Abstract
Extraintestinal pathogenic Escherichia coli (ExPEC) constitutes ongoing health concerns for women, newborns, elderly, and immunocompromised individuals due to increased numbers of urinary tract infections (UTIs), newborn meningitis, abdominal sepsis, and septicemia. E. coli remains the leading cause of UTIs, with recent investigations reporting the emergence of E. coli as the predominant cause of nosocomial and neonatal sepsis infections. This shift from the traditional Gram-positive bacterial causes of nosocomial and neonatal sepsis infections could be attributed to the use of intrapartum chemoprophylaxis against Gram-positive bacteria and the appearance of antibiotic (ATB) resistance in E. coli. While ExPEC strains cause significant healthcare concerns, these bacteria also infect chickens and cause the poultry industry economic losses due to costs of containment, mortality, and disposal of carcasses. To circumvent ExPEC-related costs, ATBs are commonly used in the poultry industry to prevent/treat microbial infections and promote growth and performance. In an unfortunate linkage, chicken products are suspected to be a source of foodborne ExPEC infections and ATB resistance in humans. Therefore, the emergence of multidrug resistance (MDR) (resistance to three or more classes of antimicrobial agents) among avian E. coli has created major economic and health concerns, affecting both human healthcare and poultry industries. Increased numbers of immunocompromised individuals, including the elderly, coupled with MDR among ExPEC strains, will continue to challenge the treatment of ExPEC infections and likely lead to increased treatment costs. With ongoing complications due to emerging ATB resistance, novel treatment strategies are necessary to control ExPEC infections. Recognizing and treating the zoonotic risk posed by ExPEC would greatly enhance food safety and positively impact human health.
Introduction
The number of extraintestinal pathogenic Escherichia coli (ExPEC) infections with higher case fatality rates are dramatically increasing worldwide, leading to a tremendous burden on public health (Pitout, 2012). In addition to causing human diseases, ExPEC strains are responsible for significant economic losses in animal production, particularly within the poultry industry (Barnes et al., 2003), one of the fastest growing industries in the United States and worldwide.
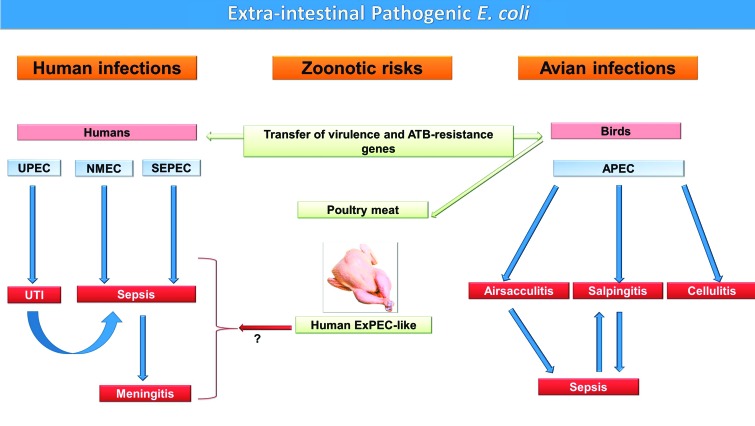
Recently, numerous studies have highlighted similarities between human and avian ExPEC, particularly in their virulence genes, suggesting that poultry products could serve as a source of ExPEC that causes sepsis infections in humans (Manges and Johnson, 2012) (Fig. 1). Since poultry meat exhibits the highest overall levels of E. coli contamination, and E. coli strains isolated from poultry are often more extensively multidrug resistant (MDR) than E. coli recovered from other meats (Manges and Johnson, 2012), the increased poultry meat consumption worldwide could have contributed to the appearance of antibiotic (ATB) resistance in ExPEC and the emergence of ExPEC infections in humans (Pitout, 2012). Global trades and travels also contribute to the worldwide spread of these infections (van der Bij and Pitout, 2012), thus making it difficult to implement infection-control measures.
FIG. 1.
Avian and human extraintestinal pathogenic Escherichia coli (ExPEC), their infections, and zoonotic potential. The schematic diagram illustrates the major ExPEC pathotypes and their infections in humans and birds. ExPEC can cause localized infections that can become systemic (urinary tract infection [UTI] in humans and airsacculitis in birds), a systemic infection that localizes (meningitis), both a local and systemic infection (salpingitis in birds), or localized only (cellulitis in birds). The diagram also shows the potential of poultry and their products to transfer antibiotic (ATB) resistance and ExPEC to humans and cause zoonotic diseases. UPEC, uropathogenic Escherichia coli; NMEC, neonatal meningitis E. coli; SEPEC, sepsis E. coli; APEC, avian pathogenic E. coli. Color images available online at www.liebertpub.com/fpd
Although ExPEC infections have previously been easily treatable with ATB therapy, traditional treatment regimens are currently challenged by the appearance of ATB-resistant strains. Developing alternative strategies to prevent/eliminate these ExPEC infections requires fully understanding the virulence and zoonotic risk, as well as the constant epidemiological surveillance of ATB-resistance trends of these bacteria in both humans and animals. The goal of this review is to summarize the importance of ExPEC infections in humans and chickens, their zoonotic risks, and the current trends in ExPEC ATB resistance.
General Characteristics of ExPEC
ExPEC strains have acquired specific virulence attributes that confer an ability to survive in different niches outside of their normal intestinal habitat in both mammals and birds. ExPEC are phylogenetically distinct from commensal and intestinal pathogenic E. coli. There are four main phylogenetic groups of E. coli: A, B1, B2, and D. ExPEC strains belong mainly to group B2, with some located in group D (Smith et al., 2007). Phylogenic group B2 is the most predominant and the most virulent in most cases of ExPEC infections. ExPEC possess virulence factors required for extraintestinal infections (Smith et al., 2007), with some virulence factors more specific to certain ExPEC groups, such as Tsh and ColV plasmids in avian pathogenic E. coli (APEC), K1 capsule in neonatal meningitis E. coli (NMEC), and Sat and Usp in uropathogenic E. coli (UPEC) (Table 1). This provides strong evidence that certain genetic backgrounds are required for the acquisition and expression of certain virulence factors.
Table 1.
Traits Determined to Be Associated with Virulence of Extraintestinal Pathogenic Escherichia coli (ExPEC) Subgroups (Bien et al., 2012; Bonacorsi and Bingen, 2005; Dziva and Stevens, 2008; Johnson and Russo, 2005; Kim, 2012)
| |
|
|
Virulence factorsa |
||||
|---|---|---|---|---|---|---|---|
| ExPEC | Serogroups | Phylo-genetic group | Adhesins/fimbriae | Iron uptake | Toxins | Protectins/invasins | Others |
| APEC | O1, O2, O78, O18 | A, B1, B2, D | Type 1 P AC/1 Stg curli |
Aerobactin Salmochelin |
VAT-PAI ECVF |
K1 capsule LPS O78 TraT Iss IbeA, IbeB |
ColV plasmid Tsh |
| UPEC | O1, O2, O4, O6, O18, O75 | B2, D | Type 1 P Dr Afa S F1C Iha |
Aerobactin Salmochelin Hma ChuA IreA |
CNF1 HlyA Sat CDT Sat |
K 1 capsule TraT OmpT |
Sat Flagellin Usp |
| NMEC | O18, O7, O16, O1, O45 | B2, D | Type 1 S fimbriae |
Salmochelin Enterobactin |
CNF1 | K1 capsule O-LPS Ibe proteins AslA OmpA |
NlpI flagellin |
| SEPECa | O1, O2, O4, O6, O18, O45, O83 | B2, D | Type 1 P S Curli |
Salmochelin Yersiniabactin Hma ChuA |
HlyA | K1 capsule Iss TraT O-LPS |
ColV plasmid NanA |
Only factors tested in vitro and/or in vivo for their virulence association are presented; SEPEC include subsets of other ExPEC (such as UPEC, NMEC) groups that reach or enter the bloodstream.
APEC, avian pathogenic E. coli; UPEC, uropathogenic E. coli; NMEC, neonatal meningitis E. coli; SEPEC, sepsis E. coli.
ExPEC are generally very diverse, with few common virulence factors between them (Mokady et al., 2005). These findings imply that strains utilize different factors for similar roles during various stages of the infection process (Mellata et al., 2003; Mokady et al., 2005). However, other virulence factors that have not yet been identified in ExPEC and host–pathogen interaction could have a significant role in the pathogenesis of these bacteria. This assortment of virulence genes is apparently made possible by a variety of genetic factors contributing to genome plasticity, including plasmids (Mellata et al., 2010).
Early research studies of ExPEC did not establish a link between E. coli causing disease in humans and birds. Thus, human ExPEC and APEC were investigated separately, as two different groups, with regard to virulence and respective hosts. Meanwhile, the virulence of APEC has been underestimated, with APEC considered an opportunistic pathogen, causing only secondary infections that are predisposed by biological and environmental stresses (Barnes et al., 2003). More recently, the virulence status of APEC has been reassigned by studies demonstrating significant differences in the distribution of virulence factors among E. coli strains isolated from chickens with colibacillosis and those from feces of healthy chickens (Johnson et al., 2012; Schouler et al., 2012). Genotypic and phylogenic comparison of E. coli from clinical cases of colibacillosis with avian fecal E. coli (Ewers et al., 2005; Johnson et al., 2008a; Schouler et al., 2012), as well as with human pathogenic E. coli strains (Ewers et al., 2005), using a large number of isolates, has identified characteristic virulence traits that could predict the APEC subgroup. The implication of many of these virulence factors in the avian pathogenesis using experimental infection models (Dziva, 2010), and the in vivo expression of multiple virulence factors associated with APEC confirm their importance in the infection process in chickens (Dozois et al., 2003; Tuntufye et al., 2012; Dziva et al., 2013).
Contrary to human ExPEC, which have been categorized into different subpathotypes based on their ability to cause different disease syndromes and virulence genes, APEC is commonly designated as a single, heterogeneous population (Fig. 1). However, lately, some studies have suggested that APEC could also be subdivided into multiple subtypes based on their pathology and virulence factors (Maturana et al., 2011; Olsen et al., 2011; Pires-Dos-Santos et al., 2013), and their infections can lead to diverse pathogenic phenotypes and pathological symptoms in chickens, including airsacculitis, salpingitis, and cellulitis (Fig. 1) (Barnes et al., 2003).
