Abstract
Aims: The aim of the study was to determine whether the trajectory of learning and memory is modified according to an interaction between midlife or late life alcohol consumption status and the presence of one or more APOE e4 alleles. Methods: This was a secondary analysis of cognitive, genetic and alcohol consumption data collected from members of the Framingham Heart Study Offspring Cohort. Results: Light and moderate alcohol consumption during late life was associated with greater decline in learning and memory among APOE e4 carriers, whereas light and moderate alcohol consumption was associated with an increase in learning and memory among non-APOE e4 carriers. There was not a significant interaction between midlife alcohol consumption status and APOE e4 on the trajectory of learning and memory. Conclusion: Light to moderate alcohol consumption during late life may protect against a decline in learning and memory for non-APOE e4 allele carriers, but not for older adults who carry one or more APOE e4 alleles.
INTRODUCTION
The relationship between alcohol consumption and cognitive functioning during old age has been examined extensively. Several studies report findings that indicate that moderate alcohol consumption during late life is associated with higher cognitive functioning (Stampfer et al., 2005; Lang et al., 2007) and a decreased risk of dementia (Anstey et al., 2009; Weyerer et al., 2011). These findings are consistent with studies that have examined the relationship between alcohol consumption during midlife and late life cognitive functioning (Virtaa et al., 2010; Kesse-Guyot et al., 2012). There are several mechanisms that may explain the observed relationship between moderate alcohol consumption and cognition, including the anti-inflammatory properties of alcohol (Wright et al., 2006) and moderate alcohol consumption being protective against risk factors for dementia, such as stroke (Beulens et al., 2010), coronary heart disease (Hvidtfeldt et al., 2010) and type II diabetes (Wannamethe et al., 2002). This is likely due to the benefits of moderate alcohol consumption on cardiovascular health (di Giuseppe et al., 2009; Marques-Vidal et al., 2010).
While current evidence suggests that moderate alcohol consumption is beneficial for cognitive functioning among older adults, the effects of moderate alcohol consumption among older adults who carry one or more apolipoprotein (APOE) e4 alleles are less certain. APOE e4 is a widely accepted genetic risk factor for Alzheimer's disease (AD) and the risk of AD has been shown to increase with each APOE e4 allele (Corder et al., 1993). The APOE e4 allele is also associated with lower cognition among nondemented older adults (Reinvang et al., 2010). APOE encodes the protein apoE, which is a lipid transport protein (Hauser et al., 2011). The APOE e4 allele is associated with elevated serum total cholesterol (Bennet et al., 2007) and adults who carry one or more APOE e4 alleles are at an increased risk of coronary heart disease (Yang et al., 2006).
There is increasing evidence that cholesterol plays an important role in the pathogenesis of AD (Morgan, 2011). The benefits of moderate alcohol consumption for cardiovascular health and the role of apoE in cholesterol transport suggest that alcohol consumption may be able to limit the cognitive decline that has been repeatedly observed among older adults who carry one or more APOE e4 alleles. Accordingly, the purpose of the current study is to examine the relationship between the decline in learning and memory during late life, alcohol consumption during midlife and late life, and APOE e4 to determine whether moderate alcohol consumption during midlife or late life limits the severity of cognitive decline among APOE e4 carriers.
MATERIALS AND METHODS
Sample population
The Framingham Heart Study (FHS) is an ongoing longitudinal study that began in 1948 and was designed to identify traits associated with cardiovascular disease (Dawber et al., 1951). The FHS Offspring cohort was initiated in 1971 and includes the children of the Original cohort and their spouses. Details regarding the design and methods of data collection of the FHS Offspring cohort have been described previously (Feinleib et al., 1975). Investigators not affiliated with the FHS can be approved access to FHS data by submitting a research proposal to the database of genotypes and phenotypes.
Beginning in 1999, subjects still remaining in the FHS Offspring cohort were recruited to participate in a secondary study in which they received a neuropsychological battery (Massaro et al., 2004). A total of 2611 subjects were actively participating in the FHS in 1999 and 2045 (78.3%) of these subjects received a baseline neuropsychological battery. Participants received up to two neuropsychological batteries (baseline and follow-up) between 1999 and 2007. Because the purpose of the current study was to examine the relationship between alcohol consumption, APOE e4 and cognition during late life, the final sample was restricted to subjects who were 65 years of age or older upon receiving a baseline neuropsychological battery. Of the 2045 participants who received a baseline neuropsychological battery, 610 (29.8%) were 65 years of age or older. For the 610 participants included in the final sample, the average age during the first clinical examination was 44.2 years (range 35.0–59.0 years) and the average age during the eighth clinical examination was 77.1 years (range 69.0–92.0 years). These ages were used to define midlife and late life alcohol consumption and are consistent with the definitions of midlife and late life provided by previous studies (Kivipelto et al., 2001; Whitmer et al., 2005; Solomon et al., 2009).
