Abstract
Background
GLIPR1 is up-regulated by p53 in prostate cancer (PCa) cells and has preclinical anti-tumor activity. A phase I clinical trial was conducted to evaluate the safety and activity of the neoadjuvant intraprostatic injection of GLIPR1 expressing adenovirus for high-risk localized PCa before radical prostatectomy (RP).
Methods
Eligible men had localized PCa (T1-T2c) with Gleason score ≥7 or PSA ≥ 10 ng/ml, and were candidates for RP. Patients received the adenoviral vector expressing the GLIPR1 gene by a single injection into the prostate followed 4 weeks later by RP. Six viral particle dose levels were evaluated: 1010, 5 × 1010, 1011, 5 × 1011, 1012 and 5 × 1012 vp.
Results
Nineteen patients with a median age of 64 years were recruited. Nine men had T1c, 4 had T2a, and 3 had T2b and T2c clinical stage. Toxicities included urinary tract infection (n=3), flu-like syndrome (n=3), fever (n=1), dysuria (n=1) and photophobia (n=1). Laboratory toxicities were grade 1 elevated AST/ALT (n=1) and elevations of PTT (n=3, with 1 proven to be lupus anticoagulant). No pathologic complete remission was seen. Morphologic cytotoxic activity, induction of apoptosis and nuclear p27Kip1 upregulation were observed. Peripheral blood CD8+, CD4+ and CD3+ T-lymphocytes were increased, with upregulation of their HLA-DR expression, and elevations of serum IL-12.
Conclusions
The intraprostatic administration of GLIPR1 tumor suppressor gene expressed by an adenoviral vector was safe in men with localized high-risk PCa preceding RP. Preliminary evidence of biologic anti-tumor activity and systemic immune response was documented.
Keywords: GLIPR1 tumor suppressor gene, adenoviral vector, neoadjuvant intraprostatic injection, localized high-risk prostate cancer, radical prostatectomy
Introduction
Prostate cancer (PCa) is the most common solid tumor diagnosed in the United States with approximately 200,000 cases and 30,000 deaths in a year (1). PCa is commonly detected when still localized since the advent of prostate-specific antigen (PSA) screening and radical prostatectomy (RP) can improve outcomes in appropriately selected patients. Androgen deprivation therapy (ADT) is the conventional frontline systemic therapy for hormone-naïve PCa. However, ADT has deleterious effects on quality of life and bone health and progression to castration-resistant prostate cancer (CRPC) is almost unavoidable in men with advanced disease. Treatment options are limited in patients with metastatic CRPC and chemotherapy (docetaxel, cabazitaxel), immunotherapy (sipuleucel-T) and androgen synthesis inhibitors (abiraterone acetate) have demonstrated modest extensions of survival (2–6). Clearly, there is a need for more effective and tolerable agents for PCa.
Given that the vast majority of men present with localized disease, the paradigm of neoadjuvant therapy preceding RP may improve outcomes and facilitate the development of new agents for PCa by providing an early signal of activity (7). The evaluation of neoadjuvant therapy is justified in high-risk cases defined by high PSA, Gleason score and clinical stage. Since pathologic and biologic activity can be rapidly determined after surgery, the efficacy of a systemic regimen is evident with a relatively small number of patients before long-term follow-up. Neoadjuvant ADT followed by RP improves pathologic outcomes with no definitive evidence for improvement in long-term clinical outcomes (8). Ongoing phase III trials are evaluating the impact of combination chemotherapy and ADT on outcomes based on the suggestion of improved pathologic outcomes (9). Additionally, biologic and chemotherapeutic agents have been evaluated without concomitant ADT to obtain a signal of activity and provide proof of concept (10–13). The intraprostatic delivery of genes by employing a vector has also been studied (14–16).
Because of the established association between loss of p53 function and prostate cancer metastasis, we have pursued the identification, characterization and functional analysis of p53-target genes in prostate cancer (17–19). We identified the GLIPR1 (Glioma Pathogenesis Related Protein), previously termed RTVP-1 (related to testes-specific, vespid and pathogenesis proteins), mRNA as being upregulated by p53 in mouse prostate cancer cells. Both mouse and human GLIPR1 contain p53 binding elements in promoter and intronic sequences. GLIPR1 was demonstrated to have pro-apoptotic, anti-angiogenic, immunostimulatory and metastasis-suppressing activity (20). Adenoviral-vector-mediated GLIPR1delivery in vivo was capable of eradicating micrometastatic disease (21, 22). GLIPR1 is downregulated, in part by gene methylation, in PCa compared to normal prostate tissue (23). Given that GLIPR1 may confer anti-tumor activity, a phase I clinical trial was conducted to evaluate the safety and biologic activity of adenovirus delivered in situ GLIPR1 gene therapy for localized intermediate and high-risk PCa before RP.
Materials and methods
Patient eligibility
Patients were required to have clinical stage T1c - T2cN0M0 adenocarcinoma of the prostate with a Gleason score ≥ 7 or PSA ≥ 10 ng/ml. All participants had to to have a needle biopsy of the prostate (at least 12 cores) to obtain tissue for pathological analysis. A baseline chest x-ray, bone scan and CT scan of the abdomen and pelvis were mandated for staging. Patients were also required to be candidates for RP. Written informed consent was obtained from all of the patients.
Construction of adenovirus bearing GLIPR1
Clinical grade replication-defective Ad5GLIPR1 viral vector was prepared in the Baylor College of Medicine Cell and Gene Therapy GMP facility. Human GLIPR1 cDNA was PCR-amplified from pcDNA3-hRTVP1 vector with specific primers that contained sequence for XbaI and KpnI restriction enzymes (underlined), respectively (upper primer: 5’CTAGTCTAGAGCCACCATGCGTGTCACACTTGCT3’, lower primer: 5’GGGGTACCTTAGTCCAAAAGAACTAA3’). Additionally, upstream primer contained optimized Kozak sequence (GCCACC, bold font) in front of ATG codon of hRTVP-1 cDNA. The resulting 821 bp PCR fragment containing complete coding sequence of GLIPR1 was cloned into XbaI and KpnI sites of pShutlle-X (Clontech, CA) transfer vector and sequenced. The coding sequence for GLIPR1 cDNA was not modified. Three recombinant pShuttle-hRTVPk vector clones were analyzed for hRTVP-1 expression by western blot after transient transfection into A459 cell line. All three pShuttle-hRTVPk clones with modified Kozak sequence demonstrated better expression of hRTVP-1 protein compared to the original pShuttle-hRTVP-1 vector (Fig. 1). Clone pShuttle-hRTVPk1 was used for construction of the adenoviral vector.