Genetic traits that define APEC are not completely elucidated. Similar to human ExPEC, APEC strains can be genetically very diverse and have a distinct repertoire of virulence genes. In order to develop diagnostic tools, many studies have attempted to define patterns of APEC virulence genes, such as those carried on colicin V plasmids (iss, tsh, iucC, cvi, iutA, hylA, iss, iroN, and ompT) (Johnson et al., 2008a), toxin genes (astA, vat), iron acquisition system genes (irp2 and iucD), adhesin genes (papC and tsh), and the ColV genes (cva-cvi) (Ewers et al., 2005). Schouler et al. (Schouler et al., 2012) determined that detection of four associated virulence genes, including two iron acquisition systems (iutA, sitA), fimbriae P (F11), sugar metabolism (frzorf4), O-antigen O78, and T6SS (aec26, aec4), would identify 70.2% of APEC strains and should be used for diagnostic purposes.
Far too often, avian isolates are considered as APEC based solely on their polymerase chain reaction–detected genotypic profile. However, this is a misleading strategy since avian isolates should only be characterized as APEC if their virulence has been confirmed in animal models validated for avian colibacillosis (Dziva, 2010). Another common misconception about avian E. coli isolates is the assumption that intestinal isolates are nonpathogenic. Comparable to human ExPEC (Smith et al., 2007), E. coli that cause extraintestinal infections in chickens often originate from the intestines, where they can have a commensal lifestyle similar to nonpathogenic E. coli (Ewers et al., 2009), and cause a number of diseases at extraintestinal sites.
ExPEC infections in humans: Impact on health
ExPEC strains are responsible for a diverse spectrum of invasive infections in humans (Smith et al., 2007). These strains include sepsis E. coli, UPEC, and NMEC, which are etiologic agents of sepsis, urinary tract infection (UTI), and neonatal meningitis, respectively (Fig. 1).
E. coli Sepsis
ExPEC constitutes an increasing problem for human medicine, as it represents the leading cause of bloodstream infections in nursing homes, hospitals, and young children, especially newborns. Sepsis infections are a major cause of mortality, morbidity, and increased healthcare costs worldwide. In the United States, it is the seventh and 10th leading cause of death in infants and children (<5-year-old) and the elderly (>55-year-old), respectively (Anonymous, 2012). Additionally, in the United States, over 8 million people contract ExPEC infections each year, with associated healthcare costs of approximately $2 billion (Russo and Johnson, 2003). In 2001, severe sepsis due to E. coli caused 40,000 deaths (Russo and Johnson, 2003), which is more than 500 times the number of deaths caused by E. coli O157:H7 (Rangel et al., 2005).
E. coli is responsible for approximately 30% of the total number of sepsis cases, especially in the elderly (Russo and Johnson, 2003). The yearly incidence rate (per 100,000 individuals) of E. coli sepsis was estimated at 40.5 in Minnesota, USA (2003–2005) (Uslan et al., 2007), 30.3 in Calgary, Canada (2000–2006) (Laupland et al., 2008), 30.0 in Finland (1995–2002) (Skogberg et al., 2008), 32.0 in North Jutland County, Denmark (1981–1994) (Madsen et al., 1999), and 28.0 in Canberra, Australia (2000–2004) (Kennedy et al., 2008). E. coli is the first and second cause of community- and hospital-acquired sepsis infections, respectively (Diekema et al., 2003; Laupland et al., 2007) and the most common cause of community-onset sepsis infections in the elderly (≥65 years), accounting for 150 cases/100,000 persons/year in the United States (Jackson et al., 2005). Importantly, these numbers are increasing.
According to recent surveillance studies from Australia and European countries, E. coli has not only emerged as the most prevalent agent of most extraintestinal infections, but has also increased dramatically over time (Schlackow et al., 2012). This trend is particularly evident among the elderly. As examples, E. coli sepsis cases have increased by 33% from 2004 to 2008 in the United Kingdom (Wilson et al., 2011), by over 54.8% from 2001 to 2009 in Europe (de Kraker et al., 2011), and from 14.2% in 2001 to 27.3% in 2009 in Australia (Aung et al., 2012). This increase could be driven by an increase in ATB-resistant isolates (Schlackow et al., 2012).
Sepsis E. coli are also associated with some specific human diseases such as nosocomial pneumonia and surgical site infections (SSI) (Smith et al., 2007). In addition, severe sepsis, mostly caused by E. coli, is associated with cases related to transrectal ultrasound-guided prostate biopsy, with most of the organisms being resistant to multiple ATBs (Williamson et al., 2012).
E. coli UTI
UTI are among the most common bacterial infections in adults, with incidence rates four times higher in women than in men (Griebling, 2007), and approximately 25% of these women having recurrence within 6 to 12 months. Individuals particularly susceptible to UTI infections include pregnant and postmenopausal women, patients undergoing urethral catheterization, and persons with diabetes (Griebling, 2007).
The urinary tract is considered one of the most common sites of ExPEC infections. E. coli accounts for 70–90% and 50% of community- and hospital-acquired UTIs, respectively (Pitout, 2012). Catheter-associated UTIs in U.S. hospitals and nursing homes account for more than 1 million of the ExPEC infections reported each year (Smith et al., 2007).
The presence of E. coli in the vaginal flora of nonpregnant (9–28%) and pregnant (24–31%) women is associated with UTIs, obstetric and neonatal complications, very-low-birth-weight infants, early-onset neonatal septicemia, and meningitis (Obata-Yasuoka et al., 2002).
Using multiple virulence attributes (Table 1), UPEC colonize the perineum, overcome the natural host defenses, traverse the urethra, and then infect the bladder, causing cystitis. Some E. coli can infect the kidneys (pyelonephritis), which can result in organ damage (Bien et al., 2012). In some cases of severe pyelonephritis, UPEC can gain access to the bloodstream, causing sepsis (Fig. 1) and sometimes death (Al-Hasan et al., 2010).
E. coli Neonatal Meningitis
Because their immune system is immature, newborns are particularly susceptible to ExPEC infections that are acquired shortly before, during, and after delivery. Neonatal sepsis is considered one of the five leading neonatal infections worldwide (Bonacorsi and Bingen, 2005). Mortality from neonatal sepsis/meningitis is estimated to be 10% in developed countries and 40–58% in developing countries (Furyk et al., 2011). In the United States, Group B Streptococcus (GBS) and E. coli are the most common etiologic agents of these diseases, with high incidence in African American preterm infants (Stoll et al., 2011; Weston et al., 2011). Contrary to GBS that affects mostly full-term births (73%), E. coli primarily affects preterm babies (45–81%) (Gaschignard et al., 2011; Stoll et al., 2011) and is the cause of high mortality and morbidity (Gaschignard et al., 2011; Weston et al., 2011). Most infant survivors had sequelae including hydrocephalus, seizures, mental retardation, cerebral palsy, and hearing loss (Klinger et al., 2000). In early-onset infection, E. coli is acquired from the maternal genital tract in utero or during passage through the birth canal. In contrast, in late-onset infection, infants can acquire E. coli from different sources including their mother, the hospital, or from community contacts (Raymond et al., 2008).
NMEC use a set of virulence factors for adhesion, invasion, and fitness (Table 1) to cause neonatal meningitis (Kim, 2012), with 80% of them possessing the capsule K1. The different steps of NMEC infection include growth in blood (bacteremia) (Fig. 1), adhesion and invasion of human brain microvascular endothelial cells, and traversal of the blood–brain barrier to cause inflammation in the central nervous system, resulting in meningitis (Kim, 2012).
ExPEC Infections in Poultry: Impact on Poultry Industry
Bacterial infections due to APEC, a subgroup of ExPEC (Fig. 1), are responsible for significant, worldwide economic losses for the poultry farms (Barnes et al., 2003), which is considered one of the most important industries in many countries, including the United States, Brazil, and China. APEC strains cause multiple systemic infections in birds, commonly referred to as avian colibacillosis (Fig. 1).
While the intestines and the environment serve as reservoirs for APEC (Ewers et al., 2009), the clinical outcome of APEC infection in birds depends on the bacterial strain, the host, the route of infection, and predisposing environmental factors. Similar to most other pathogens, APEC strains take advantage of host weaknesses to cause infections in chickens, turkeys, and other avian species. APEC infection can lead to septicemia, fibrinous lesions of internal organs (airsacculitis, pericarditis, perihepatitis), and death. APEC strains also cause local infections in birds, such as cellulitis, salpingitis, synovitis, and omphalitis (Barnes et al., 2003). The main clinical signs associated with most of these infections are depression, fever, yellowish or greenish droppings, and lesions of internal organs. E. coli infections lead to a 1–10% mortality rate in chickens, with even higher mortality rates in broilers (Zanella et al., 2000; Omer et al., 2010) and commercial organic chickens (Stokholm et al., 2010).
The financial losses due to APEC infections in chickens are due to the cost of treatment, mortality, lost production time, containment, and carcass condemnations (Barnes et al., 2003). An estimated 36–43% of broiler carcasses condemned at processing had lesions consistent with E. coli septicemia (Hasan et al., 2011; Yogaratnam, 1995). In Europe, E. coli was considered the major cause of infection condemnation of processed chickens, particularly egg layers, in the poultry industry (Vandekerchove et al., 2004). In young chicks, omphalitis and yolk sac infection, which can lead to septicemia, are the most common causes for first-week mortality in egg layers, and E. coli has been associated with 53.5% of these cases (Olsen et al., 2012a).
Respiratory Infections
Airsacculitis is the most common form of colibacillosis. It starts as a result of inhalation of fecal-contaminated dusts. E. coli colonize avian air sacs, which are particularly vulnerable to colonization and invasion by bacteria because of their lack of resident macrophages (Stearns et al., 1987). Airsacculitis is frequently followed by a generalized infection (perihepatitis, pericarditis, and septicemia) (Fig. 1). Sites of entry of APEC into the bloodstream are determined to be the gas-exchange region of the lung and the air sacs (Pourbakhsh et al., 1997).
Salpingitis
Salpingitis, an inflammation of the oviduct in layers and broiler breeders, is mainly caused by E. coli (Jordan et al., 2005). This form of avian colibacillosis is the result of either an ascending infection of the oviduct from the cloaca or as part of a systemic infection (Fig. 1) (Ozaki and Murase, 2009). Early infection may be asymptomatic, but could result in reduced egg production and increased embryonic mortality in the hatchery. According to Monroy et al. (Monroy et al., 2005), the susceptibility of adult hens to salpingitis could be related to changes initiated during their sexual maturation period, such as cytodifferentiation of the oviduct, proteins secreted, and mucus that could favor E. coli adherence. Moreover, injuries caused by cloacal cannibalism or vent pecking could also predispose birds to infections (Fossum et al., 2009).
