Abstract
Scope
Aberrant activation of the Wingless-type mouse mammary tumor virus integration site family (Wnt)/β-catenin signaling pathway is the most common modification, and often considered, a hallmark of colorectal cancer (CRC). Typically in this pathway the β-catenin translocates from the cytoplasm to the nucleus, where it functions as a transcription regulator of several genes that support tumor formation and progression. Thus, any agent that could attenuate the translocation of β-catenin could be extremely valuable against CRC, especially the tumors that exhibit constitutively active Wnt/β-catenin signaling.
Methods & Results
Using human CRC cells that exhibit differential expression of Wnt/β-catenin signaling, we demonstrate that treatment of CRC cells with dietary triterpene lupeol results in a dose dependent i) decrease in cell viability, ii) induction of apoptosis, iii) decrease in colonogenic potential, iv) decrease in β-catenin transcriptional activity, and v) decrease in the expression of Wnt target genes. Most importantly lupeol was observed to inhibit the translocation of β-catenin from the cytoplasm to the nucleus. Importantly, all these effects of lupeol were restricted to cells that harbor constitutively active Wnt/β-catenin signaling while negligible effects were observed in cells that lack constitutively active Wnt/β-catenin signaling. Further, we also demonstrate that inhibition of Wnt signaling in cells with constitutive active Wnt/β-catenin results in loss of lupeol efficacy while inducing Wnt signaling sensitizes the cells to inhibitory effects of lupeol.
Conclusions
In summary, our data strongly advocate the efficacy of lupeol against CRC cells that exhibit constitutively active Wnt/β-catenin signaling.
1.0 INTRODUCTION
Colorectal cancer (CRC) is the third most common cause of cancer related deaths in the United States [1–3]. High mortality rates and poor prognosis of the disease, advocate the need for the development of novel approaches to prevent the initiation of premalignant lesions or their progression to cancer or cancer recurrence. Wnt/β-catenin signaling pathway is known to play an important role in normal development, stem cell maintenance and various malignancies including CRC [4–6]. In the active state, β-catenin, an important transcriptional regulator is known to interact with members of the T-cell factor (TCF) family of transcription factors to induce the transcription of important downstream target genes many of which are involved in proliferation and cellular transformation [5, 7, 8]. The hallmark of active Wnt signaling i.e. the nuclear localization of β-catenin, has been observed in a majority of CRC cases [9, 10]. Also a vast majority of CRC cases arise on account of an activating mutation in the Wnt/β-catenin signaling pathway [4, 11]. Aberrant activation in this pathway due to truncating mutations in APC is recognized as a central player in colon cancer [7]. Other common molecular alterations in tumor cells leading to disruption of β-catenin degradation are mutations that inactivate axin or activate β-catenin itself [12]. These alterations result in an accumulation of β-catenin in the nucleus resulting in transcription of downstream targets.
Lupeol, a dietary triterpene found in various fruits (olives, mangoes, grapes, figs), vegetables (green peppers) and in medicinal herbs (Aloe vera) [13–17] has been shown to possess strong anti-inflammatory, anti-arthritic, anti-mutagenic and anti-malarial activity both in vitro and in vivo [14, 16, 18–20]. In our efforts to identify bioactive food components that target Wnt/β-catenin signaling, we wanted to study the effect of lupeol on CRC since a majority of CRCs exhibit aberrant mutations in Wnt/β-catenin signaling. We previously showed the effects of lupeol on melanoma cells that exhibit constitutively active Wnt/β-catenin signaling pathway [21]. In this study, we demonstrate that lupeol has a greater efficacy against CRC cells that harbor constitutive activation of Wnt/β-catenin signaling pathway (DLD 1, HCT 116) as compared to the cells that lack constitutive activation of this pathway (RKO).
2.0 MATERIALS AND METHODS
2.1 Cell lines and cell culture
The CRC cell lines DLD 1 and HCT 116 were obtained from the American Type Culture Collection (ATCC). The RKO cells were kindly provided by Dr. Bert Vogelstein (The Johns Hopkins University School of Medicine). Cells were maintained in DMEM, supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic-antimycotic solution (PSM), containing penicillin, streptomycin and amphotericin B under standard growth conditions (5% CO2, 37°C, humidified atmosphere).
2.2 Treatment of cells with lupeol
A stock solution of lupeol (10 mM) was prepared by dissolving it in warm ethanol and diluting in DMSO in a 1:1 ratio. The cells (50% confluent) were treated with lupeol (20–40 μM) for 48 h in complete cell media. All treatment protocols and controls were prepared as described previously [22].
2.3 Cell proliferation assay
The effect of lupeol on the viability of melanoma cells was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma, St Louis, MO) as described earlier [22]. 3×103 cells/well in 200 μL complete medium were seeded in a 96-well plate and treated with lupeol as described earlier [22]. All treatment concentrations were repeated in 8 wells.
2.4 Annexin V staining
The Annexin-V-FLUOS staining kit (Roche Diagnostic Corporation, Indianapolis, IN) was used for the detection of apoptotic bodies following the vendor’s protocol. This kit uses a dual-staining protocol in which the cells show green fluorescence of Annexin-V (apoptotic cells) and red fluorescence of propidium iodide (necrotic cells or late apoptotic cells). CRC cells were grown to about 60% confluency and then treated with lupeol as described earlier [22]. The fluorescence was detected by Nikon Eclipse Ti fluorescent microscope. Images were captured with an attached camera.