Fig. 1. Modification of Kozak sequence of GLIPR1/RTVP-1 cDNA improves GLIPR1 protein expression.
Unmodified and modified GLIPR1/RTVP-1 cDNA constructs were transfected in A459 cell line and cell lysates were analyzed by western blot for GLIPR1 protein expression levels 48 hour after transfection. Lanes: 1. pcDNA3.1 (control vector); 2. pShuttle-hRTVP-1: 3. pSecTag-hRTVP-1; 4. pShuttle-hRTVPk1, 5. pShuttle-hRTVPk2, 6. pShuttle-hRTVPk3. All three pShuttle-hRTVPk clones with modified Kozak sequence (lane 4–6) demonstrated increased expression of GLIPR1/hRTVP-1 protein compared to the original pShuttle-hRTVP-1 vector (lane 2).
Clontech’s adeno-X expression system protocol was used and the I-Ceu/PI-SceI fragment containing expression cassette CMV-hRTVPk1-polyA was used to generate the adenoviral vector. The construction of Ad5hRTVPk1 was confirmed by restriction endonuclease mapping and by conventional dideoxy nucleotide sequencing. Expression of RTVP-1 was confirmed by western blot. For the production of recombinant adenoviruses, 293 cells were transfected with Ad5hRTVPk1. Once cytopathic effects were observed, cells were harvested and lysed. This lysate was used for plaque purification. Further production and characterization of a clinical grade Ad5hRTVP-1 vector was performed by Baylor College of Medicine Cell and Gene Therapy GMP facility. Comprehensive information about the clinical grade vector was provided to the FDA and the vector was approved for the current IND clinical trial. According to a recent HGNC recommendation, GLIPR1 is the preferred symbol for the RTVP-1 gene. For consistency we will use Ad5GLIPR1 symbol to indicate clinical grade vector for the remaining part of the manuscript.
Trial design and therapy administration
The protocol used in our study was approved by the Biosafety Committees and the Institutional Review Boards of the participating institutions of Baylor College of Medicine (BCM) (Veterans Affairs Medical Center, Ben Taub General Hospital, St. Luke’s Episcopal Hospital), the Recombinant DNA Advisory Committee of the NIH, and the United States Food and Drug Administration. The Data and Safety Monitoring Plan was applied under an IND by the prostate cancer SPORE program at BCM.
The trial was designed as a conventional phase I trial enrolling 3 patients per cohort and evaluating 6 doses of Viral Particles (VP): 1010, 5 × 1010, 1011, 5 × 1011, 1012 and 5 × 1012 vp. On day 1, a transrectal ultrasound (TRUS) guided single intraprostate injection of adenoviral GLIPR1 was delivered, followed by RP on Day 28. Factors such as tumor size and location determined the injectable volume, which was to be no more than 2 ml split into two separate injections of 1 ml each into the right and left lobes. An oral, broad spectrum antibiotic was administered the evening before and the morning of the intraprostatic injection and continued for 4 days. Following the procedure, the patients were admitted for a 23-hour observation period. They were then followed in the outpatient clinic at day 8, 15, 21 and day 28 (±2 days at each visit) for a history and physical exam and urinalysis, and urine culture and sensitivity if necessary. Laboratory work consisting of hematology and comprehensive metabolic panel survey was measured at baseline and upon completion of therapy before RP.
Lymphocyte phenotype by flow cytometry
Automated complete blood counts were performed by bBCM and MD Anderson Cancer Center. Flow cytometric phenotyping was performed after incubating 100µl of heparinized blood with the following dual color-labeled antibody pairs: CD45/CD14, CD3/CD19, CD3/CD8, CD3/CD4, CD8/HLA-DR, CD4/HLA-DR, CD3/HLA-DR, and CD3/CD56+CD16 (Simultest, Becton Dickinson and company, Franklin Lakes, NJ). After incubation at room temperature for 30 minutes, red blood cells were lysed with formic acid and the samples fixed with paraformaldehyde using a Coulter Q-Prep workstation. Forward and side scatter were set to distinguish lymphocyte, macrophage, and granulocyte populations from debris with a BD FACSCalibur Flow Cytometer (Becton Dickinson and Company, Franklin Lakes, NJ). Results were expressed as the percent of cells in the lymphocyte gate.
Tumor tissue, blood and plasma based correlative studies
Tumor tissue was examined by routine hematoxylin and eosin based morphologic examination by urologic pathologists at each institution. Assessment of tumor for apoptosis (TUNEL) and nuclear p27Kip1 was performed by immunohistochemistry (IHC) on the baseline biopsy and RP specimen in a subset of patients. Results from the IHC were compared with results observed in controls from a tissue microarray (TMA)containing cancer tissues from RP specimens. Plasma, serum and urine basic fibroblast growth factor (bFGF) and plasma and serum vascular endothelial growth factor (VEGF) levels at baseline, days 15, 28, and the fourth post-operative day (±2 days at each visit) were performed. Blood was drawn at screening and days 8, 15, 21 and 28, and the fourth postoperative day (±2 days at each visit) for the following immunological studies by ELISA: ELISPOT assay, NK activity, IL-2, IL-6, IL-12, IFN- γ, TGF-β, and CD4/CD8 levels. The titer of serum antibodies to adenovirus was measured at baseline and day 28 (±2 days at each visit).
Statistical considerations
A conventional phase I trial was designed with a sample size of 3–6 patients for each of 6 doses of virus. The maximum tolerated dose (MTD) was the dose for which the incidence of dose limiting toxicities (DLT) was <33%. Although the trial was not expected to have sufficient power to detect small differences in biomarkers, the preliminary analyses were performed using all patients and stratified by dose. Numerical and graphical descriptive statistics were calculated. Paired t-tests or Wilcoxon signed-rank tests were employed to evaluate the change in biomarkers from biopsy to RP. The one way analysis of variance (ANOVA) or Kruskal-Wallis tests followed by appropriate multiple comparisons procedures if the overall test was statistically significant at the 5% level of significance, were used to compare changes in biological markers between the dose groups to assess differences.
Results
Patient characteristics
Nineteen patients were enrolled with a median age of 64 (range 50–75) (Table 1). One additional patient was accrued in the second cohort administered 5 × 1010 vp since a patient in this cohort withdrew consent from participation (although he did undergo therapy followed by RP). The routine pathology and toxicity data are available for the patient who withdrew consent but correlative studies could not be performed after withdrawal of consent. Forty-seven percent of men had a clinical stage of T1c. Fifty-eight percent had a Gleason score of 7, and the remainder had a Gleason score of 8 or 9. The PSA was ≤10 ng/ml in 95% of patients.