Cellulitis
Cellulitis in chickens, characterized as a subcutaneous inflammation in the lower abdomen and thigh, is commonly caused by E. coli (Barnes et al., 2003). At the histological level, the microscopic lesions of cellulitis include the thickening of the dermis, slight hyperkeratosis, hyperplasia of the epidermis, neovascularization, and infiltration of mononuclear cells and heterophils (Messier et al., 1993). Cellulitis-affected birds do not exhibit clinical symptoms commonly associated with colisepticemia, and the infection is often detected at slaughter, which results in carcass condemnation at processing (Barnes et al., 2003). Cellulitis in chickens occurs with frequencies estimated to range between 0.197% and 6.04% (Schrader et al., 2004). The development of this infection is associated with skin surface injuries, often caused by close contact among birds and litter quality (Xavier et al., 2010). Cellulitis has been successfully reproduced in laboratory chicken models, by subcutaneous injection and by contaminating dermal scratches (Norton et al., 1997). Peighambari et al. (Peighambari et al., 1995) showed that E. coli isolated from cellulitis lesions were more likely to induce cellulitis lesions in experimentally infected 4-week-old birds than strains of noncellulitis origin. The development of cellulitis lesions in broilers could be due to the weakness of their innate immune response, specifically their heterophils (Olkowski et al., 2005).
Zoonotic Potential of ExPEC
In recent years, much attention has been directed toward controlling zoonotic infections, which remain a major worldwide health concern. Meat and eggs are known to be a source of human pathogens such as Campylobacter, Listeria, and Salmonella, which frequently leads to a food recall of the suspected contaminated products (Greger, 2007). Recent studies on the zoonotic risk of ExPEC have prompted the Centers for Disease Control and Prevention to release information reports to caution the public on the zoonotic potential of ExPEC and their eventual transmission through chicken meat (Vincent et al., 2010; Bergeron et al., 2012). The zoonotic risk of APEC isolates was initially related to the fact that some human and avian ExPEC have similar phylogenic backgrounds and share some virulence genes (Manges and Johnson, 2012). The sequencing of the genome of the APEC strain O1:K1:H7 revealed that it is highly similar to human UPEC and NMEC (Johnson et al., 2007). Interestingly, a comparison of a large number of ExPEC from human and chicken diseases for their phylogenetic background and the presence of virulence-associated genes has shown that although most isolates fall into genetically distinct pathotype groups (APEC, NMEC, and UPEC), with distinguishable characteristics, the study identified a genotyping cluster that includes ExPEC with overlapping traits and was considered potentially zoonotic (Johnson et al., 2008b).
The role of poultry as a source of human ExPEC (Fig. 1) is suggested by multiple epidemiological studies that reveal the presence of avian ExPEC in both the intestines of healthy poultry and poultry meat from retail markets, strains that are often genetically similar to those found to be responsible for human infections (Manges and Johnson, 2012). Johnson et al. (Johnson et al., 2005) demonstrated that 92% (180/195) of poultry meat samples tested were contaminated with E. coli, with 46% (83/180) of strains having virulence factors associated with ExPEC and 15.6% (28/180) identified as UPEC.
However, according to recent reports, not all ExPEC strains have zoonotic potential. A subset of ExPEC strains from specific clonal groups, including ST95 and ST23, could have a broad host range distribution and cause diseases in both humans and chickens (Mora et al., 2009; Tivendale et al., 2010).
The claims that ExPEC have zoonotic potential are reinforced by the experimental evidence on the ability of human ExPEC to cause diseases in chicken models for colibacillosis and avian ExPEC to cause infections in animal models of human infections (Zhao et al., 2009; Tivendale et al., 2010). Zhao et al. (Zhao et al., 2009) have determined that UPEC and APEC strains sharing the same virulence gene profiles caused lesions of colibacillosis in chickens and showed the same tendency of gene expression, including iron acquisition, in a murine model of human UTI.
The correlation between E. coli UTI in humans and poultry meat consumption is strongly reinforced by recent investigations that have shown that E. coli B2 isolated from meat and intestines of healthy chickens are able to cause infection in a murine model of human UTI (Jakobsen et al., 2010). Moreover, B2 E. coli from UTI patients, poultry meat, and healthy chickens exhibiting high virulence genotypes were clonally related and were virulent in a mouse model of UTI (Jakobsen et al., 2012).
The zoonotic risk of ExPEC appears to be mainly related to their large plasmids. Growing evidence shows that APEC plasmids could be a source of virulence genes for other ExPEC strains (Johnson et al., 2012; Olsen et al., 2012b). Studies have shown that UPEC and APEC isolates have certain genes in common that are associated with large transmissible plasmids of APEC (Rodriguez-Siek et al., 2005). Some virulence genes associated with APEC plasmids (aerobactin, salmochelin, and sit operons) also occur on plasmids of UPEC (Ewers et al., 2007). Additionally, APEC and NMEC have virulence genes of ColV plasmids in common (Johnson et al., 2008b), and APEC plasmids in E. coli can contribute to the pathogenicity of urinary infection in mice (Skyberg et al., 2006) and meningitis in rats (Johnson et al., 2010).
ATB Resistance in ExPEC: Impact on Human Health
MDR-resistant ExPEC infections prolong hospital stays and exhibit higher mortality rates (Gastmeier et al., 2012). Until the late 1990s, ExPEC were highly susceptible to most widely used ATBs, such as ampicillin and trimethoprim-sulfamethoxazole (SXT). Yet, over the past decade, ExPEC emerged as an important reservoir of resistance to first-line ATBs, including cephalosporins, fluoroquinolones, and SXT (Table 2) (Pitout, 2012). For example, UPEC are often resistant to fluoroquinolones and SXT (Table 2), which are considered the drugs of choice for uncomplicated cystitis by the international clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women, (Gupta et al., 2011). In Turkey, the extensive use of SXT to treat UTI in the early 1980s has led to an increase of resistance to SXT in E. coli to above 50%, in the period of a decade. Subsequently, treatment shifted to quinolones due to treatment failures and has since generated an increase in resistance to quinolones (Karaca et al., 2005). In the United States, resistance of urinary E. coli to SXT exceeded 20% in 2010, and this ATB may no longer be acceptable for UTI treatment in this country (Sanchez et al., 2011). MDR resistance among UPEC has been reported from different regions of the globe (Table 2), and the rate of ATB resistance is higher in E. coli from recurrent UTIs (Schmiemann et al., 2012). The appearance and increase of ATB resistance among ExPEC strains complicate the therapeutic management of ExPEC infections (Smith et al., 2007) and has led to the increased use of last-resort antimicrobial drugs, such as carbapenems, and the appearance of the resistance to these ATBs in ExPEC (Table 2).
Table 2.
Selected Worldwide Reports on Phenotype and Genotype of Antibiotic Resistance in Human and Avian Extraintestinal Pathogenic Escherichia coli (ExPEC) Isolates
| |
|
|
Antibiotic resistancea |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
β-lactams |
Aminoglycosides |
Macrolides |
Fluoro/Quinolones |
Others |
|||||
| Country (reference) | ExPEC group (period of isolation) | Selection criteria | Phenotype (%) | Genes (N+/N tested) | Phenotype (%) | Genes (N+/N tested) | Phenotype (%) | Genes (N+/N tested) | Phenotype (%) | Genes (N+/N tested) | Phenotype (%) | Genes (N+/N tested) |
| Cambodia (Vlieghe et al., 2013) | SEPEC (07/2007–03/2010) | None | ESBL (+) (47.7) AMC (49) AMP (94) CAZ (36) CEP (46) CTX (51.5) PIT (9) |
NT | AMK (3.8) GEN (56.2) |
NT | NT | NT | CIP (65.4) | NT | SXT (95.4) COL (0.8) |
NT |
| India (Haque et al., 2012) | SEPEC (NR) | ESBL-producing | ESBL (+) (7.3) AMP (100) CEP (100) CFZ (100) CLX (100) COX (100) CRO (100) CTX (100) MEM (9.7) PN (100) |
blaTEM (3/3) blaCTX-M (3/3) blaSHV (0/3) |
AMK (43.9) GEN (43.9) TOB (51) |
NT | NT | NT | CIP (100) GAT (31.7) NAL (100) |
NT | SXT (100) TET (95.1) |
NT |
| India (Hussain et al., 2012) | UPEC (NR) | ESBL-producing ST131 genotype |
ESBL(+) (23) |
blaCTX-M15-O26b (16/16) blaTEM-1 (8/16) blaOXA-1 (11/16) |
GEN (69) | NT | CIP (81) |
aac(6’)-lb-cr (12/16) qnrA (0/16) qnrB (0/16) qnrS (0/16) |
CMP (6) SXT (69) TET (94) |