2.5 Colony formation assay
Cells (800 cells/well) were seeded in a six well plate and treated the following day with lupeol (20 and 40 μmol). The media was removed and replaced with fresh media containing lupeol every three days. After 17–20 days of incubation, colonies were fixed with formaldehyde, stained with crystal violet (Ricca Chemical Company, Arlington, TX) and counted.
To test the efficacy of lupeol upon modulation of Wnt/β-catenin signaling, CRC cells were co-transfected with pTK-puro plasmid (Addgene Inc. Cambridge, MA) and dominant-negative TCF4 (d/nTCF4) constructs while RKO cells were also co-transfected with pTK-puro, d/nTCF4 and β-cateninS33Y. The plasmids (dominant negative TCF4, β-cateninS33Y and pcDNA) were generously provided by Drs. K. Kinzler and B. Vogelstein. Transfections were performed using Lipofectamine-2000 reagent, according to manufacturer’s recommendations (Invitrogen, Carlsbad, CA). 12 hours after transfection, cells were plated and screened against puromycin. The selective doses of puromycin for RKO, HCT 116 and DLD1 cells were 1.5μg/ml, 1μg/ml and 3.5μg/ml respectively. After 48 h, the cells were treated with lupeol as described earlier. After 7–10 days of incubation, colonies were fixed with formaldehyde, stained with crystal violet (Ricca Chemical Company, Arlington, TX). For quantification of the data, the crystal violet was dissolved in 50% acetic acid and absorbance at 540nm was measured.
2.6 Luciferase reporter assay
To determine transcriptional activity of Wnt/β-catenin signaling, a luciferase reporter plasmid bearing the TCF4-binding sequence (TOPflash) generously provided by Drs. K. Kinzler and B. Vogelstein was used. Renilla luciferase (pRL-TK; Promega, Madison, WI) was used as an internal control. Transfections of CRC cells were performed using Lipofectamine-2000 reagent, as per vendor’s protocol (Invitrogen, Carlsbad, CA). Fresh media containing lupeol (20 μM and 40 μM) was added 12 h post-transfection. After 24 h, the cells were harvested and transcriptional activity was measured in terms of luciferase activity by using the dual-luciferase reporter assay system (Promega, Madison, WI) as per manufacturer’s recommendations. The transcriptional activity was measured in a dose- and time-dependent manner.
2.7 Preparation of cell lysates for immunoblot analysis
After lupeol treatment, the media was aspirated and the cells were washed with cold PBS. Whole cell, cytosolic and nuclear lysates were prepared as described earlier [22] and stored at −80°C for later use. The protein concentration was determined by the BCA protein assay kit using the manufacturer’s protocol (Pierce, Rockford, IL).
2.8 Immunoblot Analysis
Appropriate amount of protein (35–50 μg) was resolved over precast 12% tris-glycine polyacrylamide gels (Invitrogen, Carlsbad, CA) under non-reduced conditions as described earlier [22]. Antibodies against CRD-BP and β-catenin were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Antibodies against TCF-1, Axin 2, phosphor-β-catenin (Ser-552) and phosphor-β-catenin (Ser-675) were procured from Cell Signaling Technologies (Danvers, MA). Equal loading of protein was confirmed by stripping the membrane and re-probing it with monoclonal β-actin primary antibody (Sigma, St Louis, MO).
2.9 Immunocytostaining
CRC cells were cultured on chambered slides and treated with lupeol as indicated previously for 48 h. The cells were fixed in cold acetone for 5 minutes and blocked with 2.5% normal serum. Slides were incubated overnight with anti-β-catenin (Santa Cruz, CA) followed by fluorescence tagged secondary antibody, covered with ProLong Gold antifade reagent with DAPI (Invitrogen) and visualized using Nikon Eclipse Ti microscope. Images were captured with an attached camera.
2.10 Densitometry analysis
Immunoblots were scanned by HP Precisionscan Pro 3.13 (Hewlett-Packard Co., Palo Alto, CA, USA). Densitometry measurements of the scanned bands were performed using digitalized scientific software program UN-SCAN-IT (Silk Scientific Corporation, Orem, UT, USA). Data were normalized to β-actin or suitable loading controls and expressed as mean±SEM followed by appropriate statistical analysis.
3.0 Statistical analysis
Student’s t-test for independent analysis was applied to evaluate significance between different groups using S-plus software (Insightful, Seattle, WA). Values of p < 0.05 were considered to be statistically significant.