Subjects who received a baseline neuropsychological battery consumed more alcohol during midlife (6.8 drinks per week vs. 5.4 drinks per week, P < 0.0001) and late life (4.6 drinks per week vs. 3.4 drinks per week, P < 0.0001) compared with subjects who did not receive a neuropsychological battery. These differences likely reflect the overall worse health of participants who did not receive a baseline neuropsychological battery (Massaro et al., 2004). Additional health and demographic characteristics of participants who received a neuropsychological battery have been reported by Massaro et al. (2004). Subjects who were younger than 65 years upon receiving a baseline neuropsychological battery (n = 1435) reported consuming more alcohol per week (5.2 vs. 3.5; P < 0.0001) and were more likely to be current smokers (11.6 vs. 4.2%; P < 0.0001) during the eighth clinical examination compared with subjects who were included in the final sample. These differences are to be expected given the declines in the prevalence of alcohol consumption and smoking with increasing age (Mendez et al., 1998; Moore et al., 2005). There were no differences in smoking status or the amount of alcohol consumed per week during the first clinical examination. There were also no differences in educational attainment or in the proportion of males and females between subjects who were younger than 65 and those included in the final sample.
Assessment of alcohol consumption
Alcohol consumption was assessed during each clinical examination using three open-ended questions administered as part of a medical history interview (MHI): (a) During the past year, how many bottles, cans or glasses of beer did you consume per week? (b) During the past year, how many glasses of wine did you consume per week? and (c) During the past year, how many cocktails (i.e. drinks containing liquor) did you consume per week? This assessment was compared with a validated food frequency questionnaire (FFQ; Rimm et al., 1992) administered during the eighth clinical examination. The correlations between the FFQ and MHI for weekly beer (r = 0.84), wine (r = 0.78) and liquor (r = 0.80) consumption were all highly significant (P < 0.001). Paired t-tests were used to compare the average weekly consumption of beer, wine and liquor. There were no significant differences between the FFQ and MHI for the amounts of beer, wine or liquor consumed per week.
Midlife and late life alcohol consumption was determined by summing the amounts of beer, wine and liquor consumed per week as reported by participants during the first and eighth clinical examinations, respectively. Alcohol consumption data collected during clinical examinations two through seven were not included in any analyses. Participants were grouped into the following categories to define midlife and late life alcohol consumption status: (a) abstinent; (b) light, 1–6 drinks per week; (c) moderate, 7–14 drinks per week and (d) heavy, >14 drinks per week. These alcohol consumption categories are consistent with the guidelines established by the National Institute on Alcohol Abuse and Alcoholism (Dufour, 1999). Abstainers were treated as the reference category in all analyses.
Assessment of learning and memory
The FHS neuropsychological battery included six subtests of the Wechsler Memory Scale (Wechsler, 1945; immediate and delayed recall of logical memory, paired-associate learning, and visual reproductions). These subtests are commonly used measures of verbal memory, new learning and visual memory (Au et al., 2004). The raw scores from each assessment were standardized according to the sample mean and standard deviation; the standardized scores were then averaged to create a summary score of learning and memory. This method has been used previously (Wilson et al., 2011).
Covariates
Covariates were chosen according to those that have been included in previous studies that have examined alcohol consumption (Anttila et al., 2004; Britton et al., 2004; Deng et al., 2006). Education status was determined during the first neuropsychological battery and was defined as no high school degree, high school degree, some college and college degree. During the first clinical examination participants were asked whether they had smoked regularly for at least 1 year (no, yes and former). During the eighth clinical examination participants were asked whether they had smoked regularly in the last year (yes or no). APOE e4 status was defined as e4+ or e4− due to the low prevalence of APOE e4 homozygotes in the final sample. APOE e4− was the reference group in all analyses. Sex and age were also included as covariates.
Statistical analysis
The current study utilized a retrospective design. Differences in demographic characteristics according to midlife and late life alcohol consumption status were examined using analysis of variance (ANOVA) and Fisher's exact tests. Multiple linear regression was used to test for associations between midlife alcohol consumption and baseline learning and memory and late life alcohol consumption and baseline learning and memory. All models controlled for age, sex, education, smoking status and APOE e4.
Linear mixed modeling (Laird and Ware, 1982) was used to examine the relationship between alcohol consumption during midlife and late life, APOE e4, and trajectories in learning and memory. This approach was used because it provides valid estimates when data are highly unbalanced due to differences in the number and timing at which subjects are observed (Cnaan et al., 1997). Random effects for age and intercept were also included in all models to allow for the trajectory of learning and memory, as well as baseline learning and memory, to vary for each subject. Two-way interaction terms between age and alcohol consumption status and age and APOE e4 were included to determine whether the trajectory of learning and memory differed according to alcohol consumption or APOE e4 status. A three-way interaction term between age, APOE and alcohol consumption status was included to determine whether the relationship between alcohol consumption and the trajectory of learning and memory differed according to the presence of one or more APOE e4 alleles. All analyses were performed using the statistical package R (R Core Team, 2013).