tet(A) (15/16) sul1 (13/16) sul1 (10/16) str(A) (16/16) str(B) (5/16) dfrA12-aadA2 (2/16) dfrA17-aadA5 (6/16) |
||
| China (Qin et al., 2013) | UPEC (01/2008–12/2010) | None | ESBL(+) (53) CEP (21) CFZ (79) CTX (57) CXM (61) PIP (86) PIT (9) |
blaCTX-M-1 (21/36) blaCTX-M-9 (15/36) Unknown (1/36) |
AMK (10) GEN (57) |
NT | NT | NT | CIP (69) | NT | NIT (1) | NT |
| China (Sun et al., 2012) | APEC (03/2003–10/2010) | None | NT |
BlaTEM-1 (17/224) blaCTX-M-24 (16/17) blaCTX-M-14 (1/17) |
AMK (43.8) GEN (51.8) KAN (55.8) NEO (47.3) STR (84.4) |
rmtB (26/224) | NT | NT | NT | NT | NT | fosA3 (17/224) |
| Japan (Asai et al., 2011) | APEC (2001–2006) | CFZ- resistance | CFZ (32.6) |
blaCTX-M-25 (4/29) blaSHV-2 (3/29) blaCTX-M-15/TEM-1 (1/29) blaCTX-M-2 (1/29) blaCMY-2 (10/29) blaTEM-1 (7/29) unknown (2/29) |
NT | NT | NT | NT | NT | NT | NT | NT |
| Nigeria (Iroha et al., 2012) | UPEC (NR) | ESBL-producing | ESBL (+) (100) AMP (NR) ATM (NR) CAZ (NR) CTX (NR) |
blaCTX-M-1 (44/44) blaCTX-M-15 (3/3) blaTEM (41/44) blaOXA-1 (41/44) |
GEN (NR) KAN (NR) TOB (NR) |
NT | NT | NT | NT | aac(6’)-lb-cr (43/44) | CMP (NR) DOX (NR) SXT (NR) TMP (NR) |
NT |
| Italy (Giufre et al., 2012) | UPEC (01/2009–12/2009) | CIP-resistance | ESBL (+) (14.4) AMP (86.20) CFX+CZC (26.4) |
blaCTX-M-15 (20/27) blaCTX-M-15/SHV-12 (1/27) blaCTX-M-1 (2/27) blaTEM-20 (1/27) blaTEM-52 (1/27) blaSHV-12 (1/27) unknown (1/27) |
GEN (27.6) | NT | NT | NT | CIP (46.5) | NT | SXT (61) | NT |
| SEPEC (01/2009-12/2009) | CIP-resistance | ESBL(+) (23.3) AMP (100) CFX+CZC (41.7) |
blaCTX-M-15 (19/27) blaCTX-M-27 (1/27) |
GEN (16.7) | NT | NT | NT | CIP (53.3) | NT | SXT (48) | NT | |
| Germany (Heideking et al., 2013) | NMEC (01/2009–12/2010) | None | AMP (44.9) CTX (3.8) CZC (4.4) PIP (27.9) |
blaCTX-M-15/TEM-1 (1/6) blaCTX-M-14/TEM-1 (1/6) blaCTX-M-1 (1/6) blaCTX-M-3 (1/6) blaTEM-52 (2/6) |
GEN (4.4) | NT | NT | NT | CIP (4.4) | NT | SXT (19) TET (30.4) |
NT |
| Netherland (van der Bij et al., 2011) | SEPEC (2008–2009) | ESBL-producing | ESBL(+) (4.9) AMC (90) PIT (61) |
blaCTX-M-15 (22/41) blaCTX-M-14 (6/41) blaCTX-M-2 (2/41) blaCTX-M-3 (2/41) blaCTX-M-9 (1/41) blaCTX-M-27 (1/41) blaCTX-M-1 (1/41) blaSHV-5 (2/41) blaSHV-12 (3/41) blaTEM-52 (1/41) |
AMK (24) GEN (32) TOB (51) |
NT | NT | NT | CIP (63) | NT | SXT (90) | NT |
| France (Courpon-Claudinon et al., 2011) | SEPEC (2005) | None | ESBL(+) (1.8) AmpC (+) (1.7) |
blaCTX-M-1 (2/41) blaCTX-M-9 (1/41) blaCTX-M-14 (6/41) blaCTX-M-15 (3/41 blaCTX-M-15/ OXA-1 (6/41) blaTEM-52 (1/41) blaCMY-2 (1/41) blaAmpC (13/41) blaAmpC/CMY-2 (4/41) blaOXA-1 (4/41) |
AMK (1.3) GEN (4.5) |
NT | NT | NT | CIP (11.9) | NT | SXT (29.8) | NT |
| Spain (Guiral et al., 2012) | NMEC (1995–2008) | None | AMC (21) AMP (55.5) CAZ (6.5) CEP (5) CFX (6.5) CFZ (13) CPO (1.6) CTX (6.5) MEZ (62.3) PIP (20.3) PIT (6.6) SAM (46) |
blaTEM-1 (35/42) blaSHV-1 (1/42) blaCARB (1/42) blaCTX-M-14 (1/42) blaCTX-M-15 (1/42) blaAmpC (1/42) unknown (2/42) |
GEN (13) TOB (14.8) |
CIP (6.6) LVX (3.3) MXF (8.2) |
aac(3)-IV (2/8) aac(3)-II (6/8) qnrS (1/11) |
CMP (24.6) SXT (32.8) TET (35.4) |
catA2 (11/15) cmlA (2/15) floR (1/15) tetA (15/29) tetB (8/29) tetC (2/29) tetD (2/29) tetE (1/29) tetG (3/29) dfrAIa (10/20) dfrB (0/20) dfrA12 (2/20) dfrA17 (3/20) sulI (2/22) sulII (18/22) sulIII (1/22) |
|||
| Mexico (Rodriguez-Bano et al., 2012) | SEPEC (09/2004–01/2006) SEPEC+UPEC (2007–2011) |
ESBL-producing | AMC (38) CAZ (37) CEP (65) CTX (96) PIT (8) |
blaCTX-M9 (122/191) blaCTX-M1 (42/191) blaSHV (33/191) blaTEM (1/191) |
AMK (2) GEN (20) TOB (18) |
NT | NT | NT | CIP (68) | NT | SXT (60) | NT |
| Canada (Denisuik et al., 2013) | ESBL-producing | ESBL(+) (4.2) AMC (3.9) CAZ (56.2) CEP (21.9) CFZ (100) COX (8.2) CRO (97) ETP (1.3) PIT (2.2) |
blaCTX-M-15 (21/231) blaCTX-M-15/TEM-1 (18/231) blaCTX-M-15/OXA-1 (84/231) blaCTX-M-15/TEM-1/OXA-1 (30/231) blaCTX-M-14 (15/231) blaCTX-M-14/TEM-1 (28/231) blaCTX-M-14/OXA-1 (2/231) blaCTX-M-27 (13/231) blaCTX-M-27/TEM-1 (2/231) blaCTX-M-24 (1/231) blaCTX-M-24/TEM-1 (1/231) blaCTX-M-3/TEM-1 (1/231) blaCTX-M-3/TEM-1/OXA-1 (1/231) blaCTX-M-65 (1/231) blaSHV-12 (1/231) blaSHV-12/TEM-1 (3/231) blaSHV-2a (3/231) blaTEM-12 (1/231) unknown (2/231) unknown/TEM-1 (3/231) |
AMK (0.4) GEN (48.5) |
NT | NT | NT | CIP (88.3) | NT | COL (0.4) SXT (70.1) |
NT | |
| AmpC-producing | AmpC (+) (2.6) AMC (49.6) CAZ (51.4) CEP (2.3) CFZ (95.7) COX (100) CRO (57.4) PIT (1.7) |
BlaCMY-2 (64/115) BlaFOX-5 (1/115) |
AMK (1.7) GEN (16.5) |
NT | NT | NT | CIP (37.4) | NT | SXT (33.9) | NT | ||
| USA (Peirano et al., 2010) | UPEC/SEPEC (2008) | ESBL-producing | PIT (6.7) |
blaTM-1/OXA-1/CTX-M-15 (9/30) blaTM-1/CTX-M-15 (3/30) blaOXA-1/CTX-M-15 (3/30) blaTM-1/CTX-M-14 (2/30) blaCTX-M-15 (7/30) blaCTX-M-14 (1/30) blaSHV-2 (4/30) |
AMK (20) GEN (33.33) TOB (50) |
NT | NT | NT | CIP (93) | aac(6’)-lb-cr (16/30) | NIT (10) SXT (56.7) |
NT |
| USA (Adams-Sapper et al., 2013) | SEPEC (07/2007–09/2010) | None | CTX (12) ATM (12) |
blaCTX-M-15 (15/246) blaCTX-M-1 (3/246) blaCTX-M-14 (6/246) blaCTX-M-14/CTX-M-15 (6/246) blaTM-1 (8/246) blaOXA-1 (15/246) blaKPC-2 (2/246) |
NT | NT | NT | NT | CIP/LVX/MXF (27) | NT | SXT (44) | NT |
N, number of isolates; NT, not tested; NR, not reported; SEPEC, sepsis E. coli; UPEC, uropathogenic E. coli; APEC, avian pathogenic E. coli; NMEC, neonatal meningitis E. coli.
AMC, amoxicillin/clavulanic acid; AMK, Amikacin; AMP, ampicillin; ATM, aztreonam; CARB, carbenicillin; CAZ, ceftazidime; CEP, cefepime; CFZ; cefazolin; CFX, cefotaxime; CIP, ciprofloxacin; CLX, clinafloxacin; CMP, chloramphenicol; COL, colistin; COX, cefoxitin; CPO, cefpodoxime; CRO, ceftriaxone; CTX, cefotaxime; CXM, cefuroxime; CZC, ceftazidime+clavulanic acid; DOX, doxycycline; ETP, ertapenem; ESBL, extended-spectrum β-lactamases; GAT, gatifloxacin; GEN, gentamicin; KAN, kanamycin; LVX, levofloxacin; MEM, meropenem; MEZ, mezlocillin; MXF, moxifloxacin; NAL, nalidixic acid; NIT, nitrofurantoin; NEO, neomycin; OXA, oxacillin; PIP, piperacillin; PIT, piperacillin/tazobactam; PN, penicillin; SAM, amipicillin-sulbactam; STR, streptomycin; SXT, sulfamethoxazole/trimethoprim; TET, tetracycline; TOB, tobramycin; TMP, trimethoprim.
In 2007, 15,183 episodes of third-generation cephalosporin-resistant E. coli were associated with 2712 deaths and 120,065 extra hospital days in Europe (de Kraker et al., 2011). Based on prevailing trends, the number of sepsis cases caused by these E. coli is likely to rapidly increase, potentially outnumbering the number of methicillin-resistant Staphylococcus aureus (MRSA) sepsis cases in the near future (de Kraker et al., 2011).
Although the introduction of intrapartum ATB prophylaxis has reduced cases of neonatal GBS infection in the United States, it may also have resulted in a shift of pathogens and their resistance to ATBs, shifting to an increased incidence of Gram-negative bacteria, including E. coli (Furyk et al., 2011). In premature infants, an increased trend in the incidence of early-onset neonatal sepsis caused by ATB-resistant E. coli is observed (Bizzarro et al., 2008). Of particular concern is the recent apparition of NMEC producing cefotaxime (CTX)-M-type or TEM-type extended-spectrum β-lactamases (Table 2).
ExPEC producing “newer β-lactamases,” such as plasmid-mediated among class C cephalosporinases (AmpC) β-lactamases (e.g., cephamycinase [CMY] types), extended-spectrum β-lactamases (ESBLs) (e.g., TEM-, sulfhydryl variable [SHV]-, CTX-M-, oxacillin-types), and carbapenemases (e.g., imipenem-, Verona integron-encoded metallo-β-lactamase [VIM]-, New Delhi metallo-β-lactamase [NDM]-types), are widespread (Table 2) (Pitout, 2012). The most prevalent is CTX-M-15, which is the most associated with human ExPEC isolates, especially UPEC, in many regions of the world (Table 2), with many belonging to specific clones. However, it has been speculated that certain sequence types (ST38, ST131, ST405, and ST648) could have contributed to their worldwide distribution (Pitout, 2012). A recent study monitoring ATB-resistance trends among human sepsis Enterobacteriaceae, mainly sepsis E. coli (63.4%, 443/699), from multiple countries of the Asian Pacific region during the period 2008–2009 (Sheng et al., 2013), has determined that geographic genetic distribution of ESBLs, AmpCs, and carbapenemases was variable. The most dominant among ESBLs were CTX-M-14 (China, South Korea, and Taiwan), CTX-M-15 (India, Malaysia, the Philippines, and Singapore), and SHV-12 (Taiwan and the Philippines). Among AmpCs were CMY-2 (India, Taiwan, South Korea, and Vietnam), Dhahran Hospital in Saudi Arabia (DHA) (Philippines and Singapore), AmpC type (ACT)–Miriam Hospital in Providence, RI (MIR) (New Zealand and South Korea), and DHA-1 (Taiwan). Finally in this study, carbapenemases (mostly NDM-1) were exclusively detected in India.