4.0 RESULTS
4.1 Effect of lupeol on cell viability, induction of apoptosis and colonogenic potential in human CRC cells
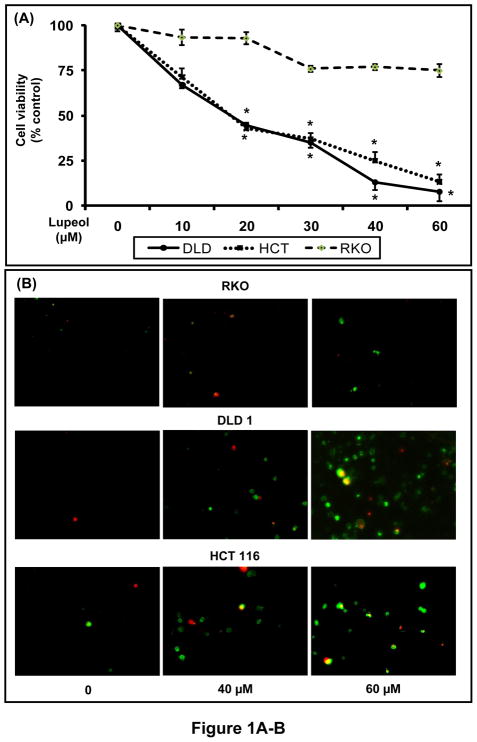
Using MTT assay, we first evaluated the effect of lupeol (0 – 60 μM; 48 h) on the viability of human CRC cells. Lupeol treatment resulted in a significant dose-dependent decrease in the viability of DLD 1 and HCT 116 CRC cells that exhibit constitutively active Wnt/β-catenin signaling. These effects were not observed in RKO cells that lack constitutively active Wnt/β-catenin signaling (Figure 1A). We next determined the effect of lupeol on induction of apoptosis in CRC cells using annexin-V/Propidium iodide staining 48 h after treatment. We observed increased staining of annexin-V in DLD 1 and HCT 116 cells while minimal staining was observed in RKO cells indicating that lupeol treatment resulted in increased apoptosis in DLD 1 and HCT 116 cells but not in RKO cells (Figure 1B).
Figure 1. Effect of lupeol on cell viability, induction of apoptosis and colonogenic potential.
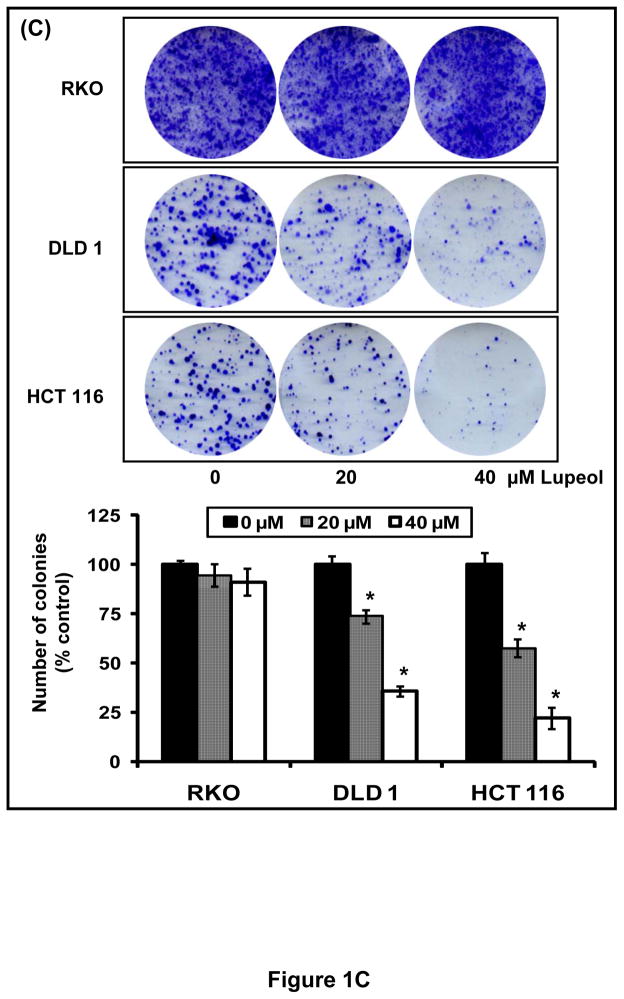
(A) CRC cells (RKO, DLD 1, HCT 116) were treated for 48 h with lupeol (0–60 μM) and viability was determined by MTT assay. Points; mean of three separate experiments wherein each treatment was repeated in 8 wells; bars ± SE. * p<0.05 compared with vehicle-treated controls. (B) Representative micrographs of DLD 1 and HCT 116 cells undergoing apoptosis after 48 h lupeol treatment. Annexin-V staining (green) represents the cells undergoing apoptosis and propidium iodide (red) represents cells in late apoptosis. (C) 800 CRC cells (RKO, DLD 1, HCT 116) were seeded and treated with lupeol (20–40 μM) for 20 days. The colonies formed were fixed, stained with crystal violet, counted and plotted as a bar graph. Each bar represents percent colonies ± SE (* p< 0.05), where colonies in untreated cells were regarded as 100%.
Next, we asked whether treatment with lupeol could exert greater effect on the formation of colonies, which allows an investigation over a longer period of time. A significant decrease in the colonogenic potential was observed in DLD 1 and HCT 116 cells that exhibit constitutively active Wnt/β-catenin signaling while no effect was observed in RKO cells that lack constitutively active Wnt/β-catenin signaling pathway (Figure 1C) indicating that lupeol selectively inhibits the proliferation of CRC that harbor constitutively active Wnt/β-catenin signaling.