Table 1.
Clinical and pathologic characteristics
| Patient characteristic | Number of patients (%) | |
|---|---|---|
| Total number of patients | 19 | |
| Median age | 64 (Range 50–75) | |
| Clinical stage | T1c | 9 (47%) |
| T2a | 4 (21%) | |
| T2b | 3 (16%) | |
| T2c | 3 (16%) | |
| Gleason Score | 7 | 11 (58%) |
| 8 | 5 (26%) | |
| 9 | 3 (16%) | |
| PSA | ≤10 | 18 (95%) |
| 10–20 | 1 (5%) | |
| >20 | 0 (0%) | |
| Radical prostatectomy pathologic stage | ||
| pT0 | 0 (0%) | |
| pT1-2 | 12 (63%) | |
| pT3 | 7 (37%) | |
| Node+ | 2 (11%) | |
| Seminal vesicle invasion | 5 (26%) | |
| Margin + | 3 (16%) | |
Safety and feasibility
All 6 doses were feasible with no grade 4 or higher toxicities. Symptomatic toxicities included urinary tract infection (n=3; grade 3 in 2 men), flu-like syndrome (n=3), grade 1 fever (n=1), dysuria (n=1) and photophobia (n=1). Asymptomatic laboratory toxicities were grade 1 elevated AST/ALT (n=1) and elevations of PTT (n=3; 2 were transient, 1 prolonged elevation proven to be lupus anticoagulant in the 1 ×1012 cohort). No excess in post-operative complications was observed.
Histopathological evaluation of radical prostatectomy specimens
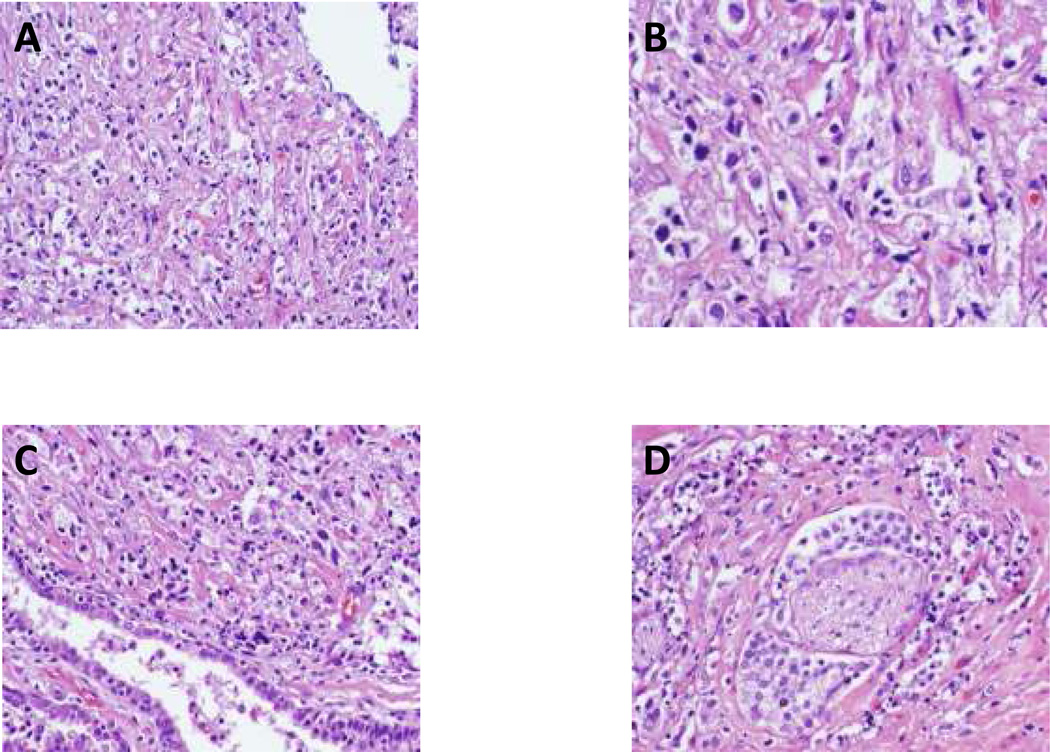
Pathologic stage at RP included pT2N0 (n=12), pT3N0 (n=6) and pT3N1 (n=1), with focal positive margins in 3 patients (Table 1). No pathologic complete remission (pCR) was seen, but morphologic evidence of biologic activity was observed including cytopathic effects and inflammatory infiltrates across all doses. For example, compared with the pretreatment biopsy, hemotoxylin and eosin staining from the RP tissue from the first patient treated with the lowest dose (1×1010 vp) revealed extensive cytotoxic activity (Fig. 2 A-D). The benign prostatic epithelium within the tumor appeared stressed but did not show the cytotoxic effect (Fig. 2C). These morphological alterations suggest a tumor-specific cytotoxic effect of AdGLIPR1. Interestingly, a protective perineural effect was observed; the cancer cells surrounding a nerve tended to survive the AdGLIPR1 induced cytotoxic effect (Fig. 2D).
Fig. 2. Cytopathological examination of prostatectomy specimens following AdGLIPR1 therapy.
Cytopathic apoptotic effects of AdGLIPR1 in prostate cancer RP specimen (1×1010 vp) demonstrated in low power (× 100) by hematoxylin- eosin staining obtained 4 weeks after a single injection. A. Extensive cell death was documented in a large (60–70%) portion of the RP tissues; B. high power (× 400) micrograph of A; C. Non-viable tumor tissue juxtaposed to compromised but viable normal prostate epithelium suggesting tumor cell specificity of cytotoxic effects; D. Viable prostate cancer cells in the perineurium surrounding a nerve (in contrast to non-viable adjacent prostate cancer) suggesting a protective perineural effect.