A recent Canadian epidemiology study that covered a 5-year period (2007–2011) has shown a significant national increase in the proportion of ESBL- and AmpC-producing isolates among human ExPEC and identified carbapenemase-producing isolates (Denisuik et al., 2013). The emergence and widespread dissemination of CTX-M-15, CMY-1, and NDM-producing E. coli is especially alarming as they often confer resistance to multiple other antibiotic classes, leaving few or no other treatment options (Table 2).
Clinicians are aware that the increase in ATB resistance is a great concern for public health as well as the economy. Treatment failures due to ATB resistance increase the cost of care and result in prolonged morbidity for patients. As the proportion of elderly and immunocompromised patients increases, the number of ExPEC infections will likely increase, while associated ATB resistance will make treatment strategies more challenging (Pitout, 2012). Therefore, the prevention of ExPEC infections is a pressing concern, and vaccines are necessary to manage ExPEC infections in the future.
Because of the significance of colibacillosis in the worldwide poultry industry, ATB treatments with tetracyclines, fluoroquinolones, and sulfonamides are primarily used to target E. coli, thus explaining the high level of resistance of avian E. coli to these ATBs (Zhao et al., 2005). In the United States, MDR resistance was found in 92% (87/95) of APEC isolates from northern Georgia (Zhao et al., 2005). Studies performed in China have shown that 80% of the 71 E. coli strains isolated from the livers of deceased chickens from 10 different poultry farms were resistant to eight or more ATBs, including fluoroquinolones, and all of them were resistant to nalidixic acid and tetracycline (Yang et al., 2004). The Li et al. (Li et al., 2007) study found comparable results, whereby 100% of APEC isolates tested were resistant to tetracycline and trimethoprim/sulfonamide, and 79–83% of the isolates exhibited resistance to chloramphenicol, ampicillin, ciprofloxacin, and enrofloxacin.
ESBL genes have also been detected in APEC and fecal E. coli of healthy poultry (Asai et al., 2011; Bortolaia et al., 2010). The emergence of ESBL genes in poultry could be associated with the use of third-generation cephalosporins in chickens, particularly ceftiofur, which is injected into eggs to control E. coli omphalitis in broiler chickens (Dutil et al., 2010). However, this assumption would not be the only explanation, as ESBL-producing E. coli were also detected in birds that were not treated with cephalosporins (Bortolaia et al., 2010). Additionally, recent reports have detected ESBL-producing E. coli in both the environment and wildlife, especially birds (Guenther et al., 2011). This could show the ability of ESBL-producing E. coli to transfer from one ecosystem to another and highlight the importance of life vectors in dissemination of these resistance genes to different environments.
Bacteria found in the poultry environment are important reservoirs for ATB resistance (Nandi et al., 2004). The presence of ATB-resistant bacteria in poultry can lead to the contamination of poultry products, which could increase the risk of the transfer of these bacteria or their ATB genes to humans. In fact, a recent study determined that women who were infected with MDR-resistant E. coli reported more frequent chicken consumption (Manges et al., 2007).
As the poultry industry has developed, ATBs have been used extensively for disease prevention and treatment. With the exception of the European Union, ATBs are administered in poultry feed or drinking water for growth promotion. Although ATBs have contributed to poultry health and welfare for several decades, extensive use and misuse has generated worldwide concern about the development of ATB resistance and transfer to humans and the environment.
It was speculated that ATB-resistant human ExPEC likely originated from poultry through both direct contact with birds and the consumption of poultry products (Fig. 1) (Manges and Johnson, 2012). According to a recent study in the Netherlands, retail chicken meat has the highest rate of ESBL-contamination compared to other meats and involves many of the same ESBL genes present in colonized and infected humans (Overdevest et al., 2011). Identification of a major multilocus sequence–type clone associated with ciprofloxacin resistance/multiresistance in both human and avian E. coli isolates confirms the zoonotic risk of avian isolates (Giufre et al., 2012). These ATB-resistance genes in E. coli may be transferred vertically to other species of bacteria. In fact, most Salmonella Kentucky avian isolates have acquired resistance to streptomycin and tetracycline through acquisition of an APEC-like plasmid, which could be responsible for their emergence as a dominant serovar in chickens (Fricke et al., 2009).
Conclusions
ExPEC is a leading cause of infections in both humans and poultry. ATB use in the poultry industry and increases in poultry product consumption could have caused the emergence, dissemination, and persistence of ATB resistance, which is a serious health concern for both animals and humans. Due to the variability and adaptability of ExPEC, constant surveillance of their epidemiology in both humans and chickens is required and rigorous controls of their contaminants are needed.
Acknowledgments
Special thanks are given to Dr. Shelley Haydel and Ms. Natalie Mitchell (Biodesign Institute, Arizona State University), for their critical review of the manuscript. The author gratefully acknowledges the funding supports of the National Institutes of Health (1R21AI090416-01 NIH/NIAID) and the U.S. Department of Agriculture (2011-67005-30182 USDA/NIFA).
Disclosure Statement
No competing financial interests exist.
References
- Adams-Sapper S. Diep BA. Perdreau-Remington F. Riley LW. Clonal composition and community clustering of drug-susceptible and -resistant Escherichia coli isolates from bloodstream infections. Antimicrob Agents Chemother. 2013;57:490–497. doi: 10.1128/AAC.01025-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasan MN. Eckel-Passow JE. Baddour LM. Bacteremia complicating gram-negative urinary tract infections: A population-based study. J Infect. 2010;60:278–285. doi: 10.1016/j.jinf.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Atlanta, GA: Centers for Disease Control and Prevention (CDC); 2012. 10 Leading Causes of Death by Age Group, United States–2010. [Google Scholar]
- Asai T. Masani K. Sato C. Hiki M. Usui M. Baba K. Ozawa M. Harada K. Aoki H. Sawada T. Phylogenetic groups and cephalosporin resistance genes of Escherichia coli from diseased food-producing animals in Japan. Acta Vet Scand. 2011;53:52. doi: 10.1186/1751-0147-53-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung AK. Skinner MJ. Lee FJ. Cheng AC. Changing epidemiology of bloodstream infection pathogens over time in adult non-specialty patients at an Australian tertiary hospital. Commun Dis Intell. 2012;36:333–341. [PubMed] [Google Scholar]
- Barnes HJ. Vaillancourt J. Gross WB. Colibacillosis. In: Saif YM, editor. Diseases of Poultry. Ames, IA: Iowa State University Press; 2003. pp. 631–652. [Google Scholar]
- Bergeron CR. Prussing C. Boerlin P. Daignault D. Dutil L. Reid-Smith RJ. Zhanel GG. Manges AR. Chicken as reservoir for extraintestinal pathogenic Escherichia coli in humans, Canada. Emerg Infect Dis. 2012;18:415–421. doi: 10.3201/eid1803.111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bien J. Sokolova O. Bozko P. Role of uropathogenic Escherichia coli virulence factors in development of urinary tract infection and kidney damage. Int J Nephrol. 2012;2012:1–15. doi: 10.1155/2012/681473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzarro MJ. Dembry LM. Baltimore RS. Gallagher PG. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics. 2008;121:689–696. doi: 10.1542/peds.2007-2171. [DOI] [PubMed] [Google Scholar]
- Bonacorsi S. Bingen E. Molecular epidemiology of Escherichia coli causing neonatal meningitis. Int J Med Microbiol. 2005;295:373–381. doi: 10.1016/j.ijmm.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Bortolaia V. Guardabassi L. Trevisani M. Bisgaard M. Venturi L. Bojesen AM. High diversity of extended-spectrum beta-lactamases in Escherichia coli isolates from Italian broiler flocks. Antimicrob Agents Chemother. 2010;54:1623–1626. doi: 10.1128/AAC.01361-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courpon-Claudinon A. Lefort A. Panhard X. Clermont O. Dornic Q. Fantin B. Mentre F. Wolff M. Denamur E. Branger C. Grp C. Bacteraemia caused by third-generation cephalosporin-resistant Escherichia coli in France: Prevalence, molecular epidemiology and clinical features. Clin Microbiol Infect. 2011;17:557–565. doi: 10.1111/j.1469-0691.2010.03298.x. [DOI] [PubMed] [Google Scholar]
- de Kraker ME. Davey PG. Grundmann H. Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: Estimating the burden of antibiotic resistance in Europe. PLoS Med. 2011;8:e1001104. doi: 10.1371/journal.pmed.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisuik AJ. Lagace-Wiens PR. Pitout JD. Mulvey MR. Simner PJ. Tailor F. Karlowsky JA. Hoban DJ. Adam HJ. Zhanel GG. Molecular epidemiology of extended-spectrum beta-lactamase-, AmpC beta-lactamase- and carbapenemase-producing Escherichia coli and Klebsiella pneumoniae isolated from Canadian hospitals over a 5 year period: CANWARD 2007-11. J Antimicrob Chemother. 2013;68:57–65. doi: 10.1093/jac/dkt027. [DOI] [PubMed] [Google Scholar]