RESULTS
Sample characteristics
A total of 610 participants received the baseline neuropsychological battery and 494 (81.0%) received a second neuropsychological battery. Participants who did not receive a second neuropsychological battery were less likely to have a high school degree (P = 0.0002) and had a lower summary score of learning and memory (P < 0.0001). There were no differences between participants who received two neuropsychological batteries and those who received only a baseline battery for age, APOE e4, sex or alcohol consumption during midlife and late life. The APOE allele frequencies of the final sample were 8.0, 81.2 and 10.8% for the e2, e3 and e4 alleles, respectively. These allele frequencies did not violate Hardy–Weinberg equilibrium. There were 126 (20.7%) subjects with one or more APOE e4 alleles and 484 (79.3%) subjects with no APOE e4 alleles. During the first clinical examination there were 59 (9.6%) abstainers, 308 (50.5%) light, 156 (25.6%) moderate and 87 (14.3%) heavy alcohol consumers. Alcohol consumption declined with increasing age 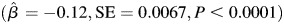 . During the eighth clinical examination there were 339 (55.6%) abstainers, 115 (18.9%) light, 128 (21.0%) moderate and 28 (4.6%) heavy alcohol consumers.
. During the eighth clinical examination there were 339 (55.6%) abstainers, 115 (18.9%) light, 128 (21.0%) moderate and 28 (4.6%) heavy alcohol consumers.
Comparisons for demographic characteristics during the baseline neuropsychological battery according to midlife and late life alcohol consumption status are provided in Table 1 (approximate location: page 10). Males were more likely than females to be heavy alcohol consumers during midlife (P < 0.0001) and late life (P < 0.0001). Heavy alcohol consumption during midlife was associated with being a current smoker during midlife (P < 0.0001), but there was not a significant relationship between alcohol consumption during late life and smoking status during late life (P = 0.78). Subjects with one or more APOE e4 alleles were less likely to be abstainers during midlife (P = 0.044) and consumed more drinks per week during midlife compared with subjects with no APOE e4 alleles (e4+ 9.6 drinks/week; e4− 7.4 drinks/week; P = 0.087). Light alcohol consumption during late life was associated with higher education obtainment (P < 0.0001).
Table 1.
Demographic characteristics of subjects who received a neuropsychological battery between 1999 and 2005 according to midlife and late life alcohol consumption status
| Variable | Abstainer | Light | Moderate | Heavy | P-value |
|---|---|---|---|---|---|
| n = 59 | n = 308 | n = 156 | n = 87 | ||
| Midlife alcohol consumption status | |||||
| Agea, midlife (SD) | 43.9 (4.4) | 44.1 (4.6) | 44.6 (4.5) | 44.1 (4.5) | 0.72 |
| Agea, late life (SD) | 76.7 (4.3) | 77.1 (4.4) | 77.4 (4.4) | 76.7 (4.3) | 0.58 |
| Number of years between NP examinationsb | 6.3 (0.79) | 6.2 (1.2) | 6.1 (0.97) | 6.0 (1.0) | 0.46 |
| Midlife smoking status (%) | |||||
| Current smoker | 10 (16.9) | 98 (31.9) | 63 (40.4) | 36 (41.4) | <0.0001 |
| Former smoker | 11 (18.6) | 76 (24.8) | 46 (29.5) | 42 (48.3) | |
| Non-smoker | 38 (64.4) | 133 (43.3) | 47 (30.1) | 9 (10.3) | |
| Late life smoking status (%) | |||||
| Non-smoker | 55 (93.2) | 293 (95.4) | 150 (96.2) | 84 (96.6) | 0.78 |
| Current smoker | 4 (6.8) | 14 (4.6) | 6 (3.8) | 3 (3.4) | |
| Drinks per week (SD) | 0.0 (0.0) | 2.8 (1.4) | 10.1 (2.4) | 27.1 (12.9) | <0.0001 |
| Education (%) | |||||
| Less than high school | 5 (8.5) | 18 (5.8) | 11 (7.1) | 6 (6.9) | 0.48 |