Modulation of tumor tissue biomarkers
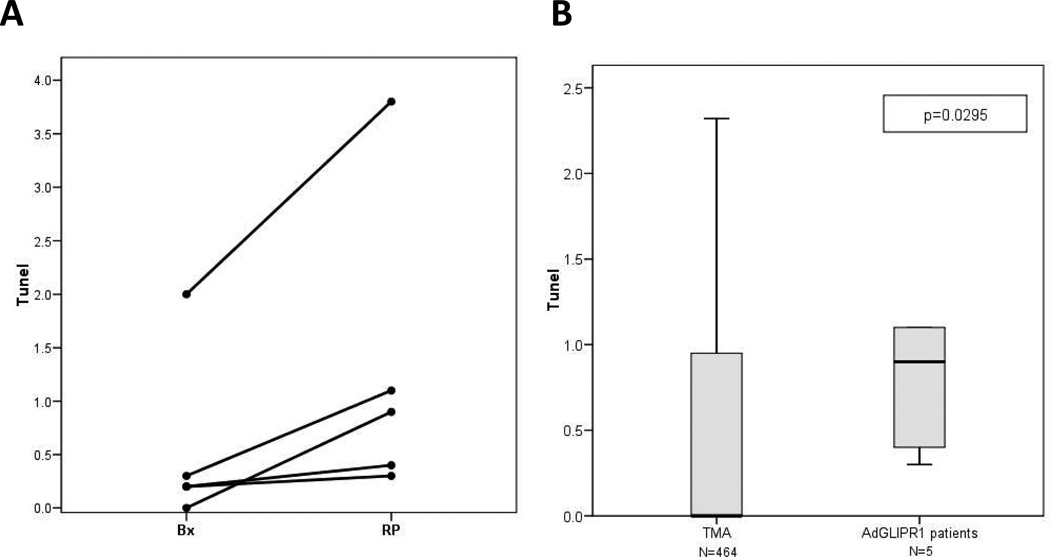
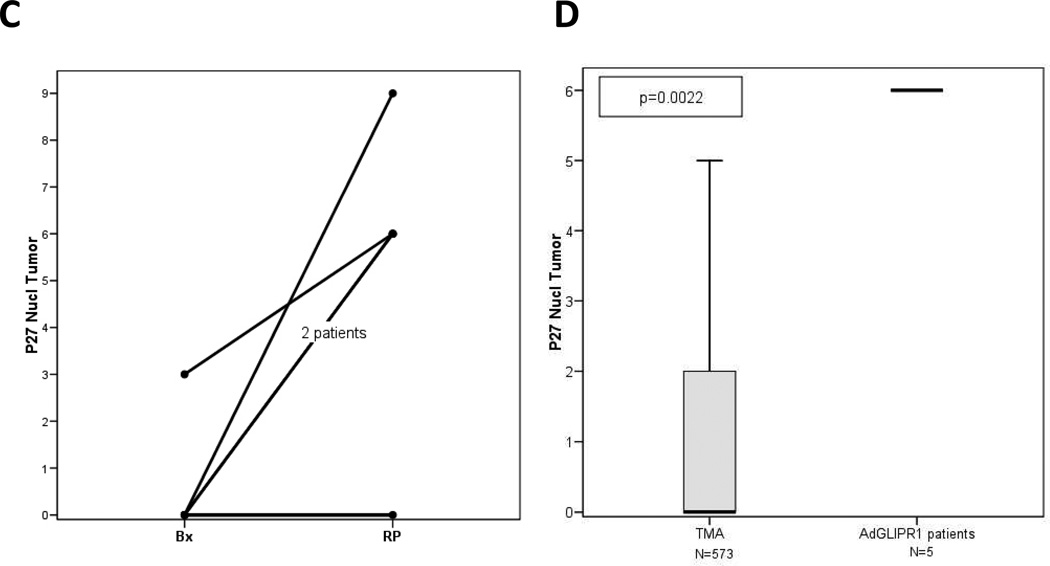
The apoptotic activity was measured by IHC for TUNEL performed in 5 patients in the first 2 cohorts (Fig. 3). The results demonstrate induction of apoptosis in these patients when pre-treatment biopsy and RP tumor specimens are compared (Fig. 3A) or when RP specimens were compared to a large number of individual RP tumor specimens (n=464) assembled on a tissue microarray (TMA) (Fig 3B). Further evidence of AdGLIPR1 activity was documented in similar studies that showed increased levels by IHC of nuclear p27Kip1 in the same 5 patients from the first 2 cohorts (Fig. 3C). AdGLIPR1 mediated translocation of p27 to the nucleus in treated patients was demonstrated in comparative analysis of pre-treatment biopsies with RP specimens (Fig. 3C) or when RP specimens were compared to RP specimens (n=573) assembled on a tissue microarray (TMA) (Fig. 3D).
Fig. 3. Immunohistochemical evaluation of prostatectomy specimens following AdGLIPR1.
Results for 5 patients 1×1010 –5×1010 vp for Cohort 1,2 are shown. A Apoptotic indices (TUNEL) in tumors of patients treated with AdGLIPR1 are significantly increased compared to preoperative biopsies [Wilcoxon Signed Rank Test, p=0.0431, N=5]; B Apoptotic indices were in patients treated AdGLIPR1 (RP) was significantly higher than in retrospective (TMA) controls [Mann-Whitney Test, p=0.0295].. C. Intranuclear p27Kip1 expression in prostate cancer tissues was increased after treatment with AdGLIPR1 (RP) in 4 out of 5 patients compared to preoperative biopsies [Wilcoxon Signed Rank Test, p=0.0656]; D. Intranuclear p27Kip1 expression in patients treated AdGLIPR1 was significantly higher than in retrospective (TMA) controls [Mann-Whitney Test, p=0.0022]. Box plots were used for nonparametric data and show the median (line), interquartile range (the box includes the middle 50% of observations), and whiskers extend out to the farthest points that are not outliers. These plots allow the reader to visually compare distributions of the variables.