- Diekema DJ. Beekmann SE. Chapin KC. Morel KA. Munson E. Doern GV. Epidemiology and outcome of nosocomial and community-onset bloodstream infection. J Clin Microbiol. 2003;41:3655–3660. doi: 10.1128/JCM.41.8.3655-3660.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dozois CM. Daigle F. Curtiss R., III Identification of pathogen-specific and conserved genes expressed in vivo by an avian pathogenic Escherichia coli strain. Proc Natl Acad Sci USA. 2003;100:247–252. doi: 10.1073/pnas.232686799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutil L. Irwin R. Finley R. Ng LK. Avery B. Boerlin P. Bourgault AM. Cole L. Daignault D. Desruisseau A. Demczuk W. Hoang L. Horsman GB. Ismail J. Jamieson F. Maki A. Pacagnella A. Pillai DR. Ceftiofur resistance in Salmonella enterica serovar Heidelberg from chicken meat and humans, Canada. Emerg Infect Dis. 2010;16:48–54. doi: 10.3201/eid1601.090729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziva F. Deciphering the infection biology of avian pathogenic Escherichia coli: Role of experimental infection models. In: Mendez-Vilas A, editor. Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology. Spain: Formatex Research Center; 2010. pp. 746–753. [Google Scholar]
- Dziva F. Hauser H. Connor TR. van Diemen PM. Prescott G. Langridge GC. Eckert S. Chaudhuri RR. Ewers C. Mellata M. Mukhopadhyay S. Curtiss R., III Dougan G. Wieler LH. Thomson NR. Pickard DJ. Stevens MP. Sequencing and functional annotation of avian pathogenic Escherichia coli serogroup O78 strains reveal the evolution of E. coli lineages pathogenic for poultry via distinct mechanisms. Infect Immun. 2013;81:838–849. doi: 10.1128/IAI.00585-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziva F. Stevens MP. Colibacillosis in poultry: Unravelling the molecular basis of virulence of avian pathogenic Escherichia coli in their natural hosts. Avian Pathol. 2008;37:355–366. doi: 10.1080/03079450802216652. [DOI] [PubMed] [Google Scholar]
- Ewers C. Antao EM. Diehl I. Philipp HC. Wieler LH. Intestine and environment of the chicken as reservoirs for extraintestinal pathogenic Escherichia coli strains with zoonotic potential. Appl Environ Microbiol. 2009;75:184–192. doi: 10.1128/AEM.01324-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers C. Janssen T. Kiessling S. Philipp HC. Wieler LH. Rapid detection of virulence-associated genes in avian pathogenic Escherichia coli by multiplex polymerase chain reaction. Avian Dis. 2005;49:269–273. doi: 10.1637/7293-102604R. [DOI] [PubMed] [Google Scholar]
- Ewers C. Li G. Wilking H. Kiessling S. Alt K. Antao EM. Laturnus C. Diehl I. Glodde S. Homeier T. Bohnke U. Steinruck H. Philipp HC. Wieler LH. Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: How closely related are they? Int J Med Microbiol. 2007;297:163–176. doi: 10.1016/j.ijmm.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Fossum O. Jansson DS. Etterlin PE. Vagsholm I. Causes of mortality in laying hens in different housing systems in 2001 to 2004. Acta Vet Scand. 2009;51:3. doi: 10.1186/1751-0147-51-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke WF. McDermott PF. Mammel MK. Zhao S. Johnson TJ. Rasko DA. Fedorka-Cray PJ. Pedroso A. Whichard JM. Leclerc JE. White DG. Cebula TA. Ravel J. Antimicrobial resistance-conferring plasmids with similarity to virulence plasmids from avian pathogenic Escherichia coli strains in Salmonella enterica serovar Kentucky isolates from poultry. Appl Environ Microbiol. 2009;75:5963–5971. doi: 10.1128/AEM.00786-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furyk JS. Swann O. Molyneux E. Systematic review: Neonatal meningitis in the developing world. Trop Med Int Health. 2011;16:672–679. doi: 10.1111/j.1365-3156.2011.02750.x. [DOI] [PubMed] [Google Scholar]
- Gaschignard J. Levy C. Romain O. Cohen R. Bingen E. Aujard Y. Boileau P. Neonatal bacterial meningitis: 444 cases in 7 years. Pediatr Infect Dis J. 2011;30:212–217. doi: 10.1097/inf.0b013e3181fab1e7. [DOI] [PubMed] [Google Scholar]
- Gastmeier P. Schwab F. Meyer E. Geffers C. Excess mortality and prolongation of stay due to bloodstream infections caused by multiresistant pathogens in Germany. Dtsch Med Wochenschr. 2012;137:1689–1692. doi: 10.1055/s-0032-1305246. (In German.) [DOI] [PubMed] [Google Scholar]
- Giufre M. Graziani C. Accogli M. Luzzi I. Busani L. Cerquetti M. Escherichia coli of human and avian origin: Detection of clonal groups associated with fluoroquinolone and multidrug resistance in Italy. J Antimicrob Chemother. 2012;67:860–867. doi: 10.1093/jac/dkr565. [DOI] [PubMed] [Google Scholar]
- Greger M. The human/animal interface: Emergence and resurgence of zoonotic infectious diseases. Crit Rev Microbiol. 2007;33:243–299. doi: 10.1080/10408410701647594. [DOI] [PubMed] [Google Scholar]
- Griebling TL. Urinary tract infection in women. In: Litwin MS, editor; Saigal CS, editor. Urology Diseases in America. Washington, DC: NIH Publication; 2007. pp. 589–619. [Google Scholar]
- Guenther S. Ewers C. Wieler LH. Extended-spectrum beta-lactamases producing E. coli in wildlife, yet another form of environmental pollution? Front Microbiol. 2011;2:1–13. doi: 10.3389/fmicb.2011.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiral E. Bosch J. Vila J. Soto SM. Antimicrobial resistance of Escherichia coli strains causing neonatal sepsis between 1998 and 2008. Chemotherapy. 2012;58:123–128. doi: 10.1159/000337062. [DOI] [PubMed] [Google Scholar]
- Gupta K. Hooton TM. Naber KG. Wullt B. Colgan R. Miller LG. Moran GJ. Nicolle LE. Raz R. Schaeffer AJ. Soper DE. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- Haque SF. Ali SZ. Tp M. Khan AU. Prevalence of plasmid mediated bla(TEM-1) and bla(CTX-M-15) type extended spectrum beta-lactamases in patients with sepsis. Asian Pac J Trop Med. 2012;5:98–102. doi: 10.1016/S1995-7645(12)60003-0. [DOI] [PubMed] [Google Scholar]
- Hasan B. Faruque R. Drobni M. Waldenstrom J. Sadique A. Ahmed KU. Islam Z. Parvez M B. Olsen B. Alam M. High prevalence of antibiotic resistance in pathogenic Escherichia coli from large- and small-scale poultry farms in Bangladesh. Avian Dis. 2011;55:689–692. doi: 10.1637/9686-021411-Reg.1. [DOI] [PubMed] [Google Scholar]
- Heideking M. Lander F. Hufnagel M. Pfeifer Y. Wicker E. Krause G. Berner R. Antibiotic susceptibility profiles of neonatal invasive isolates of Escherichia coli from a 2-year nationwide surveillance study in Germany, 2009-2010. Eur J Clin Microbiol Infect Dis. 2013 2013 Apr 5; doi: 10.1007/s10096-013-1871-3. e-pub. [DOI] [PubMed] [Google Scholar]
- Hussain A. Ewers C. Nandanwar N. Guenther S. Jadhav S. Wieler LH. Ahmed N. Multiresistant uropathogenic Escherichia coli from a region in India where urinary tract infections are endemic: Genotypic and phenotypic characteristics of sequence type 131 isolates of the CTX-M-15 extended-spectrum-beta-lactamase-producing lineage. Antimicrob Agents Chemother. 2012;56:6358–6365. doi: 10.1128/AAC.01099-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iroha IR. Esimone CO. Neumann S. Marlinghaus L. Korte M. Szabados F. Gatermann S. Kaase M. First description of Escherichia coli producing CTX-M-15- extended spectrum beta lactamase (ESBL) in out-patients from south eastern Nigeria. Ann Clin Microbiol Antimicrob. 2012;11:19. doi: 10.1186/1476-0711-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LA. Benson P. Neuzil KM. Grandjean M. Marino JL. Burden of community-onset Escherichia coli bacteremia in seniors. J Infect Dis. 2005;191:1523–1529. doi: 10.1086/429344. [DOI] [PubMed] [Google Scholar]
- Jakobsen L. Garneau P. Bruant G. Harel J. Olsen SS. Porsbo LJ. Hammerum AM. Frimodt-Moller N. Is Escherichia coli urinary tract infection a zoonosis? Proof of direct link with production animals and meat. Eur J Clin Microbiol Infect Dis. 2012;31:1121–1129. doi: 10.1007/s10096-011-1417-5. [DOI] [PubMed] [Google Scholar]
- Jakobsen L. Spangholm DJ. Pedersen K. Jensen LB. Emborg HD. Agerso Y. Aarestrup FM. Hammerum AM. Frimodt-Moller N. Broiler chickens, broiler chicken meat, pigs and pork as sources of ExPEC related virulence genes and resistance in Escherichia coli isolates from community-dwelling humans and UTI patients. Int J Food Microbiol. 2010;142:264–272. doi: 10.1016/j.ijfoodmicro.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Johnson JR. Kuskowski MA. Smith K. O'Bryan TT. Tatini S. Antimicrobial-resistant and extraintestinal pathogenic Escherichia coli in retail foods. J Infect Dis. 2005;191:1040–1049. doi: 10.1086/428451. [DOI] [PubMed] [Google Scholar]
- Johnson JR. Russo TA. Molecular epidemiology of extraintestinal pathogenic (uropathogenic) Escherichia coli. Int J Med Microbiol. 2005;295:383–404. doi: 10.1016/j.ijmm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Johnson TJ. Jordan D. Kariyawasam S. Stell AL. Bell NP. Wannemuehler YM. Alarcon CF. Li G. Tivendale KA. Logue CM. Nolan LK. Sequence analysis and characterization of a transferable hybrid plasmid encoding multidrug resistance and enabling zoonotic potential for extraintestinal Escherichia coli. Infect Immun. 2010;78:1931–1942. doi: 10.1128/IAI.01174-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TJ. Kariyawasam S. Wannemuehler Y. Mangiamele P. Johnson SJ. Doetkott C. Skyberg JA. Lynne AM. Johnson JR. Nolan LK. The genome sequence of avian pathogenic Escherichia coli strain O1:K1:H7 shares strong similarities with human extraintestinal pathogenic E. coli genomes. J Bacteriol. 2007;189:3228–3236. doi: 10.1128/JB.01726-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TJ. Logue CM. Johnson JR. Kuskowski MA. Sherwood JS. Barnes HJ. DebRoy C. Wannemuehler YM. Obata-Yasuoka M. Spanjaard L. Nolan LK. Associations between multidrug resistance, plasmid content, and virulence potential among extraintestinal pathogenic and commensal Escherichia coli from humans and poultry. Foodborne Pathog Dis. 2012;9:37–46. doi: 10.1089/fpd.2011.0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TJ. Wannemuehler Y. Doetkott C. Johnson SJ. Rosenberger SC. Nolan LK. Identification of minimal predictors of avian pathogenic Escherichia coli virulence for use as a rapid diagnostic tool. J Clin Microbiol. 2008a;46:3987–3996. doi: 10.1128/JCM.00816-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TJ. Wannemuehler Y. Johnson SJ. Stell AL. Doetkott C. Johnson JR. Kim KS. Spanjaard L. Nolan LK. Comparison of extraintestinal pathogenic Escherichia coli strains from human and avian sources reveals a mixed subset representing potential zoonotic pathogens. Appl Environ Microbiol. 2008b;74:7043–7050. doi: 10.1128/AEM.01395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan FT. Williams NJ. Wattret A. Jones T. Observations on salpingitis, peritonitis and salpingoperitonitis in a layer breeder flock. Vet Rec. 2005;157:573–577. doi: 10.1136/vr.157.19.573. [DOI] [PubMed] [Google Scholar]