| High school | 28 (47.5) | 126 (40.9) | 53 (34.0) | 36 (41.4) | |
| Some college | 15 (25.4) | 71 (23.1) | 41 (26.3) | 15 (17.2) | |
| College | 11 (18.6) | 93 (30.8) | 51 (32.7) | 30 (34.5) | |
| Gender (%) | |||||
| Male | 16 (27.1) | 104 (33.8) | 90 (57.7) | 70 (80.5) | <0.0001 |
| Female | 43 (72.9) | 204 (33.4) | 66 (42.3) | 17 (19.5) | |
| APOE e4 | |||||
| e4− | 53 (89.8) | 250 (81.2) | 116 (74.4) | 65 (74.7) | 0.044 |
| e4+ | 6 (10.2) | 58 (18.8) | 40 (25.6) | 22 (25.3) | |
| n = 339 | n = 115 | n = 128 | n = 28 | ||
| Late life alcohol consumption status | |||||
| Agea, midlife (SD) | 44.3 (4.6) | 44.1 (4.6) | 43.8 (4.1) | 44.6 (4.3) | 0.72 |
| Agea, late life (SD) | 77.2 (4.4) | 76.9 (4.5) | 76.7 (4.0) | 77.0 (4.2) | 0.65 |
| Number of years between NP examinationsb | 6.2 (1.1) | 6.0 (1.2) | 6.0 (1.1) | 6.0 (0.92) | 0.12 |
| Smoking, midlife (%) | |||||
| Yes | 105 (31.1) | 40 (34.8) | 48 (37.5) | 14 (50.0) | 0.0005 |
| Former | 81 (24.0) | 39 (33.9) | 45 (35.2) | 10 (35.7) | |
| No | 152 (45.0) | 36 (31.3) | 35 (27.3) | 4 (14.3) | |
| Smoking, late life (%) | |||||
| Current smoker | 17 (5.0) | 4 (3.5) | 6 (4.7) | 0 (0) | 0.78 |
| Non-smoker | 322 (95.0) | 110 (96.5) | 122 (95.3) | 28 (100.0) | |
| Drinks per week (SD) | 0.0 (0.0) | 3.1 (1.7) | 9.4 (2.8) | 22.1 (4.5) | <0.0001 |
| Education (%) | |||||
| Less than high school | 32 (9.4) | 3 (2.6) | 4 (3.1) | 1 (3.6) | <0.0001 |
| High school | 157 (46.3) | 37 (32.2) | 39 (30.5) | 10 (35.7) | |
| Some college | 78 (23.0) | 22 (19.1) | 35 (27.3) | 7 (25.0) | |
| College | 72 (21.2) | 53 (46.1) | 50 (39.1) | 10 (35.7) | |
| Gender (%) | |||||
| Male | 125 (36.9) | 60 (52.2) | 70 (54.7) | 25 (89.3) | <0.0001 |
| Female | 214 (63.1) | 55 (47.8) | 58 (45.3) | 3 (10.7) | |
| APOE e4 allele status | |||||
| e4− | 269 (79.4) | 90 (78.3) | 102 (79.7) | 23 (82.1) | 0.98 |
| e4+ | 79 (20.6) | 25 (21.7) | 26 (20.3) | 5 (17.9) | |
Bold values indicate statistical significance P < 0.05. ANOVA was used for continuous characteristics and Fisher's exact test for categorical characteristics.
aMidlife was defined as the age during the first examination. Late life was defined as the age during the eighth examination.
bBased on 494 subjects (273 abstainers, 93 light, 104 moderate and 24 heavy).
Relationship between alcohol consumption and baseline learning and memory
There were no significant differences in baseline learning and memory according to midlife or late life alcohol consumption status or the presence of one or more APOE e4 alleles after adjusting for age, sex, smoking status and education. When APOE and alcohol consumption status were removed from the model, higher scores on measures for learning and memory were associated with younger age, higher education and female gender (Table 2; approximate location page 11).
Table 2.
Baseline learning and memory according to age, gender, smoking status and education
| Variable | Point estimate | Standard error | P-value |
|---|---|---|---|
| Age | −0.020 | 0.0063 | 0.0011 |
| Gender | |||
| Male (ref) | – | – | – |
| Female | 0.25 | 0.055 | <0.0001 |
| Smoking, late life | |||
| Non-smoker (ref) | – | – | – |
| Smoker | −0.13 | 0.13 | 0.31 |
| Smoking, midlife | |||
| Non-smoker (ref) | – | – | – |
| Smoker | 0.041 | 0.066 | 0.54 |
| Former smoker | 0.090 | 0.067 | 0.18 |
| Education | |||
| < High school (ref) | – | – | – |
| High school | 0.37 | 0.11 | 0.00087 |
| Some college | 0.53 | 0.12 | <0.0001 |
| College degree | 0.87 | 0.11 | <0.0001 |
Bold values indicate statistical significance P < 0.05. All models adjusted for age, gender, smoking status and education.