Immunophenotyping analysis of peripheral blood lymphocytes
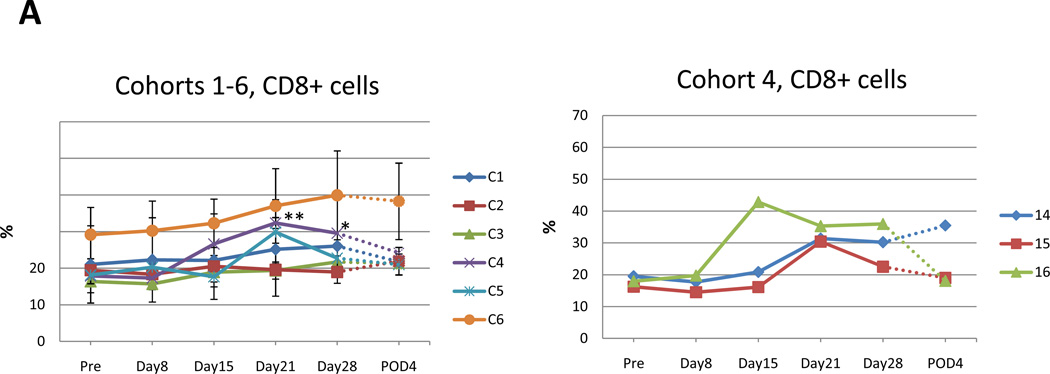
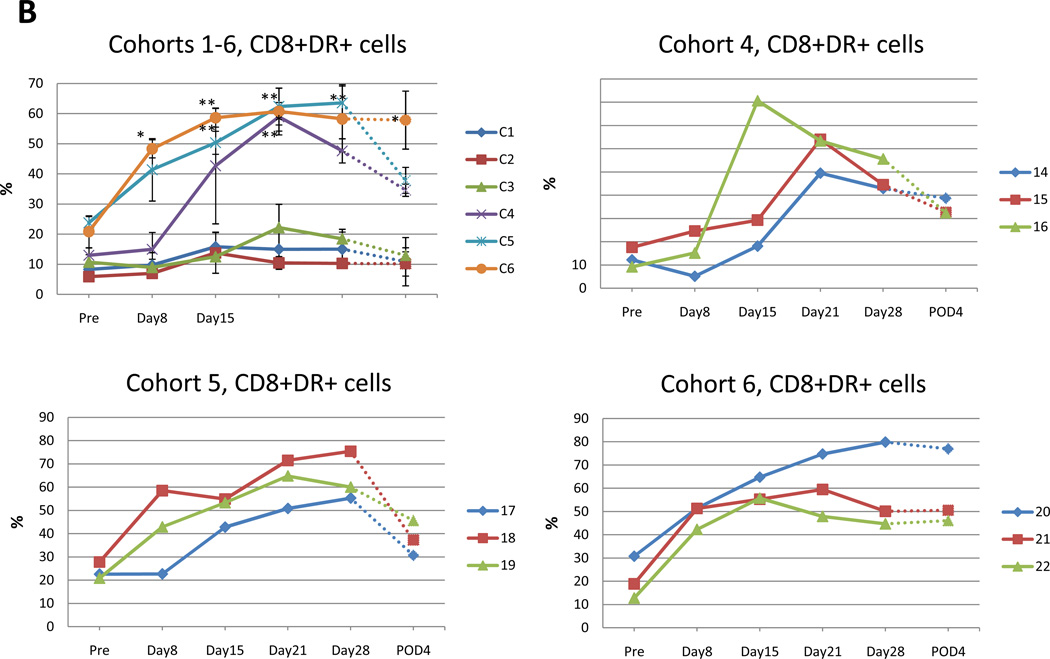
Cohorts 1–3 did not demonstrate a clear increase in CD8+ lymphocytes (Fig. 4A). There was moderate increase of CD8+ lymphocytes from 17.9%, 18.1%, 29.2% in cohorts 4–6 at pretreatment to 32.4%, 29.9%, 39.9% at 2 or 3 weeks after treatment (Fig. 4A). This increase reached statistical significance on day 21 & day 28 of cohort 4 (p=0.0012, p=0.0440 respectively; unpaired t-test) (Fig. 4 A, right).
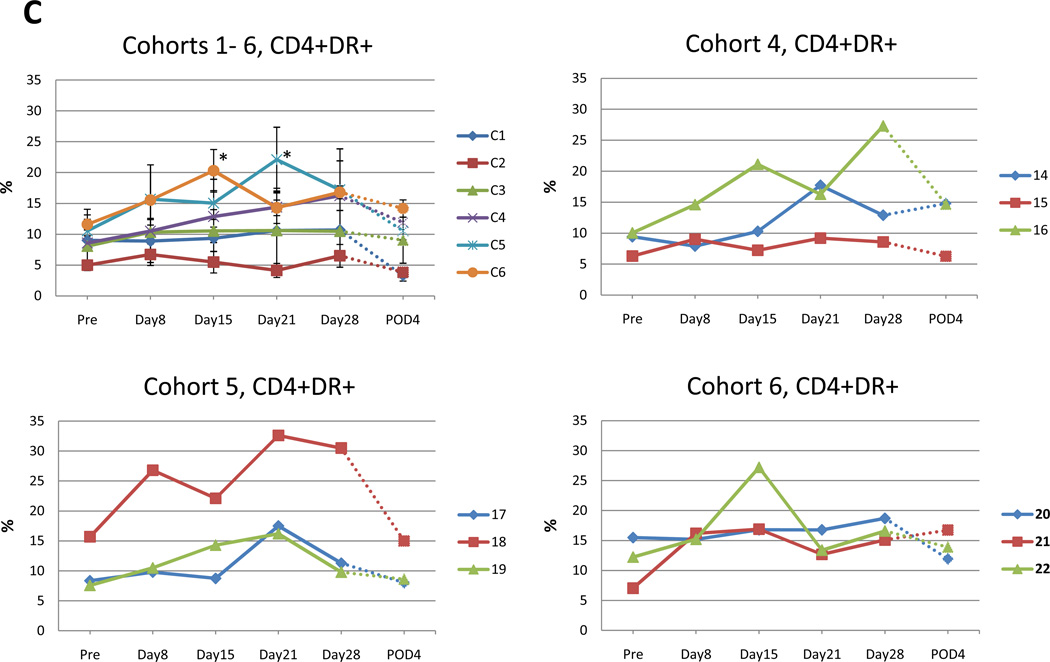
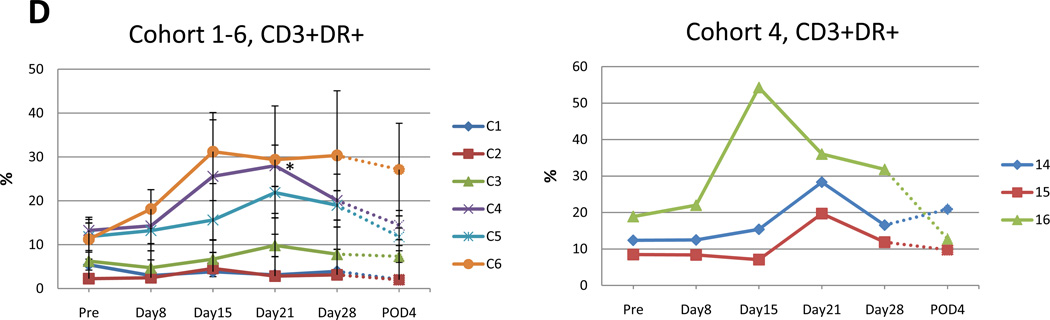
Fig. 4. Flow cytometry analysis of patient samples of circulating peripheral blood lymphocytes at indicated time points.