- Karaca Y. Coplu N. Gozalan A. Oncul O. Citil BE. Esen B. Co-trimoxazole and quinolone resistance in Escherichia coli isolated from urinary tract infections over the last 10 years. Int J Antimicrob Agents. 2005;26:75–77. doi: 10.1016/j.ijantimicag.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Kennedy KJ. Roberts JL. Collignon PJ. Escherichia coli bacteraemia in Canberra: Incidence and clinical features. Med J Aust. 2008;188:209–213. doi: 10.5694/j.1326-5377.2008.tb01586.x. [DOI] [PubMed] [Google Scholar]
- Kim KS. Current concepts on the pathogenesis of Escherichia coli meningitis: Implications for therapy and prevention. Curr Opin Infect Dis. 2012;25:273–278. doi: 10.1097/QCO.0b013e3283521eb0. [DOI] [PubMed] [Google Scholar]
- Klinger G. Chin CN. Beyene J. Perlman M. Predicting the outcome of neonatal bacterial meningitis. Pediatrics. 2000;106:477–482. doi: 10.1542/peds.106.3.477. [DOI] [PubMed] [Google Scholar]
- Laupland KB. Gregson DB. Church DL. Ross T. Pitout JD. Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region. Clin Microbiol Infect. 2008;14:1041–1047. doi: 10.1111/j.1469-0691.2008.02089.x. [DOI] [PubMed] [Google Scholar]
- Laupland KB. Gregson DB. Flemons WW. Hawkins D. Ross T. Church DL. Burden of community–onset bloodstream infection: A population-based assessment. Epidemiol Infect. 2007;135:1037–1042. doi: 10.1017/S0950268806007631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XS. Wang GQ. Du XD. Cui BA. Zhang SM. Shen JZ. Antimicrobial susceptibility and molecular detection of chloramphenicol and florfenicol resistance among Escherichia coli isolates from diseased chickens. J Vet Sci. 2007;8:243–247. doi: 10.4142/jvs.2007.8.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KM. Schonheyder HC. Kristensen B. Sorensen HT. Secular trends in incidence and mortality of bacteraemia in a Danish county 1981-1994. APMIS. 1999;107:346–352. doi: 10.1111/j.1699-0463.1999.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Manges AR. Johnson JR. Food-borne origins of Escherichia coli causing extraintestinal infections. Clin Infect Dis. 2012;55:712–719. doi: 10.1093/cid/cis502. [DOI] [PubMed] [Google Scholar]
- Manges AR. Smith SP. Lau BJ. Nuval CJ. Eisenberg JNS. Dietrich PS. Riey LW. Retail meat consumption and the acquisition of antimicrobial resistant Escherichia coli causing urinary tract infections: A case-control study. Foodborne Pathog Dis. 2007;4:419–431. doi: 10.1089/fpd.2007.0026. [DOI] [PubMed] [Google Scholar]
- Maturana VG. de Pace F. Carlos C. Mistretta Pires M. Amabile de Campos T. Nakazato G. Guedes Stheling E. Logue CM. Nolan LK. Dias da Silveira W. Subpathotypes of avian pathogenic Escherichia coli (APEC) exist as defined by their syndromes and virulence traits. Open Microbiol J. 2011;5:55–64. doi: 10.2174/1874285801105010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellata M. Dho-Moulin M. Dozois CM. Curtiss R., III Brown PK. Arne P. Bree A. Desautels C. Fairbrother JM. Role of virulence factors in resistance of avian pathogenic Escherichia coli to serum and in pathogenicity. Infect Immun. 2003;71:536–540. doi: 10.1128/IAI.71.1.536-540.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellata M. Ameiss K. Mo H. Curtiss R., III Characterization of the contribution to virulence of three large plasmids of avian pathogenic Escherichia coli chi7122 (O78:K80:H9) Infect Immun. 2010;78:1528–1541. doi: 10.1128/IAI.00981-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier S. Quessy S. Robinson Y. Devriese LA. Hommez J. Fairbrother JM. Focal dermatitis and cellulitis in broiler chickens: Bacteriological and pathological findings. Avian Dis. 1993;37:839–844. [PubMed] [Google Scholar]
- Mokady D. Gophna U. Ron EZ. Extensive gene diversity in septicemic Escherichia coli strains. J Clin Microbiol. 2005;43:66–73. doi: 10.1128/JCM.43.1.66-73.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy MA. Knobl T. Bottino JA. Ferreira CS. Ferreira AJ. Virulence characteristics of Escherichia coli isolates obtained from broiler breeders with salpingitis. Comp Immunol Microbiol Infect Dis. 2005;28:1–15. doi: 10.1016/j.cimid.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Mora A. Lopez C. Dabhi G. Blanco M. Blanco JE. Alonso MP. Herrera A. Mamani R. Bonacorsi S. Moulin-Schouleur M. Blanco J. Extraintestinal pathogenic Escherichia coli O1:K1:H7/NM from human and avian origin: Detection of clonal groups B2 ST95 and D ST59 with different host distribution. BMC Microbiol. 2009;9:132. doi: 10.1186/1471-2180-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi S. Maurer JJ. Hofacre C. Summers AO. Gram-positive bacteria are a major reservoir of Class 1 antibiotic resistance integrons in poultry litter. Proceedings of the National Academy of Sciences of the United States of America; 2004. pp. 7118–7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton RA. Bilgili SF. McMurtrey BC. A reproducible model for the induction of avian cellulitis in broiler chickens. Avian Dis. 1997;41:422–428. [PubMed] [Google Scholar]
- Obata-Yasuoka M. Ba-Thein W. Tsukamoto T. Yoshikawa H. Hayashi H. Vaginal Escherichia coli share common virulence factor profiles, serotypes and phylogeny with other extraintestinal E. coli. Microbiology. 2002;148:2745–2752. doi: 10.1099/00221287-148-9-2745. [DOI] [PubMed] [Google Scholar]
- Olkowski AA. Wojnarowicz C. Chirino-Trejo M. Wurtz BM. Kumor L. The role of first line of defense mechanisms in the pathogenesis of cellulitis in broiler chickens: Skin structural, physiological and cellular response factors. J Vet Med, Series A—Physiol Pathol Clin Med. 2005;52:517–524. doi: 10.1111/j.1439-0442.2005.00768.x. [DOI] [PubMed] [Google Scholar]
- Olsen RH. Chadfield MS. Christensen JP. Scheutz F. Christensen H. Bisgaard M. Clonality and virulence traits of Escherichia coli associated with haemorrhagic septicaemia in turkeys. Avian Pathol. 2011;40:587–595. doi: 10.1080/03079457.2011.618942. [DOI] [PubMed] [Google Scholar]
- Olsen RH. Frantzen C. Christensen H. Bisgaard M. An investigation on first-week mortality in layers. Avian Dis. 2012a;56:51–57. doi: 10.1637/9777-051011-Reg.1. [DOI] [PubMed] [Google Scholar]
- Olsen RH. Christensen H. Bisgaard M. Comparative genomics of multiple plasmids from APEC associated with clonal outbreaks demonstrates major similarities and identifies several potential vaccine-targets. Vet Microbiol. 2012b;158:384–393. doi: 10.1016/j.vetmic.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Omer MM. Abusalab SM. Gumaa MM. Mulla SA. Omer EA. Jeddah IE. Al-Hassan AM. Hussein MA. Ahmed AM. Outbreak of colibacillosis among broiler and layer flocks in intensive and semi intensive poultry farms in Kassala State, Eastern Sudan. Asian J Poultry Sci. 2010;4:173–181. [Google Scholar]
- Overdevest I. Willemsen I. Rijnsburger M. Eustace A. Xu L. Hawkey P. Heck M. Savelkoul P. Vandenbroucke-Grauls C. van der Zwaluw K. Huijsdens X. Kluytmans J. Extended-spectrum beta-lactamase genes of Escherichia coli in chicken meat and humans, The Netherlands. Emerg Infect Dis. 2011;17:1216–1222. doi: 10.3201/eid1707.110209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H. Murase T. Multiple routes of entry for Escherichia coli causing colibacillosis in commercial layer chickens. J Vet Med Sci. 2009;71:1685–1689. doi: 10.1292/jvms.001685. [DOI] [PubMed] [Google Scholar]
- Peighambari SM. Julian RJ. Vaillancourt JP. Gyles CL. Escherichia coli cellulitis: Experimental infections in broiler chickens. Avian Dis. 1995;39:125–134. [PubMed] [Google Scholar]
- Peirano G. Costello M. Pitout JD. Molecular characteristics of extended-spectrum beta-lactamase-producing Escherichia coli from the Chicago area: High prevalence of ST131 producing CTX-M-15 in community hospitals. Int J Antimicrob Agents. 2010;36:19–23. doi: 10.1016/j.ijantimicag.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Pires-Dos-Santos T. Bisgaard M. Christensen H. Genetic diversity and virulence profiles of Escherichia coli causing salpingitis and peritonitis in broiler breeders. Vet Microbiol. 2013;162:873–880. doi: 10.1016/j.vetmic.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Pitout JD. Extraintestinal pathogenic Escherichia coli: A combination of virulence with antibiotic resistance. Front Microbiol. 2012;3:1–7. doi: 10.3389/fmicb.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourbakhsh SA. Boulianne M. Martineau-Doize B. Dozois CM. Desautels C. Fairbrother JM. Dynamics of Escherichia coli infection in experimentally inoculated chickens. Avian Dis. 1997;41:221–233. [PubMed] [Google Scholar]