4.2 Lupeol inhibits Wnt/β-catenin signaling in CRC cells
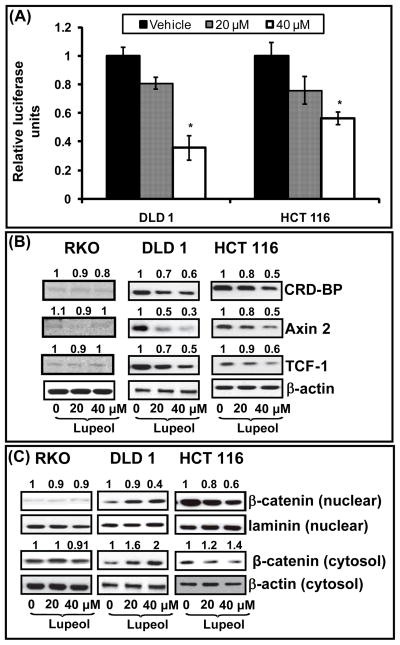
In our next set of experiments, we tested whether lupeol could inhibit the transcriptional activity of Wnt/β-catenin signaling which in turn would ultimately lead to the inhibition of its downstream targets. In DLD 1 and HCT 116 cells treated with lupeol (20 and 40 μM) for 24 h, we observed over 50% decrease in β-catenin transcriptional activity (Figure 2A), an effect persistent for up to 48 h while no change was observed in RKO cells (data not shown). We further analyzed the effect of lupeol treatment on the protein expression of various Wnt target genes. DLD 1 and HCT 116 cells treated with lupeol showed a dose-dependent decrease in Wnt target genes, coding region determinant-binding protein (CRD-BP), Axin 2 and TCF 1 while no decrease in the expression of Wnt target genes was observed in RKO cells that were also treated with lupeol (Figure 2B).
Figure 2. Effect of lupeol on Wnt/β-catenin signaling in CRC cells.
(A) CRC cells were treated as indicated in Materials and Methods. The β-catenin/Tcf responsive luciferase activity was measured and normalized against renilla luciferase activity. Each bar represents relative luciferase activity ± SE. * p<0.05 compared with vehicle-treated controls. (B) CRC cells were treated as described in Materials and Methods. Whole cell lysates were subjected to SDS-PAGE electrophoresis followed by immunoblot analysis and chemiluminescence detection, Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. Numbers on top represent relative density normalized to β-actin. (C–D) Cytosolic and nuclear fractions were analyzed by immunoblotting for β-catenin levels. Equal protein loading was confirmed by stripping the blots and reprobing with β-actin. All the immunoblots shown here are representative of three independent experiments with similar results. Numbers on top represent relative density of the bands normalized to β-actin.
4.3 Lupeol treatment decreases nuclear accumulation of β-catenin in CRC cells
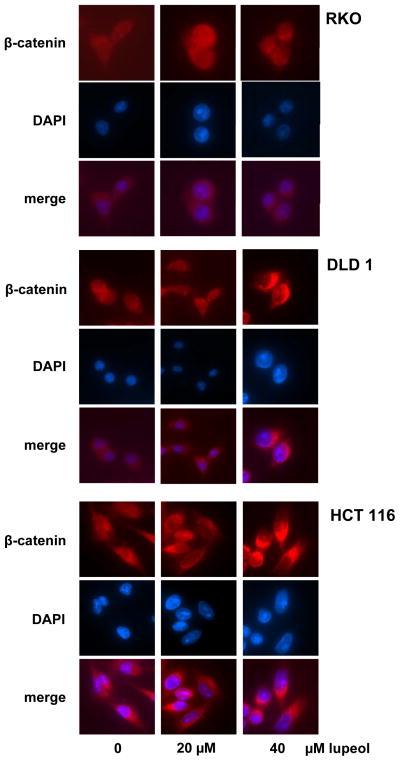
The presence of β-catenin in the nucleus is key for the effects on downstream Wnt targets. We therefore studied the localization of β-catenin after lupeol treatment. In DLD 1 and HCT 116 cells, we observed a decrease in β-catenin protein expression in the nucleus with a corresponding increase in the cytosol while no change in the localization of β-catenin was observed in RKO cells (Figure 2C). We further studied β-catenin localization using immunofluorescence assay and observed a decrease in the localization of β-catenin in the nucleus in CRC cells that exhibit constitutively active Wnt signaling (DLD 1 and HCT 116 cells) while no significant change in β-catenin localization was observed in RKO cells that do not harbor constitutive activation of Wnt/β-catenin signaling (Figure 3).
Figure 3. Effect of lupeol on β-catenin localization in CRC cells.
Photomicrographs showing β-catenin localization in CRC cells treated with lupeol. CRC cells (RKO, DLD 1 and HCT 116) were seeded in chamber slides and treated as indicated in Materials and Methods. After lupeol treatment, the slides were incubated overnight with anti-β-catenin antibody followed by fluorescence tagged secondary antibody, covered with ProLong Gold antifade reagent with DAPI (Invitrogen) and analyzed by Nikon Ti fluorescent microscope.
