson FR, Kilambi V, Hauber AB. Eliciting benefit-risk preferences and probability-weighted utility using choice-format conjoint analysis. Med Decis Making. 2011;31:469–480. doi: 10.1177/0272989X10386116. [DOI] [PubMed] [Google Scholar]
- 111.Apter AJ, Paasche-Orlow MK, Remillard JT, et al. Numeracy and communication with patients: they are counting on us. J Gen Intern Med. 2008;23:2117–2124. doi: 10.1007/s11606-008-0803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fagerlin A, Ubel PA, Smith DM, Zikmund-Fisher BJ. Making numbers matter: present and future research in risk communication. Am J Health Behav. 2007;31(Suppl 1):S47–S56. doi: 10.5555/ajhb.2007.31.supp.S47. [DOI] [PubMed] [Google Scholar]
- 113.Elting LS, Martin CG, Cantor SB, Rubenstein EB. Influence of data display formats on physician investigators' decisions to stop clinical trials: prospective trial with repeated measures. BMJ. 1999;318:1527–1531. doi: 10.1136/bmj.318.7197.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reyna VF, Brainerd CJ. Numeracy, ratio bias, and denominator neglect in judgments of risk and probability. Learn Individ Differ. 2008;18:89–107. [Google Scholar]
- 115.Ancker JS, Senathirajah Y, Kukafka R, Starren JB. Design features of graphs in health risk communication: a systematic review. J Am Med Inform Assoc. 2006;13:608–618. doi: 10.1197/jamia.M2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Garcia-Retamero R, Galesic M. Communicating treatment risk reduction to people with low numeracy skills: a cross-cultural comparison. Am J Public Health. 2009;99:2196–2202. doi: 10.2105/AJPH.2009.160234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Burkell J. What are the chances? Evaluating risk and benefit information in consumer health materials. J Med Libr Assoc. 2004;92:200–208. [PMC free article] [PubMed] [Google Scholar]
- 118.Brase GL. Which statistical formats facilitate what decisions? The perception and influence of different statistical information formats. J Behav Decis Making. 2002;15:381–401. [Google Scholar]
- 119.Yamagishi K. When a 12.86% Mortality is More Dangerous then 24.14%: Implications for Risk Communication. Appl Cogn Psychol. 1997;11:495–506. [Google Scholar]
- 120.Galesic M, Gigerenzer G, Straubinger N. Natural frequencies help older adults and people with low numeracy to evaluate medical screening tests. Med Decis Making. 2009;29:368–371. doi: 10.1177/0272989X08329463. [DOI] [PubMed] [Google Scholar]
- 121.Cavanaugh K, Wallston KA, Gebretsadik T, et al. Addressing literacy and numeracy to improve diabetes care: two randomized controlled trials. Diabetes Care. 2009;32:2149–2155. doi: 10.2337/dc09-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people's health care decisions: is a picture worth a thousand statistics? Med Decis Making. 2005;25:398–405. doi: 10.1177/0272989X05278931. [DOI] [PubMed] [Google Scholar]
- 123.Hawley ST, Zikmund-Fisher B, Ubel P, Jancovic A, Lucas T, Fagerlin A. The impact of the format of graphical presentation on health-related knowledge and treatment choices. Patient Educ Couns. 2008;73:448–455. doi: 10.1016/j.pec.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 124.Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM. Measuring numeracy without a math test: development of the Subjective Numeracy Scale. Med Decis Making. 2007;27:672–680. doi: 10.1177/0272989X07304449. [DOI] [PubMed] [Google Scholar]
- 125.Woloshin S, Schwartz LM, Moncur M, Gabriel S, Tosteson AN. Assessing values for health: numeracy matters. Med Decis Making. 2001;21:382–390. doi: 10.1177/0272989X0102100505. [DOI] [PubMed] [Google Scholar]
