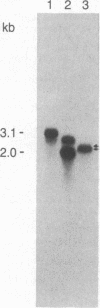
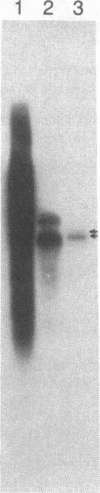
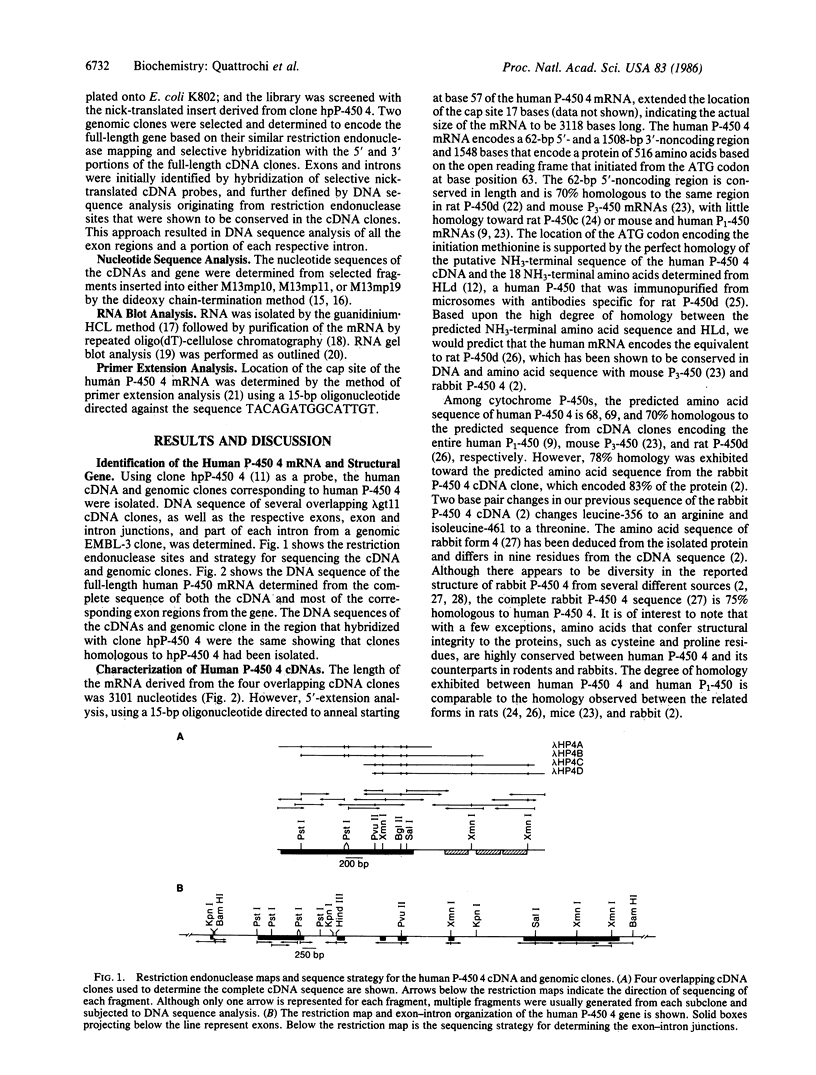
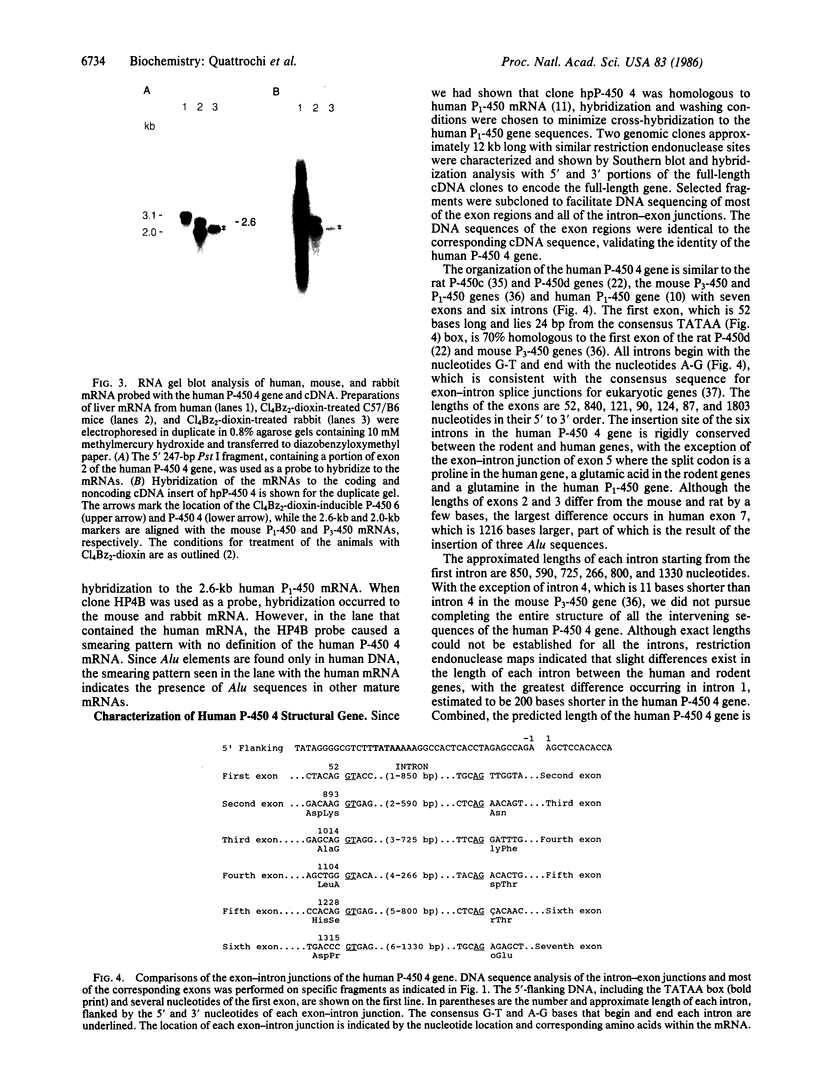
Abstract
Several overlapping lambda gt11 cDNA clones have been sequenced and shown to encode for the full-length human cytochrome P-450 4. The structure and location of the exons and flanking intron regions were also identified from a lambda EMBL-3 human genomic clone that encodes the full-length human P-450 4 gene. The human P-450 4 mRNA is flanked by 62 base pairs of 5'- and 1508 base pairs of 3'-noncoding sequence, with 1548 bases that encode a protein of 516 amino acids (Mr, 58,376). The predicted amino acid sequence of human P-450 4 is 69% and 70% homologous to its equivalent in mouse and rat, respectively, 75% homologous to rabbit P-450 4, and 68% homologous to human P1-450. The 7.6-kilobase gene encodes 3118 nucleotides of exon sequence that is separated by six introns into seven exons. Exon 7, which is 1802 nucleotides, contains three inverse/complement Alu sequences that are organized in tandem. Comparison of the genomic DNA sequence of the human P-450 4 gene with the human P1-450 and related genes in rat and mouse and the identification of the amino acid residues and triplet codon at each exon-intron junction show that the location of each intron in the human P-450 4 gene is conserved within this gene family. Although the length and homology of the introns within a related gene family may not be conserved, the location of intronic sequences may be an important determinant in the identification of related P-450 genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conney A. H. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982 Dec;42(12):4875–4917. [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Deininger P. L., Jolly D. J., Rubin C. M., Friedmann T., Schmid C. W. Base sequence studies of 300 nucleotide renatured repeated human DNA clones. J Mol Biol. 1981 Sep 5;151(1):17–33. doi: 10.1016/0022-2836(81)90219-9. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Sogawa K., Nishi C., Fujii-Kuriyama Y. Regulatory DNA elements localized remotely upstream from the drug-metabolizing cytochrome P-450c gene. Nucleic Acids Res. 1986 Feb 11;14(3):1465–1477. doi: 10.1093/nar/14.3.