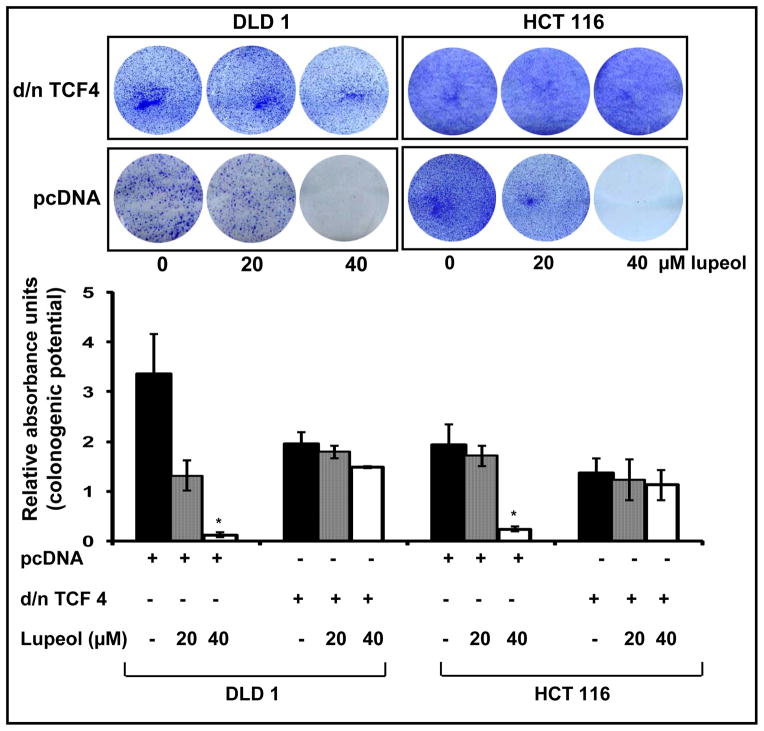
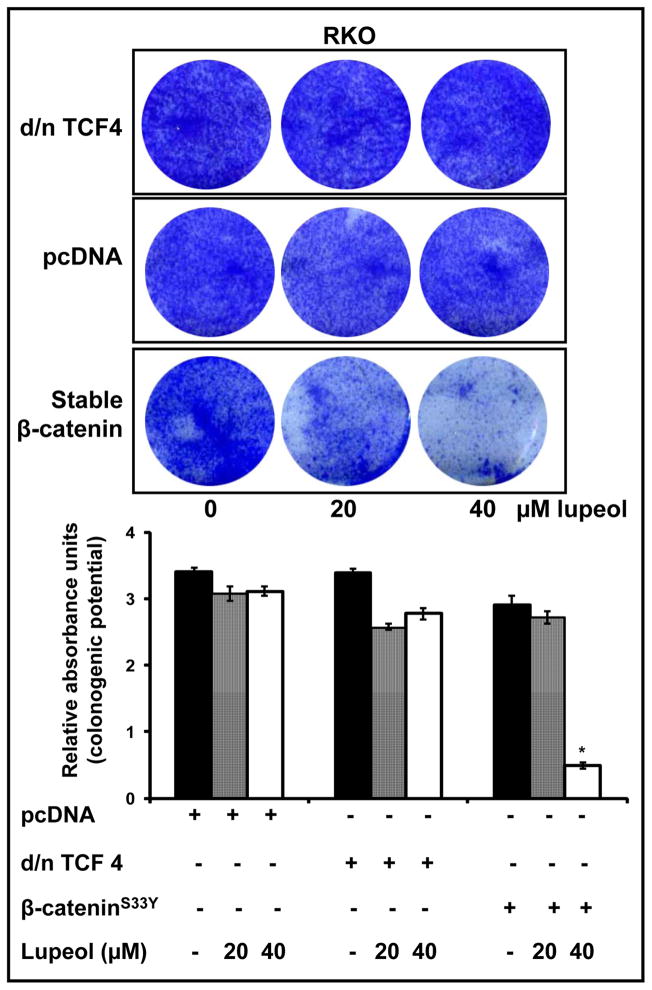
4.4 Modulation of Wnt/β-catenin signaling pathway alters the efficacy of lupeol on the colonogenic potential of CRC cells
To further verify that Wnt/β-catenin signaling is involved in lupeol-mediated effects in CRC cells, we modulated the activity of this pathway. First, we investigated the effect of lupeol on the colonogenic potential of CRC cells with down-regulated Wnt/β-catenin signaling. Our data demonstrated that when Wnt signaling was inhibited by dominant-negative TCF4, the efficacy of lupeol was lost and no significant decrease in number of colonies was observed (Figure 4). For our next set of experiments, by transfecting cells with β-catenin carrying stabilizing mutation (β-cateninS33Y), we induced Wnt/β-catenin signaling in RKO cells (that lack constitutively active Wnt/β-catenin signaling). Lupeol treatment to these cells resulted in a dose dependent inhibition of colony numbers, suggesting that activation of Wnt/β-catenin signaling sensitizes the cells to lupeol mediated effects (Figure 5).
Figure 4. Effect of down-regulation of Wnt/β-catenin signaling and lupeol treatment on the colonogenic potential of CRC cells that exhibit constitutively active Wnt/β-catenin signaling.
Wnt signaling was down-regulated in CRC cells (DLD 1, HCT 116) using dominant-negative TCF4. The cells were screened against puromycin and treated with lupeol. The colonies formed were stained as described in Materials and Methods. The crystal violet was dissolved in 50% acetic acid and absorbance was measured at 540 nm. The results are from a representative experiment repeated thrice with similar results. Data is mean ± SE of 3 samples. * p<0.05 compared with vehicle treated cells.
Figure 5. Effect of induction of Wnt/β-catenin signaling and lupeol treatment on the colonogenic potential of CRC cells that do not harbor constitutively active Wnt signaling.
RKO cells were also transfected with stable-β-catenin (β-cateninS33Y) to induce the effects of active Wnt/β-catenin signaling. The transfected cells were selected for puromycin resistance and treated with lupeol. The colonies formed were stained with crystal violet. The crystal violet was dissolved in 50% acetic acid and the absorbance was read at 540 nm. The results are from a representative experiment repeated thrice with similar results. Data is mean ± SE of 3 samples. * p<0.05 compared with vehicle-treated controls.