Data for each cohort are presented as mean +/−SE. Value that reach statistical significance compare to the pretreatment levels are indicated by * p ≤ 0.05, ** p ≤ 0.01. A. Percent of CD8+ positive cells at indicated time points in cohort 1–6 (left panel). Increase in percentage of CD8+ cells in the 3 patients in cohort 4 attained statistical significance compared to pretreatment value indicated by * p ≤ 0.05, ** p ≤ 0.01 at day 28 and day 21, respectively. Percent of CD8+ cells for individual patients in cohort 4 (right panel). B. Percent of CD8+DR+ positive cells in cohorts 1–6 at indicated time points during the trial (top left panel). Increase in percentage of CD8+DR+ cells that reach statistical significance compared to pretreatment value in cohort 4 (top right), 5 (bottom left) and 6 (bottom right) indicated. C. Percent of CD4+DR+ positive cells at indicated time points in cohort 1–6 (top left). Increase in percentage of CD4+DR+ cells that reach statistical significance compared to pretreatment value in cohort 5 and 6 indicated by * p ≤ 0.05. Additionally, CD4+DR+ values are presented for individual patient in cohort 4 (top right) and 5, 6 (bottom left and right). D. Percent of CD4+DR+ positive cells at indicated time points in cohort 1–6 (top left). Increase in percentage of CD4+DR+ cells that reach statistical significance compared to pretreatment value in cohort 5 and 6 indicated by * p ≤ 0.05. Additionally, CD4+DR+ values are presented for individual patient in cohort 4 (top right) and 5, 6 (bottom left and right).
The HLA-DR marker of activation was used to double label CD4 or CD8+ lymphocytes as a relative measure of activated T cells. There was a moderate increase in the percentage of double positive CD8+ DR+ T cells for patients in cohort 4–6 (Fig. 4B). The pretreatment mean percentage of CD8+ T cells positive for the HLA-DR marker of activation was 12.9%, 23.8%, 20.8% (cohort 4, 5, 6). In cohort 4, for day 21 and day 28 post treatment and the fourth postoperative day, the mean percent of CD8+ DR+ T cells increased by 58.9%, 47.6% and 34.6%, which were statistically significant (p=0.001, p=0.0018, p=0.0025 respectively) (Fig. 4B, top right). In cohort 5, for day 14, day 21, day 28 and the fourth postoperative day, the mean percentage of CD8+ DR+ T cells significantly increased by 50.3%, 62.4%, 63.6% and 37.9% (p=0.0036, p=0.0039, p=0.0034, p=0.0421, respectively) (Fig. 4B, bottom left). In cohort 6, on day 7, the mean percentage of CD8+ DR+ T cells was already significantly increased to 48.3% (p=0.0107), and on day 14, the mean percent of CD8+ DR+ T cells was gradually increased to 58.6% (p=0.0035). It reached a peak at day 21 (60.7% p=0.0133). After that, the percent of DR+CD8+ T cells remained high until post-operative day 4 (day 28; 58.2% p=0.0370, fourth postoperative day; 57.8% p=0.0280) (Fig. 4B, bottom right).
In cohort 4, 5 and 6, the post treatment mean percentage of CD4+ T cells positive for the HLA-DR marker of activation was increased compared to pretreatment levels (Fig. 4C). The pretreatment mean percentage of CD4+ DR+ T cells was 8.6%, 10.6%, 11.6% for cohorts 4, 5 and 6, respectively, which increased to 16.3%, 22.1% and 20.3%, respectively. In cohorts 5 and 6, these increases were statistically significant (cohort 5; p=0.0495, cohort 6; p=0.0495, Mann-Whitney U test).
The post treatment mean percentage of CD3+ T cells positive for the HLA-DR marker of activation increased compared to pretreatment for cohort 4 (Fig. 4D). The pretreatment mean percentage of CD3+ DR+ T cells was 13.3%, 11.8%, 11.3% (cohort 4, 5, 6, respectively), which increased to 28.0%, 21.9% and 31.2%, respectively. These increases attained statistical significance on day 21 of cohort 4 (p=0.0495, Mann-Whitney U test) and day 14 and 21 of cohort 6 (p=0.0495, Mann-Whitney U test).
Analysis of serum cytokines
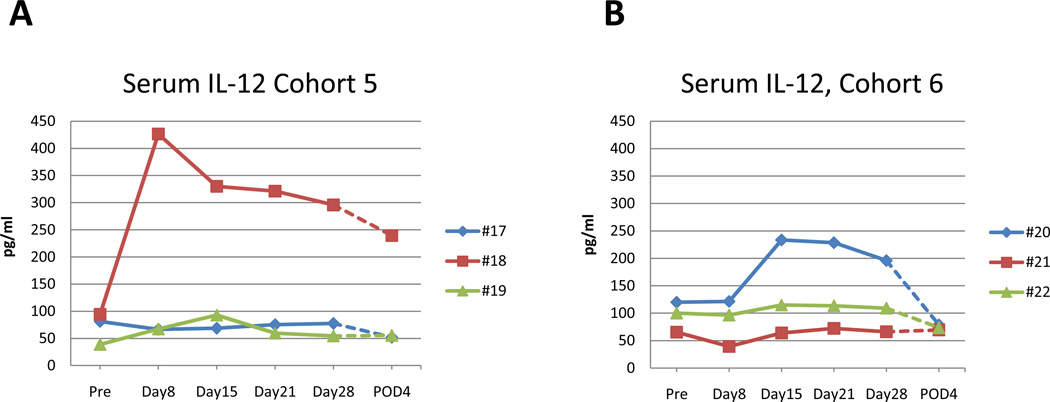
The levels of serum TGF-β and IFN-γ remained unchanged throughout the trial in all cohorts. The level of IL-6 remained at pretreatment levels and was only elevated at the fourth postoperative day, probably as a result of the surgery, in all patients regardless of treatment groups (data not shown). Serum IL-12 levels also did not demonstrate any significant changes in the majority of the patients and was <200 µg/ml with two exceptions. For patient # 18 (cohort 5) serum IL-12 levels increased to 450 ug/ml at day 7 after treatment that gradually declined to 250 µg/ml on the fourth postoperative day (Fig. 5 A). Patient # 20 (cohort 6) had a moderate increase in serum IL-12 to 239 ug/ml at day 14 after treatment that declined gradually to 79 µg/ml on the fourth postoperative day (Fig. 5 B).
Fig. 5. Serum levels of IL-12 for patients in cohort 5 and 6.