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita V. S., Black S. D., Tarr G. E., Koop D. R., Coon M. J. On the amino acid sequence of cytochrome P-450 isozyme 4 from rabbit liver microsomes. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4260–4264. doi: 10.1073/pnas.81.14.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. J., Kimura S., Nebert D. W. Comparison of the flanking regions and introns of the mouse 2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible cytochrome P1-450 and P3-450 genes. J Biol Chem. 1985 Apr 25;260(8):5040–5049. [PubMed] [Google Scholar]
- Gonzalez F. J., Nebert D. W. Autoregulation plus upstream positive and negative control regions associated with transcriptional activation of the mouse P1(450) gene. Nucleic Acids Res. 1985 Oct 25;13(20):7269–7288. doi: 10.1093/nar/13.20.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbüchle O., Bovey R., Young R. A. Tissue-specific expression of mouse-alpha-amylase genes: nucleotide sequence of isoenzyme mRNAs from pancreas and salivary gland. Cell. 1980 Aug;21(1):179–187. doi: 10.1016/0092-8674(80)90125-7. [DOI] [PubMed] [Google Scholar]
- Hood L., Steinmetz M., Malissen B. Genes of the major histocompatibility complex of the mouse. Annu Rev Immunol. 1983;1:529–568. doi: 10.1146/annurev.iy.01.040183.002525. [DOI] [PubMed] [Google Scholar]
- Jaiswal A. K., Gonzalez F. J., Nebert D. W. Human P1-450 gene sequence and correlation of mRNA with genetic differences in benzo[a]pyrene metabolism. Nucleic Acids Res. 1985 Jun 25;13(12):4503–4520. doi: 10.1093/nar/13.12.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal A. K., Gonzalez F. J., Nebert D. W. Human dioxin-inducible cytochrome P1-450: complementary DNA and amino acid sequence. Science. 1985 Apr 5;228(4695):80–83. doi: 10.1126/science.3838385. [DOI] [PubMed] [Google Scholar]
- Jones P. B., Galeazzi D. R., Fisher J. M., Whitlock J. P., Jr Control of cytochrome P1-450 gene expression by dioxin. Science. 1985 Mar 22;227(4693):1499–1502. doi: 10.1126/science.3856321. [DOI] [PubMed] [Google Scholar]