4.5 Lupeol inhibits the phosphorylation of β-catenin at S552 and S675 sites
Recent studies suggest that phosphorylation of β-catenin at sites Ser-552 and Ser-675 supports β-catenin to enter the nucleus and enable transcription of downstream targets [23–25]. Immunoblot analysis of lupeol treated colorectal carcinoma cells demonstrated a dose-dependent decrease in the phosphorylation of β-catenin at these two serine sites with no significant change in β-catenin protein expression (Figure 6).
Figure 6. Effect of lupeol treatment on the phosphorylation of β-catenin at sites Ser552 and Ser675.
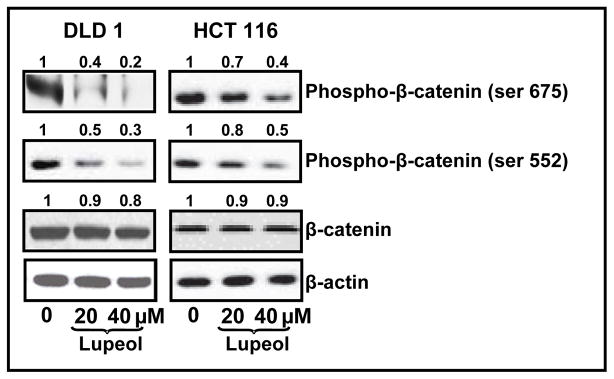
β-catenin phosphorylation in CRC cells treated with lupeol. CRC cells were treated with lupeol for 48 h. Whole cell lysates of treated cells were subjected to SDS-PAGE electrophoresis followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. Numbers on top represent relative density normalized to β-actin.
5.0 DISCUSSION
Few effective treatment options exist for patients suffering from advanced form of CRC. Several natural agents, including lupeol, with high anti-cancer efficacy and relatively minimal toxicity to normal tissues are suggested as possible candidates for chemoprevention [16, 26–29]. In vitro as well as in vivo studies from our laboratory and elsewhere have suggested that lupeol could impart chemopreventive as well as chemotherapeutic effect against a variety of cancers like melanoma [21, 30–32], prostate [33, 34], hepatocarcinoma [35]. In a previous study [22], we demonstrated the efficacy of lupeol against metastatic melanoma 451 Lu cells that exhibit constitutively active Wnt/β-catenin signaling which is supported by our recent observations demonstrating the differential response of lupeol on cells differing in the activity of Wnt/β-catenin signaling pathway. Since a vast majority of CRC cases are also known to exhibit constitutive activation of Wnt/β-catenin signaling pathway, we wanted to evaluate the effect of lupeol treatment in CRC cells. In this study, we provide evidence that lupeol is significantly more effective against CRC cells that exhibit constitutively active Wnt/β-catenin signaling as compared to those that lack active Wnt signaling. We employed HCT 116 cell line which carries a stabilizing mutation of β-catenin, DLD 1 that expresses wild-type β-catenin, but no detectable adenomatosis polyposis coli (APC) and RKO, containing both intact β-catenin and APC. DLD 1 and HCT 116 cells are characterized by elevated levels of β-catenin and constitutive activation of β-catenin/TCF-dependent transcription [9, 36].
Our data suggests that lupeol treatment causes a dose-dependent decrease in viability and colonogenic potential and an increase in apoptosis (expression of annexin V) in CRC cells that harbor constitutive activation of Wnt/β-catenin signaling.
Through the binding of a complex of β-catenin and TCF to specific promoter elements, β-catenin acts as an important transcriptional regulator for numerous genes [37]. Our data clearly demonstrates that lupeol treatment decreases β-catenin transcriptional activity and expression of Wnt target genes such as CRD-BP [38], Axin 2 [5, 39, 40] and TCF 1 [41]. Decreased expression of β-catenin in the nucleus with a corresponding increase in the cytoplasm indicates that lupeol plays a role in the translocation of β-catenin from the cytoplasm to the nucleus. In addition to CRC, a variety of other cancers including a subset of melanomas demonstrate constitutive activation of Wnt/β-catenin signaling and this subset of cancers have very poor prognosis [21, 42]. Previous studies have suggested that tumor cells show activation of genes that directly or indirectly cross talk with Wnt/β-catenin signaling forming a network allowing CRC cells to survive, proliferate and acquire highly aggressive characteristics [9, 10]. Constitutively active S33Y mutation in β-catenin results in increased accumulation and localization of beta-catenin in the nucleus resulting in activated Wnt signaling and an increase in the expression of Wnt targets.
Recent studies suggest that phosphorylation of β-catenin at Ser-552 or Ser-675 abets β-catenin to localize to the nucleus and induce transcription of various downstream targets [23–25]. We observed that lupeol treatment results in a significant decrease in the phosphorylation of β-catenin at these sites possibly contributing to decreased accumulation of β-catenin in the nucleus thereby resulting in decreased protein expression of Wnt target genes.
Taken together, our present findings demonstrate the anticancer efficacy of lupeol against a subset of CRCs that exhibit constitutively active Wnt/β-catenin signaling. These observations assume significance since a majority of CRCs have aberrant Wnt/β-catenin signaling. Several small molecules that inhibit β-catenin have recently been identified [43, 44]. Our data is significant since we utilize a naturally occurring bioactive food component to demonstrate our results. It is worth mentioning that in recent years the emphasis is on natural agents capable of selective/preferred elimination of cancer cells while sparing normal cells. In addition, from the data it can be speculated that lupeol has the potential to target advanced stage CRC cells that exhibit constitutively active Wnt/β-catenin signaling. It is important to mention that this subset of CRC has poor prognosis [36]. Validation of these cell culture data in animal model systems could pave the way for developing new strategies for chemoprevention of cancers that exhibit active Wnt/β-catenin signaling.