Relationship between alcohol consumption and trajectory of learning and memory
Subjects with one or more APOE e4 alleles exhibited greater decline in learning and memory compared with subjects with no APOE e4 alleles 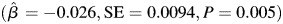 . The trajectory of learning and memory was not associated with midlife or late life alcohol consumption status and there were no differences in the trajectory of learning and memory according to gender or education. Furthermore, there was not a statistically significant three-way interaction between age, APOE and midlife alcohol consumption status. The findings from the model that included the interaction between age, APOE and late life alcohol consumption status indicated that the relationship between late life alcohol consumption status and learning and memory was modified according to APOE e4 status (P = 0.021; Table 3; approximate location page 11). When subjects were stratified according to APOE e4 status, moderate alcohol consumption was associated with greater decline in learning and memory among those who were APOE e4+,
. The trajectory of learning and memory was not associated with midlife or late life alcohol consumption status and there were no differences in the trajectory of learning and memory according to gender or education. Furthermore, there was not a statistically significant three-way interaction between age, APOE and midlife alcohol consumption status. The findings from the model that included the interaction between age, APOE and late life alcohol consumption status indicated that the relationship between late life alcohol consumption status and learning and memory was modified according to APOE e4 status (P = 0.021; Table 3; approximate location page 11). When subjects were stratified according to APOE e4 status, moderate alcohol consumption was associated with greater decline in learning and memory among those who were APOE e4+, 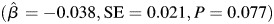 compared with abstainers, whereas among subjects who were APOE e4−, moderate alcohol consumption was associated with an increase in learning and memory
compared with abstainers, whereas among subjects who were APOE e4−, moderate alcohol consumption was associated with an increase in learning and memory 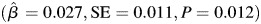 compared with abstainers. A similar trend was observed for light alcohol consumers. Light alcohol consumption was associated with greater decline in learning and memory compared with abstainers among APOE e4+ older adults
compared with abstainers. A similar trend was observed for light alcohol consumers. Light alcohol consumption was associated with greater decline in learning and memory compared with abstainers among APOE e4+ older adults 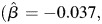 SE = 0.022, P = 0.091), whereas light alcohol consumption was associated with an increase in learning and memory among those who were APOE e4−
SE = 0.022, P = 0.091), whereas light alcohol consumption was associated with an increase in learning and memory among those who were APOE e4− 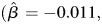 SE = 0.011, P = 0.29). Heavy alcohol consumption was associated with an increase in learning and memory among subjects who were APOE e4+. It is possible that this finding is due to a small sample size because only five subjects were APOE e4+ and heavy alcohol consumers during late life. There was not a significant three-way interaction between age, APOE and midlife alcohol consumption status on the trajectory of learning and memory.
SE = 0.011, P = 0.29). Heavy alcohol consumption was associated with an increase in learning and memory among subjects who were APOE e4+. It is possible that this finding is due to a small sample size because only five subjects were APOE e4+ and heavy alcohol consumers during late life. There was not a significant three-way interaction between age, APOE and midlife alcohol consumption status on the trajectory of learning and memory.
Table 3.
Interaction between age, APOE and late life alcohol consumption status on the trajectory of learning and memory
| Alcohol consumption | Point estimate | Standard error | P-value |
|---|---|---|---|
| APOE e4+ | |||
| Abstainer (ref), n = 70 | – | – | – |
| Light, n = 25 | −0.037 | 0.022 | 0.091 |
| Moderate, n = 26 | −0.038 | 0.021 | 0.077 |
| Heavy, n = 5 | 0.055 | 0.036 | 0.13 |
| APOE e4− | |||
| Abstainer (ref), n = 269 | – | – | – |
| Light, n = 90 | 0.012 | 0.011 | 0.29 |
| Moderate, n = 102 | 0.027 | 0.011 | 0.012 |
| Heavy, n = 23 | 0.016 | 0.02 | 0.43 |
Bold values indicate statistical significance P < 0.05. All models adjusted for age, gender, smoking status and education. The interaction between age, APOE and midlife alcohol consumption status on the trajectory of learning and memory was not statistically significant.
DISCUSSION
In the present study we did not find evidence that the trajectory of learning and memory during late life is modified according to midlife alcohol consumption status. However, our findings provide evidence that the relationship between late life alcohol consumption and the decline in learning and memory is modified according to APOE e4 status. Moderate alcohol consumption during late life was associated with an increase in learning and memory among subjects who were APOE e4−, whereas moderate alcohol consumption during late life was associated with greater decline among subjects who were APOE e4+. These findings are consistent with previous research on the effect of APOE e4 and alcohol consumption on cognitive decline and the risk of Alzheimer's disease. In one such study, Dufouil et al. (2000) observed that the risk for decline on the MMSE decreased with greater alcohol consumption during late life for older adults with no APOE e4 alleles, but the opposite trend was observed for older adults with one or more APOE e4 alleles. In a second study, Anttila et al. (2004) reported the risk of dementia increased with greater alcohol consumption for APOE e4 carriers, but not for non-APOE e4 carriers. Similarly, Harwood et al. (2010) observed an additive effect of APOE e4 and heavy alcohol consumption on the age of onset for AD.