- Kawajiri K., Gotoh O., Sogawa K., Tagashira Y., Muramatsu M., Fujii-Kuriyama Y. Coding nucleotide sequence of 3-methylcholanthrene-inducible cytochrome P-450d cDNA from rat liver. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1649–1653. doi: 10.1073/pnas.81.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Gonzalez F. J., Nebert D. W. The murine Ah locus. Comparison of the complete cytochrome P1-450 and P3-450 cDNA nucleotide and amino acid sequences. J Biol Chem. 1984 Sep 10;259(17):10705–10713. [PubMed] [Google Scholar]
- Liem H. H., Muller-Eberhard U., Johnson E. F. Differential induction by 2,3,7,8-tetrachlorodibenzo-p-dioxin of multiple forms of rabbit microsomal cytochrome P-450: evidence for tissue specificity. Mol Pharmacol. 1980 Nov;18(3):565–570. [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Norman R. L., Johnson E. F., Muller-Eberhard U. Identification of the major cytochrome P-450 form transplacentally induced in neonatal rabbits by 3,3,7,8-tetrachlorodibenzo-p-dioxin. J Biol Chem. 1978 Dec 10;253(23):8640–8647. [PubMed] [Google Scholar]
- Okino S. T., Quattrochi L. C., Barnes H. J., Osanto S., Griffin K. J., Johnson E. F., Tukey R. H. Cloning and characterization of cDNAs encoding 2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible rabbit mRNAs for cytochrome P-450 isozymes 4 and 6. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5310–5314. doi: 10.1073/pnas.82.16.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols J. Complete amino acid sequence of a cytochrome P-450 isolated from beta-naphthoflavone-induced rabbit liver microsomes. Comparison with phenobarbital-induced and constitutive isozymes and identification of invariant residues. J Biol Chem. 1986 Mar 25;261(9):3965–3979. [PubMed] [Google Scholar]
- Quattrochi L. C., Okino S. T., Pendurthi U. R., Tukey R. H. Cloning and isolation of human cytochrome P-450 cDNAs homologous to dioxin-inducible rabbit mRNAs encoding P-450 4 and P-450 6. DNA. 1985 Oct;4(5):395–400. doi: 10.1089/dna.1985.4.395. [DOI] [PubMed] [Google Scholar]
- Reik L. M., Levin W., Ryan D. E., Thomas P. E. Immunochemical relatedness of rat hepatic microsomal cytochromes P-450c and P-450d. J Biol Chem. 1982 Apr 10;257(7):3950–3957. [PubMed] [Google Scholar]