Acknowledgments
We would like to thank Drs. K Kinzler and B Vogelstein (Sidney Kimmel Comprehensive Cancer Center at The Johns Hopkins University, Baltimore, MD) for providing various plasmids. This work was supported by the National Institutes of Health (R01-120451; R01-CA121851). IAS was supported by a postdoctoral fellowship by U.S. PHS Grant (T32AR055893) and in part by Mentored Research Scholar Grant (MRSG-11-019-01-CNE) from the American Cancer Society.
References
- 1.Phelps RA, Broadbent TJ, Stafforini DM, Jones DA. New perspectives on APC control of cell fate and proliferation in colorectal cancer. Cell Cycle. 2009;8:2549–2556. doi: 10.4161/cc.8.16.9278. [DOI] [PubMed] [Google Scholar]
- 2.Chandra SH, Wacker I, Appelt UK, Behrens J, Schneikert J. A common role for various human truncated adenomatous polyposis coli isoforms in the control of beta-catenin activity and cell proliferation. PloS one. 2012;7:e34479. doi: 10.1371/journal.pone.0034479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 5.Lustig B, Behrens J. The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol. 2003;129:199–221. doi: 10.1007/s00432-003-0431-0. [DOI] [PubMed] [Google Scholar]
- 6.Weeraratna AT. A Wnt-er wonderland--the complexity of Wnt signaling in melanoma. Cancer Metastasis Rev. 2005;24:237–250. doi: 10.1007/s10555-005-1574-z. [DOI] [PubMed] [Google Scholar]
- 7.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 8.Elcheva I, Tarapore RS, Bhatia N, Spiegelman VS. Overexpression of mRNA-binding protein CRD-BP in malignant melanomas. Oncogene. 2008;27:5069–5074. doi: 10.1038/onc.2008.141. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs SY, Ougolkov AV, Spiegelman VS, Minamoto T. Oncogenic beta-catenin signaling networks in colorectal cancer. Cell Cycle. 2005;4:1522–1539. doi: 10.4161/cc.4.11.2129. [DOI] [PubMed] [Google Scholar]
- 10.Kim TH, Xiong H, Zhang Z, Ren B. beta-Catenin activates the growth factor endothelin-1 in colon cancer cells. Oncogene. 2005;24:597–604. doi: 10.1038/sj.onc.1208237. [DOI] [PubMed] [Google Scholar]
- 11.Taketo MM. Shutting down Wnt signal-activated cancer. Nat Genet. 2004;36:320–322. doi: 10.1038/ng0404-320. [DOI] [PubMed] [Google Scholar]
- 12.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 13.Beveridge TH, Li TS, Drover JC. Phytosterol content in American ginseng seed oil. J Agric Food Chem. 2002;50:744–750. doi: 10.1021/jf010701v. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez MA, de las Heras B, Garcia MD, Saenz MT, Villar A. New insights into the mechanism of action of the anti-inflammatory triterpene lupeol. J Pharm Pharmacol. 2001;53:1533–1539. doi: 10.1211/0022357011777909. [DOI] [PubMed] [Google Scholar]
- 15.Imam S, Azhar I, Hasan MM, Ali MS, Ahmed SW. Two triterpenes lupanone and lupeol isolated and identified from Tamarindus indica linn. Pak J Pharm Sci. 2007;20:125–127. [PubMed] [Google Scholar]
- 16.Saleem M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009;285:109–115. doi: 10.1016/j.canlet.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You YJ, Nam NH, Kim Y, Bae KH, Ahn BZ. Antiangiogenic activity of lupeol from Bombax ceiba. Phytother Res. 2003;17:341–344. doi: 10.1002/ptr.1140. [DOI] [PubMed] [Google Scholar]
- 18.Lima LM, Perazzo FF, Tavares Carvalho JC, Bastos JK. Anti-inflammatory and analgesic activities of the ethanolic extracts from Zanthoxylum riedelianum (Rutaceae) leaves and stem bark. J Pharm Pharmacol. 2007;59:1151–1158. doi: 10.1211/jpp.59.8.0014. [DOI] [PubMed] [Google Scholar]
- 19.Saleem M, Kaur S, Kweon MH, Adhami VM, Afaq F, Mukhtar H. Lupeol, a fruit and vegetable based triterpene, induces apoptotic death of human pancreatic adenocarcinoma cells via inhibition of Ras signaling pathway. Carcinogenesis. 2005;26:1956–1964. doi: 10.1093/carcin/bgi157. [DOI] [PubMed] [Google Scholar]
- 20.Vasconcelos JF, Teixeira MM, Barbosa-Filho JM, Lucio AS, Almeida JR, de Queiroz LP, Ribeiro-Dos-Santos R, Soares MB. The triterpenoid lupeol attenuates allergic airway inflammation in a murine model. Int Immunopharmacol. 2008;8:1216–1221. doi: 10.1016/j.intimp.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Tarapore RS, Siddiqui IA, Saleem M, Adhami VM, Spiegelman VS, Mukhtar H. Specific targeting of Wnt/beta-catenin signaling in human melanoma cells by a dietary triterpene lupeol. Carcinogenesis. 2010;31:1844–1853. doi: 10.1093/carcin/bgq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saleem M, Maddodi N, Abu Zaid M, Khan N, bin Hafeez B, Asim M, Suh Y, Yun JM, Setaluri V, Mukhtar H. Lupeol inhibits growth of highly aggressive human metastatic melanoma cells in vitro and in vivo by inducing apoptosis. Clin Cancer Res. 2008;14:2119–2127. doi: 10.1158/1078-0432.CCR-07-4413. [DOI] [PubMed] [Google Scholar]