The findings of the current study should be interpreted with some caution because of the relatively small size of the final sample, in particular for participants who were APOE e4+ and heavy alcohol consumers during late life. However, the finding that the relationship between moderate alcohol consumption during late life and the trajectory of learning and memory is modified according to APOE e4 is consistent with previous research, and there are biological mechanisms that support these results. First, moderate alcohol consumption has been shown to be beneficial for cardiovascular health (Mukamal and Rimm, 2008), but moderate alcohol consumption is associated with higher LDL cholesterol among APOE e4 carriers when compared with non-carriers (Corella et al., 2001). Elevated serum cholesterol during midlife is a risk factor for AD and vascular dementia (Solomon et al., 2009) and may contribute to the observed decline in learning and memory among moderate alcohol consumers who are APOE e4+ in the current study. Second, the apoE protein is involved in neuronal repair and carriers of the APOE e4 allele have an impaired neuronal repair mechanism (Mahley and Huang, 2012). Even moderate alcohol consumption can lead to increased brain atrophy (Anstey et al., 2006) and APOE e4 carriers may be more susceptible to the neurotoxic effects of alcohol consumption due to an impaired neuronal repair mechanism (Kim et al., 2012).
The strengths of the present study include the ability to analyze both midlife and late life alcohol consumption, the use of multiple questions to assess alcohol consumption and the use of multiple cognitive measures to create a summary score of learning and memory. Despite these strengths there are limitations that need to be addressed. First, the final sample may not represent the general population due to a survivor effect among participants who remained in the FHS and were healthy enough to receive a neuropsychological battery. Both heavy alcohol consumption (Roerecke et al., 2011) and the APOE e4 allele (Christensen et al., 2006) are associated with an increased incidence of mortality. This may have contributed to the very low number of subjects included in the final sample who were APOE e4+ and heavy alcohol consumers during late life. Furthermore, the small size of the final sample increases the likelihood for spurious results. There is strong biological evidence to support the findings of the current study and the findings are consistent with previous research. This decreases the likelihood that our finding that moderate alcohol consumption limits the decline in learning and memory among APOE e4− subjects but not APOE e4+ subjects is due to chance. Additional research using larger sample populations is necessary to replicate these findings. A second limitation is the use of self-reported measures of alcohol consumption. Alcohol consumption data collected during the eighth clinical examination were compared with a FFQ that has been shown to be valid in other sample populations but biases may have been introduced into the analysis. Finally, the FHS only examined cognitive functioning during two time periods. It is necessary to have more than two repeated measures of cognition in order to determine the long-term effects that late life alcohol consumption, APOE e4 and interactions between these factors have on cognitive decline.
The findings from the current study provide evidence that moderate alcohol consumption during late life is associated with less decline in learning and memory for older adults with no APOE e4 alleles, but not for those who carry one or more APOE e4 alleles. Additional research using larger sample populations that includes several repeated measures of cognitive functioning is necessary to replicate these findings and to determine whether an interaction between alcohol consumption and APOE e4 extends to other cognitive domains and the long-term trajectories of cognitive decline according to alcohol consumption status and APOE e4 allele status.
Funding
This work was supported by theNational Institutes of Health, D.W.F. [5P30AG028383-07 and 8P20GM103436-12] and F.Z. [1K01DA031764]
Conflict of interest statement
None declared.
Acknowledgments
The Framingham Heart Study is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with Boston University. The opinions and conclusions are solely of the authors and do not reflect the FHS or NHLBI.
REFERENCES
- Anstey KJ, Jorm AF, Reglade-Meslin C, et al. Weekly alcohol consumption, brain atrophy, and white matter hyperintensities in a community-based sample aged 60 to 64 years. Psychosom Med. 2006;68:778–85. doi: 10.1097/01.psy.0000237779.56500.af. doi:10.1097/01.psy.0000237779.56500.af. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Mack HA, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry. 2009;17:542–55. doi: 10.1097/JGP.0b013e3181a2fd07. doi:10.1097/JGP.0b013e3181a2fd07. [DOI] [PubMed] [Google Scholar]