A. For patient #18 (cohort 5) serum IL-12 levels increased to 450 pg/ml at 1st week after treatment that gradually drop to 300 pg/ml at week 4 and to 250 pg/ml the 4th postoperative day. B. Patient # 20 (cohort 6) had moderate increase in serum IL-12 to 239 pg/ml at 2nd week after treatment that drop to 196 pg/ml at week 4 and to 79 pg/ml the 4th postoperative day.
Discussion
The intraprostatic administration of GLIPR1 tumor suppressor gene expressed by an adenoviral vector was safe in men with localized intermediate and high-risk PCa preceding RP. Preliminary evidence of local biologic antitumor activity accompanied by systemic immune responses were observed. Increased levels of tumor cell TUNEL (measuring apoptosis) and nuclear p27Kip1 (a cyclin dependent kinase inhibitor) provided corroborative evidence for biologic anti-tumor activity. Systemic induction of an immune response was observed with an increase in serum IL-12 and circulating CD8+, CD4+ and CD3+ T cells coupled with increased HLA-DR upregulation on these cells, a marker of activation. Indications of a dose response were observed, with serum IL-12 increases in a patient in each of the 2 highest dose cohorts and more robust increases in peripheral blood circulating activated T lymphocytes in the higher dose cohorts. Additionally, the prolongation of PTT in 3 men (1 proven to be lupus anticoagulant) also suggests the generation of a systemic immune response. These data provide proof of concept and suggest a role for the further development of intraprostatic injection of adenoviral vector-delivered GLIPR1 in the perioperative setting for prostate cancer.
Other previously reported trials have established the feasibility and biologic activity (both local and systemic) of the intraprostatic delivery of favorable disease-modifying genes. INGN 201 (Ad-p53), a replication-defective adenoviral vector that encodes a wild-type p53 gene driven by the cytomegalovirus promoter, was administered in a neoadjuvant trial of patients with high-risk localized PCa (14). Of 11 patients with negative baseline p53 expression, 10 had expressed p53 and 8 had an increase in apoptosis. To explore the activity of IL-2 expressing adenovirus in PCa, another Phase I clinical trial was conducted in patients with localized high-risk disease (15). An inflammatory response consisting predominantly of CD3+CD8+ T lymphocytes with areas of tumor necrosis was observed. Increases in both γ-interferon and IL-4 secreting T cells were observed. In another small neoadjuvant phase I trial, a DNA-lipid complex encoding the IL-2 gene was administered intraprostatically (16). Evidence of immune activation was observed, reflected by an increase in T cell infiltration seen in tissue and increased proliferation of peripheral blood lymphocytes cocultured with patient serum.
A limitation of the neoadjuvant paradigm evaluating biologic activity with a novel agent is that the level of biologic activity that may translate to enhanced objective clinical outcomes (progression-free or overall survival) is unknown. Therefore, while our trial provides evidence of biologic activity and systemic immune responses, complementary information in terms of improved clinical outcomes in the setting of a randomized trial is necessary. The pathologic stages at RP of patients enrolled on our trial are difficult to interpret in the setting of a small phase I trial. A randomized trial design employing a control arm receiving intraprostatic adenovirus vector not carrying GLIPR1 may have been more optimal, but was considered impractical and beyond the scope of available resources. The evaluation of tumor tissue correlative studies was not possible in all patients due to resource constraints, although the demonstration of biologic activity in the 2 lowest dose cohorts suggests that activity would probably have been observed in the higher dose cohorts too. Demonstration of an upregulation of GLIPR1 in tumor tissue (following therapy) correlating with biologic activity may have been desirable, but was considered beyond the scope of this trial. Moreover, this was a phase I trial with the primary goal of demonstrating feasibility and biologic activity, and upregulation of GLIPR1 has been demonstrated in previous preclinical studies (20, 21). Additionally, long-term follow-up with biochemical and clinical recurrence data are not available, but will also probably be uninterpretable in the absence of a randomized design. While the sparing of perineural tumor cells was based on visual interpretation and was not quantified objectively, a tumor growth promoting microenvironment provided by neurons has been previously described (24).
The appropriate dose to further develop AdGLIPR1 therapy is unclear, since no maximum tolerated dose was established. Therefore, the optimal biologic dose should be based on further analysis of correlative studies, and the study of larger doses of viral particles may be warranted. While our study did not evaluate the potentially deleterious effects of transduction of non-malignant prostate cells, we have previously demonstrated substantially less preclinical proapoptotic activity of GLIPR1 delivered to non-transformed fibroblasts (20). The simultaneous induction of a suppressive immune response was not evaluated, but the overall profile strongly supports an immune stimulatory response. Although PSA changes were not monitored following the intraprostatic injection, such changes in the 4 weeks between the injection and RP are unlikely to be informative, since PSA alterations are known to occur from prostatic procedures. Circulating tumor cells were not available at the time of conduct of this trial, but are also unlikely to have added information since they are seldom detected in early disease. Additionally, biomarkers predictive of response need to be studied; e.g. tumors with p53 mutations, attenuated p53 activities, or GLIPR1 hypermethylation may preferentially respond, since GLIPR1 may be expected to be down-regulated.
To conclude, this phase I trial of neoadjuvant intraprostatic GLIPR1 tumor suppressor gene delivered by an adenoviral vector employed a resource-friendly and small number of patients and demonstrated biologic anti-tumor activity and favorable modulation of blood based biomarkers of immune stimulation. Potentially, the biologic activity of neoadjuvant adenovirus delivered GLIPR1 into early tumor tissue may translate into improved perioperative outcomes as well as activity in more advanced settings. Additionally, the therapeutic index appears excellent, and combinations with other classes of tolerable and active biologic agents may warrant exploration, e.g. sipuleucel-T or abiraterone acetate (5, 6). Additionally, the combination of adenovirus delivered GLIPR1 with conventional ADT, chemotherapy and radiotherapy may also warrant exploration.
Translational significance.
The intraprostatic administration of a single injection of GLIPR1, a p53-regulated tumor suppressor gene, expressed by an adenoviral vector was safe and demonstrated morphologic cytotoxicity, biologic anti-tumor activity (upregulation of apoptosis and a cyclin dependent kinase inhibitor) and systemic immune responses (increased activated T lymphocytes and IL-12) in men with localized intermediate and high-risk prostate cancer preceding prostatectomy. No dose limiting toxicities were observed, suggesting that higher or more frequent doses may warrant evaluation. Given the excellent tolerability profile in this elderly population with frequent comorbidities, the further development of this therapeutic modality, both alone and in combination approaches may be justified.