- Rothkopf G. S., Telakowski-Hopkins C. A., Stotish R. L., Pickett C. B. Multiplicity of glutathione S-transferase genes in the rat and association with a type 2 Alu repetitive element. Biochemistry. 1986 Mar 11;25(5):993–1002. doi: 10.1021/bi00353a007. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schmid C. W., Jelinek W. R. The Alu family of dispersed repetitive sequences. Science. 1982 Jun 4;216(4550):1065–1070. doi: 10.1126/science.6281889. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Conversion of RNA to DNA in mammals: Alu-like elements and pseudogenes. Nature. 1983 Feb 10;301(5900):471–472. doi: 10.1038/301471a0. [DOI] [PubMed] [Google Scholar]
- Sogawa K., Gotoh O., Kawajiri K., Fujii-Kuriyama Y. Distinct organization of methylcholanthrene- and phenobarbital-inducible cytochrome P-450 genes in the rat. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5066–5070. doi: 10.1073/pnas.81.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogawa K., Gotoh O., Kawajiri K., Harada T., Fujii-Kuriyama Y. Complete nucleotide sequence of a methylcholanthrene-inducible cytochrome P-450 (P-450d) gene in the rat. J Biol Chem. 1985 Apr 25;260(8):5026–5032. [PubMed] [Google Scholar]
- Suwa Y., Mizukami Y., Sogawa K., Fujii-Kuriyama Y. Gene structure of a major form of phenobarbital-inducible cytochrome P-450 in rat liver. J Biol Chem. 1985 Jul 5;260(13):7980–7984. [PubMed] [Google Scholar]
- Tukey R. H., Nebert D. W., Negishi M. Structural gene product of the [Ah] complex. Evidence for transcriptional control of cytochrome P1-450 induction by use of a cloned DNA sequence. J Biol Chem. 1981 Jul 10;256(13):6969–6974. [PubMed] [Google Scholar]
- Tuteja N., Gonzalez F. J., Nebert D. W. Developmental and tissue-specific differential regulation of the mouse dioxin-inducible P1-450 and P3-450 genes. Dev Biol. 1985 Nov;112(1):177–184. doi: 10.1016/0012-1606(85)90131-9. [DOI] [PubMed] [Google Scholar]
- Wrighton S. A., Campanile C., Thomas P. E., Maines S. L., Watkins P. B., Parker G., Mendez-Picon G., Haniu M., Shively J. E., Levin W. Identification of a human liver cytochrome P-450 homologous to the major isosafrole-inducible cytochrome P-450 in the rat. Mol Pharmacol. 1986 Apr;29(4):405–410. [PubMed] [Google Scholar]
- Yabusaki Y., Shimizu M., Murakami H., Nakamura K., Oeda K., Ohkawa H. Nucleotide sequence of a full-length cDNA coding for 3-methylcholanthrene-induced rat liver cytochrome P-450MC. Nucleic Acids Res. 1984 Mar 26;12(6):2929–2938. doi: 10.1093/nar/12.6.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]