- Anttila T, Helkala EL, Viitanen M, et al. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. BMJ. 2004;329:539–42. doi: 10.1136/bmj.38181.418958.BE. doi:10.1136/bmj.38181.418958.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au R, Seshadri S, Wolf PA, et al. New norms for a new generation: cognitive performance in the framingham offspring cohort. Exp Aging Res. 2004;30:333–58. doi: 10.1080/03610730490484380. doi:10.1080/03610730490484380. [DOI] [PubMed] [Google Scholar]
- Bennet AM, Di Angelantonio E, Ye Z, et al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007;298:1300–11. doi: 10.1001/jama.298.11.1300. doi:10.1001/jama.298.11.1300. [DOI] [PubMed] [Google Scholar]
- Beulens JW, Algra A, Soedamah-Muthu SS, et al. Alcohol consumption and risk of recurrent cardiovascular events and mortality in patients with clinically manifest vascular disease and diabetes mellitus: the Second Manifestations of ARTerial (SMART) disease study. Atherosclerosis. 2010;212:281–86. doi: 10.1016/j.atherosclerosis.2010.04.034. doi:10.1016/j.atherosclerosis.2010.04.034. [DOI] [PubMed] [Google Scholar]
- Britton A, Singh-Manoux A, Marmot A. Alcohol consumption and cognitive function in the Whitehall II Study. Am J Epidemiol. 2004;160:240–47. doi: 10.1093/aje/kwh206. doi:10.1093/aje/kwh206. [DOI] [PubMed] [Google Scholar]
- Christensen K, Johnson TE, Vaupel JW. The quest for genetic determinants of human longevity: challenges and insights. Nat Rev Genet. 2006;7:436–48. doi: 10.1038/nrg1871. doi:10.1038/nrg1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16:2349–80. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. doi:10.1002/(SICI)1097-0258(19971030)16:20<2349::AID-SIM667>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–23. doi: 10.1126/science.8346443. doi:10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Corella D, Tucker K, Lahoz C, et al. Alcohol drinking determines the effect of the APOE locus on LDL-cholesterol concentrations in men: the Framingham Offspring Study. Am J Clin Nutr. 2001;73:736–45. doi: 10.1093/ajcn/73.4.736. [DOI] [PubMed] [Google Scholar]
- Dawber TR, Meadors GF, Moore FE. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–81. doi: 10.2105/ajph.41.3.279. doi:10.2105/AJPH.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Zhou DH, Li J, et al. A 2-year follow-up study of alcohol consumption and risk of dementia. Clin Neurol Neurosurg. 2006;108:378–83. doi: 10.1016/j.clineuro.2005.06.005. doi:10.1016/j.clineuro.2005.06.005. [DOI] [PubMed] [Google Scholar]
- di Giuseppe R, de Lorgeril M, Salen P, et al. Alcohol consumption and n-3 polyunsaturated fatty acids in healthy men and women from 3 European populations. Am J Clin Nutr. 2009;89:354–62. doi: 10.3945/ajcn.2008.26661. doi:10.3945/ajcn.2008.26661. [DOI] [PubMed] [Google Scholar]
- Dufouil C, Tzourio C, Brayne C, et al. Influence of apolipoprotein E genotype on the risk of cognitive deterioration in moderate drinkers and smokers. Epidemiology. 2000;11:280–84. doi: 10.1097/00001648-200005000-00009. doi:10.1097/00001648-200005000-00009. [DOI] [PubMed] [Google Scholar]
- Dufour M. What is moderate drinking? Defining ‘drinks’ and drinking levels. Alcohol Res Health. 1999;23:5–14. [PMC free article] [PubMed] [Google Scholar]
- Feinleib M, Kannel WB, Garrison RJ, et al. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–25. doi: 10.1016/0091-7435(75)90037-7. doi:10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- Harwood DG, Kalechstein A, Barker WW, et al. The effect of alcohol and tobacco consumption, and apolipoprotein E genotype, on the age of onset in Alzheimer's disease. Int J Geriatr Psychiatry. 2010;25:511–18. doi: 10.1002/gps.2372. doi:10.1002/gps.2372. [DOI] [PubMed] [Google Scholar]
- Hauser PS, Narayanaswami V, Ryan RO. Apolipoprotein E: from lipid transport to neurobiology. Prog Lipid Res. 2011;50:62–74. doi: 10.1016/j.plipres.2010.09.001. doi:10.1016/j.plipres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvidtfeldt UA, Tolstrup JS, Jakobsen MU. Alcohol intake and risk of coronary heart disease in younger, middle-aged, and older adults. Circulation. 2010;121:1589–97. doi: 10.1161/CIRCULATIONAHA.109.887513. doi:10.1161/CIRCULATIONAHA.109.887513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesse-Guyot E, Andreeva VA, Jeandel C, et al. Alcohol consumption in midlife and cognitive performance assessed 13 years later in the SU.VI.MAX 2 cohort. PLoS One. 2012;7:e52311. doi: 10.1371/journal.pone.0052311. doi:10.1371/journal.pone.0052311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Lee DY, Lee BC, et al. Alcohol and cognition in the elderly: a review. Psychiatry Investig. 2012;9:8–16. doi: 10.4306/pi.2012.9.1.8. doi:10.4306/pi.2012.9.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Helkal EL, Hanninen T, et al. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology. 2001;56:1683–89. doi: 10.1212/wnl.56.12.1683. doi:10.1212/WNL.56.12.1683. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. doi:10.2307/2529876. [PubMed] [Google Scholar]