- 126.Zikmund-Fisher BJ, Smith DM, Ubel PA, Fagerlin A. Validation of the Subjective Numeracy Scale: effects of low numeracy on comprehension of risk communications and utility elicitations. Med Decis Making. 2007;27:663–671. doi: 10.1177/0272989X07303824. [DOI] [PubMed] [Google Scholar]
- 127.Johnson FR, Banzhaf MR, Desvousges WH. Willingness to pay for improved respiratory and cardiovascular health: a multiple-format, stated-preference approach. Health Econ. 2000;9:295–317. doi: 10.1002/1099-1050(200006)9:4<295::aid-hec520>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 128.Phillips KA, Maddala T, Johnson FR. Measuring preferences for health care interventions using conjoint analysis: an application to HIV testing. Health Serv Res. 2002;37:1681–1705. doi: 10.1111/1475-6773.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ryan M, Hughes J. Using conjoint analysis to assess women's preferences for miscarriage management. Health Econ. 1997;6:261–273. doi: 10.1002/(sici)1099-1050(199705)6:3<261::aid-hec262>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 130.Hauber AB, Johnson FR, Grotzinger KM, Ozdemir S. Patients' benefit-risk preferences for chronic idiopathic thrombocytopenic purpura therapies. Ann Pharmacother. 2010;44:479–488. doi: 10.1345/aph.1M567. [DOI] [PubMed] [Google Scholar]
- 131.Johnson FR, Van Houtven G, Ozdemir S, et al. Multiple sclerosis patients' benefit-risk preferences: serious adverse event risks versus treatment efficacy. J Neurol. 2009;256:554–562. doi: 10.1007/s00415-009-0084-2. [DOI] [PubMed] [Google Scholar]
- 132.Johnson FR, Ozdemir S, Hauber B, Kauf TL. Women's willingness to accept perceived risks for vasomotor symptom relief. J Womens Health (Larchmt) 2007;16:1028–1040. doi: 10.1089/jwh.2006.0218. [DOI] [PubMed] [Google Scholar]
- 133.Johnson FR, Manjunath R, Mansfield CA, Clayton LJ, Hoerger TJ, Zhang P. High-risk individuals' willingness to pay for diabetes risk-reduction programs. Diabetes Care. 2006;29:1351–1356. doi: 10.2337/dc05-2221. [DOI] [PubMed] [Google Scholar]
- 134.Lichtenstein G, Waters H, Kelly J, et al. Assessing Drug Treatment Preferences of Patients with Crohn's Disease, A Conjoint Analysis. Patient. 2010;3:113–123. [Google Scholar]
- 135.Johnson FR, Ozdemir S, Mansfield C, et al. Crohn's disease patients' risk-benefit preferences: serious adverse event risks versus treatment efficacy. Gastroenterology. 2007;133:769–779. doi: 10.1053/j.gastro.2007.04.075. [DOI] [PubMed] [Google Scholar]
- 136.Johnson FR, Ozdemir S, Mansfield C, Hass S, Siegel CA, Sands BE. Are adult patients more tolerant of treatment risks than parents of juvenile patients? Risk Anal. 2009;29:121–136. doi: 10.1111/j.1539-6924.2008.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Johnson FR, Hauber B, Ozdemir S, Siegel CA, Hass S, Sands BE. Are gastroenterologists less tolerant of treatment risks than patients? Benefit-risk preferences in Crohn's disease management. J Manag Care Pharm. 2010;16:616–628. doi: 10.18553/jmcp.2010.16.8.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hodgkins P, Swinburn P, Solomon D, Yen L, Dewilde S, Lloyd A. Patient preferences for first-line oral treatment for mild-to-moderate ulcerative colitis: a discrete-choice experiment. Patient. 2012;5:33–44. doi: 10.2165/11595390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 139.Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health--a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14:403–413. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 140.Johnson F, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16:3–13. doi: 10.1016/j.jval.2012.08.2223. [DOI] [PubMed] [Google Scholar]