- Qin X. Hu F. Wu S. Ye X. Zhu D. Zhang Y. Wang M. Comparison of adhesin genes and antimicrobial susceptibilities between uropathogenic and intestinal commensal Escherichia coli strains. PloS ONE. 2013 Apr 9;8:e61169. doi: 10.1371/journal.pone.0061169. e-pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel JM. Sparling PH. Crowe C. Griffin PM. Swerdlow DL. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerg Infect Dis. 2005;11:603–609. doi: 10.3201/eid1104.040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond J. Lopez E. Bonacorsi S. Poyart C. Moriette G. Jarreau PH. Bingen E. Evidence for transmission of Escherichia coli from mother to child in late-onset neonatal infection. Pediatr Infect Dis J. 2008;27:186–188. doi: 10.1097/INF.0b013e31815b1b03. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Bano J. Mingorance J. Fernandez-Romero N. Serrano L. Lopez-Cerero L. Pascual A Esbl-Reipi Grp. Virulence profiles of bacteremic extended-spectrum beta-lactamase-producing Escherichia coli: Association with epidemiological and clinical features. PloS ONE. 2012 Sep 7;7(9):e44238. doi: 10.1371/journal.pone.0044238. e-pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Siek KE. Giddings CW. Doetkott C. Johnson TJ. Fakhr MK. Nolan LK. Comparison of Escherichia coli isolates implicated in human urinary tract infection and avian colibacillosis. Microbiology. 2005;151:2097–2110. doi: 10.1099/mic.0.27499-0. [DOI] [PubMed] [Google Scholar]
- Russo TA. Johnson JR. Medical and economic impact of extraintestinal infections due to Escherichia coli: Focus on an increasingly important endemic problem. Microbes Infect. 2003;5:449–456. doi: 10.1016/s1286-4579(03)00049-2. [DOI] [PubMed] [Google Scholar]
- Sanchez GV. Master RN. Bordon J. Trimethoprim-sulfamethoxazole may no longer be acceptable for the treatment of acute uncomplicated cystitis in the United States. Clin Infect Dis. 2011;53:316–317. doi: 10.1093/cid/cir345. [DOI] [PubMed] [Google Scholar]
- Schlackow I. Stoesser N. Walker AS. Crook DW. Peto TE. Wyllie DH. Increasing incidence of Escherichia coli bacteraemia is driven by an increase in antibiotic-resistant isolates: Electronic database study in Oxfordshire 1999–2011. J Antimicrob Chemother. 2012;67:1514–1524. doi: 10.1093/jac/dks082. [DOI] [PubMed] [Google Scholar]
- Schmiemann G. Gagyor I. Hummers-Pradier E. Bleidorn J. Resistance profiles of urinary tract infections in general practice—An observational study. BMC Urol. 2012;12:33. doi: 10.1186/1471-2490-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouler C. Schaeffer B. Bree A. Mora A. Dahbi G. Biet F. Oswald E. Mainil J. Blanco J. Moulin-Schouleur M. Diagnostic strategy for identifying avian pathogenic Escherichia coli based on four patterns of virulence genes. J Clin Microbiol. 2012;50:1673–1678. doi: 10.1128/JCM.05057-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader JS. Singer RS. Atwill ER. A prospective study of management and litter variables associated with cellulitis in California broiler flocks. Avian Dis. 2004;48:522–530. doi: 10.1637/7125. [DOI] [PubMed] [Google Scholar]
- Sheng WH. Badal RE. Hseuh PR. Distribution of extended-spectrum beta-lactamases (ESBLs), AmpC beta-lactamases, and carbapenemases among Enterobacteriaceae isolates causing intra-abdominal infections in Asia-Pacific: The study for monitoring antimicrobial resistance trends (SMART) Antimicrob Agents Chemother. 2013;57:2981–2988. doi: 10.1128/AAC.00971-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogberg K. Lyytikainen O. Ruutu P. Ollgren J. Nuorti JP. Increase in bloodstream infections in Finland, 1995-2002. Epidemiol Infect. 2008;136:108–114. doi: 10.1017/S0950268807008138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skyberg JA. Johnson TJ. Johnson JR. Clabots C. Logue CM. Nolan LK. Acquisition of avian pathogenic Escherichia coli plasmids by a commensal E. coli isolate enhances its abilities to kill chicken embryos, grow in human urine, and colonize the murine kidney. Infect Immun. 2006;74:6287–6292. doi: 10.1128/IAI.00363-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL. Fratamico PM. Gunther NW. Extraintestinal pathogenic Escherichia coli. Foodborne Pathog Dis. 2007;4:134–163. doi: 10.1089/fpd.2007.0087. [DOI] [PubMed] [Google Scholar]
- Stearns RC. Barnas GM. Walski M. Brain JD. Deposition and phagocytosis of inhaled particles in the gas exchange region of the duck, Anas platyrhynchos. Respir Physiol. 1987;67:23–36. doi: 10.1016/0034-5687(87)90004-1. [DOI] [PubMed] [Google Scholar]
- Stokholm NM. Permin A. Bisgaard M. Christensen JP. Causes of mortality in commercial organic layers in Denmark. Avian Dis. 2010;54:1241–1250. doi: 10.1637/9375-041910-Reg.1. [DOI] [PubMed] [Google Scholar]
- Stoll BJ. Hansen NI. Sanchez PJ. Faix RG. Poindexter BB. Van Meurs KP. Bizzarro MJ. Goldberg RN. Frantz ID., III Hale EC. Shankaran S. Kennedy K. Carlo WA. Watterberg KL. Bell EF. Walsh MC. Schibler K. Laptook AR. Shane AL. Schrag SJ. Das A. Higgins RD. Early onset neonatal sepsis: The burden of group B streptococcal and E. coli disease continues. Pediatrics. 2011;127:817–826. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H. Li S. Xie Z. Yang F. Sun Y. Zhu Y. Zhao X. Jiang S. A novel multidrug resistance plasmid isolated from an Escherichia coli strain resistant to aminoglycosides. J Antimicrob Chemother. 2012;67:1635–1638. doi: 10.1093/jac/dks107. [DOI] [PubMed] [Google Scholar]
- Tivendale KA. Logue CM. Kariyawasam S. Jordan D. Hussein A. Li G. Wannemuehler Y. Nolan LK. Avian-pathogenic Escherichia coli strains are similar to neonatal meningitis E. coli strains and are able to cause meningitis in the rat model of human disease. Infect Immun. 2010;78:3412–3419. doi: 10.1128/IAI.00347-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuntufye HN. Lebeer S. Gwakisa PS. Goddeeris BM. Identification of avian pathogenic Escherichia coli genes that are induced in vivo during infection in chickens. Appl Environ Microbiol. 2012;78:3343–3351. doi: 10.1128/AEM.07677-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslan DZ. Crane SJ. Steckelberg JM. Cockerill FR., III St Sauver JL. Wilson WR. Baddour LM. Age- and sex-associated trends in bloodstream infection: A population-based study in Olmsted County, Minnesota. Arch Intern Med. 2007;167:834–839. doi: 10.1001/archinte.167.8.834. [DOI] [PubMed] [Google Scholar]
- van der Bij AK. Peirano G. Goessens WH. van der Vorm ER. van Westreenen M. Pitout JD. Clinical and molecular characteristics of extended-spectrum-beta-lactamase-producing Escherichia coli causing bacteremia in the Rotterdam Area, Netherlands. Antimicrob Agents Chemother. 2011;55:3576–3578. doi: 10.1128/AAC.00074-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bij AK. Pitout JD. The role of international travel in the worldwide spread of multiresistant Enterobacteriaceae. J Antimicrob Chemother. 2012;67:2090–2100. doi: 10.1093/jac/dks214. [DOI] [PubMed] [Google Scholar]
- Vandekerchove D. De Herdt P. Laevens H. Pasmans F. Colibacillosis in caged layer hens: Characteristics of the disease and the aetiological agent. Avian Pathol. 2004;33:117–125. doi: 10.1080/03079450310001642149. [DOI] [PubMed] [Google Scholar]
- Vincent C. Boerlin P. Daignault D. Dozois CM. Dutil L. Galanakis C. Reid-Smith RJ. Tellier PP. Tellis PA. Ziebell K. Manges AR. Food reservoir for Escherichia coli causing urinary tract infections. Emerg Infect Dis. 2010;16:88–95. doi: 10.3201/eid1601.091118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlieghe ER. Phe T. De Smet B. Chhun Veng H. Kham C. Lim K. Koole O. Lynen L. Peetermans WE. Jacobs JA. Bloodstream infection among adults in Phnom Penh, Cambodia: Key pathogens and resistance patterns. PLoS ONE. 2013;8:e59775. doi: 10.1371/journal.pone.0059775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston EJ. Pondo T. Lewis MM. Martell-Cleary P. Morin C. Jewell B. Daily P. Apostol M. Petit S. Farley M. Lynfield R. Reingold A. Hansen NI. Stoll BJ. Shane AJ. Zell E. Schrag SJ. The burden of invasive early-onset neonatal sepsis in the United States, 2005-2008. Pediatr Infect Dis J. 2011;30:937–941. doi: 10.1097/INF.0b013e318223bad2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DA. Roberts SA. Paterson DL. Sidjabat H. Silvey A. Masters J. Rice M. Freeman JT. Escherichia coli bloodstream infection after transrectal ultrasound-guided prostate biopsy: Implications of fluoroquinolone-resistant sequence type 131 as a major causative pathogen. Clin Infect Dis. 2012;54:1406–1412. doi: 10.1093/cid/cis194. [DOI] [PubMed] [Google Scholar]
- Wilson J. Elgohari S. Livermore DM. Cookson B. Johnson A. Lamagni T. Chronias A. Sheridan E. Trends among pathogens reported as causing bacteraemia in England, 2004-2008. Clin Microbiol Infect. 2011;17:451–458. doi: 10.1111/j.1469-0691.2010.03262.x. [DOI] [PubMed] [Google Scholar]
- Xavier DB. Broom DM. McManus CM. Torres C. Bernal FE. Number of flocks on the same litter and carcase condemnations due to cellulitis, arthritis and contact foot-pad dermatitis in broilers. Br Poult Sci. 2010;51:586–591. doi: 10.1080/00071668.2010.508487. [DOI] [PubMed] [Google Scholar]
- Yang H. Chen S. White DG. Zhao S. McDermott P. Walker R. Meng J. Characterization of multiple-antimicrobial-resistant Escherichia coli isolates from diseased chickens and swine in China. J Clin Microbiol. 2004;42:3483–3489. doi: 10.1128/JCM.42.8.3483-3489.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogaratnam V. Analysis of the causes of high rates of carcase rejection at a poultry processing plant. Vet Rec. 1995;137:215–217. doi: 10.1136/vr.137.9.215. [DOI] [PubMed] [Google Scholar]
- Zanella A. Alborali GL. Bardotti M. Candotti P. Guadagnini PF. Martino PA. Stonfer M. Severe Escherichia coli O111 septicaemia and polyserositis in hens at the start of lay. Avian Pathol. 2000;29:311–317. doi: 10.1080/03079450050118430. [DOI] [PubMed] [Google Scholar]
- Zhao L. Gao S. Huan H. Xu X. Zhu X. Yang W. Gao Q. Liu X. Comparison of virulence factors and expression of specific genes between uropathogenic Escherichia coli and avian pathogenic E. coli in a murine urinary tract infection model and a chicken challenge model. Microbiology. 2009;155:1634–1644. doi: 10.1099/mic.0.024869-0. [DOI] [PubMed] [Google Scholar]
- Zhao S. Maurer JJ. Hubert S. De Villena JF. McDermott PF. Meng J. Ayers S. English L. White DG. Antimicrobial susceptibility and molecular characterization of avian pathogenic Escherichia coli isolates. Vet Microbiol. 2005;107:215–224. doi: 10.1016/j.vetmic.2005.01.021. [DOI] [PubMed] [Google Scholar]