- Lang I, Wallace RB, Huppert FA, et al. Moderate alcohol consumption in older adults is associated with better cognition and well-being than abstinence. Age Aging. 2007;36:256–61. doi: 10.1093/ageing/afm001. doi:10.1093/ageing/afm001. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y. Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron. 2012;76:871–85. doi: 10.1016/j.neuron.2012.11.020. doi:10.1016/j.neuron.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Vidal P, Bochud M, Paccaud F, et al. No interaction between alcohol consumption and HDL-related genes on HDL cholesterol levels. Atherosclerosis. 2010;211:551–57. doi: 10.1016/j.atherosclerosis.2010.04.001. doi:10.1016/j.atherosclerosis.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Massaro JM, D'Agostino RB, Sullivan LM. Managing and analysing data from a large-scale study on Framingham Offspring relating brain structure to cognitive function. Stat Med. 2004;23:351–67. doi: 10.1002/sim.1743. doi:10.1002/sim.1743. [DOI] [PubMed] [Google Scholar]
- Mendez D, Warner KE, Courant PN. Has smoking cessation ceased? Expected trends in the prevalence of smoking in the United States. Am J Epidemiol. 1998;148:249–58. doi: 10.1093/oxfordjournals.aje.a009632. doi:10.1093/oxfordjournals.aje.a009632. [DOI] [PubMed] [Google Scholar]
- Moore AA, Gould R, Reuben DB, et al. Longitudinal patterns and predictors of alcohol consumption in the United States. Am J Public Health. 2005;95:458–65. doi: 10.2105/AJPH.2003.019471. doi:10.2105/AJPH.2003.019471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K. The three new pathways leading to Alzheimer's disease. Neuropathol Appl Neurobiol. 2011;37:353–57. doi: 10.1111/j.1365-2990.2011.01181.x. doi:10.1111/j.1365-2990.2011.01181.x. [DOI] [PubMed] [Google Scholar]
- Mukamal KJ, Rimm EB. Alcohol consumption: risks and benefits. Curr Atheroscler Rep. 2008;10:536–43. doi: 10.1007/s11883-008-0083-2. doi:10.1007/s11883-008-0083-2. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. ISBN 3-900051-07-0 URL http://www.R-project.org/ [Google Scholar]
- Reinvang I, Winjevoll IL, Rootwelt H, et al. Working memory deficits in healthy APOE epsilon 4 carriers. Neuropsychologia. 2010;48:566–73. doi: 10.1016/j.neuropsychologia.2009.10.018. doi:10.1016/j.neuropsychologia.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–36. doi: 10.1093/oxfordjournals.aje.a116211. doi: [DOI] [PubMed] [Google Scholar]
- Roerecke M, Greenfield TK, Kerr WC, et al. Heavy drinking occasions in relation to ischaemic heart disease mortality—an 11–22 year follow-up of the 1984 and 1995 US National Alcohol Surveys. Int J Epidemiol. 2011;40:1401–10. doi: 10.1093/ije/dyr129. doi:10.1093/ije/dyr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A, Kivipelto M, Wolozin B, et al. Midlife serum cholesterol and increased risk of Alzheimer's and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28:75–80. doi: 10.1159/000231980. doi:10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer MJ, Kang JH, Chen J, et al. Effects of moderate alcohol consumption on cognitive function in women. N Engl J Med. 2005;352:245–53. doi: 10.1056/NEJMoa041152. doi:10.1056/NEJMoa041152. [DOI] [PubMed] [Google Scholar]
- Virtaa JJ, Jarvenpaa T, Heikkila K, et al. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22:939–48. doi: 10.3233/JAD-2010-100870. [DOI] [PubMed] [Google Scholar]
- Wannamethe SC, Shaper AG, Perry IJ, et al. Alcohol consumption and the incidence of type II diabetes. J Epidemiol Community Health. 2002;56:542–48. doi: 10.1136/jech.56.7.542. doi:10.1136/jech.56.7.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. A standardized memory scale for clinical use. JPIA. 1945;19:87–95. [Google Scholar]
- Weyerer S, Schaufele M, Wiese B, et al. Current alcohol consumption and its relationship to incident dementia: results from a 3-year follow-up study among primary care attenders aged 75 years and older. Age Aging. 2011;40:456–63. doi: 10.1093/ageing/afr007. doi:10.1093/ageing/afr007. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Sidney S, Selby J, et al. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–81. doi: 10.1212/01.WNL.0000149519.47454.F2. doi:10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Leurgans SE, Boyle PA, et al. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch Neurol . 2011;68:351–56. doi: 10.1001/archneurol.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CB, Sacco RL, Rundek T, et al. Interleukin-6 is associated with cognitive function: the Northern Manhattan Study. J Stroke Cerebrovasc Dis. 2006;15:34–8. doi: 10.1016/j.jstrokecerebrovasdis.2005.08.009. doi:10.1016/j.jstrokecerebrovasdis.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Gao Z, Zhou J, et al. Plasma homocysteine thiolactone adducts associated with risk of coronary heart disease. Clin Chim Acta. 2006;364:230–34. doi: 10.1016/j.cccn.2005.07.007. doi:10.1016/j.cccn.2005.07.007. [DOI] [PubMed] [Google Scholar]