Acknowledgements
This work was supported by grants from the National Institutes of Health (SPORE P50-CA58204, SPORE P50-CA140388, and R0150588). The authors thank Dr. Malcolm K. Brenner for critical review of the manuscript.
Footnotes
Presented in part as a poster at the Genitourinary Cancer Symposium March 2010, San Francisco, CA
Conflicts of interest: T.C.T. and C.R. are co-inventors on patents involving therapeutic applications of GLIPR1. These patents are held by Baylor College of Medicine, and licensed to Progression Therapeutics Inc., a private biotechnology start-up.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus rednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 3.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 4.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 5.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 6.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonpavde G, Palapattu GS. Neoadjuvant therapy preceding prostatectomy for prostate cancer: rationale and current trials. Expert Rev Anticancer Ther. 2010;10:439–450. doi: 10.1586/era.10.17. [DOI] [PubMed] [Google Scholar]
- 8.Gleave ME, Goldenberg SL, Chin JL, Warner J, Saad F, Klotz LH, et al. Randomized comparative study of 3 versus 8-month neoadjuvant hormonal therapy before radical prostatectomy: biochemical and pathological effects. J Urol. 2001;166:500–506. discussion 6-7. [PubMed] [Google Scholar]
- 9.Chi KN, Chin JL, Winquist E, Klotz L, Saad F, Gleave ME. Multicenter phase II study of combined neoadjuvant docetaxel and hormone therapy before radical prostatectomy for patients with high risk localized prostate cancer. J Urol. 2008;180:565–570. doi: 10.1016/j.juro.2008.04.012. discussion 70. [DOI] [PubMed] [Google Scholar]
- 10.Magi-Galluzzi C, Zhou M, Reuther AM, Dreicer R, Klein EA. Neoadjuvant docetaxel treatment for locally advanced prostate cancer: a clinicopathologic study. Cancer. 2007;110:1248–1254. doi: 10.1002/cncr.22897. [DOI] [PubMed] [Google Scholar]
- 11.Febbo PG, Richie JP, George DJ, Loda M, Manola J, Shankar S, et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2005;11:5233–5240. doi: 10.1158/1078-0432.CCR-05-0299. [DOI] [PubMed] [Google Scholar]
- 12.Antonarakis ES, Heath EI, Walczak JR, Nelson WG, Fedor H, De Marzo AM, et al. Phase II, randomized, placebo-controlled trial of neoadjuvant celecoxib in men with clinically localized prostate cancer: evaluation of drug-specific biomarkers. J Clin Oncol. 2009;27:4986–4993. doi: 10.1200/JCO.2009.21.9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chi KN, Eisenhauer E, Fazli L, Jones EC, Goldenberg SL, Powers J, et al. A phase I pharmacokinetic and pharmacodynamic study of OGX-011, a 2'-methoxyethyl antisense oligonucleotide to clusterin, in patients with localized prostate cancer. J Natl Cancer Inst. 2005;97:1287–1296. doi: 10.1093/jnci/dji252. [DOI] [PubMed] [Google Scholar]
- 14.Pisters LL, Pettaway CA, Troncoso P, McDonnell TJ, Stephens LC, Wood CG, et al. Evidence that transfer of functional p53 protein results in increased apoptosis in prostate cancer. Clin Cancer Res. 2004;10:2587–2593. doi: 10.1158/1078-0432.ccr-03-0388. [DOI] [PubMed] [Google Scholar]
- 15.Trudel S, Trachtenberg J, Toi A, Sweet J, Li ZH, Jewett M, et al. A phase I trial of adenovector-mediated delivery of interleukin-2 (AdIL-2) in high-risk localized prostate cancer. Cancer Gene Ther. 2003;10:755–763. doi: 10.1038/sj.cgt.7700626. [DOI] [PubMed] [Google Scholar]
- 16.Belldegrun A, Tso CL, Zisman A, Naitoh J, Said J, Pantuck AJ, et al. Interleukin 2 gene therapy for prostate cancer: phase I clinical trial and basic biology. Hum Gene Ther. 2001;12:883–892. doi: 10.1089/104303401750195854. [DOI] [PubMed] [Google Scholar]
- 17.Thompson TC, Park SH, Timme TL, Ren C, Eastham JA, Donehower LA, et al. Loss of p53 function leads to metastasis in ras+myc-initiated mouse prostate cancer. Oncogene. 1995;10:869–879. [PubMed] [Google Scholar]
- 18.Eastham JA, Stapleton AM, Gousse AE, Timme TL, Yang G, Slawin KM, et al. Association of p53 mutations with metastatic prostate cancer. Clin Cancer Res. 1995;1:1111–1118. [PubMed] [Google Scholar]
- 19.Yang G, Stapleton AM, Wheeler TM, Truong LD, Timme TL, Scardino PT, et al. Clustered p53 immunostaining: a novel pattern associated with prostate cancer progression. Clin Cancer Res. 1996;2:399–401. [PubMed] [Google Scholar]
- 20.Ren C, Li L, Goltsov AA, Timme TL, Tahir SA, Wang J, et al. mRTVP-1, a novel p53 target gene with proapoptotic activities. Mol Cell Biol. 2002;22:3345–3357. doi: 10.1128/MCB.22.10.3345-3357.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satoh T, Timme TL, Saika T, Ebara S, Yang G, Wang J, et al. Adenoviral vector-mediated mRTVP-1 gene therapy for prostate cancer. Hum Gene Ther. 2003;14:91–101. doi: 10.1089/104303403321070793. [DOI] [PubMed] [Google Scholar]
- 22.Naruishi K, Timme TL, Kusaka N, Fujita T, Yang G, Goltsov A, et al. Adenoviral vector-mediated RTVP-1 gene-modified tumor cell-based vaccine suppresses the development of experimental prostate cancer. Cancer Gene Ther. 2006;13:658–663. doi: 10.1038/sj.cgt.7700919. [DOI] [PubMed] [Google Scholar]
- 23.Ren C, Li L, Yang G, Timme TL, Goltsov A, Ji X, et al. RTVP-1, a tumor suppressor inactivated by methylation in prostate cancer. Cancer Res. 2004;64:969–976. doi: 10.1158/0008-5472.can-03-2592. [DOI] [PubMed] [Google Scholar]
- 24.Ayala GE, Dai H, Ittmann M, Li R, Powell M, Frolov A, et al. Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer Res. 2004;64:6082–6090. doi: 10.1158/0008-5472.CAN-04-0838. [DOI] [PubMed] [Google Scholar]