- 23.Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J Biol Chem. 2006;281:9971–9976. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- 25.Hino S, Tanji C, Nakayama KI, Kikuchi A. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase stabilizes beta-catenin through inhibition of its ubiquitination. Mol Cell Biol. 2005;25:9063–9072. doi: 10.1128/MCB.25.20.9063-9072.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afaq F, Adhami VM, Ahmad N, Mukhtar H. Botanical antioxidants for chemoprevention of photocarcinogenesis. Front Biosci. 2002;7:d784–792. doi: 10.2741/afaq. [DOI] [PubMed] [Google Scholar]
- 27.Becker JC, Kirkwood JM, Agarwala SS, Dummer R, Schrama D, Hauschild A. Molecularly targeted therapy for melanoma: current reality and future options. Cancer. 2006;107:2317–2327. doi: 10.1002/cncr.22273. [DOI] [PubMed] [Google Scholar]
- 28.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 29.Nichenametla SN, Taruscio TG, Barney DL, Exon JH. A review of the effects and mechanisms of polyphenolics in cancer. Crit Rev Food Sci Nutr. 2006;46:161–183. doi: 10.1080/10408390591000541. [DOI] [PubMed] [Google Scholar]
- 30.Hata K, Mukaiyama T, Tsujimura N, Sato Y, Kosaka Y, Sakamoto K, Hori K. Differentiation-inducing activity of lupane triterpenes on a mouse melanoma cell line. Cytotechnology. 2006;52:151–158. doi: 10.1007/s10616-007-9069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogiwara K, Hata K. Melanoma cell differentiation induced by lupeol separates into two stages: morphological and functional changes. J Nat Med. 2009;63:323–326. doi: 10.1007/s11418-009-0319-7. [DOI] [PubMed] [Google Scholar]
- 32.Tarapore RS, Siddiqui IA, Mukhtar H. Modulation of Wnt/beta-catenin signaling pathway by bioactive food components. Carcinogenesis. 2012;33:483–491. doi: 10.1093/carcin/bgr305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prasad S, Kalra N, Shukla Y. Induction of apoptosis by lupeol and mango extract in mouse prostate and LNCaP cells. Nutr Cancer. 2008;60:120–130. doi: 10.1080/01635580701613772. [DOI] [PubMed] [Google Scholar]
- 34.Saleem M, Murtaza I, Witkowsky O, Kohl AM, Maddodi N. Lupeol triterpene, a novel diet-based microtubule targeting agent: disrupts survivin/cFLIP activation in prostate cancer cells. Biochem Biophys Res Commun. 2009;388:576–582. doi: 10.1016/j.bbrc.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Zhang Y, Yang X, Lv Z. Lupeol, a dietary triterpene, inhibited growth, and induced apoptosis through down-regulation of DR3 in SMMC7721 cells. Cancer Invest. 2009;27:163–170. doi: 10.1080/07357900802210745. [DOI] [PubMed] [Google Scholar]
- 36.Deng J, Miller SA, Wang HY, Xia W, Wen Y, Zhou BP, Li Y, Lin SY, Hung MC. beta-catenin interacts with and inhibits NF-kappa B in human colon and breast cancer. Cancer Cell. 2002;2:323–334. doi: 10.1016/s1535-6108(02)00154-x. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z, Deb A, Pachori A, He W, Guo J, Pratt R, Dzau VJ. Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. J Mol Cell Cardiol. 2009;46:370–377. doi: 10.1016/j.yjmcc.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noubissi FK, Elcheva I, Bhatia N, Shakoori A, Ougolkov A, Liu J, Minamoto T, Ross J, Fuchs SY, Spiegelman VS. CRD-BP mediates stabilization of betaTrCP1 and c-myc mRNA in response to beta-catenin signalling. Nature. 2006;441:898–901. doi: 10.1038/nature04839. [DOI] [PubMed] [Google Scholar]
- 39.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, Guo L, Sakamoto D, Caothien RH, Fuller JH, Reinhard C, et al. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors. Proc Natl Acad Sci U S A. 2001;98:14973–14978. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roose J, Huls G, van Beest M, Moerer P, van der Horn K, Goldschmeding R, Logtenberg T, Clevers H. Synergy between tumor suppressor APC and the beta-catenin-Tcf4 target Tcf1. Science. 1999;285:1923–1926. doi: 10.1126/science.285.5435.1923. [DOI] [PubMed] [Google Scholar]
- 42.Elcheva I, Tarapore RS, Bhatia N, Spiegelman VS. Overexpression of mRNA-binding protein CRD-BP in malignant melanomas. Oncogene. 2008 doi: 10.1038/onc.2008.141. [DOI] [PubMed] [Google Scholar]
- 43.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]